ABSTRACT
rRNAs of dormant spores of Bacillus subtilis were >95% degraded during extended incubation at 50°C, as reported previously (E. Segev, Y. Smith, and S. Ben-Yehuda, Cell 148:139–114, 2012, doi:http://dx.doi.org/10.1016/j.cell.2011.11.059), and this was also true of spores of Bacillus megaterium. Incubation of spores of these two species for ∼20 h at 75 to 80°C also resulted in the degradation of all or the great majority of the 23S and 16S rRNAs, although this rRNA degradation was slower than nonenzymatic hydrolysis of purified rRNAs at these temperatures. This rRNA degradation at high temperature generated almost exclusively oligonucleotides with minimal levels of mononucleotides. RNase Y, suggested to be involved in rRNA hydrolysis during B. subtilis spore incubation at 50°C, did not play a role in B. subtilis spore rRNA breakdown at 80°C. Twenty hours of incubation of Bacillus spores at 70°C also decreased the already minimal levels of ATP in dormant spores 10- to 30-fold, to ≤0.01% of the total free adenine nucleotide levels. Spores depleted of rRNA were viable and germinated relatively normally, often even faster than starting spores. Their return to vegetative growth was also similar to that of untreated spores for B. megaterium spores and slower for heat-treated B. subtilis spores; accumulation of rRNA took place only after completion of spore germination. These findings thus strongly suggest that protein synthesis is not essential for Bacillus spore germination.
IMPORTANCE A recent report (L. Sinai, A. Rosenberg, Y. Smith, E. Segev, and S. Ben-Yehuda, Mol Cell 57:3486–3495, 2015, doi:http://dx.doi.org/10.1016/j.molcel.2014.12.019) suggested that protein synthesis is essential for early steps in the germination of dormant spores of Bacillus subtilis. If true, this would be a paradigm shift in our understanding of spore germination. We now show that essentially all of the rRNA can be eliminated from spores of Bacillus megaterium or B. subtilis, and these rRNA-depleted spores are viable and germinate as well as or better than spores with normal rRNA levels. Thus, protein synthesis is not required in the process of spore germination.
INTRODUCTION
Spores of Bacillus species formed in sporulation are metabolically dormant and extremely resistant to many severe treatments, including heat, radiation, and toxic chemicals (1–3). Although these spores can survive in this dormant resistant state for many years, if given the appropriate stimulus, generally small molecules that are found in environments that are appropriate for the growth of these bacteria, the spores can rapidly break dormancy in the process of spore germination and then outgrowth (1, 4, 5). Notably, spores' extreme resistance properties are lost in the process of germination and early outgrowth, and thus, germinated spores are much easier to kill than dormant spores. This property has significant applied implications, as dormant spores are the vectors for much food spoilage, as well as some foodborne and other human diseases. Consequently, there is significant interest in mechanisms that determine rates of spore germination and how sporulation conditions, as well as storage conditions, modify spores' resistance properties and their rates of germination.
In addition to the applied interest in spore resistance and germination, there has also long been much basic interest in the mechanism of spore germination, in particular, the signal transduction pathways operating during the process (4, 5). The major proteins involved in Bacillus spore germination are as follows. (i) Germinant receptors (GRs) in spores' inner membrane (IM) recognize and respond to the physiological germinants specific for spores of each species/strain. (ii) The IM SpoVA proteins form a channel for the release of the major spore core small molecule pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]), present in spores as a 1:1 chelate with divalent cations, normally Ca2+ (Ca-DPA); GRs trigger opening of the SpoVA protein channel and Ca-DPA release early in germination and its replacement with water. (iii) Cortex-lytic enzymes (CLEs) degrade spores' large peptidoglycan cortex, allowing the spore core to swell and take up much water. As a result of cortex hydrolysis and core swelling, the germinated spore core wet weight as water reaches a value of ∼80%, similar to that of growing cells. This change allows resumption of enzyme activity in the spore core, including metabolism and macromolecular synthesis. CLE action in Bacillus spore germination is triggered by Ca-DPA release, both directly and indirectly (4, 5).
A variety of evidence has accumulated over many years indicating that spore germination requires minimal, if any, high energy in the form of ATP. In particular, spores have ∼0.1% of the ATP levels of growing cells, although the levels of free adenine nucleotides are similar to those in growing cells (6). Other studies have shown that (i) detectable ATP generation takes place only after completion of spore germination, (ii) spore germination can take place relatively normally even if ATP production from spores' endogenous energy reserves is blocked, (iii) there is no evidence of significant metabolism of spores' endogenous energy reserves until after completion of spore germination, and (iv) protein synthesis is also minimal, if it takes place at all, until after completion of spore germination (6–11).
In spite of the evidence noted above, most of which is from work published 30 to 40 years ago, there are recent reports suggesting that metabolic activity does take place in dormant spores, including RNA synthesis and degradation, and that some protein synthesis takes place in and is required for the process of spore germination (12, 13). Equally importantly, one of these reports suggested that these events in dormant spores could modify their germination properties significantly, in particular when spores were incubated for extended times at 37 to 50°C, leading to degradation of much of spores' rRNA (12).
In the present work, we examined rRNA levels in dormant spores of Bacillus subtilis and Bacillus megaterium by first incubating them for long periods at 37 to 80°C. The incubated spores were then purified to eliminate any spores that had lost Ca-DPA either because of spore germination or by high-temperature-induced loss. These purified heat-treated spores were largely viable, and their levels of phosphorus-containing small molecules, including ATP and rRNA, were determined; this was also done with untreated spores. The germination and outgrowth of untreated and high-temperature-treated spores were also measured, as well as the rRNA levels during the outgrowth of high-temperature-treated spores. The results obtained in this work strongly indicate that neither ATP nor protein synthesis is required to complete spore germination and are consistent with older work on this topic.
MATERIALS AND METHODS
Bacillus strains and spore preparation and purification.
The isogenic B. subtilis 168 strains used in this study were PS533 (wild type) (14), a derivative of laboratory strain PS832 carrying plasmid pUB110 encoding resistance to kanamycin (10 μg/ml); FB73 (15), a PS832 derivative lacking the genes for the three functional GRs in B. subtilis (this strain is identical to strain FB72); and PS4432, a PS832 derivative with the gene encoding RNase Y under the control of a xylose-inducible promoter and with chloramphenicol resistance (Cmr) (5 μg/ml). Strain PS4432 was constructed by transforming strain PS832 to Cmr with genomic DNA from strain ES83 (a gift from S. Ben-Yehuda) and selecting for transformants on plates containing 0.5% xylose, as well as chloramphenicol (12, 16). Spores of B. subtilis strains were routinely prepared at 37°C on 2×SG medium agar plates without antibiotics, isolated and purified as described previously, and stored at 4°C in water and protected from light (17). The inocula used to prepare spores of PS4432 contained 0.5% xylose, but no xylose was present in or on sporulation medium (12, 15). In some experiments, B. subtilis spores were prepared in 2×SG liquid medium, harvested immediately after most sporangia had lysed, and purified as described previously (11). The spent medium from liquid sporulation was filter sterilized and stored at 4°C.
The B. megaterium strains used were QMB1551 (wild type; originally obtained from H. S. Levinson) and its isogenic derivative GC614 (18) that lacks genes for all functional GRs. Spores of these strains were prepared in liquid supplemented nutrient broth at 30°C, and spores were purified as described previously (11), as was spent sporulation medium, and spores and spent sporulation medium were stored as described above. All of the spores used in this work were free (>98%) from growing or sporulating cells, germinated spores, or cell debris, as shown by phase-contrast microscopy and by the uniformity of all spore pellets at the final purification steps.
Spore incubation and analysis.
Purified spores were routinely incubated at 37 to 80°C in water, 100 mM Na-HEPES buffer (pH 7.2), or spent sporulation medium isolated as described above. Spores in these incubations were at an optical density at 600 nm (OD600) of ∼10 (∼109 spores/ml). Following all incubations, spore preparations were centrifuged and washed with water and final pellets were suspended in 0.2 ml of 20% HistoDenz (Sigma Chemical Co., St. Louis, MO), applied to the top of 1.9 ml of 50 or 55% HistoDenz in water for B. subtilis and B. megaterium spores, respectively, and centrifuged at 4°C at ∼8,000 × g for 10 min. Under these centrifugation conditions, dormant spores that retain their DPA pellet while spores that have lost DPA and are germinated, dead, or both float (11, 19, 20). Spore pellets from these centrifugations were isolated, washed several times with 1.5 ml of water to remove HistoDenz, suspended in water at an OD600 of ∼5, and stored at 4°C prior to analyses. The viability of the spores after incubation at elevated temperature and subsequent purification was assessed by spotting 10-μl aliquots of appropriate dilutions of treated and untreated PS533, PS4432, and B. megaterium QMB1551 spores on L broth (LB) medium agar plates (21) with the appropriate antibiotics and incubating them for ∼16 h at 37°C (B. subtilis) or 30°C (B. megaterium). B. megaterium spore viability was determined at 30°C to minimize mucoid colony formation, which interferes with the formation of isolated colonies. The viability of treated and purified FB73 and GC614 spores was not measured, as these spores germinate extremely poorly with nutrient germinants (15, 18).
Spore germination and outgrowth.
Germination of spores of B. megaterium and B. subtilis was monitored by measuring DPA release in a multiwell fluorescence plate reader (Molecular Devices, Sunnyvale, CA) at 37°C as described previously (22). Briefly, spores were germinated at an OD600 of 0.5 in 200 μl of 25 mM K-HEPES buffer (pH 7.4) plus 50 μM TbCl3. The GR-dependent germinants used were as follows: B. subtilis spores, 10 mM l-valine or a mixture of 10 mM l-asparagine, 10 mM d-glucose, 10 mM d-fructose, and 10 mM KCl (AGFK); B. megaterium spores, 10 mM d-glucose or 50 mM KBr. Tb-DPA fluorescence was read every 5 min for 30 min to 2 h. Untreated spores were heat activated prior to germination as follows: 30 min or 2 h at 75°C for B. subtilis spores germinating with l-valine or AGFK, respectively, and 30 min at 60°C for B. megaterium spores (18, 23). After heat activation, spores were cooled on ice for at least 15 min prior to germination experiments.
For spore germination and outgrowth, spores not incubated at 75 to 80°C overnight were heat activated prior to germination as described above. Spores at an OD600 of ∼0.8 were incubated at 37°C in liquid LB medium (21) plus 10 mM l-valine (B. subtilis) or 10 mM d-glucose (B. megaterium), the OD600 of the culture was measured periodically, and aliquots were observed by phase-contrast microscopy. In one experiment with heat-treated spores of each species, samples (∼25 ml) were taken at various times and chilled by ice addition, spores were harvested, and RNA was isolated as described below.
Analytical procedures.
DPA was extracted from spores at an OD600 of 1 to 5 by boiling them in water for 30 min, the suspension was cooled and centrifuged, and DPA was assayed in the supernatant fluid by its fluorescence with Tb as described above and previously (22).
Small molecules including adenine nucleotides were extracted from spores essentially as described previously (6, 11). Spores (1 ml) at an OD600 of 10 to 20 in water were added to 4 ml of boiling 1-propanol, and the suspension was boiled for 10 min, cooled on ice, and flash evaporated to dryness. Prior to ATP assays, the dry residue from the 1-propanol extract was suspended in 1 ml of water at 4°C for ∼30 min on ice and the suspension was centrifuged for ∼5 min in a microcentrifuge. ATP was assayed in this supernatant fluid by the luciferase assay essentially as described previously (24), with an ATP bioluminescence assay kit (Sigma-Aldrich, St. Louis, MO). ATP determinations were all done with reference to a standard curve of known ATP concentrations, all of the values reported are from multiple determinations with several amounts of various extracts, and all of the determinations were repeated with two different samples of untreated and treated spores.
In experiments to determine the levels of all of the phosphorus-containing small molecules in spores, extracts of spores were prepared as described above but with the following modifications. Two 1-ml aliquots of HistoDenz-purified spores with an OD600 of 125 were added separately to tubes containing 4 ml of boiling 1-propanol and boiled for 5 min; this was followed by flash evaporation. The two dry residues were extracted with ∼1 ml of cold water, and after centrifugation, the supernatant fluid was passed through a Chelex column to remove divalent cations, in particular Mn2+. Finally, the material passing through the column was lyophilized and dissolved in D2O-containing buffer prior to 31P nuclear magnetic resonance (NMR) analysis to determine the levels of various small molecules as described previously (11).
RNA was initially isolated from dormant and germinated spores with a FastRNA Pro Blue kit (MP Biomedicals, Solon, OH) and a RiboPure-Bacteria kit (Life Technologies, Grand Island, NY) (12, 25, 26). Both kits use spore/cell disruption by shaking with glass beads, and a Mini-Beadbeater (Biospec Products, Bartlesville, OK) was used for 1 min, followed by 1 min on ice with a total of two disruption periods. Initial comparisons showed that the two kits gave similar RNA yields but the RiboPure-Bacteria kit gave better-quality RNA that gave sharper bands on agarose gel electrophoresis and was therefore used in all subsequent RNA extractions. RNA concentrations were determined with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Denaturing agarose gel electrophoresis of RNA samples was used to separate RNA as described in the RiboPure-Bacteria kit manual. Generally ∼1 ml of dormant or germinated spores at an OD600 of 25 was pelleted by centrifugation; RNA was extracted, giving ∼10 μg of total RNA, although outgrown spores gave more and spores that had undergone extensive rRNA degradation gave up to 50% less. For example, recoveries of RNA measured by NanoDrop analysis from B. megaterium spores incubated for 20 h at 75°C and B. subtilis spores incubated for 20 h at 80°C were 80 and 50%, respectively, of the values of untreated spores (see Results). Presumably, the lower RNA recoveries from heat-treated spores were because some RNA had been cleaved to fragments of ≤120 nucleotides (nt), which are not well recovered by the RNA purification method used, as noted in the information provided in the RiboPure-Bacteria kit manual. Unless otherwise noted, RNA from equal volumes of culture (1 to 2 μg) was loaded onto gels, which were stained with 60 μg/ml ethidium bromide in the formaldehyde loading dye mixture provided in the kit and photographed. RNA was also isolated from B. megaterium cells grown at 37°C in LB medium to an OD600 of 1.2 as described above. In some experiments, the band intensities of rRNAs in stained gels were determined by ImageJ analysis.
RESULTS
Changes in spore rRNA during long incubations at 37 to 50°C.
Previous work (12) found that incubation of wild-type B. subtilis spores for 3 to 7 days at 37 or 50°C results in the loss of much 16S and 23S rRNA species. We repeated this experiment with wild-type B. megaterium spores, but the great majority of spores had germinated at the end of the experiment, as reported previously (11) (data not shown). Indeed, with wild-type B. megaterium spores, there was so much spore germination during extended incubations at 37 and 50°C that we did not get sufficient dormant spores for RNA analyses (data not shown), again as found previously (11). Consequently, we turned to B. megaterium and B. subtilis spores that lacked all functional GRs for long incubations at 37 and 50°C (Fig. 1), as used in previous experiments with long spore incubations at these temperatures (11). With B. megaterium spores, incubation in either water or spent sporulation medium, in particular at 50°C, led to almost complete loss of both 16S and 23S rRNAs, with slightly faster loss in spores incubated in spent medium (Fig. 1A). For unknown reasons, the B. subtilis 23S rRNA isolated from spores consistently appeared as a doublet running on gel electrophoresis at both 23S and slightly faster even in extracts from spores held at 4°C (Fig. 1B). However, again almost all of the 23S rRNA and some of the 16S rRNA disappeared from extracts of B. subtilis spores incubated for 7 days at 37 or 50°C in water or spent medium. Notably, both B. megaterium and B. subtilis GR-less spores incubated at 37 and 50°C accumulated much rRNA smaller than 16S, as expected. It is important to emphasize that in all of these experiments, the spores analyzed following the long incubations at elevated temperatures were purified to remove the small amount of germinated spores or spores that had lost DPA. In addition, the purified heat-treated spores always had ≥80% of the viability of the untreated spores.
FIG 1.

Changes in rRNA during long incubation of B. megaterium and B. subtilis spores at moderate temperatures. Spores of B. megaterium GC614 (A) or B. subtilis FB73 (B), both lacking all nutrient GRs, were prepared in liquid medium, harvested immediately after most spores were released from the sporangium, and purified, and the spent medium was saved as described in Materials and Methods. The spores were then incubated at 4, 37, or 50°C in 20 ml of either water (W) or spent medium (SM) at an OD600 of 5; 5 to 6 ml was harvested after 0, 4, or 7 days of incubation and purified again; RNA was extracted from spores at an OD600 of 20 to 25 and purified; and 2 μg was analyzed by agarose gel electrophoresis as described in Materials and Methods. The values to the left of the panels show the migration positions of various RNA markers (lanes M) in kilobases, and the arrows to the right of the panels show the migration positions of the 23S and 16S rRNAs.
Changes in spore rRNA during long incubation at 60 to 80°C.
While the loss of spores' rRNA was clear during extended incubation at 37 to 50°C, this experiment with wild-type spores, in particular B. megaterium spores, could only be carried out with spores lacking GRs to ensure that enough spores remained dormant and viable to get sufficient spores for RNA analysis. However, use of these GR-less spores precluded measurement of the effects of these long incubations on the germination of the treated spores. Given spores' high wet heat resistance and the minimal GR-dependent germination of B. megaterium and B. subtilis spores at temperatures of >60°C (27), we decided to also examine the fate of wild-type spore rRNA during incubation for 18 to 20 h at 60 to 80°C, temperatures that should leave much of the treated spores still viable. However, to ensure that all of the spores that were analyzed for rRNA were neither germinated nor dead, spores incubated at these temperatures were layered on a high-density solution and centrifuged. The density of the solution was chosen such that spores that retain DPA pellet because of their high core wet density while spores that have lost DPA either through germination or heat-induced release float because their wet density has gone down significantly (11, 19, 20). Previous work has shown that spores treated in water at even higher temperatures that have lost all DPA are dead, while spores that have retained DPA retain significant viability (19, 20). We felt that this purification step after heat treatment would be essential for ultimate analysis of rRNA only in spores that are still dormant and not dead. Using this postincubation purification procedure, we found that with wild-type B. subtilis or B. megaterium spores, the viability of the purified treated spores was always ≥80% of that of spores held at 4°C (data not shown). Importantly, the rRNA levels in the purified heat-treated spores of both species decreased dramatically and the rRNA was almost completely gone after 20 h at 70°C (Fig. 2A). It was also notable that purified B. megaterium rRNA incubated in either water or 100 mM Na-HEPES buffer (pH 7.2) was also lost completely upon incubation for 20 h at 70 or 80°C, and the purified rRNAs were lost faster during incubation in water than during incubation in buffer (Fig. 2B).
FIG 2.
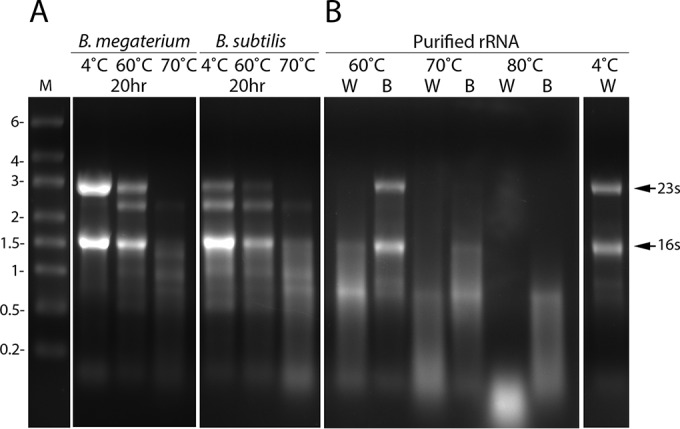
Changes in rRNAs upon incubation of spores or purified RNA at high temperatures. (A) Purified spores of B. megaterium QMB1551 (wild type) or B. subtilis PS533 (wild type) prepared in liquid or on plates, respectively, were incubated in water for 20 h at various temperatures; spores were harvested and purified to remove any germinated spores or those that had lost Ca-DPA because of the heat treatments; and RNA was extracted, purified, and subjected to agarose gel electrophoresis as described in Materials and Methods. (B) Purified B. megaterium growing cell rRNA was incubated for 20 h in water (W) or buffer (B), and analyzed by agarose gel electrophoresis as described in Materials and Methods. The values to the left show the migration positions of RNA markers (lane M) in kilobases, and the arrows to the right show the migration positions of B. megaterium growing cell 23S and 16S rRNAs.
Previous work analyzing rRNA loss during extended incubation of B. subtilis spores at 37 and 50°C indicated that this rRNA loss could be due to RNase action (12). Indeed, removal of one specific RNase, RNase Y, decreased the loss of spore rRNA during incubation at these temperatures. However, it seemed unlikely that this enzyme would work effectively at 70 to 80°C. Indeed, removal of RNase Y from otherwise wild-type B. subtilis spores by mutation had no effect on the kinetics of rRNA loss during incubation at 80°C (Fig. 3).
FIG 3.
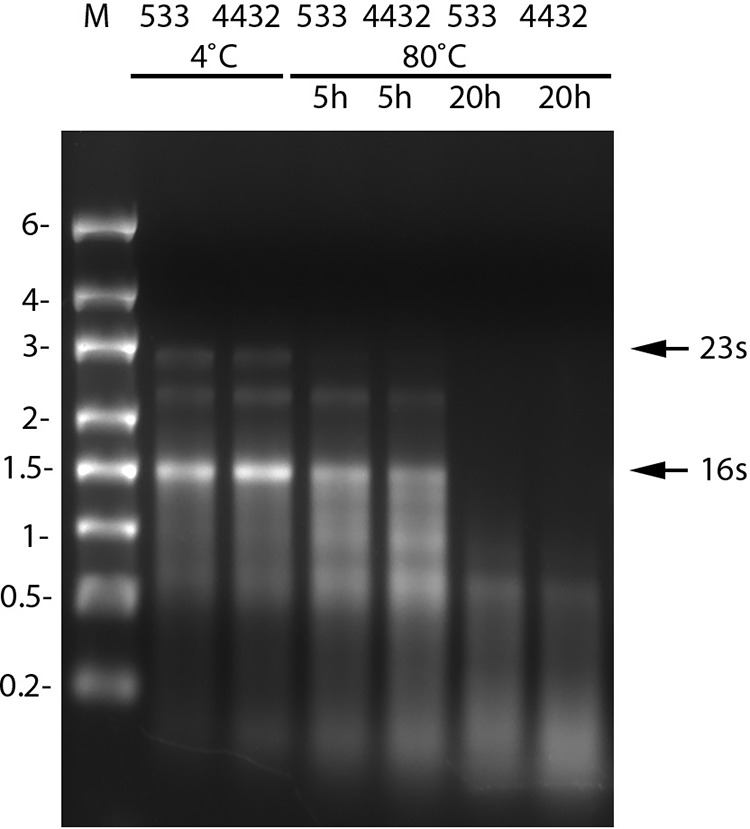
Effect of loss of RNase Y on rRNA levels during incubation of B. subtilis spores at 80°C. Spores of B. subtilis strain PS533 (wild type) or PS4432 (rny) were incubated for 0, 5, or 20 h at 80°C and purified, and RNA was extracted and analyzed by agarose gel electrophoresis as described in Materials and Methods. Values to the left denote migration positions of RNA markers (lane M) in kilobases, and the arrows to the right denote the migration positions of the 23S and 16S rRNAs from B. megaterium. The values above lanes indicate the hours of incubation of various samples at 80°C.
Changes in spore-associated ATP levels upon extended incubation at various temperatures.
Work done a number of years ago showed that spores of several Bacillus species have levels of total free adenine nucleotides that are similar to those in growing cells (6, 28). However, levels of ATP in spores made up only ∼0.1% of the total adenine pool, while ATP comprises ∼85% of the free adenine nucleotide pool in growing cells. Given the large amount of rRNA breakdown in spores upon high-temperature incubation, this could well increase the free adenine nucleotide pool in dormant spores. In addition, it is possible that the high-temperature treatment could somehow activate energy metabolism in these dormant spores. Indeed, levels of at least one endogenous spore energy reserve, 3-phosphoglyceric acid (3PGA), decrease somewhat during extended incubation at 50°C of GR-less spores of B. megaterium and B. subtilis under some conditions (11). Consequently, we assayed extracts of both B. megaterium and B. subtilis GR-less spores left untreated or treated at 37 to 70°C for ATP with a luciferase-based assay (Table 1). The results of this analysis showed that levels of ATP associated with density centrifugation-purified spores were still minimal in spores incubated at 37 or 50°C for up to a week and actually went down ∼10-fold following 20 h of incubation at 70°C. The small amount of material in extracts from untreated and treated spores that reacted in the luciferase assay did appear to be ATP, as a brief incubation of selected extracts with glucose and hexokinase eliminated all reactivity in the luciferase assay (Table 1).
TABLE 1.
ATP levels in spores incubated under various conditionsa
| Incubation conditions | ATP levelb in: |
|
|---|---|---|
| B. megaterium | B. subtilis | |
| Just harvested | 1.3 (<0.01) | 0.25 |
| 3 days, 4°C H2O | 1.5 | NDc |
| 3 days, 4°C, spent medium | 1.1 | 0.06 |
| 3 days, 37°C, H2O | 1.1 | 0.06 |
| 3 days, 37°C, spent medium | 0.2 | 0.16 |
| 3 days, 50°C, H2O | 0.3 | 0.07 |
| 3 days, 50°C, spent medium | 0.14 | <0.05 |
| 7 days, 4°C, H2O | 1.2 | 0.11 |
| 7 days, 4°C, spent medium | 0.5 | 0.10 |
| 7 days, 37°C, H2O | 0.8 (<0.01) | 0.08 |
| 7 days, 37°C, spent medium | 0.9 (<0.01) | 0.15 |
| 7 days, 50°C, H2O | <0.1 | 0.09 |
| 7 days, 50°C, spent medium | 0.2 | <0.05 |
| 18–20 h, 70°C, H2O | 0.05 | 0.03 |
Spores of B. megaterium GC614 and B. subtilis FB73 were prepared, isolated, incubated, and then purified and extracted, and extracts were processed and assayed for ATP as described in Materials and Methods.
The values shown are in picomoles per milliliter of spores at an OD600 of 1.0. Those in parentheses are for samples that were incubated with glucose and hexokinase prior to ATP determination.
ND, not determined.
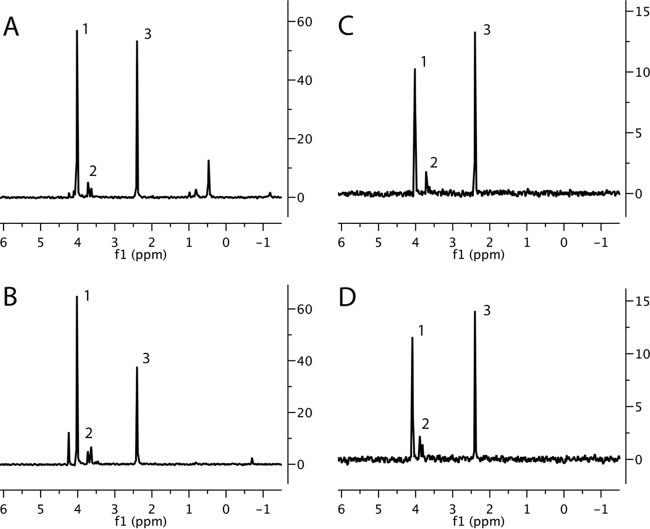
To determine the levels of all phosphorus-containing small molecules in high-temperature-treated spores, purified untreated and heat-treated wild-type spores were extracted with boiling 1-propanol, the extracts were flash evaporated, the dry residue was extracted with water and centrifuged, and divalent metal ions in the supernatant fluid, in particular the Mn2+ that interferes with NMR, were removed by Chelex column chromatography (11). The final samples were lyophilized and dissolved in D2O with buffer and EDTA prior to 31P-NMR analysis on an 800-MHz instrument (11). In some experiments, known amounts of compounds such as 3PGA, AMP, and inorganic phosphate (Pi) were added to spore extracts just prior to 31P-NMR analysis to allow definitive identification of 31P-NMR peaks due to these compounds. As found previously (11), the three most abundant low-molecular-weight phosphorus-containing compounds in B. megaterium and B. subtilis spores were 3PGA, AMP, and Pi (Fig. 4A and C). Strikingly, the levels of most of the phosphorus-containing small molecules in extracts from high-temperature-treated spores of B. subtilis and, to a lesser extent, of B. megaterium were almost identical to the levels in spores held at 4°C (Fig. 4B and D). However, the levels of Pi and an unknown phosphate-containing small molecule went down significantly after the incubation of B. megaterium spores at 75°C for 20 h (Fig. 4A and C). The decrease in the Pi level may well be due to the release of loosely bound Pi in spores' outer layers, as was seen previously in B. megaterium spores but not in B. subtilis spores (11). Interestingly, the loss of the peak produced by an unknown phosphate-containing small molecule from untreated B. megaterium spores was almost paralleled by the appearance of a new peak also from an unknown phosphate-containing small molecule in B. megaterium spores treated at 75°C. Notably, there were only minimal increases in the intensities of peaks produced by ribomononucleotides, indicating that rRNA degradation in dormant spores, at least for the times used in these experiments, generates only minimal levels of mononucleotides. However, the small size of much of the RNA species in heat-treated spores, ≤200 nt (Fig. 3) (see below), and the expected and observed poor recovery of RNA of this size from heat-treated spores strongly suggest that some rRNA is cleaved into several fragments of ≤100 nt by heat treatment.
FIG 4.
Levels of phosphorus-containing small molecules in spores as determined by 31P-NMR. Spores of B. megaterium QMB B1551 (wild type) (A, B) or B. subtilis PS533 (wild type) (C, D) either left untreated (A, C) or incubated at 75°C (B) or 80°C (D) for 20 h were purified and extracted, the extracts were treated, and samples were analyzed by 31P-NMR as described in Materials and Methods. Peaks 1, 2, and 3 are due to 3PGA, AMP, and Pi, respectively, as determined by the addition of appropriate amounts of the pure compounds to spore extracts. Note that AMP produced the largest peak in the region around peak 2 in untreated spores; other peaks in this region are due to other ribomononucleotides. The peaks at ∼0.5 ppm in panel A and 4 ppm in panel B have not been identified.
Germination of rRNA-depleted spores.
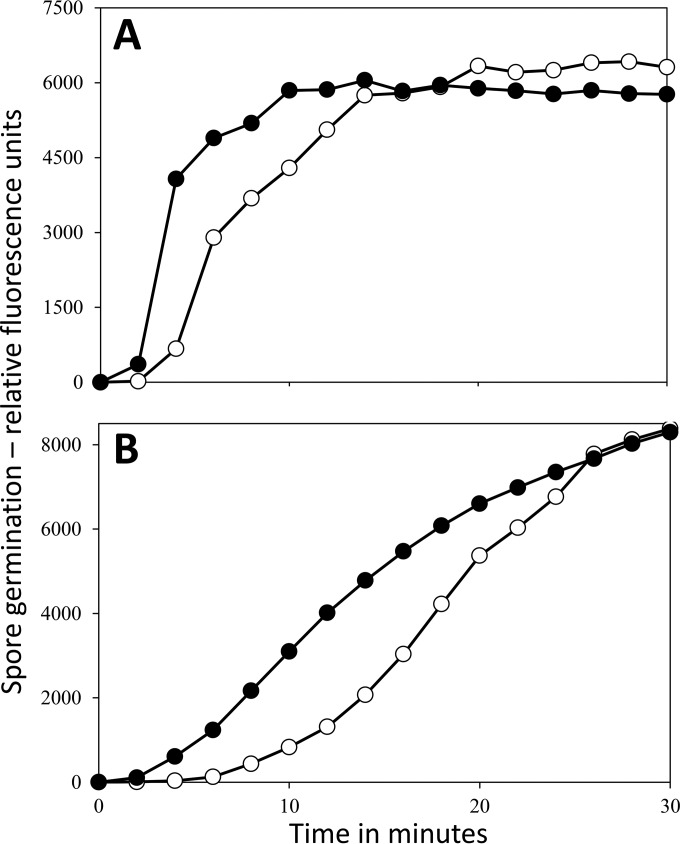
Given the almost complete absence of rRNA from wild-type spores of several Bacillus species treated at 70 to 80°C for ∼20 h and the retention of almost normal viability levels by the purified spores, these heat-treated spores must be able to germinate. However, an obvious question is how rapidly do these spores germinate. Indeed, there is one report that spores incubated at lower temperatures for longer periods germinate more slowly than untreated spores (12). In addition, spores heat treated at even higher temperatures (∼90°C) that retain DPA and at least some viability germinated more slowly than untreated spores (19, 20, 29). However, the surprising result obtained with purified wild-type B. megaterium spores that had been incubated at 75°C was that they germinated more rapidly than untreated spores (Fig. 5). This was the case for two GR-dependent germinants of B. megaterium spores (17), d-glucose and KBr (Fig. 5). Heat-treated B. subtilis spores germinated similarly to untreated spores with l-valine via the GR GerA and even faster than untreated spores with the AGFK mixture via the GerB and GerK GRs (4, 5) (Fig. 6). The similar or faster germination of the heat-treated spores was seen even though the untreated spores were given the heat activation treatments prior to germination shown to give maximal increases in spore germination (23, 30).
FIG 5.
Germination of spores of B. megaterium with and without treatment at 75°C for 18 h. Spores of B. megaterium QMB1551 (wild type) with or without incubation at 75°C for 18 h were purified, purified spores were germinated with either d-glucose (A) or KBr (B), and spore germination was monitored by measuring DPA release as described in Materials and Methods. Untreated spores were heat activated for 30 min at 60°C and then cooled prior to germinations. Symbols: ○, untreated spores; ●, spores incubated at 75°C for 18 h.
FIG 6.
Germination of spores of B. subtilis with and without heat treatment for 18 h at 80°C. Spores of B. subtilis PS533 (wild type) with and without incubation at 80°C for 18 h were purified, purified untreated and heat-treated spores were germinated with either l-valine (A) or AGFK (B), and spore germination was monitored by measuring DPA release, all as described in Materials and Methods. Untreated spores were heat activated for 30 min or 2 h at 75°C prior to l-valine or AGFK germination, respectively, and then cooled; these heat activation conditions have been shown to be optimal for eliciting maximum spore germination with l-valine or AGFK (23). Symbols: ○, untreated spores; ●, spores incubated at 80°C for 18 h.
Outgrowth and return of rRNA levels in spores treated at 70 to 80°C.
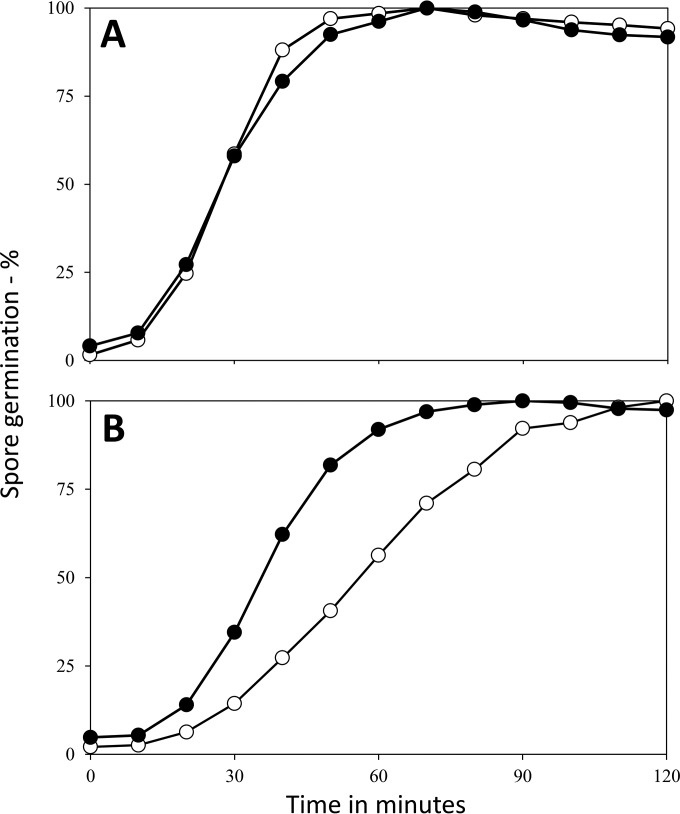
While wild-type spores treated for ∼20 h at 75 to 80°C retained almost normal viability even though almost all of their rRNA was lost, obviously rRNA must be synthesized in order for spores to grow out, since there is much protein synthesis in this period of spore development (13, 31–34). To compare these wild-type spores' outgrowth kinetics, spores left untreated and spores treated at 75 or 80°C were incubated in medium that promoted spore germination, outgrowth, and subsequent vegetative growth and these processes were monitored by measuring the OD600 of the incubated spores (Fig. 7). The relative rates of germination of untreated and heat-treated B. megaterium spores (Fig. 7A) were more difficult to monitor in this experiment, since outgrowth began so soon after germination. More importantly, as measured by the increase in the OD600 of spore cultures due to spore swelling and elongation following germination, the return to vegetative growth (e.g., outgrowth) was approximately the same for untreated and heat-treated B. megaterium spores (Fig. 7A). The germination of heat-treated B. subtilis spores was also only slightly slower than that of untreated spores in the complete outgrowth medium, although the outgrowth of the heat-treated spores was much slower.
FIG 7.
Germination and outgrowth of B. megaterium and B. subtilis spores with and without heat treatment at 75 or 80°C. Spores of B. megaterium QMB1551 (wild type) (A) or B. subtilis PS533 (wild type) (B) either left untreated or incubated for ∼18 h at 75°C (B. megaterium) or 80°C (B. subtilis) were purified as described in Materials and Methods. Purified spores, with heat activation for untreated spores as described in the legends to Fig. 5 and 6, were incubated at 37°C in LB medium plus either 10 mM d-glucose (B. megaterium) or 10 mM l-valine (B. subtilis), and spore germination and outgrowth were monitored by measuring the OD600 of the culture as described in Materials and Methods. Symbols: ○, untreated spores; ●, spores incubated at 75 or 80°C for 18 h.
The relatively similar rates of outgrowth of untreated and heat-treated B. megaterium spores were somewhat surprising, since the heat-treated spores needed to synthesize more rRNA than the untreated spores early in outgrowth. However, the heat-treated spores probably germinated more rapidly than the untreated spores and thus had a head start in outgrowth. These spores were also not treated as severely as the heat-treated B. subtilis spores that were significantly slower in outgrowth than untreated spores. Slower outgrowth of even more severely heat-treated B. subtilis spores has been seen previously and has been suggested to be due to partial inactivation of spore core proteins essential for metabolism essential for spore outgrowth, with more complete inactivation of such proteins leading to spore death (19, 20). Notably, heat-treated spores of a number of species can often exhibit conditional survival on poor or stressful medium, even medium with small amounts of added salt (35–37).
The results noted above indicated that an early event following germination of the high-temperature-treated spores must be the synthesis of rRNA. Indeed, rRNA has been shown to be the earliest RNA species made after B. subtilis spore germination is completed (33). To determine when rRNA accumulation took place following the germination of heat-treated wild-type spores, samples were isolated at various times from spores treated at 75 or 80°C incubated in a medium that allowed both germination and outgrowth. RNA was isolated from the isolated samples, and the RNA was analyzed by gel electrophoresis (Fig. 8 and 9). ImageJ analysis of the separated RNAs showed that ≤3% of the 23S rRNA remained in heat-treated B. megaterium spores and ≤1% of the 23S and 16S rRNA remained in heat-treated B. subtilis spores. While RNA isolated at the earliest time points in germination and outgrowth showed no or minimal levels of intact 23S and 16S rRNA, as expected, these RNA species did reappear, but only after germination was complete, although before the OD600 of germinating spore cultures began to increase significantly.
FIG 8.

(A) Levels of rRNA in germinating and outgrowing B. megaterium spores previously incubated at high temperature. Spores of B. megaterium QMB1551 (wild type) were incubated for 18 h at 75°C and purified as described in Materials and Methods. The purified spores were incubated in LB medium plus 10 mM d-glucose, and germination and outgrowth were monitored by measuring the OD600 of the culture. (B) At various times during germination and outgrowth, samples were isolated and RNA was extracted from equal volumes of culture and analyzed by gel electrophoresis as described in Materials and Methods. In this experiment, the amount of RNA recovered from the initial heat-treated spores was ∼50% of that recovered from untreated spores. The arrows to the right of panel B denote the migration positions of the 16S and 23S rRNAs, the values to the left of panel B indicate the migration positions of RNA markers (lane M) in kilobases, and the values above various lanes indicate the times in minutes when the samples for RNA isolation were harvested from the experiment shown in panel A. The pre sample is from untreated spores, and the 0-min sample is from heat-treated spores prior to addition to germination/outgrowth medium.
FIG 9.
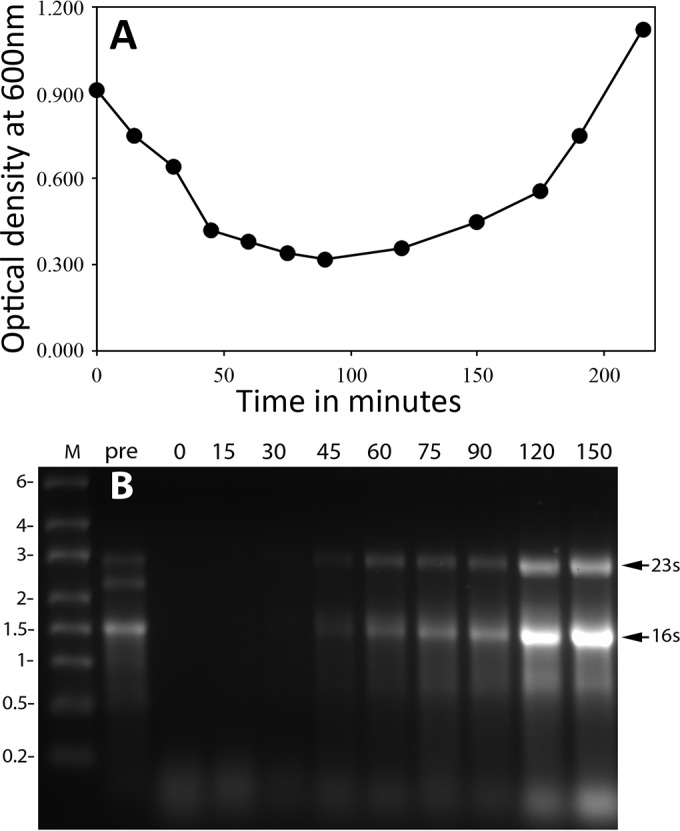
(A) Levels of rRNA in germinating and outgrowing B. subtilis spores previously incubated at high temperature. Spores of B. subtilis PS533 (wild type) were incubated for 18 h at 80°C and purified as described in Materials and Methods. The purified spores were incubated in LB medium plus 10 mM l-valine, and germination and outgrowth were monitored by measuring the OD600 of the culture. (B) At various times of germination and outgrowth, samples were isolated and RNA was extracted from equal volumes of culture and analyzed by gel electrophoresis as described in Materials and Methods. In this experiment, the amount of RNA recovered from the initial heat-treated spores was ∼50% of that recovered from untreated spores. The arrows to the right of panel B denote the migration positions of the 16S and 23S rRNAs, the values to the left of panel B indicate the migration positions of RNA markers (lane M) in kilobases, and the values above the various lanes in panel B are the times in minutes when the samples for RNA isolation were harvested from the experiment shown in panel A. The pre sample is from untreated spores, and the 0-min sample is from heat-treated spores prior to addition to germination/outgrowth medium.
DISCUSSION
Several observations in the work reported here indicate that protein synthesis is not required for spore germination. One observation is that ATP is present at only minute levels in spores that can clearly germinate well. It is, of course, possible that following germinant addition, ATP to drive protein synthesis is generated soon after the event that commits spores to germinate but prior to DPA release. However, much previous work suggests this is not the case (6–10). The rapid germination of rRNA-less B. megaterium and B. subtilis spores that takes place well before rRNA synthesis begins is even stronger evidence that protein synthesis is not required for spore germination. One cannot, of course, rule out the possibility that (i) there is a minute amount of rRNA remaining in heat-treated spores that have lost most, if not all, of their rRNA and it is this minute amount of rRNA that is used to make some protein(s) essential for spore germination or (ii) ribosomes can exhibit a minimal amount of translation capacity even when all of the 23S and 16S rRNA is degraded to fragments of ≤200 nt. While these arguments are impossible to refute, the more likely conclusion from the new results is that protein synthesis is not essential for spore germination.
A second notable conclusion is that, as reported previously (12), some reactions can go on in dormant spores, as the present work has confirmed that rRNA species are degraded in spores incubated at temperatures of 37 to 80°C. The major question about their degradation, however, is whether this is enzymatic or nonenzymatic, especially given the lability of RNA. It is possible that enzymes work very slowly even at the extremely low spore core water content in the spore core, perhaps with minimal levels of free water (38–40). Indeed, there are multiple RNases that participate in rRNA degradation in bacteria, in particular under starvation conditions (41, 42). However, at least one spore core protein that is freely mobile in germinated spores has a mobility that is at least 6 orders of magnitude lower in dormant spores (43). Thus, spore core enzyme activity would be expected to be minimal. This would also be expected in the spore core environment at elevated temperatures well above the optimal temperatures for the activity of enzymes of mesophilic spores. Indeed, the heat stability of enzymes in the dormant spore core is ∼40°C higher than that of soluble enzymes (38), consistent with these proteins being immobilized in the spore core at even 70 to 80°C, such that thermal motions do not result in protein denaturation at these temperatures. Thus, a simple conclusion at this time is that the rRNA degradation in spores incubated at 70 to 80°C is most probably nonenzymatic and certainly does not involve RNase Y at 80°C. Indeed, there is a precedent for rapid RNA nonenzymatic degradation at the higher temperatures used in the present work (44, 45). However, a definitive conclusion that this rRNA degradation at high temperatures in spores is not enzymatic may require analysis of rRNA degradation in spores of conditional mutants lacking various RNases that degrade rRNA.
With the conclusions noted above, a number of questions remain about the work in this communication. (i) What is the fate of rRNA degradation products generated by incubation of dormant spores at temperatures of 37 to 80°C? Examination of agarose gels of RNA isolated from spores incubated at high temperatures indicates that increasing amounts of low-molecular-weight RNAs accumulate during incubation at elevated temperatures (12) (Fig. 1 to 3). Presumably, these are oligonucleotides generated largely from rRNA, and these might be stabilized significantly by binding to ribosomal proteins. However, previous work indicates that minimal free mononucleotides are generated during extended incubation of spores at 37 to 50°C (11), and there was also only a minimal increase in free mononucleotide levels in spores incubated at 75 to 80°C. Notably, if all of the dormant spore rRNA were degraded to mononucleotides, this would increase the levels of AMP in B. megaterium and B. subtilis spores 20- or 30-fold, respectively, and increase the levels of CMP, GMP, and UMP even more (11, 28). Presumably, the oligonucleotides generated by rRNA degradation are only degraded to mononucleotides rapidly when spores' core water content rises as germination proceeds and is completed, and these mononucleotides are then used to resynthesize new RNAs. However, this has not yet been examined. It is theoretically possible that rRNA in heat-treated spores was degraded not to mononucleotides but rather to mononucleosides and Pi, although this seems extremely unlikely since the Pi levels in heat-treated spores did not increase. (ii) What is the fate of the many ribosomal proteins (46) following rRNA degradation during spore treatment at 75 to 80°C? Presumably, at least some ribosomal proteins dissociate from rRNA fragments during heat treatment, and these are most likely stable in dormant spores even at high temperatures, as are most spore enzymes (1–3), during the heating regimens used. Spore outgrowth begins soon after completion of germination even with heat-treatment, and this requires protein synthesis and thus functional ribosomes (1, 32, 33). When all intact rRNAs are absent from spores, they can clearly be synthesized soon after completion of germination by using the RNA polymerase stored in spores. However, ribosomes can only be generated and protein synthesis can only begin if ribosomal proteins are also present to generate functional ribosomes with newly synthesized rRNA. Consequently, we predict that ribosomal proteins must remain in heat-treated spores even if all of the full-length rRNA species are lost. (iii) What is the fate of 3PGA during high-temperature incubation? Previous work showed that 3PGA levels in dormant spores fall only slightly during long incubations at 37 to 50°C, and then only in spent medium and with the core pH elevated to ∼8 to facilitate phosphoglycerate mutase activity in dormant spores (11). In the present study, 3PGA levels fell minimally, if at all, in spores incubated at 75 to 80°C. The lack of 3PGA loss under these conditions is in contrast to the rapid loss of all 3PGA in just a few minutes late in the germination of B. megaterium and B. subtilis spores, even though enzymes for rapid 3PGA metabolism are present in the spore core (6). (iv) Dormant spores with or without extended incubation at 37 to 80°C had minimal ATP levels and are known to lack significant membrane potential (10). However, some luciferase-reactive material was detected in dormant spores, even if at extremely low levels, and the loss of this reactive material by reaction with hexokinase and glucose suggests that this material is ATP. Could this minimal level of ATP be used to drive metabolic reactions in a dormant spore, even if slowly? This question is almost impossible to answer definitively, as it requires that spores extracted for ATP analysis be 100% free of germinated spores or growing/sporulating cells, as a minute amount of dormant spore contamination with the latter cell types would result in some ATP in spore extracts. However, even if the ATP in dormant spore extracts were all from dormant spores, the free energy available in ATP when this species is only 0.1 to 0.01% of the total adenine nucleotide pool could be too low to allow this ATP to be used for many reactions that would be favorable when ATP is 85% of the adenine nucleotide pool. (v) Finally, it was striking that spores incubated at 75 to 80°C for ∼20 h germinated as well as, and in most cases better than, untreated spores. Why is this the case? The simple answer is that we do not know. Incubation of B. megaterium and B. subtilis spores at temperatures higher than those used in the present work does generate spores that germinate more poorly than untreated spores (47). The reason for this lower germination rate is also not clear, but at least one CLE, CwlJ, is relatively heat labile, at least compared to other spores' germination proteins (48), and loss of CwlJ is known to slow germination (49). But why would spores germinate faster after extended incubation at 75 to 80°C? Possible explanations for this include (i) release or inactivation of some autoinhibitor of germination from treated spores, (ii) weakening of the gating of spores' SpoVA protein channel for Ca-DPA such that Ca-DPA is released more readily from the heat-treated spores, and (iii) some effect on GRs or the auxiliary GerD protein such that they are transiently more active or functional in spores treated at 75 to 80°C. However, while some questions remain about the behavior of Bacillus spores given extended incubations at elevated temperatures, the major conclusion of this work—that spore germination most likely does not require protein synthesis and energy metabolism—seems to be significantly strengthened.
Finally, the instability of rRNA in dormant spores incubated at high temperatures for extended periods that did not kill spores suggests that the small amounts of mRNA in dormant spores of both bacilli and clostridia (12, 50–52) will also be unstable under these types of conditions. Indeed, at least one study suggests that this is the case (50), and since rRNA is likely stabilized to some degree by binding to ribosomal proteins, mRNAs might even be degraded more rapidly than rRNA. If this is the case, then spores incubated at 75 to 80°C that are still viable and germinate and grow out relatively normally do so in the absence of all or almost all of their initial complement of mRNAs. Thus, dormant spore mRNAs are also probably not essential for normal spore germination and outgrowth, at least under the conditions used in the present work, in which outgrowth was in a rich medium. However, it is possible that spores lacking all mRNAs will exhibit outgrowth defects in more minimal medium, and this is under investigation.
ACKNOWLEDGMENTS
P.S. conceived the idea for this work, and G.K., B.S., L.R., and Q.L. carried out the experiments. P.S. and G.K. wrote the manuscript and made the figures, and all of the authors edited the manuscript.
We are grateful to S. Ben-Yehuda for the gift of strain ES83, to M. P. Deutscher for helpful discussions, and to the reviewers for helpful suggestions.
Footnotes
For a commentary on this article, see doi:10.1128/JB.00721-16.
REFERENCES
- 1.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food microbiology: fundamentals and frontiers, 4th edition ASM Press, Washington, DC. [Google Scholar]
- 2.Setlow P. 2016. Spore resistance properties, p 201–216. In Eichenberger P, Driks A (ed), The bacterial spore. ASM Press, Washington, DC. [Google Scholar]
- 3.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 4.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 5.Setlow P. 2014. The germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setlow P, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem 245:3637–3644. [PubMed] [Google Scholar]
- 7.Scott IR, Ellar DJ. 1978. Metabolism and the triggering of germination of Bacillus megaterium. Concentrations of amino acids, organic acids, adenine nucleotides and nicotinamide nucleotides during germination. Biochem J 174:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott IR, Ellar DJ. 1978. Metabolism and the triggering of germination of Bacillus megaterium. Use of l-[3H]alanine and tritiated water to detect metabolism. Biochem J 174:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vary JC. 1978. Glucose-initiated germination in Bacillus megaterium spores, p 104–108. In Chambliss G, Vary JC (ed), Spores VII. American Society for Microbiology, Washington, DC. [Google Scholar]
- 10.Magge A, Setlow B, Cowan AE, Setlow P. 2009. Analysis of dye binding by and membrane potential in spores of Bacillus species. J Appl Microbiol 106:814–821. doi: 10.1111/j.1365-2672.2008.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S, Korza G, Maciejewski M, Setlow P. 2015. Analysis of metabolism in dormant spores of Bacillus species by 31P-NMR of low-molecular-weight compounds. J Bacteriol 197:992–1001. doi: 10.1128/JB.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segev E, Smith Y, Ben-Yehuda S. 2012. RNA dynamics in aging bacterial spores. Cell 148:139–149. doi: 10.1016/j.cell.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 13.Sinai L, Rosenberg A, Smith Y, Segev E, Ben-Yehuda S. 2015. The molecular timeline of a reviving bacterial spore. Mol Cell 57:695–707. doi: 10.1016/j.molcel.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. doi: 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Commichau FM, Rothe FM, Herzberg C, Wagner E, Hellwig D, Lehnik-Habrink M, Hammer E, Völker U, Stülke J. 2009. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol Cell Proteomics 8:1350–1360. doi: 10.1074/mcp.M800546-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 18.Gupta S, Ustok FI, Johnson CL, Bailey DMD, Lowe CR, Christie G. 2013. Investigating the functional hierarchy of Bacillus megaterium PV361 spore germinant receptors. J Bacteriol 195:3045–3053. doi: 10.1128/JB.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coleman WH, Zhang P, Li YQ, Setlow P. 2010. Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50:507–514. doi: 10.1111/j.1472-765X.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 20.Coleman WH, Chen D, Li YQ, Cowan AE, Setlow P. 2007. How moist heat kills spores of Bacillus subtilis. J Bacteriol 189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luu S, Cruz-Mora J, Setlow B, Feeherry FE, Doona CJ, Setlow P. 2015. The effects of heat activation on Bacillus spore germination with nutrients or under high pressure, with or without various germination proteins. Appl Environ Microbiol 81:2927–2938. doi: 10.1128/AEM.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setlow B, Melly E, Setlow P. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J Bacteriol 183:4894–4899. doi: 10.1128/JB.183.16.4894-4899.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller R, Hornek G, Rettberg G, Mollenkopf H-J, Stackebrandt E, Nicholson WL. 2006. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr Microbiol 53:227–231. doi: 10.1007/s00284-006-0099-1. [DOI] [PubMed] [Google Scholar]
- 26.Rump VL, Asamoah B, Gonzalez-Escalona N. 2010. Comparison of commercial RNA extraction kits for preparation of DNA-free total RNA from Salmonella cells. BMC Res Notes 3:211. doi: 10.1186/1756-0500-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaysi G. 1965. Maximal temperatures of the two stages of germination in several mesophilic members of the genus Bacillus. Appl Microbiol 13:500–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson DL, Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem 245:1137–1145. [PubMed] [Google Scholar]
- 29.Wang G, Paredes-Sabja D, Sarker MR, Green C, Setlow P, Li YQ. 2012. Effects of wet heat-treatment on the germination of individual spores of Clostridium perfringens. J Appl Microbiol 113:824–836. doi: 10.1111/j.1365-2672.2012.05387.x. [DOI] [PubMed] [Google Scholar]
- 30.Hyatt MT, Levinson HS. 1961. Interaction of heat, glucose, l-alanine, and potassium nitrate in spore germination of Bacillus megaterium. J Bacteriol 81:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setlow P, Primus G. 1975. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem 250:623–630. [PubMed] [Google Scholar]
- 32.Rodenberg S, Steinberg W, Piper J, Nickerson K, Vary J, Epstein R, Halvorson HO. 1968. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol 96:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong RL, Sueoka N. 1968. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A 59:153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albertini AM, Galizzi A. 1975. Mutant of Bacillus subtilis with a temperature-sensitive lesion in ribonucleic acid synthesis during germination. J Bacteriol 124:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busta FF. 1978. Introduction to injury and repair of microbial cells. Adv Appl Microbiol 23:195–201. [DOI] [PubMed] [Google Scholar]
- 36.Hurst A. 1977. Bacterial injury: a review. Can J Microbiol 23:935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- 37.Feeherry FE, Munsey DT, Rowley DN. 1987. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl Environ Microbiol 53:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerhardt P, Marquis RE. 1989. Spore thermoresistance mechanisms, p 43–63. In Smith I, Slepecky RA, Setlow P (ed), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC. [Google Scholar]
- 39.Kaieda S, Setlow B, Setlow P, Halle B. 2013. Mobility of core water in Bacillus subtilis spores by 2H NMR. Biophys J 105:2016–2023. doi: 10.1016/j.bpj.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunde EP, Setlow P, Hederstedt L, Halle B. 2009. The physical state of water in bacterial spores. Proc Natl Acad Sci U S A 106:19334–19339. doi: 10.1073/pnas.0908712106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sulthana S, Basturea GN, Deutscher MP. 2016. Elucidation of pathways of ribosomal RNA degradation: an essential role for RNase E. RNA 22:1163–1171. doi: 10.1261/rna.056275.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiväli Ü Paier A, Tenson T. 2013. When stable RNA becomes unstable: the degradation of ribosomes in bacteria and beyond. Biol Chem 394:845–855. doi: 10.1515/hsz-2013-0133. [DOI] [PubMed] [Google Scholar]
- 43.Cowan AE, Koppel DE, Setlow B, Setlow P. 2003. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc Natl Acad Sci U S A 100:4209–4214. doi: 10.1073/pnas.0636762100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaga E, Nakagomi O, Uesugi S. 1992. Thermal degradation of RNA-RNA hybrids during hybridization in solution. Mol Cell Probes 6:261–264. doi: 10.1016/0890-8508(92)90026-T. [DOI] [PubMed] [Google Scholar]
- 45.Tenhunen J. 1989. Hydrolysis of single-stranded RNA in aqueous solutions—effect on quantitative hybridizations. Mol Cell Probes 3:391–396. doi: 10.1016/0890-8508(89)90018-2. [DOI] [PubMed] [Google Scholar]
- 46.Shajani Z, Sykes MT, Williamson JR. 2011. Assembly of bacterial ribosomes. Annu Rev Biochem 80:501–526. doi: 10.1146/annurev-biochem-062608-160432. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Zhang P, Setlow P, Li YQ. 2011. Kinetics of germination of wet-heat-treated individual spores of Bacillus species, monitored by Raman spectroscopy and differential interference contrast microscopy. Appl Environ Microbiol 77:3368–3379. doi: 10.1128/AEM.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atrih A, Foster SJ. 2001. In vivo roles of the germination-specific lytic enzymes of Bacillus subtilis. Microbiology 147:2925–2932. doi: 10.1099/00221287-147-11-2925. [DOI] [PubMed] [Google Scholar]
- 49.Peng L, Chen D, Setlow P, Li YQ. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows the monitoring of spore germination dynamics. Anal Chem 81:4035–4042. doi: 10.1021/ac900250x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergman NH, Anderson EC, Swenson EE, Niemeyer MW, Miyoshi AD, Hanna PC. 2006. Transcriptional profiling of the Bacillus anthracis life cycle in vitro and an implied model for regulation of spore formation. J Bacteriol 188:6092–6100. doi: 10.1128/JB.00723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keijser BJF, Ter Beek A, Rauwerda H, Schuren F, Montijn R, van der Spek H, Brul S. 2007. Analysis of temporal gene expression during Bacillus subtilis spore germination and outgrowth. J Bacteriol 189:3624–3634. doi: 10.1128/JB.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bettegowda C, Huang X, Lin J, Cheong I, Kohli M, Szabo SA, Zhang X, Diaz LA Jr, Velculescu VE, Parmigiani G, Kinzler KW, Vogelstein B, Zhou S. 2006. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol 24:1573–1580. doi: 10.1038/nbt1256. [DOI] [PMC free article] [PubMed] [Google Scholar]