ABSTRACT
Proteus mirabilis is a social bacterium that is capable of self (kin) versus nonself recognition. Swarming colonies of this bacterium expand outward on surfaces to centimeter-scale distances due to the collective motility of individual cells. Colonies of genetically distinct populations remain separate, while those of identical populations merge. Ids proteins are essential for this recognition behavior. Two of these proteins, IdsD and IdsE, encode identity information for each strain. These two proteins bind in vitro in an allele-restrictive manner. IdsD-IdsE binding is correlated with the merging of populations, whereas a lack of binding is correlated with the separation of populations. Key questions remained about the in vivo interactions of IdsD and IdsE, specifically, whether IdsD and IdsE bind within single cells or whether IdsD-IdsE interactions occur across neighboring cells and, if so, which of the two proteins is exchanged. Here we demonstrate that IdsD must originate from another cell to communicate identity and that this nonresident IdsD interacts with IdsE resident in the recipient cell. Furthermore, we show that unbound IdsD in recipient cells does not cause cell death and instead appears to contribute to a restriction in the expansion radius of the swarming colony. We conclude that P. mirabilis communicates IdsD between neighboring cells for nonlethal kin recognition, which suggests that the Ids proteins constitute a type of cell-cell communication.
IMPORTANCE We demonstrate that self (kin) versus nonself recognition in P. mirabilis entails the cell-cell communication of an identity-encoding protein that is exported from one cell and received by another. We further show that this intercellular exchange affects swarm colony expansion in a nonlethal manner, which adds social communication to the list of potential swarm-related regulatory factors.
INTRODUCTION
Bacteria, such as the swarming bacterium Proteus mirabilis, can come together in groups that move rapidly across surfaces. During this swarm migration, P. mirabilis exhibits self (kin) versus nonself recognition. Populations of genetically identical organisms merge, while populations of genetically different organisms separate and form a visible boundary (1–4). The ids operon, which encodes the six proteins IdsA to IdsF, is one of the genetic loci responsible for boundary formation (2, 5, 6). Cells lacking the Ids proteins form a boundary with their wild-type parent strain (2). A functional type VI secretion system (T6SS) is essential for boundary formation (5, 7), and three Ids proteins (IdsA, IdsB, and IdsD [D]) are exported in a T6SS-dependent manner (5). T6SSs, which are widely distributed among Gram-negative bacteria, are machines that can translocate proteins (primarily lethal) from the inside of one cell directly into another cell (8–28). The action of these transferred effector proteins is inhibited through the binding of an inhibitory immunity protein in the recipient cell (15, 16, 18, 21, 22, 28–30).
In addition to a functional T6SS, the Ids system relies on the interactions between two proteins, D and IdsE (E), which together encode strain-specific identity information (2, 31). D and E each contain a variable region (VR), a stretch of amino acids that is generally unique among strains (2, 31). D and E bind in vitro when the VRs of the two proteins originate from the same strain. Binding pairs of D and E are termed cognate (31). In contrast, when the VRs of D and E do not originate from the same strain, the proteins do not bind in vitro and the D-E pair is thus termed noncognate (31). Interestingly, swarming populations of strains producing cognate D-E pairs merge and thus recognize each other as self; however, swarms of strains producing noncognate D-E pairs form a visible boundary and are considered nonself (31). How the in vitro binding of D and E accounts for in vivo boundary behaviors remains unknown. Both D and E contain transmembrane domains (31). D has been found outside cells, and its export has been shown to be dependent on a functional T6SS (5). Consistent with these data, D contains the recently described MIX motif, which has been found among multiple T6SS effector proteins; the MIX motif is predicted to identify previously unknown substrates of the T6SS (32). In contrast, E has not been found outside cells and is predicted to be an integral inner membrane protein (5, 31). Given these data, the prevailing hypothesis is that the Ids proteins constitute a lethal effector-immunity (toxin-antitoxin) system. Within this model, D is proposed to be delivered to neighboring cells, where it can interact with E; lack of D-E binding might result in cell lethality, as seen for other effector-immunity pairs. However, there is no experimental evidence for Ids transfer between cells or for Ids-associated lethality. Furthermore, whether boundary formation results from interactions among the Ids proteins within individual cells or between cells has not been addressed.
Here we demonstrate that D-E interactions, or a lack thereof, do not cause lethality in P. mirabilis. Furthermore, we provide evidence that D is communicated from one cell to another in a T6SS-dependent manner and that interactions with E in the recipient cells determine behavior. We also present evidence that these D-E interactions impact the expansion of a swarming colony. These data demonstrate that kin recognition in P. mirabilis involves the cell-cell communication of an identity-encoding protein.
MATERIALS AND METHODS
Bacterial strains and media.
The strains and plasmids used in this study are described in Table 1. P. mirabilis strains were maintained on low swarm (LSW−) agar (33). CM55 blood agar base agar (Oxoid, Basingstoke, England) was used for swarm-permissive nutrient plates. Overnight cultures of all strains were grown at 37°C in LB broth under aerobic conditions. Kanamycin was used at a concentration of 35 μg/ml for plasmid maintenance and was added to all swarm and growth media.
TABLE 1.
Strains used in this study
| Strain | Name in this study | Description | Reference |
|---|---|---|---|
| Proteus mirabilis | |||
| BB2000 | Produces cognate DVR-BBEVR-BB pair from single allele | 33 | |
| BB2000 Δids | Δids strain | Δids::Tn-Cmr; produces neither D nor E | 2 |
| Δids strain carrying pIdsBB | CCS01 | Δids::Tn-Cmr; produces cognate DVR-BBEVR-BB pair | 2 |
| Δids strain carrying pIdsBB-DVR-HI-EVR-BB | CCS02 | Δids::Tn-Cmr; produces noncognate DVR-HIEVR-BB pair | 31 |
| Δids strain carrying pIdsBB-DVR-BB-EVR-HI | CCS03 | Δids::Tn-Cmr; produces noncognate DVR-BBEVR-HI pair | 31 |
| Δids strain carrying pIdsBB-DVR-HI-EVR-HI | CCS04 | Δids::Tn-Cmr; produces cognate DVR-HIEVR-HI pair | 31 |
| Δids strain carrying pIdsBB-E-mt | Δids::Tn-Cmr; produces DVR-BB and not E | 2 | |
| Δids strain carrying pKG101 | Δids::Tn-Cmr; carries pKG101, which carries promoterless gfp | 6 | |
| Δids strain carrying pLW101 | Δids::Tn-Cmr; carries derivative of pIdsBB with FLAG epitope inserted immediately before idsA stop codon | 5 | |
| vipA::Tn5 strain carrying pLW101 | vipA::Tn5-Cmr; carries pLW101 | 5 | |
| BB2000 Δids vipAT95G | CCS05 | Δids::Tn-Cmr vipAT95G; deficient in T6SS-mediated transport | This study |
| CCS05 carrying pLW101 | Δids::Tn-Cmr vipAT95G; carries pLW101 | This study | |
| Δids strain carrying pIdsHI | Δids::Tn-Cmr; produces cognate DHIEHI pair | 31 | |
| CCS05 carrying pIdsBB | Δids::Tn-Cmr vipAT95G; produces cognate DVR-BBEVR-BB pair | This study | |
| CCS05 carrying pIdsBB-DVR-HI-EVR-BB | Δids::Tn-Cmr vipAT95G; produces noncognate DVR-HIEVR-BB pair | This study | |
| CCS05 carrying pIdsBB-DVR-BB-EVR-HI | Δids::Tn-Cmr vipAT95G; produces noncognate DVR-BBEVR-HI pair | This study | |
| CCS05 carrying pIdsBB-DVR-HI-EVR-HI | Δids::Tn-Cmr vipAT95G; produces cognate DVR-HIEVR-HI pair | This study | |
| Δids strain carrying pIdsBB-ΔE | CCS06 | Δids::Tn-Cmr; produces DVR-BB and not E | This study |
| CCS05 carrying pIdsBB-ΔE | Δids::Tn-Cmr vipAT95G; produces DVR-BB and not E | This study | |
| Escherichia coli | |||
| S17λpir | Mating strain for moving plasmids from E. coli into P. mirabilis | 64 | |
| SM10λpir | Mating strain for moving suicide vector pKNG101 into P. mirabilis | 35 |
We employed a previously described ids expression system (2) in which the entire ids locus from P. mirabilis strain BB2000 is expressed from a low-copy-number plasmid, under the control of its native promoter (pIdsBB), in a BB2000-derived strain lacking the chromosomal copy of the ids operon (Δids) (2). We engineered alterations to the ids locus on the vector; hence, all strains are isogenic except for the ids genes.
Plasmid and strain construction. (i) Construction of pIdsBB-ΔE.
The pIdsBB-ΔE plasmid was constructed using a 390-bp gBlock (Integrated DNA Technologies, Inc., Coralville, IA) containing the last 266 bp of idsD, the last 18 bp of idsE, and the first 106 bp of idsF (gBlock sequence as follows: GCGAACAATTAAAAATGGCAAGTGAAAAAGGTGATTGGAACCCTGAAACAGGTATATTTAAATTTAGTTTGGAAGTACAGTCTCAATTAGTAAATACATATTCTGCTTTTGGTGCACATCCTAATAGCCGTATAGGTATTGAAGATTTATATTGGTATTATCAAGTCAATCCCGAGGTAACAACACCGATGCGTTATATCAATTGGGGGGGAGATACCCAAGAAAACAATCAGCTTTTAGGCTTTATTAACAGTGAGAATATCTAAATCAGGAGAAAGAACACCATGCGTAGTTTGGTAAACGGCAGAAAGATTATTTTAGAAAATGATACAACAAATACCGGCGGTACCGTACTTACCGGCTCTTCTATTGCTAAACAAACACAAGGGG). EcoNI and KpnI restriction sites within this fragment were used to replace the sequence between the EcoNI and KpnI sites in pIdsBB (2). Ligation products were transformed into One Shot OmniMax 2 T1R chemically competent Escherichia coli (Thermo Fisher Scientific, Waltham, MA). Oligonucleotides were synthesized by Integrated DNA Technologies, Inc., and DNA sequencing was performed by Genewiz (South Plainfield, NJ).
(ii) Construction of the vipA mutation.
A swarm-capable spontaneous mutant of the BB2000 Δids strain lacking full-length E (2) was isolated. This isolate was subjected to phenol-chloroform extractions to isolate genomic DNA (gDNA). gDNA was sheared using a Covaris S220 system (Covaris, Woburn, MA), and a library for whole-genome sequencing was prepared using the PrepX ILM DNA library kit (WaferGen Biosystems, Fremont, CA) for the Apollo 324 next-generation sequencing (NGS) library prep system (WaferGen Biosystems). The library was sequenced as 100-bp paired-end reads using an Illumina HiSeq 2500 system (Illumina, San Diego, CA). Reads were aligned to the P. mirabilis BB2000 genome (GenBank accession no. CP004022) using Geneious (Biomatters, Auckland, New Zealand). Suppressor-specific polymorphisms were identified by aligning the assembled genome to that of the ancestral strain, BB2000 Δids. The identified mutation mapped to a gene encoding a VipA homolog, i.e., T6SS_VipA (PFAM family PF05591). BB2000 Δids vipAT95G was then constructed by using the pCS34 forward (CGCGGGCCCGGTATTACCCCATAAATAGTGC) and reverse (CAGCTATATTTGGTTTAACTTAAGGTCTAGAGCGCGC) primers to amplify the vipA-containing fragment from gDNA of the isolated spontaneous mutant strain. Restriction digestion with ApaI and XbaI was used to introduce this sequence into the suicide vector pKNG101 (34). The resulting vector, pCS34, was introduced into the mating strain E. coli SM10λpir (35) and then mated into BB2000 Δids. Matings were subjected to antibiotic selection on LSW− agar (with 15 μg/ml tetracycline and 25 μg/ml streptomycin). Candidate strains were subjected to sucrose counterselection as described previously (36). Double recombinants were confirmed using whole-genome sequencing, as described above. The Bauer Core Facility at Harvard University performed all genome sequencing.
Colony expansion, coswarm assays, and viable cell counts.
Overnight cultures were normalized to an optical density at 600 nm (OD600) of 0.1, and swarm-permissive nutrient plates supplemented with kanamycin were inoculated with 1 μl of normalized culture. Plates were incubated at 37°C for 16 h, and the radii of actively migrating swarms were measured. Additionally, the widths of individual swarm rings within the swarm colonies were recorded. For coswarm assays, strains were processed as described and mixed at a ratio of 1:1 where indicated.
For measurement of viable cell counts after 16 h, actively migrating swarms were resuspended in 6 ml of LB medium, and 20 μl of the cell suspension was used for a 10-fold dilution series. A total of eight dilutions were prepared for each sample, and 10 μl of each dilution was spotted onto LSW− agar plates supplemented with kanamycin.
For measurement of viable cell counts over time, swarm plates were set up as described above. Viable cell counts at time zero were determined by preparing a 10-fold dilution series of the normalized overnight cultures and spotting 10 μl of each dilution on LSW− agar plates supplemented with kanamycin. Viable cell counts at 2, 4, 6, and 8 h postinoculation were determined by resuspending swarm colonies in 1 ml LB medium and preparing 10-fold dilution series as described above; 10 μl of each dilution was spotted onto LSW− agar plates supplemented with kanamycin. Dilutions with countable numbers of colonies were used to determine the viable cell counts of swarm colonies.
Swimming assay.
Overnight cultures were normalized to an OD600 of 0.1. An inoculation needle was used to inoculate 0.3% LB nutrient plates supplemented with kanamycin. Plates were incubated at 37°C for 9 h, and the diameters of swim colonies were measured.
Measurement of generation times.
Overnight cultures were normalized to an OD600 of 0.1 in LB medium supplemented with kanamycin. Normalized cultures were grown overnight at 37°C, with periodic shaking, in a Tecan Infinite 200 PRO microplate reader (Tecan, Männedorf, Switzerland). Generation times were calculated from logarithmic phase growth measurements.
Trichloroacetic acid precipitation, SDS-PAGE, Western blotting, and LC-MS/MS analysis.
Trichloroacetic acid precipitations were performed as described previously (5). Samples were normalized according to the OD600 of the liquid cultures at the time of collection, separated by gel electrophoresis using 12% Tris-tricine polyacrylamide gels, transferred onto 0.45-μm nitrocellulose membranes, and probed with monoclonal rabbit anti-FLAG (Sigma-Aldrich, St. Louis, MO) and mouse anti-σ70 (Thermo Fisher Scientific) primary antibodies, followed by polyclonal goat anti-rabbit IgG and goat anti-mouse IgG secondary antibodies (KPL, Inc., Gaithersburg, MD), respectively. Membranes were developed with the Immun-Star horseradish peroxidase (HRP) substrate kit (Bio-Rad Laboratories, Hercules, CA) and visualized using a ChemiDoc imaging system (Bio-Rad Laboratories). TIFF images were exported and figures were prepared in Adobe Illustrator (Adobe Systems, San Jose, CA).
To detect secreted proteins in liquid supernatants by mass spectrometry (MS), trichloroacetic acid precipitations were performed as described previously (5). Samples were normalized according to the OD600 of the liquid cultures at the time of collection, separated by gel electrophoresis using 12% Tris-tricine polyacrylamide gels, and stained with Coomassie blue. Gel fragments corresponding to molecular masses of approximately 70 to 150 kDa were excised and subjected to liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The Taplin Biological Mass Spectrometry Facility at Harvard Medical School performed the LC-MS/MS analyses.
Boundary assay.
Boundary assays were conducted as reported previously (5). Assays were carried out using swarm-permissive agar plates supplemented with kanamycin.
Phase-contrast microscopy.
One-millimeter-thick swarm-permissive agar pads supplemented with kanamycin were inoculated with overnight cultures. The agar pads were incubated at 37°C in a modified humidity chamber. After 6 h, the pads were imaged by phase-contrast microscopy using a Leica DM5500B microscope system (Leica Microsystems, Buffalo Grove, IL) and a CoolSnap HQ2 cooled charge-coupled-device (CCD) camera (Photometrics, Tucson, AZ). MetaMorph (version 7.8.0.0; Molecular Devices, Sunnyvale, CA) was used for image acquisition. Images were acquired every 2 s for 78 s. Image stacks were imported into Fiji (ImageJ 1.48s) (37–40), where the image stacks were cropped to show a segment of cells, combined into a single movie from four individual movies, and converted to an .AVI file with a frame rate of five frames per second.
RESULTS
Noncognate D-E pairs cause restricted swarm colony expansion but not reduced viability or apparent swarmer cell differentiation.
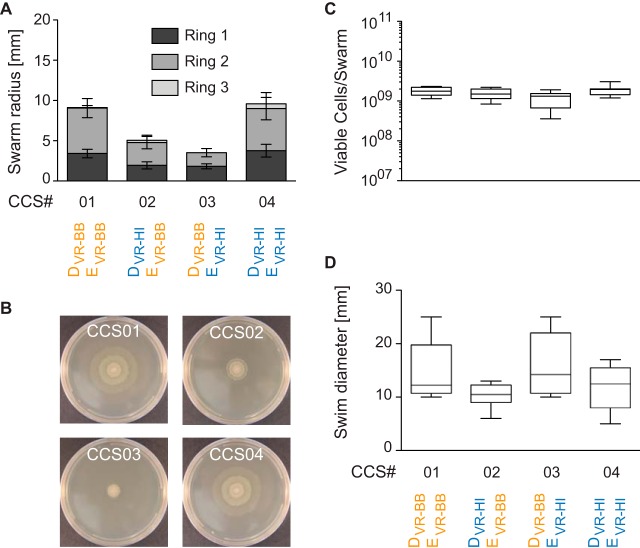
Swarming colonies of P. mirabilis strain BB2000 carrying mutations in the variable regions (VRs) of the ids operon appear unusually small in diameter (31). To investigate the effects of Ids-mediated self-recognition on swarm colony expansion, we utilized these P. mirabilis strains, which are genetically identical except for the VRs in D and E. The VRs originated either from wild-type strain BB2000 (VR-BB) or from wild-type strain HI4320 (VR-HI). The ids operon, including the genes for D and E, was maintained on a low-copy-number plasmid under the control of the native promoter in the Δids strain, which is a BB2000-derived strain lacking the chromosomal copy of the ids locus (2). This complemented Δids strain and its derivatives are the standard tools for studying the Ids system (2, 5, 31). We observed that, after 16 h on swarm-permissive agar, swarm colonies of a strain producing the cognate DVR-BBEVR-BB pair (CCS01) expanded further than swarm colonies of strains producing the noncognate DVR-HIEVR-BB (CCS02) or DVR-BBEVR-HI (CCS03) pair (Fig. 1A). In contrast, a strain producing the cognate DVR-HIEVR-HI pair (CCS04) showed recovered swarm expansion (Fig. 1A). Differences in colony expansion persisted even after 24 h (Fig. 1B). Thus, swarm colony expansion was restricted when noncognate D and E proteins were present.
FIG 1.
Binding states of D and E regulate swarm colony expansion. (A) Colony expansion of monoclonal P. mirabilis swarms after 16 h on swarm-permissive agar surfaces. Variable region (VR) identities of the produced D and E variants are indicated for each strain. VRs originate either from P. mirabilis strain BB2000 (orange) or from P. mirabilis strain HI4320 (blue). Widths of individual swarm rings within a swarm colony are marked by different shades. Error bars, standard deviations of individual swarm ring widths (n = 16). (B) Representative pictures of each strain from panel A, taken 24 h after inoculation. (C) Viable cells per monoclonal swarm colony after 16 h on swarm-permissive agar surfaces. Boxes range from the 25th to the 75th percentile, lines within boxes indicate medians, and whiskers indicate minima and maxima. Strain descriptions are found in panel D (n = 12). (D) Diameters of monoclonal colonies in 0.3% LB medium after 9 h (n = 6). Box plots are structured as described for panel C.
Whether D and E contained cognate or noncognate VRs, however, had no measurable effect on the number of swarm rings per colony (Fig. 1A), on growth on surfaces (Fig. 1C and 2A), or on growth in liquid (Fig. 2B), suggesting that growth and swarm-related developmental cycles were not impaired. Marginal differences in colony expansion during swimming in low-percentage agar were observed between these strains (Fig. 1D), and individual cells of all four strains were capable of differentiating into elongated, actively moving, swarmer cells (see Movie S1 in the supplemental material). Therefore, noncognate D-E pairs did not appear to inhibit cell viability, swimming motility, or swarm colony development, although macroscopic swarm colony expansion was impaired. We reasoned that this stark phenotype could be used to address the outstanding question of how the Ids system communicates identity information between cells within a colony.
FIG 2.

Viability on surfaces and generation times in liquid are unaltered when D and E are noncognate. (A) Viable cells per swarm colony over time on swarm-permissive agar surfaces. D and E variants produced by the different strains are indicated in panel B. Error bars, standard deviations (n = 4). (B) Generation times during logarithmic growth in liquid medium (n = 6). Boxes range from the 25th to the 75th percentile, lines within boxes indicate medians, and whiskers indicate minima and maxima.
D communicates identity between neighboring cells.
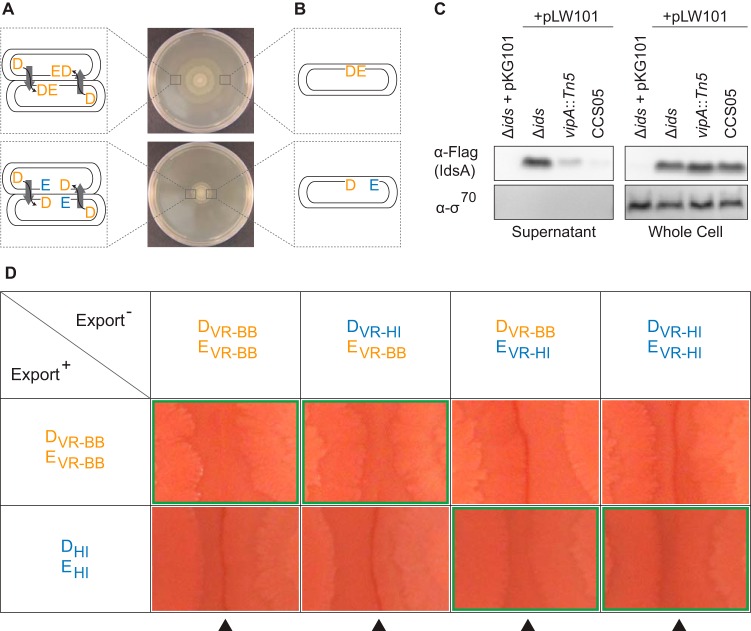
There are two prevailing mechanistic models for where the causative in vivo interactions between D and E may occur. D-E binding could happen between neighboring cells (Fig. 3A) or within single cells (Fig. 3B). To distinguish between these models, we first examined whether D export is necessary for its function. We used a Δids mutant-derived strain deficient in T6SS-mediated transport (CCS05). CCS05 carries a mutation in vipA; the encoded protein, VipA (T6SS_VipA [PFAM family PF05591]), is essential for export of T6SS-related factors (11, 41, 42). To confirm the loss of T6SS-mediated transport, IdsA (T6SS_Hcp [PFAM family PF05638]) carrying a C-terminal FLAG epitope tag (N-DYKDDDDK-C) (5) was introduced into CCS05. Export of Hcp homologs, such as IdsA, is a hallmark of T6SS-dependent protein transport and has often been used to evaluate T6SS activity (5, 8, 9, 11, 26, 43). Furthermore, the export of D, as well as IdsB, is dependent on a functional T6SS and has been correlated with IdsA export (5). The export of IdsA-FLAG from the CCS05-derived strain was markedly decreased, compared to that from the otherwise genetically equivalent strain expressing wild-type vipA (Fig. 3C). We then analyzed supernatants isolated from these strains by LC-MS/MS and found that peptides corresponding to IdsB and D were absent in the CCS05-derived strain expressing mutant vipA, in contrast to the Δids mutant-derived strain expressing wild-type vipA (Table 2). These results indicate that CCS05 is deficient in T6SS-mediated export, including the loss of IdsA, IdsB, and D transport.
FIG 3.
D is communicated between cells. (A and B) Competing mechanistic models for the mode of D-E interactions. (A) Intercellular T6SS-dependent communication (arrows) of D from one cell (double-walled oval) to a neighboring cell. Binding to E in the recipient cell allows swarm colony expansion to proceed, while lack thereof impairs colony expansion. This communication is bidirectional if both cells have a functional T6SS. (B) Swarm colony expansion depends on the binding states of the D and E variants produced within individual cells. (C) IdsA (Hcp) secretion analysis. Supernatants of strains carrying either the empty vector pKG101 (6) or pLW101, which produces IdsA (T6SS_Hcp [PFAM family PF05638]) with a C-terminal FLAG tag (5), were subjected to trichloroacetic acid precipitations. Whole-cell extracts were also obtained. All samples were analyzed using Western blot analysis. The BB2000-derived vipA::Tn5 strain contains a chromosomal transposon insertion in the gene encoding VipA (T6SS_VipA [PFAM family PF05591]) (5). Blots were probed with antibodies against FLAG to detect IdsA-FLAG and against σ70 as a cell lysis control. (D) Self-recognition behavior of export-inactive strains. Swarm-permissive agar surfaces were inoculated with export-active Δids mutant-derived donor strains on the left side and export-inactive CCS05-derived recipient strains on the right side. Each strain produces the indicated D and E variants. Variable region (VR) exchanges from BB2000 (BB) to HI4320 (HI) are indicated with the prefix “VR.” DHI and EHI are D and E variants, respectively, derived completely from HI4320. Green outlines, combinations of swarms that merged. Arrowheads, intersections of opposing swarm colonies.
TABLE 2.
LC-MS/MS results for supernatant fractions, from ∼70 to 150 kDa
| Strain | Protein | Predicted size (kDa) | No. of unique peptides | No. of total peptides | Coverage (%) |
|---|---|---|---|---|---|
| Δids strain carrying pLMW101 (export active) | σ70 | 71.11 | 3 | 3 | 4.05 |
| IdsB | 81.55 | 5 | 5 | 10.65 | |
| IdsD | 118.16 | 2 | 6 | 2.32 | |
| CCS05 carrying pLMW101 (export inactive) | σ70 | 71.11 | 2 | 2 | 2.91 |
We utilized CCS05-derived strains expressing different combinations of D and E variants, as described in Fig. 1, as indicator strains to determine whether D is received from neighboring cells. We determined boundary formation phenotypes of these CCS05-derived strains when swarmed against Δids mutant-derived (export-active donor) strains that produced D and E proteins either from strain BB2000 (DVR-BBEVR-BB) or from strain HI4320 (DHIEHI). These two export-active strains form a boundary against each other and are nonself (31). Swarming populations of the CCS05-derived strains producing EVR-BB (and either DVR-BB or DVR-HI) merged with the donor strain producing DVR-BBEVR-BB but not with the donor strain producing DHIEHI (Fig. 3D). Conversely, CCS05-derived strains producing EVR-HI (and either DVR-BB or DVR-HI) merged with the donor strain producing DHIEHI (Fig. 3D). In all cases, the D variant produced by the export-inactive CCS05-derived strain did not affect the outcome (Fig. 3D). Thus, the identities of the D variant in the donor strain and the E variant in the export-inactive CCS05-derived strain correlated with whether the populations merged or formed a boundary.
Given these data, the observed impairment in swarm colony expansion of CCS02 and CCS03, i.e., strains producing noncognate D and E proteins (Fig. 1A), could be explained by the presence of unbound D in the recipient cells (Fig. 3A). If so, then a similar defect would be expected for strains lacking E since, in a clonal population, every cell could export as well as receive D and would have no E to bind it. To test this hypothesis, we constructed a Δids mutant-derived strain complemented with an ids operon that lacks the gene encoding E (CCS06). CCS06 swarms displayed colony expansion similar to that of CCS02 and CCS03 (Fig. 4A). CCS06 did not exhibit defects in swarm rings per colony (Fig. 4A), growth on surfaces (Fig. 2A), or growth in liquid (Fig. 2B). Therefore, the presence of unbound D indeed impaired swarm colony expansion.
FIG 4.
Unbound D in a recipient cell impairs swarm colony expansion. (A) Colony expansion, as described in the legend to Fig. 1A. Strains were inoculated either as monoswarms (export-active CCS06 donor or export-inactive CCS05-derived recipients) or as coswarms (CCS06 and CCS05 derivatives at a 1:1 ratio). D and E variants produced by strains derived from CCS05 are indicated. CCS06 lacks E but produces DVR-BB. Error bars, standard deviations for each swarm ring width (n = 3 for monoswarms and coswarms of DVR-BBEVR-HI and DVR-HIEVR-HI; n = 6 for all others.). Fold changes of total colony expansion between monoswarms and coswarms are indicated. na, not applicable. (B) Representative pictures of monoswarms and coswarms, taken 24 h after inoculation. (C) Models of intercellular T6SS-dependent (arrows) communication of DVR-BB from an export-active CCS06 cell (gray) to its neighboring cell, whether export active (gray) or export inactive (CCS05 derivative) (white). For export-inactive cells, only the resident E is depicted and the resident D is omitted.
There remained, however, the question of whether D exchange between cells is crucial for this inhibition of swarm colony expansion or whether unbound self-produced D could also affect this self-recognition behavior. Therefore, we examined the swarm colony expansion of export-inactive CCS05-derived cells lacking E. In this strain, cells contain self-produced D but cannot export D, i.e., cells do not contain transferred D. This strain exhibited a rescued swarm colony expansion phenotype (Fig. 4A). Together, these results support the hypothesis that D is exported and transferred between cells (Fig. 3A). Moreover, transferred unbound D in recipient cells, rather than self-produced D, appears to impair swarm colony expansion.
Interactions between transferred D and resident E impact swarm colony expansion.
We hypothesized that the transfer of D might be sufficient to induce impaired swarm colony expansion. We investigated this hypothesis by examining the swarm colony expansion of 1:1 mixtures of two strains, i.e., coswarms. Strain CCS06 (lacking E) was coswarmed with the nearly isogenic CCS05-derived recipient strain lacking both E and a functional T6SS. We observed a 1.75-fold decrease in expansion of the coswarm colony, compared to that of a monoculture swarm of the recipient strain (Fig. 4A), indicating that transfer of D to recipient cells restricted swarm colony expansion.
We further hypothesized that transfer of D and its resulting binding state with E in recipient cells determines whether or not swarm colony expansion is restricted. Therefore, we used CCS06 as a donor of DVR-BB in 1:1 mixtures with export-inactive CCS05-derived recipient strains that produced either EVR-BB, which binds DVR-BB, or EVR-HI, which cannot bind DVR-BB. All coswarms were compared to monoculture swarms of the recipient strain. In coswarms of CCS06 with the recipient strain producing DVR-BB and EVR-HI, a 3.12-fold reduction was observed (Fig. 4). Similarly, a coswarm of CCS06 with the recipient strain producing DVR-HI and EVR-HI resulted in a 3.35-fold reduction in colony expansion (Fig. 4). In contrast, mixing CCS06 with the recipient strain producing DVR-BB and EVR-BB resulted in marginal reduction of swarm colony expansion (Fig. 4). In sum, only marginal restriction appeared when the E variant in the recipient strain was capable of binding DVR-BB from the donor strain. However, we observed an approximately 2- to 3-fold restriction in swarm colony expansion when E in the recipient strain was noncognate to DVR-BB. Thus, communication of D from donor to recipient cells causes restricted swarm colony expansion. Alleviation of this swarm restriction can be achieved by the presence of a cognate E in the recipient cells.
While observing monoclonal swarms, we unexpectedly noticed that the production of E in recipient strains, regardless of whether a cognate D was produced, resulted in marked decreases in colony expansion (average, 2.3-fold), compared to that for an otherwise identical strain that lacked E (Fig. 4A). These results raise the possibility that, independently of D, E itself contributes to repression of swarm colony expansion.
DISCUSSION
Here, we have addressed unresolved questions regarding the communication of Ids proteins within a colony of swarming P. mirabilis cells. We have shown that the self-identity protein D is communicated from one cell to another. We have also presented evidence that D from donor cells likely interacts with E in recipient cells; lack of this interaction negatively impacts swarm colony expansion but not viability. Therefore, D might represent a class of nonlethal T6SS effector proteins.
Based on the prominent T6SS models for effector-inhibitor pairs, it was expected that unbound D, whether in donor or recipient cells, should suffice to act as an effector; this was not strictly observed, however, since unbound D was active only in recipient cells. D might not be in a folded or active state in donor cells. Even more surprisingly, the presence of E in export-impaired cells appeared to have an inhibitory effect on colony expansion (Fig. 4A). Together, our observations suggest that D and E regulate swarm colony expansion, although the specific molecular mechanisms remain to be determined.
It is a bit perplexing that D is communicated between cells in a T6SS-dependent manner, as D is over 100 kDa in size and contains two predicted transmembrane segments (31). Many T6SS-exported effectors are under 50 kDa, and the inner Hcp tube constituting the channel of many T6SSs has a width of 40 Å in multiple bacteria (8, 44–47). In fact, a variety of T6SS effectors bind to the inside of the Hcp tube, which allows them to be exported (43). The size of the P. mirabilis T6SS pore has not been directly measured. Given the large size of D, it might be communicated via the T6SS by an alternative mechanism. For example, D might be exported out of the donor cell in an unfolded state and then fold into its active state before or after being received by the recipient cell. This would be consistent with the observation that D transfer is required for its activity (Fig. 4A). Clearly, the macromolecular states of D before, during, and after transfer remain to be resolved to explain this transfer.
Microbial communities frequently exhibit cell-to-cell communication, in many cases involving the exchange of information about kin group identity. Self versus nonself recognition allows that certain group behaviors occur primarily between closely related individuals and/or exclude nonkin cells from shared resources. Many of the mechanisms for the exchange of kin group identity can be distinguished on the basis of their contact dependency or effects on viability. Quorum sensing, by which groups of bacteria can roughly assess cell population density, is an example of contact-independent recognition. In that case, kin group identity information is encoded by the molecular structure of the quorum-sensing molecule and its ability to bind its protein receptor (48–51). Because quorum-sensing molecules are often diffusible across membranes (52, 53), however, recognition events do not require physical contact between cells and can occur over greater spatial distances than contact-dependent mechanisms.
In contrast, contact-dependent interactions are local. These recognition events usually require cell-to-cell contact and can involve lethal attacks on nonkin members of the community. For example, contact-dependent killing mechanisms have been described for antagonistic interactions between species and even genera, e.g., T6SS-associated killing (8–10, 13, 14, 16–20, 27, 28), and within species, e.g., contact-dependent inhibition (CDI) (54–61). From a competition perspective, this could be beneficial; if susceptible competitor cells are inhibited, fewer cells will compete for resources such as nutrients. However, the existence of contact-dependent recognition that does not involve killing, as demonstrated here for the self-identity proteins D and E, suggests that there likely are fitness and competitive advantages to recognizing cells of the same kin group. For sibling cells of the bacterium Myxococcus xanthus, fusion of outer membranes, which is mediated by a recognition protein, can contribute to overall increased colony fitness (62). For P. mirabilis, swarm expansion of the colony involves intimate interactions between individual cells (63); therefore, cooperation might be essential for long-range motility. One purpose of the Ids system, and specifically of the self-identity proteins D and E, might be to restrict cooperative motility behavior to kin cells. Therefore, the transfer of D and its subsequent interactions with E may represent an additional form of cell-cell communication within a bacterial population.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lia Cardarelli, Richard Losick, and members of the Gibbs laboratory for advice and thoughtful discussions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00402-16.
REFERENCES
- 1.Budding AE, Ingham CJ, Bitter W, Vandenbroucke-Grauls CM, Schneeberger PM. 2009. The Dienes phenomenon: competition and territoriality in swarming Proteus mirabilis. J Bacteriol 191:3892–3900. doi: 10.1128/JB.00975-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbs KA, Urbanowski ML, Greenberg EP. 2008. Genetic determinants of self identity and social recognition in bacteria. Science 321:256–259. doi: 10.1126/science.1160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senior BW. 1977. The Dienes phenomenon: identification of the determinants of compatibility. J Gen Microbiol 102:235–244. doi: 10.1099/00221287-102-2-235. [DOI] [PubMed] [Google Scholar]
- 4.Dienes L. 1947. Further observations on the reproduction of bacilli from large bodies in Proteus cultures. Proc Soc Exp Biol Med 66:97–98. doi: 10.3181/00379727-66-15994. [DOI] [PubMed] [Google Scholar]
- 5.Wenren LM, Sullivan NL, Cardarelli L, Septer AN, Gibbs KA. 2013. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. mBio 4:e00374-13. doi: 10.1128/mBio.00374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs KA, Wenren LM, Greenberg EP. 2011. Identity gene expression in Proteus mirabilis. J Bacteriol 193:3286–3292. doi: 10.1128/JB.01167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alteri CJ, Himpsl SD, Pickens SR, Lindner JR, Zora JS, Miller JE, Arno PD, Straight SW, Mobley HL. 2013. Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells. PLoS Pathog 9:e1003608. doi: 10.1371/journal.ppat.1003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A 104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basler M, Mekalanos JJ. 2012. Type 6 secretion dynamics within and between bacterial cells. Science 337:815. doi: 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basler M, Ho BT, Mekalanos JJ. 2013. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152:884–894. doi: 10.1016/j.cell.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, Leiman PG. 2013. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500:350–353. doi: 10.1038/nature12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD. 2013. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496:508–512. doi: 10.1038/nature12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitney JC, Chou S, Russell AB, Biboy J, Gardiner TE, Ferrin MA, Brittnacher M, Vollmer W, Mougous JD. 2013. Identification, structure, and function of a novel type VI secretion peptidoglycan glycoside hydrolase effector-immunity pair. J Biol Chem 288:26616–26624. doi: 10.1074/jbc.M113.488320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A 107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyata ST, Kitaoka M, Brooks TM, McAuley SB, Pukatzki S. 2011. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect Immun 79:2941–2949. doi: 10.1128/IAI.01266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S. 2013. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J Biol Chem 288:7618–7625. doi: 10.1074/jbc.M112.436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, Provenzano D, Pukatzki S. 2014. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 5:3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durand E, Derrez E, Audoly G, Spinelli S, Ortiz-Lombardia M, Raoult D, Cascales E, Cambillau C. 2012. Crystal structure of the VgrG1 actin cross-linking domain of the Vibrio cholerae type VI secretion system. J Biol Chem 287:38190–38199. doi: 10.1074/jbc.M112.390153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunet YR, Espinosa L, Harchouni S, Mignot T, Cascales E. 2013. Imaging type VI secretion-mediated bacterial killing. Cell Rep 3:36–41. doi: 10.1016/j.celrep.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Durand E, Nguyen VS, Zoued A, Logger L, Pehau-Arnaudet G, Aschtgen MS, Spinelli S, Desmyter A, Bardiaux B, Dujeancourt A, Roussel A, Cambillau C, Cascales E, Fronzes R. 2015. Biogenesis and structure of a type VI secretion membrane core complex. Nature 523:555–560. doi: 10.1038/nature14667. [DOI] [PubMed] [Google Scholar]
- 26.Hachani A, Lossi NS, Hamilton A, Jones C, Bleves S, Albesa-Jove D, Filloux A. 2011. Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J Biol Chem 286:12317–12327. doi: 10.1074/jbc.M110.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hachani A, Allsopp LP, Oduko Y, Filloux A. 2014. The VgrG proteins are “a la carte” delivery systems for bacterial type VI effectors. J Biol Chem 289:17872–17884. doi: 10.1074/jbc.M114.563429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma LS, Hachani A, Lin JS, Filloux A, Lai EM. 2014. Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 16:94–104. doi: 10.1016/j.chom.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Le Trong I, Carl MA, Larson ET, Chou S, De Leon JA, Dove SL, Stenkamp RE, Mougous JD. 2012. Structural basis for type VI secretion effector recognition by a cognate immunity protein. PLoS Pathog 8:e1002613. doi: 10.1371/journal.ppat.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyata ST, Unterweger D, Rudko SP, Pukatzki S. 2013. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog 9:e1003752. doi: 10.1371/journal.ppat.1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardarelli L, Saak C, Gibbs KA. 2015. Two proteins form a heteromeric bacterial self-recognition complex in which variable subdomains determine allele-restricted binding. mBio 6:e00251-15. doi: 10.1128/mBio.00251-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomon D, Kinch LN, Trudgian DC, Guo X, Klimko JA, Grishin NV, Mirzaei H, Orth K. 2014. Marker for type VI secretion system effectors. Proc Natl Acad Sci U S A 111:9271–9276. doi: 10.1073/pnas.1406110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belas R, Erskine D, Flaherty D. 1991. Transposon mutagenesis in Proteus mirabilis. J Bacteriol 173:6289–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaniga K, Delor I, Cornelis GR. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 35.de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in Gram-negative eubacteria. J Bacteriol 172:6568–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturgill GM, Siddiqui S, Ding X, Pecora ND, Rather PN. 2002. Isolation of lacZ fusions to Proteus mirabilis genes regulated by intercellular signaling: potential role for the sugar phosphotransferase (Pts) system in regulation. FEMS Microbiol Lett 217:43–50. doi: 10.1111/j.1574-6968.2002.tb11454.x. [DOI] [PubMed] [Google Scholar]
- 37.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. 2015. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82:518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietzsch T, Preibisch S, Tomancak P, Saalfeld S. 2012. ImgLib2: generic image processing in Java. Bioinformatics 28:3009–3011. doi: 10.1093/bioinformatics/bts543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. 2009. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J 28:315–325. doi: 10.1038/emboj.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapitein N, Bonemann G, Pietrosiuk A, Seyffer F, Hausser I, Locker JK, Mogk A. 2013. ClpV recycles VipA/VipB tubules and prevents non-productive tubule formation to ensure efficient type VI protein secretion. Mol Microbiol 87:1013–1028. doi: 10.1111/mmi.12147. [DOI] [PubMed] [Google Scholar]
- 43.Silverman JM, Agnello DM, Zheng H, Andrews BT, Li M, Catalano CE, Gonen T, Mougous JD. 2013. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol Cell 51:584–593. doi: 10.1016/j.molcel.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz FM, Santillana E, Spinola-Amilibia M, Torreira E, Culebras E, Romero A. 2015. Correction: crystal structure of Hcp from Acinetobacter baumannii: a component of the type VI secretion system. PLoS One 10:e0136978. doi: 10.1371/journal.pone.0136978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osipiuk J, Xu X, Cui H, Savchenko A, Edwards A, Joachimiak A. 2011. Crystal structure of secretory protein Hcp3 from Pseudomonas aeruginosa. J Struct Funct Genomics 12:21–26. doi: 10.1007/s10969-011-9107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douzi B, Spinelli S, Blangy S, Roussel A, Durand E, Brunet YR, Cascales E, Cambillau C. 2014. Crystal structure and self-interaction of the type VI secretion tail-tube protein from enteroaggregative Escherichia coli. PLoS One 9:e86918. doi: 10.1371/journal.pone.0086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz FM, Santillana E, Spinola-Amilibia M, Torreira E, Culebras E, Romero A. 2015. Crystal structure of Hcp from Acinetobacter baumannii: a component of the type VI secretion system. PLoS One 10:e0129691. doi: 10.1371/journal.pone.0129691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray KM, Passador L, Iglewski BH, Greenberg EP. 1994. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J Bacteriol 176:3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 50.Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, Marco SD. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J 21:4393–4401. doi: 10.1093/emboj/cdf459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang RG, Pappas KM, Brace JL, Miller PC, Oulmassov T, Molyneaux JM, Anderson JC, Bashkin JK, Winans SC, Joachimiak A. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan HB, Greenberg EP. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio-Fischeri luminescence system. J Bacteriol 163:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearson JP, Van Delden C, Iglewski BH. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol 181:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 55.Aoki SK, Malinverni JC, Jacoby K, Thomas B, Pamma R, Trinh BN, Remers S, Webb J, Braaten BA, Silhavy TJ, Low DA. 2008. Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol Microbiol 70:323–340. doi: 10.1111/j.1365-2958.2008.06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoki SK, Diner EJ, de Roodenbeke CT, Burgess BR, Poole SJ, Braaten BA, Jones AM, Webb JS, Hayes CS, Cotter PA, Low DA. 2010. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468:439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole SJ, Diner EJ, Aoki SK, Braaten BA, t' Kint de Roodenbeke C, Low DA, Hayes CS. 2011. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet 7:e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. 2012. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI). Genes Dev 26:515–525. doi: 10.1101/gad.182345.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruhe ZC, Wallace AB, Low DA, Hayes CS. 2013. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. mBio 4:e00480–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson MS, Garcia EC, Cotter PA. 2012. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet 8:e1002877. doi: 10.1371/journal.pgen.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson MS, Garcia EC, Cotter PA. 2014. Kind discrimination and competitive exclusion mediated by contact-dependent growth inhibition systems shape biofilm community structure. PLoS Pathog 10:e1004076. doi: 10.1371/journal.ppat.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vassallo C, Pathak DT, Cao P, Zuckerman DM, Hoiczyk E, Wall D. 2015. Cell rejuvenation and social behaviors promoted by LPS exchange in myxobacteria. Proc Natl Acad Sci U S A 112:E2939–E2946. doi: 10.1073/pnas.1503553112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones BV, Young R, Mahenthiralingam E, Stickler DJ. 2004. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect Immun 72:3941–3950. doi: 10.1128/IAI.72.7.3941-3950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.