Abstract
Background
Severe anemia is a major cause of sickness and death in African children, yet the causes of anemia in this population have been inadequately studied.
Methods
We conducted a case-control study of 381 preschool children with severe anemia (hemoglobin concentration, <5.0 g per deciliter) and 757 preschool children without severe anemia in urban and rural settings in Malawi. Causal factors previously associated with severe anemia were studied. The data were examined by multivariate analysis and structural equation modeling.
Results
Bacteremia (adjusted odds ratio, 5.3; 95% confidence interval [CI], 2.6 to 10.9), malaria (adjusted odds ratio, 2.3; 95% CI, 1.6 to 3.3), hookworm (adjusted odds ratio, 4.8; 95% CI, 2.0 to 11.8), human immunodeficiency virus infection (adjusted odds ratio, 2.0; 95% CI, 1.0 to 3.8), the G6PD−202/−376 genetic disorder (adjusted odds ratio, 2.4; 95% CI, 1.3 to 4.4), vitamin A deficiency (adjusted odds ratio, 2.8; 95% CI, 1.3 to 5.8), and vitamin B12 deficiency (adjusted odds ratio, 2.2; 95% CI, 1.4 to 3.6) were associated with severe anemia. Folate deficiency, sickle cell disease, and laboratory signs of an abnormal inflammatory response were uncommon. Iron deficiency was not prevalent in case patients (adjusted odds ratio, 0.37; 95% CI, 0.22 to 0.60) and was negatively associated with bacteremia. Malaria was associated with severe anemia in the urban site (with seasonal transmission) but not in the rural site (where malaria was holoendemic). Seventy-six percent of hookworm infections were found in children under 2 years of age.
Conclusions
There are multiple causes of severe anemia in Malawian preschool children, but folate and iron deficiencies are not prominent among them. Even in the presence of malaria parasites, additional or alternative causes of severe anemia should be considered.
Introduction
Severe anemia (hemoglobin concentration less than 5.0 g per deciliter) is a major cause of sickness and death among children in sub-Saharan Africa.1–4 In various settings, 12 to 29% of hospitalized children are severely anemic,1–4 and the in-hospital case fatality rate in these children is 8 to 17%.1,3,4 Little is known about the causes of severe anemia in African children. Most studies have been confined to the anemia associated with malaria5 or with other individual factors.1,2,6 As a result, the treatment guidelines advocated by the World Health Organization (WHO) deal specifically with malaria, folate deficiency, and iron deficiency, which are widely held to be the most common causes of severe anemia in African children.7 To improve our understanding of severe anemia, we conducted a case-control study in Malawi to assess the causative factors in Malawian children with severe anemia.
Methods
Study sites
We conducted the study in Malawi at Chikwawa District Hospital in a rural area where malaria infections occur throughout the year (approximately 170 infectious bites per person per year) and at Queen Elizabeth Central Hospital, a referral hospital in urban Blantyre, where malaria is seasonal, largely coinciding with the rainy season (approximately 1 infectious bite per person per year) (Mzilahowa T: personal communication). Predefined catchment areas were used; the urban area was confined to the city limits.
Study design
Between July 2002 and July 2004, a consecutive sample of children (382 case patients) who presented at the outpatient department during working hours with a primary diagnosis of severe anemia (defined as a hemoglobin concentration of <5.0 g per deciliter) were recruited into a prospective case-control study. Additional inclusion criteria were an age between 6 and 60 months and no blood transfusion within the previous 4 weeks.
For each case patient, a community control patient and a hospital control patient were enrolled. Community control patients were recruited from apparently healthy residents living within 100 to 1000 m of the case patient; hospital control patients were recruited by selecting the first child presenting at the outpatient department at the same time on the next working day after presentation of the case patient. Community and hospital control patients were eligible for recruitment if their hemoglobin level was at least 5.0 g per deciliter and they were between 6 and 60 months of age; no other matching was applied. Written informed consent was obtained from a parent or a guardian of each child in all three study groups. The study was approved by the ethics committees of the College of Medicine, Malawi, and the Liverpool School of Tropical Medicine, United Kingdom.
Clinical assessment and management
On enrollment, a clinical research form, including a medical and dietary history, sociodemographic data, and physical examination, was completed, and samples of blood, urine, and stool were collected. In case patients, if the clinical condition permitted it, a bone marrow aspirate was obtained under local anesthesia. Children requiring admission were treated in a study ward. All conditions were managed according to standard protocols.
Anthropometric measurements
Nutritional z scores were calculated according to the WHO growth reference curves8 with the use of Epi Info 2000. Weight-for-height, height-for-age, and weight-for-age z scores of less than −2 were considered to indicate wasting, stunting, and underweight, respectively; z scores of less than −3 were considered to indicate severe wasting, severe stunting, or severe underweight.
Laboratory methods
Laboratory tests (hematologic, bacteriologic, and parasitologic) were performed within 24 hours after collection, and aliquots were stored at −80°C for later analysis. The laboratory staff were unaware of the children's study groups.
Hematologic studies
Hemoglobin concentration was measured on site with a Hem°Cue system. A complete blood count, including reticulocytes, was performed by a Coulter counter. In case patients, bone marrow slides were stained with Hematognost Fe (Merck) and graded for iron content9; these results were used to validate peripheral blood markers of iron deficiency. The ratio of soluble transferrin receptor to log ferritin (TfR-F index)10 best predicted bone marrow iron status, irrespective of the presence of infection; a TfR-F index greater than 5.6 was used to define iron deficiency with a sensitivity of 70% and a specificity of 75% (unpublished data).
Chemical studies
Plasma levels of C-reactive protein, haptoglobin, transferrin, iron, ferritin, folate, and vitamin B12 were analyzed on Modular P800 and Modular Analytics E170 systems (Roche). Inflammatory cytokine profiles were measured by Cytometric Bead Array on a FACSCalibur flow cytometer (Becton Dickinson). Serum vitamin A (retinol) and soluble transferrin receptor were measured by high-performance liquid chromatography11 and enzyme-linked immunosorbent assay, respectively.
Parasitologic Studies
The number of Plasmodium falciparum asexual parasites per 200 white cells was counted and expressed as the number per microliter of blood. Malaria slides were read by two independent readers, with a third being used if the results differed by more than 25%. Malaria was defined by the presence of P. falciparum asexual parasites. Recent or current malaria was defined by the presence of P. falciparum asexual parasites in erythrocytes or malaria pigment in monocytes or macrophages.12 Hyperparasitemia was defined as more than 100,000 parasites per microliter.2 Stool samples were examined for helminths by the Kato-Katz method.13 Heavy hookworm infection was defined by the presence of more than 1000 ova per gram of feces. A polymerase-chain-reaction (PCR) test was used to confirm the microscopical results and define the species (Ancylostoma duodenale or Necator americanus).14 Urine specimens were examined for Schistosoma haematobium by a semiquantitative concentration method.15
Bacteriologic studies
A bone marrow or venous blood sample (1 to 2 ml) was inoculated into BACTEC Myco/F-Lytic culture vials and incubated in a BACTEC 9050 automated culture system (Becton Dickinson) for 56 days. Subculturing, susceptibility testing, and isolate identification were performed by standard techniques.16 Cultures were checked for mycobacteria with the use of Ziehl-Neelsen staining of smears. Mixed growths or growths of micrococci, bacillus species, or coagulase-negative staphylococci were considered contaminants.
Virologic studies
Whole-blood isolates17 were assessed for Epstein-Barr virus and cytomegalovirus infection by semiquantitative PCR18 and for parvovirus by real-time PCR.19 Infections were considered clinically important if the number of viral copies exceeded 1000 per milliliter of blood. Testing for human immunodeficiency virus (HIV) was performed by two rapid tests (Determine, Abbott Laboratories; and Uni-Gold, Trinity Biotech). Discordant or positive rapid-test results in children less than 18 months of age were resolved by PCR.20
Genetic studies
DNA was extracted with a Nucleon extraction kit (Amersham Biosciences) and genotyped by primer-extension mass spectrometry with the use of MassARRAY (Sequenom).21 The presence of sickle cell disease (homozygosity for hemoglobin S) and single-nucleotide polymorphisms in the promoter regions of the genes encoding interleukin-10 (IL10) (−1117, −3585, and +4949)21 and tumor necrosis factor (TNF) (−238, −308, and −1031)22 was determined. The term G6PD−202/−376 is used to denote boys who are hemizygous and girls who are homozygous for both the G6PD202A and the G6PD376G alleles, a condition that is strongly predictive of glucose-6-phosphate dehydrogenase deficiency.23 The Hardy-Weinberg equilibrium was applied (cutoff, P<0.001), and there was no significant population stratification. We chose the allele frequency, dominant model, or haplotype that was most strongly associated with severe anemia.
Statistical analysis
The prevalence rates of each factor were compared individually across the three groups with the use of Fisher's exact test and the chi-square test. The combined association of characteristics related to the risk of severe anemia (P≤0.10, unless the characteristics were uncommon [<5%]) was examined by an unconditional multivariate logistic-regression model corrected for potential confounding factors (age, sex, recent use [i.e., within the previous 8 weeks] of antimalarial or hematinic agents, and a history of transfusions [i.e., before the previous 4 weeks]). Missing observations were included in the analysis by creating missing-value categories. Alternative definitions for malaria, hookworm, and nutritional deficiencies and status were tested. Attributable-risk percentages were calculated with the use of adjusted odds ratios.24 The primary analysis compared all case patients with the two control groups combined. To explore the possibility that different patient characteristics were important in the two study locations, secondary analyses were performed with stratification according to location and with the community and hospital control groups separated. More complex associations and alternative strategies for handling missing data (e.g., maximum-likelihood imputation) were explored by structural equation modeling.25 All reported P values are two-sided. The data were analyzed with the use of Stata (version 9), SPSS (version 12), and Amos (version 6.0) software.
Results
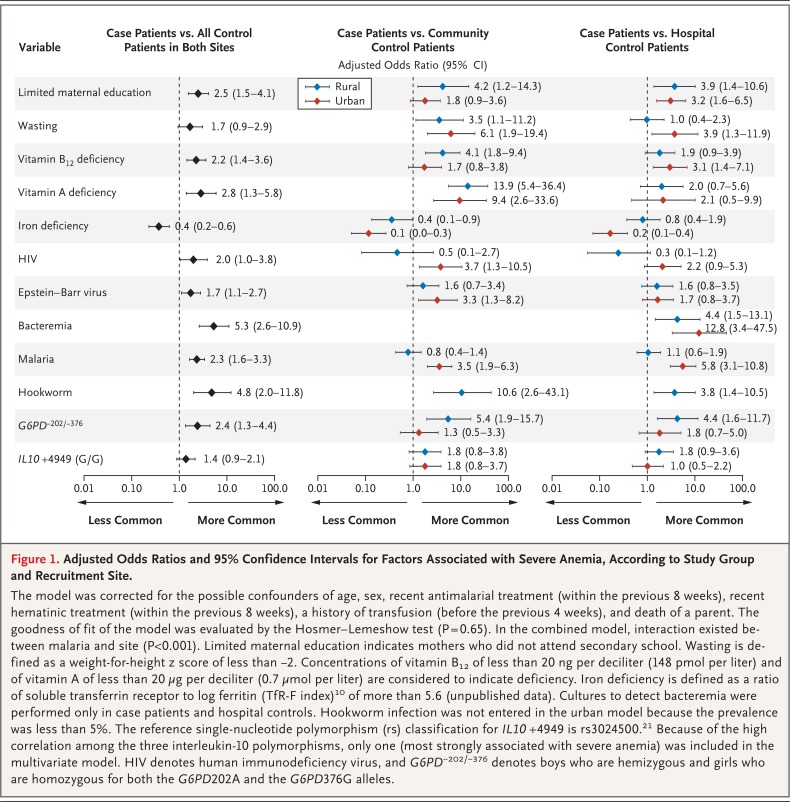
We enrolled 1141 children over a 2-year period. Five protocol violations occurred: two hospital control children had severe anemia and were redesignated as case patients, one case patient with a hemoglobin concentration of more than 5.0 g per deciliter was excluded, and two control patients under 6 months of age were excluded. Table 1 summarizes the characteristics of the 1138 children included in the analysis. Hemoglobin levels differed significantly between the case patients and each of the two control groups but were similar in the two control groups. Splenomegaly (in which >1 cm of the spleen was palpable) and severe splenomegaly (in which ≥8 cm was palpable) were more common in case patients (P<0.001 and P = 0.03, respectively). Severe splenomegaly, which was present in 11 case patients (3.0%), was not associated with thrombocytopenia or leukopenia. Jaundice was more common in case patients (5.0%) but was not associated with sickle cell disease (P = 1.00), G6PD−202/−376 (P = 0.70), or splenomegaly (P = 0.30). Twenty-four case patients (6.3%) died after admission to the hospital, nine (37.5%) before receiving a transfusion. We obtained samples of peripheral blood from 1105 subjects (97.1%), stool from 1024 subjects (90.0%), urine from 1042 subjects (91.6%), and bone marrow from 348 case patients (91.3%). Table 2 lists the features we investigated in the three groups and gives the P values for differences among the groups. Factors significantly associated with severe anemia were further explored in a multivariate and structural equation model (Fig. 1 and 2).
Table 1.
Characteristics of the Study Groups.*
| Characteristic | Case Patients (N = 381) |
Community Control Patients (N = 380) |
Hospital Control Patients (N = 377) |
| Area — no. (%)† | |||
| Urban | 205 (53.8) | 203 (53.4) | 201 (53.3) |
| Rural | 176 (46.2) | 177 (46.6) | 176 (46.7) |
| Sex — no. (%) | |||
| Female | 203 (53.3) | 191 (50.3) | 180 (47.7) |
| Male | 178 (46.7) | 189 (49.7) | 197 (52.3) |
| Age — mo‡ | 20.4±12.8 | 25.3±13.1 | 22.5±12.1 |
| Jaundice — no./total no. (%)§ | 19/379 (5.0) | 1/380 (0.3) | 0/376 |
| Splenomegaly — no./total no. (%)§¶ | 237/372 (63.7) | 108/363 (29.8) | 86/349 (24.6) |
| Fever — no./total no. (%)‖** | 189/376 (50.3) | 41/375 (10.9) | 172/374 (46.0) |
| Hemoglobin — g/dl§†† | 3.6±0.8 | 9.9±1.9 | 9.6±2.2 |
| Mean corpuscular volume — fl§‡‡ | 82.9±15.2 | 75.5±9.3 | 74.2±9.7 |
| Reticulocyte count — ×10−9/liter‡§§ | |||
| Median | 53.2 | 76.8 | 64.5 |
| Interquartile range | 30.2–91.7 | 46.4–114.7 | 43.0–103.2 |
| Admitted to hospital — no. (%)† | 381 (100) | 3 (0.8) | 17 (4.5) |
| Died in hospital — no. (%)† | 24 (6.3) | 0 | 0 |
Plus-minus values are means ±SD.
No statistical tests were applied.
P<0.05 for differences among all three groups (by Tukey post hoc test or Kruskal.Wallis test with Tukey multiple comparisons).
Community and hospital control patients were significantly different from case patients (P<0.05 by Tukey post hoc test). Case patients had a primary diagnosis of severe anemia (defined as a hemoglobin concentration of <5.0 g per deciliter).
Splenomegaly was defined as more than 1 cm of palpable spleen below the left costal margin in the midaxillary line.
Community control patients were significantly different from case patients and hospital control patients (P<0.05 by Tukey post hoc test).
Fever was defined as an axillary temperature of more than 37.5°C.
Hemoglobin values were recorded in 373 community control patients and 375 hospital control patients.
Mean corpuscular volume was recorded in 316 case patients 322 community control patients and 314 hospital control patients.
Reticulocyte values were recorded in 266 case patients, 284 community control patients, and 279 hospital control patients.
Table 2.
Distribution of Possible Etiologic and Confounding Factors among Study Groups According to Recruitment Sites.*
| Variable | Both Sites | Rural Site | Rural Site | Urban Site | |||||||||
| Case Patients (N = 381) |
Community and Hospital Control Patients (N = 757) |
P Value | Case Patients (N = 176) |
Community Control Patients (N = 177) |
P Value | Hospital Control Patients (N = 176) |
P Value | Case Patients (N = 205) |
Community Control Patients (N = 203) |
P Value | Hospital Control Patients (N = 201) |
P Value | |
| no./total no. (%) | no./total no. (%) | no./total no. (%) | no./total no. (%) | no./total no. (%) | |||||||||
| History | |||||||||||||
| Mother did not attend secondary school |
323/366 (88.3) | 554/753 (73.6) | <0.001 | 162/172 (94.2) | 154/176 (87.5) | 0.03 | 143/176 (81.2) | <0.001 | 161/194 (83.0) | 142/200 (71.0) | 0.005 | 115/201 (57.2) | <0.001 |
| Death of a parent | 25/284 (8.8) | 22/554 (4.0) | 0.004 | 13/168 (7.7) | 5/163 (3.1) | 0.06 | 3/164 (1.8) | 0.01 | 12/116 (10.3) | 6/109 (5.5) | 0.18 | 8/118 (6.8) | 0.33 |
| Recent hematinic treatment | 85/376 (22.6) | 61/754 (8.1) | <0.001 | 40/176 (22.7) | 7/177 (4.0) | <0.001 | 13/176 (7.4) | <0.001 | 45/200 (22.5) | 19/202 (9.4) | <0.001 | 22/199 (11.1) | 0.002 |
| Recent antimalarial treatment | 232/375 (61.9) | 346/755 (45.8) | <0.001 | 107/176 (60.8) | 79/177 (44.6) | 0.002 | 90/176 (51.1) | 0.07 | 125/199 (62.8) | 77/203 (37.9) | <0.001 | 100/199 (50.3) | 0.01 |
| History of transfusion | 57/378 (15.1) | 38/756 (5.0) | <0.001 | 24/176 (13.6) | 9/177 (5.1) | 0.006 | 13/176 (7.4) | 0.06 | 33/202 (16.3) | 7/203 (3.4) | <0.001 | 9/200 (4.5) | <0.001 |
| Malnutrition† | |||||||||||||
| Wasting | 52/330 (15.8) | 43/695 (6.2) | <0.001 | 24/169 (14.2) | 9/174 (5.2) | 0.005 | 19/175 (10.9) | 0.35 | 28/161 (17.4) | 6/182 (3.3) | <0.001 | 9/164 (5.5) | <0.001 |
| Iron deficiency | 97/208 (46.6) | 288/415 (69.4) | <0.001 | 71/101 (70.3) | 76/95 (80.0) | 0.12 | 63/92 (68.5) | 0.78 | 26/107 (24.3) | 76/113 (67.3) | <0.001 | 73/115 (63.5) | <0.001 |
| Vitamin B12 deficiency | 95/312 (30.4) | 94/603 (15.6) | <0.001 | 46/142 (32.4) | 20/143 (14.0) | <0.001 | 30/149 (20.1) | 0.02 | 49/170 (28.8) | 24/157 (15.3) | 0.003 | 20/154 (13.0) | <0.001 |
| Vitamin A deficiency | 228/247 (92.3) | 172/262 (65.6) | <0.001 | 113/126 (89.7) | 44/83 (53.0) | <0.001 | 60/74 (81.1) | 0.09 | 115/121 (95.0) | 32/60 (53.3) | <0.001 | 36/45 (80.0) | 0.003 |
| Viral infections | |||||||||||||
| HIV | 45/357 (12.6) | 41/682 (6.0) | <0.001 | 7/176 (4.0) | 5/176 (2.8) | 0.56 | 9/172 (5.2) | 0.58 | 38/181 (21.0) | 9/171 (5.3) | <0.001 | 18/163 (11.0) | 0.01 |
| Parvovirus B19 | 5/294 (1.7) | 2/609 (0.3) | 0.03 | 2/143 (1.4) | 0/147 | 0.15 | 1/146 (0.7) | 0.55 | 3/151 (2.0) | 1/157 (0.6) | 0.30 | 0/159 | 0.07 |
| Epstein-Barr virus | 89/269 (33.1) | 102/566 (18.0) | <0.001 | 43/128 (33.6) | 34/133 (25.6) | 0.16 | 24/127 (18.9) | 0.008 | 46/141 (32.6) | 14/148 (9.5) | <0.001 | 30/158 (19.0) | 0.007 |
| Bacteremia | 54/359 (15.0) | 14/353 (4.0) | <0.001 | 20/171 (11.7) | ND | 9/166 (5.4) | 0.04 | 34/188 (18.1) | ND | 5/187 (2.7) | <0.001 | ||
| Parasitic infections | |||||||||||||
| Plasmodium falciparum | 226/380 (59.5) | 321/750 (42.8) | <0.001 | 91/176 (51.7) | 93/175 (53.1) | 0.79 | 93/176 (52.8) | 0.83 | 135/204 (66.2) | 74/199 (37.2) | <0.001 | 61/200 (30.5) | <0.001 |
| Hyperparasitemic P. falciparum‡ | 45/380 (11.8) | 24/750 (3.2) | <0.001 | 17/176 (9.7) | 3/175 (1.7) | 0.001 | 11/176 (6.2) | 0.24 | 28/204 (13.7) | 3/199 (1.5) | <0.001 | 7/200 (3.5) | <0.001 |
| Recent or current P. falciparum | 243/334 (72.8) | 336/696 (48.3) | <0.001 | 113/169 (66.9) | 98/171 (57.3) | 0.07 | 98/175 (56.0) | 0.04 | 130/165 (78.8) | 83/180 (46.1) | <0.001 | 57/170 (33.5) | <0.001 |
| Hookworm | 29/296 (9.8) | 12/642 (1.9) | <0.001 | 27/154 (17.5) | 4/160 (2.5) | <0.001 | 8/156 (5.1) | <0.001 | 2/142 (1.4) | 0/164 | 0.13 | 0/162 | 0.13 |
| Schistosoma mansoni | 2/296 (0.7) | 8/643 (1.2) | 0.43 | 2/154 (1.3) | 4/160 (2.5) | 0.44 | 4/156 (2.6) | 0.42 | 0/142 | 0/164 | 0/163 | ||
| S. haematobium | 4/307 (1.3) | 8/669 (1.2) | 0.89 | 4/159 (2.5) | 6/168 (3.6) | 0.58 | 1/162 (0.6) | 0.17 | 0/148 | 0/171 | 1/168 (0.6) | 0.35 | |
| Genetic disorders§ | |||||||||||||
| G6PD−202/−376 | 44/318 (13.8) | 54/601 (9.0) | 0.02 | 21/152 (13.8) | 11/141 (7.8) | 0.10 | 9/145 (6.2) | 0.03 | 23/166 (13.9) | 20/161 (12.4) | 0.70 | 14/154 (9.1) | 0.18 |
| Sickle cell disease | 4/238 (1.7) | 4/404 (1.0) | 0.45 | 2/118 (1.7) | 2/101 (2.0) | 0.88 | 0/86 | 0.23 | 2/120 (1.7) | 0/106 | 0.18 | 2/111 (1.8) | 0.94 |
| IL10 −1117 (C/C+C/T vs. T/T) | 196/324 (60.5) | 332/607 (54.7) | 0.09 | 98/155 (63.2) | 75/141 (53.2) | 0.08 | 83/147 (56.5) | 0.23 | 98/169 (58.0) | 92/162 (56.8) | 0.83 | 82/157 (52.2) | 0.30 |
| IL10 −3585 (A/A vs. A/T+T/T) | 22/308 (7.1) | 25/575 (4.3) | 0.08 | 8/148 (5.4) | 6/134 (4.5) | 0.72 | 5/140 (3.6) | 0.45 | 14/160 (8.8) | 4/153 (2.6) | 0.02 | 10/148 (6.8) | 0.51 |
| IL10 +4949 (G/G vs. G/A+A/A) | 97/322 (30.1) | 134/606 (22.1) | 0.007 | 48/155 (31.0) | 31/140 (22.1) | 0.09 | 34/147 (23.1) | 0.13 | 49/167 (29.3) | 34/162 (21.0) | 0.08 | 35/157 (22.3) | 0.15 |
| Abnormal ratio of interleukin-10 to TNF-α |
4/276 (1.4) | NA | 1/122 (0.8) | NA | NA | 3/154 (1.9) | NA | NA | |||||
All P values are for the comparisons between case patients and community control patients, hospital control patients, or both. Among variables not included in the table (because they did not meet the preset cutoff for significance) are parental unemployment, household assets, folate deficiency, trichuriasis, ascariasis, cytomegalovirus infection, hemoglobin C, and tumor necrosis factor α (TNF-α) alleles or genotypes (−238, −308, and −1031). Recent is defined as within 8 weeks before recruitment. HIV denotes human immunodeficiency virus, IL10 the gene encoding interleukin-10, NA not available, and ND not done. G6PD−202/−376 denotes boys who are hemizygous and girls who are homozygous for both the G6PD202A and the G6PD376G alleles.
Wasting is defined as a weight-for-height z score of less than −2. Iron deficiency is defined as a ratio of soluble transferrin receptor to log ferritin (TfR-F index)10 that is greater than 5.6 (unpublished data). Folate concentrations of less than 0.3 µg per deciliter (6.8 nmol per liter), vitamin B12 concentrations of less than 20 ng per deciliter (148 pmol per liter), and vitamin A concentrations of less than 20 µg per deciliter (0.7 µmol per liter) are considered to indicate deficiencies.
Hyperparasitemia is defined as more than 100,000 parasites per microliter of blood.
The rs classifications for the genetic markers are as follows: IL10 −1117, rs1800896; IL10 −3585, rs1800890; IL10 +4949, rs3024500; TNF −238, rs361525; TNF −308, rs1800629; and TNF −1031, rs1799964. A ratio of interleukin-10 to TNF-α that is less than 1 is considered abnormal.22
Figure 1.
Adjusted Odds Ratios and 95% Confidence Intervals for Factors Associated with Severe Anemia, According to Study Group and Recruitment Site.
The model was corrected for the possible confounders of age, sex, recent antimalarial treatment (within the previous 8 weeks), recent hematinic treatment (within the previous 8 weeks), a history of transfusion (before the previous 4 weeks), and death of a parent. The goodness of fit of the model was evaluated by the Hosmer-Lemeshow test (P = 0.65). In the combined model, interaction existed between malaria and site (P<0.001). Limited maternal education indicates mothers who did not attend secondary school. Wasting is defined as a weight-for-height z score of less than −2. Concentrations of vitamin B12 of less than 20 ng per deciliter (148 pmol per liter) and of vitamin A of less than 20 µg per deciliter (0.7 µmol per liter) are considered to indicate deficiency. Iron deficiency is defined as a ratio of soluble transferrin receptor to log ferritin (TfR-F index)10 of more than 5.6 (unpublished data). Cultures to detect bacteremia were performed only in case patients and hospital controls. Hookworm infection was not entered in the urban model because the prevalence was less than 5%. The reference single-nucleotide polymorphism (rs) classification for IL10 +4949 is rs3024500.21 Because of the high correlation among the three interleukin-10 polymorphisms, only one (most strongly associated with severe anemia) was included in the multivariate model. HIV denotes human immunodeficiency virus, and G6PD−202/−376 denotes boys who are hemizygous and girls who are homozygous for both the G6PD202A and the G6PD376G alleles.
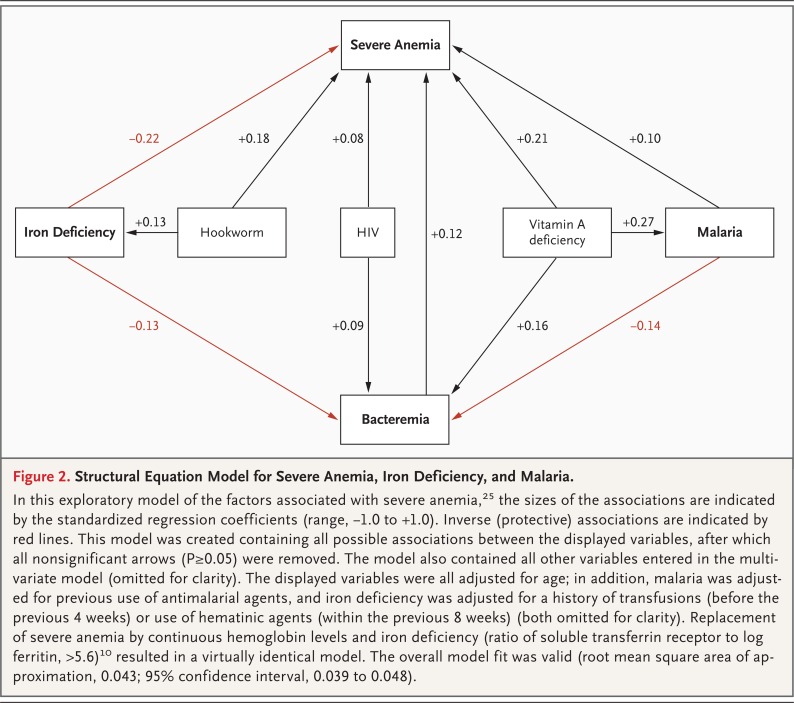
Figure 2.
Structural Equation Model for Severe Anemia, Iron Deficiency, and Malaria.
In this exploratory model of the factors associated with severe anemia,25 the sizes of the associations are indicated by the standardized regression coefficients (range, −1.0 to +1.0). Inverse (protective) associations are indicated by red lines. This model was created containing all possible associations between the displayed variables, after which all nonsignificant arrows (P≥0.05) were removed. The model also contained all other variables entered in the multivariate model (omitted for clarity). The displayed variables were all adjusted for age; in addition, malaria was adjusted for previous use of antimalarial agents, and iron deficiency was adjusted for a history of transfusions (before the previous 4 weeks) or use of hematinic agents (within the previous 8 weeks) (both omitted for clarity). Replacement of severe anemia by continuous hemoglobin levels and iron deficiency (ratio of soluble transferrin receptor to log ferritin, >5.6)10 resulted in a virtually identical model. The overall model fit was valid (root mean square area of approximation, 0.043; 95% confidence interval, 0.039 to 0.048).
Malaria
P. falciparum was identified in 226 case patients (59.5%) and 321 control patients (42.8%) and was the predominant malarial species overall (97.5%). P. malariae was found in 1.6% and a mixed infection in 0.9% of the study patients. The attributable risk of severe anemia associated with P. falciparum was 33.5% overall and 47.3% in the urban setting. In the rural setting, a significant association between malaria and severe anemia was found only in the subgroup of patients who had hyperparasitemia (9.7%) (adjusted odds ratio for case patients vs. community controls, 7.1; 95% confidence interval [CI], 1.4 to 34.6).
HIV
HIV infection was found in 86 children (12.6% of case patients and 6.0% of controls). The attributable risk of severe anemia associated with HIV was 6.2% overall and 15.4% in the urban setting. Among severely anemic children, significantly more HIV-infected than HIV-uninfected children had Epstein-Barr virus infection (15 of 30 [50.0%] vs. 69 of 226 [30.5%], P=0.03) or bacteremia (11 of 42 [26.2%] vs. 38 of 300 [12.7%], P=0.02), whereas significantly fewer HIV-infected than HIV-uninfected children had hyperparasitemia (2 of 44 [4.5%] vs. 42 of 312 [13.5%], P=0.09) or vitamin B12 deficiency (5 of 39 [12.8%] vs. 85 of 254 [33.5%], P=0.009).
Bacteremia
Fifty-four case patients (15.0%) and 14 controls (4.0%) had bacteremia. The attributable risk of severe anemia associated with bacteremia was 12.2%. The most common pathogen was nontyphoid salmonella, which was present in 38 of the case patients (70.4%) and 11 of the controls (78.6%) who had bacteremia (P=0.54). No mycobacteria were isolated from any of the specimens. Fever was absent in 36.8% of children with bacteremia. Among case patients, bacteremia was less common in children with malaria than in those without malaria (21 of 208 [10.1%] vs. 32 of 150 [21.3%], P=0.003). Among control patients, bacteremia was also less common in children with malaria than in those without (3 of 146 [2.1%] vs. 11 of 207 [5.3%], P=0.12).
Nutrition
Fifty-two case patients (15.8%) and 43 control patients (6.2%) had wasting; the attributable risk of severe anemia associated with wasting was 6.2%. Severely anemic children were commonly stunted (53.2%) or underweight (49.2%), but for both conditions the unadjusted and adjusted odds ratios were similar to those for wasting (data not shown). Severe wasting occurred in 3.7% of severely anemic children. Vitamin B12 deficiency was found in 95 case patients (30.4%) and 94 controls (15.6%) and was severe (<13.6 ng per deciliter [100 pmol per liter]) in 11.2% of case patients and 2.8% of controls (adjusted odds ratio, 4.3; 95% CI, 1.9 to 9.9). Macrocytosis was more common in children with vitamin B12 deficiency than in children with normal vitamin B12 levels (P=0.02), although the sensitivity for vitamin B12 deficiency was low (17.5%). Severely anemic children with vitamin B12deficiency ate fewer meals with meat than those not deficient (1.9 vs. 2.7 per month, P=0.02). Folate deficiency was not found in any child enrolled in the study. Vitamin B12 and folate levels were inversely correlated with each other among severely anemic children (Pearson correlation coefficient, −0.22; P=0.01).
Vitamin A deficiency was found in 92.3% of case patients and 65.6% of controls and was considered severe (less than 10 µg per deciliter) in 32.8% of case patients and 14.9% of controls (adjusted odds ratio, 1.6; 95% CI, 0.91 to 2.76). Vitamin A deficiency was associated with malaria and bacteremia in the structural equation model. Iron deficiency was found in 46.6% of case patients and 69.4% of controls. Further exploration indicated this finding was not affected by the definition used (Table 3). In the structural equation model, iron deficiency was found to be inversely associated with bacteremia (P=0.006).
Table 3.
Prevalence of Iron Deficiency in Relation to the Development of Severe Anemia, According to Several Peripheral Blood Markers.*
| Marker | Prevalence | Multivariate Analysis | |
| Case Patients | Control Patients | Odds Ratio (95% CI)† | |
| no./total no. (%) | |||
| TfR-F index‡ | 97/208 (46.6) | 288/415 (69.4) | 0.37 (0.22–0.60) |
| Alternative definitions | |||
| CRP-containing index§ | 35/208 (16.8) | 212/415 (51.1) | 0.29 (0.16–0.53) |
| Microcytosis¶ | 48/316 (15.2) | 182/636 (28.6) | 0.47 (0.29–0.76) |
| Hypochromasia‖ | 137/314 (43.6) | 294/637 (46.2) | 0.61 (0.41–0.91) |
| Microcytosis and hypochromasia | 26/316 (8.2) | 108/638 (16.9) | 0.40 (0.22–0.72) |
Other markers that were assessed but not presented because they predicted iron deficiency less well (with sensitivity, specificity, or both under 40%) were ferritin, transferrin receptor, serum iron, serum transferrin, total iron-binding capacity, and transferrin saturation. CI denotes confidence interval, CRP C-reactive protein, and TfR-F index the ratio of soluble transferrin receptor to log ferritin.
The odds ratios were obtained by replacing the original variable for iron deficiency in the multivariate analysis with the alternative definition.
The original definition of iron deficiency was a TfR-F index greater than 5.6.
Iron deficiency is defined as a CRP-containing index (0.34 + 0.0043 × ferritin − [2.7 × TfR] ÷ ferritin + 0.0696 × CRP + 0.05 × TfR) of less than 0.26
Microcytosis is defined as a mean corpuscular volume of less than 67 fl in children under 2 years of age and less than 73 fl in children 2 to 5 years of age.27
Hypochromasia is defined as a mean corpuscular hemoglobin concentration of less than 32 g per liter.27
Hookworm
Hookworm was the most common helminth infection. Thirty-one (75.6%) of the hookworm infections occurred in children less than 2 years old. The attributable risk of severe anemia associated with hookworm in the rural site, where 95.1% of infections were seen, was 15.9%. In this site, 10.4% of case patients and 0.6% of controls had heavy infections (adjusted odds ratio, 9.4; 95% CI, 2.0 to 44.8). PCR was performed on 36 of 41 positive samples (87.8%). A. duodenale was found in 80.6% of these samples, N. americanus in 8.3%, and a mixed infection in 11.1%. Hookworm infection was associated with iron deficiency (P=0.003) (Figure 2).
Sickle cell and G6PD variants
No association was found between severe anemia and sickle cell disease or between severe anemia and sickle cell trait (P=0.45 and P=0.20, respectively). Jaundice was uncommon in severely anemic children with sickle cell disease (0%) or G6PD−202/−376 (2.3%). Haptoglobin levels were commonly decreased (<0.30 g per liter) in case patients with G6PD−202/−376 (78.4%). Boys accounted for 68.2% of children with G6PD−202/−376, but after stratification, G6PD−202/−376 remained significantly associated with severe anemia in girls (adjusted odds ratio, 4.1; 95% CI, 1.2 to 13.3) and boys (adjusted odds ratio, 2.2; 95% CI, 1.1 to 4.7).
Discussion
In many African hospitals, severe anemia is a leading reason for admission and a major contributor to death. The cause of the anemia has not been comprehensively investigated, but we found several important associations in this study.
Malaria is commonly considered to be a principal cause of severe anemia in Africa.7 In this study, P. falciparum parasitemia was strongly associated with severe anemia in the area with seasonal transmission but not in the area with holoendemic transmission. However, the cumulative effect of malaria on an individual person is difficult to assess in holoendemic settings where children are repeatedly infected. Our findings therefore do not exclude malaria as a predisposing cause of severe anemia in the rural area but indicate that additional or alternative diagnoses should be considered in severely anemic children who receive a diagnosis of malaria infection. In the structural equation model, malaria and bacteremia were identified as variables that modify the association between vitamin A deficiency and severe anemia. This is in line with earlier observations that vitamin A deficiency is associated with an increased susceptibility to infection.28 A vitamin A supplementation trial showed a reduction in the incidence of malaria,29 although this and another study failed to show that vitamin A supplementation reduced the incidence of severe anemia.29,30
We found an inverse association between iron deficiency and severe anemia. The structural equation model partly explains this finding by indicating that iron deficiency was inversely associated with bacteremia. This finding supports the hypothesis that iron deficiency protects against infection by creating an unfavorable environment for bacterial growth.31,32 It is also in agreement with observations of increased morbidity and mortality during iron-supplementation studies in areas where bacterial infections are common.32,33 Although iron supplementation may have a role in preventing anemia,33 supplementation after severe anemia of malaria had no hematologic benefits and resulted in increased morbidity in Tanzanian children.34
In rural children with severe anemia, we found an increased prevalence and intensity of hookworm infections, with A. duodenale being the predominant species. Three quarters of the hookworm-infected children were less than 2 years of age. This age group would have remained untreated according to the current guidelines.7 Although hookworm is usually more frequent among older children, younger children might be more vulnerable to severe hematologic complications, especially in the presence of heavy infections with A. duodenale.35
Bacteremia, most commonly due to nontyphoid salmonella, was strongly associated with severe anemia. This association has been described previously16,36,37 but is not reflected in current management guidelines for severe anemia in children.7 In the structural equation model, bacteremia was also identified as a mediating variable of the effect of HIV on severe anemia. Although bacteremia might not necessarily be a cause of severe anemia, its high prevalence may justify antibiotic treatment in the standard management of severe anemia in settings where HIV is prevalent and blood-culture facilities are not available.
Although folate supplementation is recommended by the WHO, folate deficiency was not found in our study groups. We may have underestimated its prevalence, because folate deficiency can be masked by vitamin B12 deficiency,38 and we measured plasma concentrations of folate rather than erythrocyte concentrations. However, our findings concur with previous reports39 and observations that folate supplementation in anemic children with malaria failed to raise hemoglobin concentrations.40 Unlike folate, vitamin B12 is not recommended in the management of severe anemia. In our population, vitamin B12 deficiency was found in 30.4% of case patients and was associated with severe anemia. This is in agreement with findings among adults in this region41,42 and may be explained by the lack of animal products in the diet of Malawian children.
G6PD−202/−376 was found in 13.8% of case patients and was associated with severe anemia, whereas sickle cell disease was uncommon in our study. The roles of these mutations may be different in West and Central Africa. Possible associations of interleukin-10 and tumor necrosis factor α with severe anemia of malaria have been described,22,43 but in our study an imbalance in circulating plasma levels of interleukin-10 and tumor necrosis factor a was uncommon.
We found that several independent yet overlapping conditions are associated with severe anemia in Malawian children: bacteremia, malaria, hookworm, HIV infection, G6PD−202/−376, vitamin A deficiency, and vitamin B12 deficiency. Our findings indicate that even in the presence of malaria parasites, additional or alternative diagnoses should be considered. Current treatment recommendations that promote iron and folate supplements and ignore bacteremia, vitamin B12 deficiency, and, in young children, hookworm infections appear to be of limited applicability in our setting. Our findings, if confirmed in different settings, will contribute to the assessment of new therapeutic and preventive strategies for Africa.
Footnotes
This article originally appeared in the New England Journal of Medicine
Citation: Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, de Haan RJ, Phiri AI, Malange P, Khoka M, Hulshof PJ, van Lieshout L, Beld MG, Teo YY, Rockett KA, Richardson A, Kwiatkowski DP, Molyneux ME, van Hensbroek MB. Severe anemia in Malawian children. N Engl J Med. 2008 Feb 28;358(9):888–99.
doi: 10.1056/NEJMoa072727
(Published 28 February 2008)
© 2008 Massachusetts Medical Society
Republished with permission from the Massachusetts Medical Society
Supported by a grant (064722) from the Wellcome Trust and by independent grants from the Nutricia Research Foundation and the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences.
No potential conflict of interest relevant to this article was reported.
We thank the parents and guardians of the children admitted to the study; the Severe Anemia (SevAna) study team; and the staffs of the Queen Elizabeth Central Hospital, the Chikwawa District Hospital, and the Wellcome Trust Research Laboratories, especially S.M. Graham, E.M. Molyneux, T.N. Williams, M. Cornelissen, H. van den Berg, R.J.W.M. Vet, Z. Ayde, A. van Breda, J.J. Verweij, B.J.E. van Geenen, M.A. Sanjoaquin, R.J. Kraaijenhagen, F.A. Wijnberg, and W.J. van Lüling, for their contribution to the study.
Copyright © 2008 Massachusetts Medical Society.
References
- 1.English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494–495. doi: 10.1016/S0140-6736(02)07666-3. [DOI] [PubMed] [Google Scholar]
- 2.Koram KA, Owusu-Agyei S, Utz G, et al. Severe anemia in young children after high and low malaria transmission seasons in the Kassena-Nankana district of northern Ghana. Am J Trop Med Hyg. 2000;62:670–674. doi: 10.4269/ajtmh.2000.62.670. [DOI] [PubMed] [Google Scholar]
- 3.Lackritz EM, Campbell CC, Ruebush TK, II, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 4.Newton CR, Warn PA, Winstanley PA, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health. 1997;2:165–178. doi: 10.1046/j.1365-3156.1997.d01-238.x. [DOI] [PubMed] [Google Scholar]
- 5.Biemba G, Dolmans D, Thuma PE, Weiss G, Gordeuk VR. Severe anaemia in Zambian children with Plasmodium falciparum malaria. Trop Med Int Health. 2000;5:9–16. doi: 10.1046/j.1365-3156.2000.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Dicko A, Klion AD, Thera MA, et al. The etiology of severe anemia in a village and a periurban area in Mali. Blood. 2004;104:1198–200. doi: 10.1182/blood-2003-11-3884. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. Geneva: World Health Organization; 1998. [February 4, 2008]. http://www.who.int/nutrition/publications/anaemia_iron_pub/en/index.html. [Google Scholar]
- 8.Dibley MJ, Goldsby JB, Staehling NW, Trowbridge FL. Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr. 1987;46:736–748. doi: 10.1093/ajcn/46.5.736. [DOI] [PubMed] [Google Scholar]
- 9.Gale E, Torrence J, Bothwell T. The quantitative estimation of total iron stores in human bone marrow. J Clin Invest. 1963;42:1076–1082. doi: 10.1172/JCI104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 11.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan AD, Ittarat I, Meshnick SR. Patterns of haemozoin accumulation in tissue. Parasitology. 1996;112:285–294. doi: 10.1017/s003118200006580x. [DOI] [PubMed] [Google Scholar]
- 13.Katz N, Coelho PM, Pellegrino J. Evaluation of Kato's quantitative method through the recovery of Schistosoma mansoni eggs added to human feces. J Parasitol. 1970;56:1032–1033. [PubMed] [Google Scholar]
- 14.de Gruijter JM, van Lieshout L, Gasser RB, et al. Polymerase chain reactionbased differential diagnosis of Ancylostoma duodenale and Necator americanus infections in humans in northern Ghana. Trop Med Int Health. 2005;10:574–580. doi: 10.1111/j.1365-3156.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaker ZA, Hassan SI, el-Attar GM, et al. Use of Kato and nucleopore techniques for qualitative diagnosis of schistosomiasis. J Egypt Soc Parasitol. 1994;24:656–662. [PubMed] [Google Scholar]
- 16.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–314. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 17.Boom R, Sol C, Beld M, Weel J, Goudsmit J, Wertheim-van Dillen P. Improved silica-guanidiniumthiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J Clin Microbiol. 1999;37:615–619. doi: 10.1128/jcm.37.3.615-619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boom R, Sol C, Weel J, Gerrits Y, de Boer M, Wertheim-van Dillen P. A highly sensitive assay for detection and quantitation of human cytomegalovirus DNA in serum and plasma by PCR and electrochemiluminescence. J Clin Microbiol. 1999;37:1489–1497. doi: 10.1128/jcm.37.5.1489-1497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppelman MH, Cuypers HT, Emrich T, Zaaijer HL. Quantitative real-time detection of parvovirus B19 DNA in plasma. Transfusion. 2004;44:97–103. doi: 10.1046/j.0041-1132.2004.00610.x. [DOI] [PubMed] [Google Scholar]
- 20.Molyneux EM, Tembo M, Kayira K, et al. The effect of HIV infection on paediatric bacterial meningitis in Blantyre, Malawi. Arch Dis Child. 2003;88:1112–1118. doi: 10.1136/adc.88.12.1112. [Erratum, Arch Dis Child 2004;89:395.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JN, Rockett K, Jallow M, et al. Analysis of IL10 haplotypic associations with severe malaria. Genes Immun. 2005;6:462–466. doi: 10.1038/sj.gene.6364227. [DOI] [PubMed] [Google Scholar]
- 22.May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. Plasma interleukin-10:Tumor necrosis factor (TNF)-alpha ratio is associated with TNF promoter variants and predicts malarial complications. J Infect Dis. 2000;182:1570–1573. doi: 10.1086/315857. [DOI] [PubMed] [Google Scholar]
- 23.Hirono A, Beutler E. Molecular cloning and nucleotide sequence of cDNA for human glucose-6-phosphate dehydrogenase variant A(−) Proc Natl Acad Sci U S A. 1988;85:3951–3954. doi: 10.1073/pnas.85.11.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 25.Arbuckle JL. Amos 60 user's guide. Chicago: SPSS; 2007. [Google Scholar]
- 26.van den Broek NR, Letsky EA, White SA, Shenkin A. Iron status in pregnant women: which measurements are valid? Br J Haematol. 1998;103:817–824. doi: 10.1046/j.1365-2141.1998.01035.x. [DOI] [PubMed] [Google Scholar]
- 27.Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva: World Health Organization; 2001. [February 4, 2008]. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. [Google Scholar]
- 28.Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983;2:585–588. doi: 10.1016/s0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- 29.Shankar AH, Genton B, Semba RD, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. 1999;354:203–209. doi: 10.1016/S0140-6736(98)08293-2. [DOI] [PubMed] [Google Scholar]
- 30.Villamor E, Mbise R, Spiegelman D, Ndossi G, Fawzi WW. Vitamin A supplementation and other predictors of anemia among children from Dar Es Salaam, Tanzania. Am J Trop Med Hyg. 2000;62:590–597. doi: 10.4269/ajtmh.2000.62.590. [DOI] [PubMed] [Google Scholar]
- 31.Chlosta S, Fishman DS, Harrington L, et al. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–3067. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. BMJ. 1978;2:1113–1115. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [Erratum, Lancet 2006;367:302.] [DOI] [PubMed] [Google Scholar]
- 34.van den Hombergh J, Dalderop E, Smit Y. Does iron therapy benefit children with severe malaria-associated anaemia? A clinical trial with 12 weeks supplementation of oral iron in young children from the Turiani Division, Tanzania. J Trop Pediatr. 1996;42:220–227. doi: 10.1093/tropej/42.4.220. [DOI] [PubMed] [Google Scholar]
- 35.Albonico M, Stoltzfus RJ, Savioli L, et al. Epidemiological evidence for a differential effect of hookworm species, Ancylostoma duodenale or Necator americanus, on iron status of children. Int J Epidemiol. 1998;27:530–537. doi: 10.1093/ije/27.3.530. [DOI] [PubMed] [Google Scholar]
- 36.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J. 2006;25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 37.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 38.Dierkes J, Domrose U, Ambrosch A, et al. Supplementation with vitamin B12 decreases homocysteine and methylmalonic acid but also serum folate in patients with end-stage renal disease. Metabolism. 1999;48:631–635. doi: 10.1016/s0026-0495(99)90062-8. [DOI] [PubMed] [Google Scholar]
- 39.Abdalla SH. Iron and folate status in Gambian children with malaria. Ann Trop Paediatr. 1990;10:265–272. doi: 10.1080/02724936.1990.11747441. [DOI] [PubMed] [Google Scholar]
- 40.Van Hensbroek MB, Morris-Jones S, Meisner S, et al. Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:672–676. doi: 10.1016/0035-9203(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 41.Savage DG, Allen RH, Gangaidzo IT, et al. Pancytopenia in Zimbabwe. Am J Med Sci. 1999;317:22–32. doi: 10.1097/00000441-199901000-00004. van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr 2000;72:Suppl:247S–256S. [DOI] [PubMed] [Google Scholar]
- 42.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72(Suppl):247S–256S. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 43.Kurtzhals JA, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351:1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [Errata, Lancet 1998;352:242, 1999;353:848.] [DOI] [PubMed] [Google Scholar]