Abstract
Background
Rotavirus is the most common cause of severe gastroenteritis among young children worldwide. Data are needed to assess the efficacy of the rotavirus vaccine in African children.
Methods
We conducted a randomized, placebo-controlled, multicenter trial in South Africa (3166 infants; 64.1% of the total) and Malawi (1773 infants; 35.9% of the total) to evaluate the efficacy of a live, oral rotavirus vaccine in preventing severe rotavirus gastroenteritis. Healthy infants were randomly assigned in a 1:1:1 ratio to receive two doses of vaccine (in addition to one dose of placebo) or three doses of vaccine — the pooled vaccine group — or three doses of placebo at 6, 10, and 14 weeks of age. Episodes of gastroenteritis caused by wild-type rotavirus during the first year of life were assessed through active follow-up surveillance and were graded with the use of the Vesikari scale.
Results
A total of 4939 infants were enrolled and randomly assigned to one of the three groups; 1647 infants received two doses of the vaccine, 1651 infants received three doses of the vaccine, and 1641 received placebo. Of the 4417 infants included in the per-protocol efficacy analysis, severe rotavirus gastroenteritis occurred in 4.9% of the infants in the placebo group and in 1.9% of those in the pooled vaccine group (vaccine efficacy, 61.2%; 95% confidence interval, 44.0 to 73.2). Vaccine efficacy was lower in Malawi than in South Africa (49.4% vs. 76.9%); however, the number of episodes of severe rotavirus gastroenteritis that were prevented was greater in Malawi than in South Africa (6.7 vs. 4.2 cases prevented per 100 infants vaccinated per year). Efficacy against all-cause severe gastroenteritis was 30.2%. At least one serious adverse event was reported in 9.7% of the infants in the pooled vaccine group and in 11.5% of the infants in the placebo group.
Conclusions
Human rotavirus vaccine significantly reduced the incidence of severe rotavirus gastroenteritis among African infants during the first year of life. (ClinicalTrials.gov number, NCT00241644.)
Introduction
Rotavirus is the most important cause of severe gastroenteritis among children worldwide. The World Health Organization (WHO) estimates that globally 527,000 deaths occur each year among children as a result of rotavirus infection1; more than 230,000 of the deaths occur in sub-Saharan Africa. Six of the seven countries with the highest mortality due to rotavirus diarrhea are located in Africa.2 Similarly, data generated from global rotavirus surveillance networks highlight the burden of hospitalizations for rotavirus3; among young children hospitalized for acute diarrhea, the median detection rate for rotavirus was 40% globally and 41% in Africa. Therefore, measures to prevent rotavirus diarrhea in African children are urgently needed.
Vaccines represent the best hope for preventing the severe consequences of rotavirus infection, especially in impoverished regions where resources and access to care may be limited. Two oral, live attenuated rotavirus vaccines, Rotarix (GlaxoSmithKline Biologicals) and RotaTeq (Merck), have shown excellent protective efficacy against severe rotavirus gastroenteritis in trials conducted mainly in Latin America, Europe, and the United States.4–6 The WHO's Strategic Advisory Group of Experts (SAGE) first reviewed data on these vaccines in 2005 and strongly recommended the inclusion of rotavirus vaccination in the national immunization programs of countries and regions in which, according to available data, severe rotavirus gastroenteritis has a substantial public health impact.7 But SAGE noted that live oral vaccines may not be as effective in protecting the poorest children in developing countries as they are in protecting children in industrialized countries and therefore recommended that efficacy trials be conducted in representative populations in Africa and Asia before the recommendation is extended.1,7 In response to this mandate, we conducted a clinical trial to determine whether Rotarix, an attenuated human rotavirus vaccine containing a G1P[8] strain, could protect African children against severe rotavirus gastroenteritis.
Methods
Study design and participants
We conducted a double-blind, randomized, placebocontrolled multicenter study in South Africa and Malawi to assess the efficacy, safety, and immunogenicity of Rotarix. A placebo-controlled design was chosen because the vaccine was not licensed or available in these countries at the time the study was initiated, and data were needed to inform policy decisions in low-resource countries. Children who were infected with human immunodeficiency virus (HIV) and children who had been exposed to HIV were included in the trial on the basis of the absence of serious immunosuppression in infants at the age at which these vaccines are first given (6 weeks), the experience with other live vaccines in HIV-infected children, and the need to inform decisions on the introduction of vaccine in settings with high prevalences of HIV. The study protocol and the informed consent form were approved by the ethics committee at the WHO and by the ethics committees at all study centers. The trial was conducted in accordance with Good Clinical Practice guidelines. The parents or legal representatives of the infants participating in the study provided written informed consent before the initiation of any study-related procedures. All the investigators shared responsibility for the design of the study and the accrual and analysis of the data. All the authors participated in the preparation of the manuscript and made the decision to submit the manuscript for publication.
In South Africa, healthy infants, 5 to 10 weeks of age, were enrolled from October 2005 through January 2006 and from November 2006 through early February 2007, before the anticipated rotavirus seasons of 2006 and 2007, respectively.8,9 Since rotavirus is known to circulate year-round in Malawi,10 infants in Malawi were enrolled from October 2006 through July 2007.
Infants were randomly assigned individually, in a 1:1:1 ratio, into three groups to receive two doses of the rotavirus vaccine at 10 and 14 weeks of age; three doses of the vaccine at 6, 10, and 14 weeks of age; or three doses of placebo. Infants in the two-dose vaccine group received a dose of placebo at 6 weeks of age. Vaccines that are administered routinely according to the guidelines of the Expanded Program on Immunization (EPI) were concomitantly administered with the vaccine or placebo. No restrictions were imposed on the breast-feeding of infants around the time of vaccination.
Testing for HIV
The parents or legal representatives of the infants were given the opportunity to have the infants tested for HIV at the time the first dose of vaccine or placebo was administered or 1 month after the last dose, and testing was performed when consent was given. Detailed information regarding the testing and treatment of infants is included in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Vaccine
The study vaccine, the calcium carbonate buffer, and the placebo were developed and manufactured by GlaxoSmithKline Biologicals. The composition of the vaccine was the same as the commercial formulation, and the placebo was the same formulation without the viral antigen.11
Analysis of stool samples during episodes of gastroenteritis
An episode of gastroenteritis was identified by the occurrence of diarrhea, whether or not it was accompanied by vomiting; diarrhea was defined as the passage of three or more stools that were looser than normal within a 24-hour period. Stool samples were collected during each episode of gastroenteritis that occurred between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age. Stool samples were tested for rotavirus with the use of an enzyme-linked immunosorbent assay (ELISA) (Rotaclone, Meridian Bioscience). All stool samples that were positive for rotavirus were examined further with the use of a reverse-transcriptase-polymerase-chain-reaction (PCR) assay, followed by a reverse hybridization assay to determine the G and P types.12
Assessment of vaccine efficacy
The efficacy of the vaccine was assessed during the period from 2 weeks after the last dose of vaccine or placebo was administered until the child reached 1 year of age. Active surveillance for all gastroenteritis episodes was conducted by members of the study staff through weekly visits to parents or guardians to collect diary cards and through the collection of data from health clinics that served the study populations. The severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more.
Assessment of safety
All serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age. The site investigator, who was unaware of the group assignments of the children, determined whether the serious adverse events appeared to have any causal association with vaccination.
Assessment of immunogenicity
Blood samples were collected from approximately 10% of the infants immediately before the first dose of vaccine or placebo was administered and from all infants 1 month after the last dose to determine the serum concentrations of antirotavirus IgA antibody. The blood samples were analyzed with the use of an ELISA (GlaxoSmithKline Biologicals), with the assay cutoff point set at 20 U per milliliter.14
Statistical analysis
The primary study analysis compared findings from the pooled vaccine group with those from the placebo group. The secondary end points were the efficacy of each vaccine dose (i.e., the two-dose vaccine and the three-dose vaccine) as compared with placebo. A supplementary analysis was performed to evaluate the efficacy, immunogenicity, and safety of the vaccine according to country.
Infants who had received the complete vaccination course and had entered the efficacy surveillance period, which began 2 weeks after the last dose, were included in the prespecified primary efficacy analysis (per-protocol efficacy cohort). Infants in the pooled vaccine and placebo groups who had at least one episode of severe rotavirus gastroenteritis caused by wild-type rotavirus strains during the period from 2 weeks after the last dose was administered until the infants reached 1 year of age were considered as having achieved the primary outcome. The efficacy analysis was also performed on data from the total cohort, which included infants who received at least one dose of vaccine or placebo. The safety analysis was performed on data from the total cohort. The immunogenicity analysis was performed on data from infants in the per-protocol efficacy cohort for whom immunogenicity data were available. The method used to calculate the sample size and specific information about the statistical analysis are shown in the Supplementary Appendix.
Results
Study population
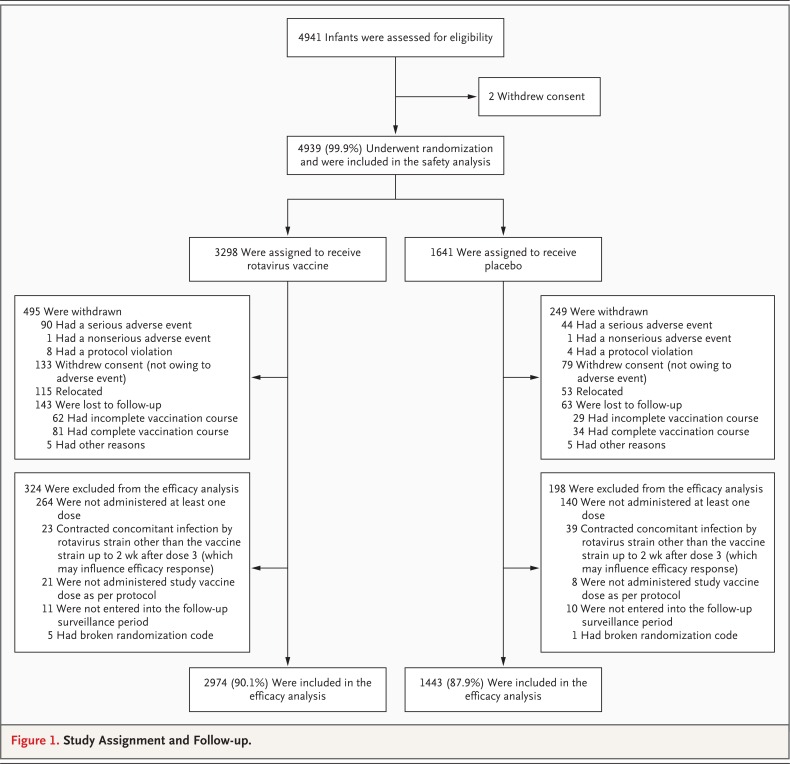
A total of 4939 infants were enrolled and randomly assigned to one of three groups (Figure 1); 1647 infants were assigned to the two-dose group, 1651 to the three-dose group (for a total of 3298 in pooled vaccine group), and 1641 to the placebo group. A total of 4417 infants were included in the primary efficacy analysis — 2974 in the pooled vaccine group and 1443 in the placebo group. The reasons for withdrawal from the study are listed in Figure 1. The demographic characteristics of the infants and the proportion of children who were infected with HIV were similar across the study groups. Almost all infants (≥99%) received oral poliovirus vaccine concomitantly with each dose of rotavirus vaccine or placebo (Table 1 in the Supplementary Appendix).
Figure 1.
Study Assignment and Follow-up.
Efficacy
Severe gastroenteritis caused by circulating rotavirus was detected in 70 of 1443 infants in the placebo group (4.9%) as compared with 56 of 2974 infants in the pooled vaccine group (1.9%), resulting in a vaccine efficacy against the primary outcome of severe rotavirus gastroenteritis of 61.2% (95% confidence interval [CI], 44.0 to 73.2; P<0.001) (Table 1). Vaccination with the rotavirus vaccine prevented 5.0 episodes of severe rotavirus gastroenteritis per 100 infant-years (Table 2). The vaccine showed efficacy against severe rotavirus gastroenteritis both in infants who received two doses of vaccine (58.7% efficacy; 95% CI, 35.7 to 74.0) and in those who received three doses (63.7% efficacy; 95% CI, 42.4 to 77.8). In South Africa, the efficacy of the vaccine was 76.9% (95% CI, 56.0 to 88.4), and in Malawi the vaccine efficacy was 49.4% (95% CI, 19.2 to 68.3); 4.2 and 6.7 episodes of severe rotavirus gastroenteritis per 100 infant-years were prevented by vaccination in South Africa and Malawi, respectively (Table 1 and Table 2). The efficacy of the rotavirus vaccine against rotavirus gastroenteritis of any severity is presented in Table 2 in the Supplementary Appendix.
Table 1.
Efficacy of Rotavirus Vaccine with Respect to the Development of Severe Rotavirus Gastroenteritis and Hospitalization for Rotavirus Gastroenteritis.*
| Variable | Infants with at Least One Event | Vaccine Efficacy | P Value† | |||
| Rotavirus Vaccine | Placebo | |||||
| no./total no. | % (95% CI) | no./total no. | % (95% CI) | % (95% CI) | ||
| Severe rotavirus gastroenteritis | ||||||
| Overall | ||||||
| Pooled | 56/2974 | 1.9 (1.4–2.4) | 70/1443 | 4.9 (3.8–6.1) | 61.2 (44.0–73.2) | <0.001 |
| Two-dose | 30/1496 | 2.0 (1.4–2.9) | — | — | 58.7 (35.7–74.0) | <0.001 |
| Three-dose | 26/1478 | 1.8 (1.2–2.6) | — | — | 63.7 (42.4–77.8) | <0.001 |
| Malawi | ||||||
| Pooled | 41/1030 | 4.0 (2.9–5.4) | 38/483 | 7.9 (5.6–10.6) | 49.4 (19.2–68.3) | 0.003 |
| Two-dose | 21/525 | 4.0 (2.5–6.0) | — | — | 49.2 (11.1–71.7) | 0.01 |
| Three-dose | 20/505 | 4.0 (2.4–6.1) | — | — | 49.7 (11.3–72.2) | 0.01 |
| South Africa | ||||||
| Pooled | 15/1944 | 0.8 (0.4–1.3) | 32/960 | 3.3 (2.3–4.7) | 76.9 (56.0–88.4) | <0.001 |
| Two-dose | 9/971 | 0.9 (0.4–1.8) | — | — | 72.2 (40.4–88.3) | <0.001 |
| Three-dose | 6/973 | 0.6 (0.2–1.3) | — | — | 81.5 (55.1–93.7) | <0.001 |
| Rotavirus-type-specific severe rotavirus gastroenteritis‡ |
||||||
| Overall | ||||||
| G1 strain | 17/2974 | 0.6 (0.3–0.9) | 23/1443 | 1.6 (1.0–2.4) | 64.1 (29.9–82.0) | 0.002 |
| Non-G1 strain | 39/2974 | 1.3 (0.9–1.8) | 47/1443 | 3.3 (2.4–4.3) | 59.7 (37.1–74.4) | <0.001 |
| Malawi | ||||||
| G1 strain | 6/1030 | 0.6 (0.2–1.3) | 5/483 | 1.0 (0.3–2.4) | 43.7 (<0–85.7) | 0.34 |
| Non-G1 strain | 35/1030 | 3.4 (2.4–4.7) | 33/483 | 6.8 (4.7–9.5) | 50.3 (17.4–70.0) | 0.005 |
| South Africa | ||||||
| G1 strain | 11/1944 | 0.6 (0.3–1.0) | 18/960 | 1.9 (1.1–2.9) | 69.8 (32.5–87.1) | 0.002 |
| Non-G1 strain | 4/1944 | 0.2 (0.1–0.5) | 14/960 | 1.5 (0.8–2.4) | 85.9 (55.1–96.6) | <0.001 |
| Hospitalization for rotavirus gastroenteritis‡ |
14/2974 | 0.5 (0.3–0.8) | 16/1443 | 1.1 (0.6–1.8) | 57.5 (7.2–80.8) | 0.02 |
A total of 4417 infants were included in the efficacy analysis — 2974 in the pooled vaccine group and 1443 in the placebo group.
P values were calculated with the use of a two-sided Fisher's exact test. P values of less than 0.05 were considered to indicate a statistically significant difference.
Data in the rotavirus vaccine group are for the pooled vaccine cohort.
Table 2.
Risk of Severe Rotavirus Gastroenteritis in the Pooled Vaccine Group and the Placebo Group, According to Dose, Country, and Rotavirus Strain.*
| Cohort | Rotavirus Vaccine | Placebo | Difference in Rate (95% CI)† |
||
| No. in Cohort |
Episodes/ 100 Infants/Yr (95% CI) |
No. in Cohort |
Episodes/ 100 Infants/Yr (95% CI) |
||
| Severe rotavirus gastroenteritis | |||||
| Pooled | 2974 | 3.0 (2.3–3.9) | 1443 | 8.0 (6.3–10.1) | 5.0 (3.1–7.2) |
| Two-dose | 1496 | 3.2 (2.2–4.6) | — | — | 4.8 (2.6–7.1) |
| Three-dose | 1478 | 2.8 (1.9–4.1) | — | — | 5.2 (3.0–7.5) |
| Country‡ | |||||
| Malawi | 1030 | 6.5 (4.8–8.8) | 483 | 13.1 (9.6–18.0) | 6.7 (2.4–11.9) |
| South Africa | 1944 | 1.2 (0.7–2.0) | 960 | 5.4 (3.8–7.7) | 4.2 (2.4–6.5) |
| Rotavirus type‡ | |||||
| Total cohort | 2974 | 1443 | |||
| G1 strain | 0.9 (0.6–1.5) | 2.6 (1.7–3.9) | 1.7 (0.6–3.0) | ||
| Non-G1 strain | 2.1 (1.5–2.9) | 5.3 (4.0–7.1) | 3.2 (1.7–5.1) | ||
| Malawi | 1030 | 483 | |||
| G1 strain | 0.9 (0.4–2.1) | 1.7 (0.7–4.0) | 0.7 (<0–3.1) | ||
| Non-G1 strain | 5.5 (4.0–7.7) | 11.3 (8.1–16.0) | 5.8 (1.9–10.7) | ||
| South Africa | 1944 | 960 | |||
| G1 strain | 0.9 (0.5–1.6) | 3.0 (1.9–4.8) | 2.1 (0.8–3.9) | ||
| Non-G1 strain | 0.3 (0.1–0.9) | 2.4 (1.4–4.0) | 2.0 (0.9–3.7) | ||
The analyses are based on the efficacy cohort, which comprised 4417 infants — 2974 in the pooled vaccine group and 1443 in the placebo group.
The difference in rate is calculated as the episodes per 100 infants per year in the placebo group minus the episodes per 100 infants per year in the rotavirus vaccine group.
Data in the rotavirus vaccine group are for the pooled vaccine cohort.
The distribution of rotavirus G and P types differed between South Africa and Malawi (Figure 1 in the Supplementary Appendix). The G1P[8] strain was detected in 57.0% of the episodes among recipients of the placebo in South Africa and in 12.9% of the episodes among recipients of the placebo in Malawi. The type-specific efficacy against severe rotavirus gastroenteritis and the difference in incidence rates between the vaccine groups and the placebo group are shown in Table 1 and Table 2, respectively.
The incidence rate of severe gastroenteritis from any cause was 8.6% in the pooled vaccine group as compared with 12.3% in the placebo group, corresponding to a reduction in the rate with vaccination of 30.2% (95% CI, 15.0 to 42.6; P<0.001) (Table 3). The reductions in all-cause severe gastroenteritis were statistically significant in both countries. The efficacy of the vaccine in the total vaccinated cohort was similar to that in the per-protocol efficacy cohort (Tables 3 to 6 in the Supplementary Appendix).
Table 3.
Efficacy of Rotavirus Vaccine against All-Cause Severe Gastroenteritis.*
| Cohort | Infants with at Least One Event of All-Cause Severe Gastroenteritis | Vaccine Efficacy | P Value† | |||
| Rotavirus Vaccine | Placebo | |||||
| no./total no. | % (95% CI) | no./total no. | % (95% CI) | % (95% CI) | ||
| Overall | 256/2974 | 8.6 (7.3–9.7) | 178/1443 | 12.3 (10.7–14.1) | 30.2 (15.0–42.6) | <0.001 |
| Malawi | 187/1030 | 18.2 (15.8–20.6) | 117/483 | 24.2 (20.5–28.3) | 25.1 (4.7–40.8) | 0.007 |
| South Africa | 69/1944 | 3.5 (2.8–4.5) | 61/960 | 6.4 (4.9–8.1) | 44.1 (19.8–61.0) | <0.001 |
The analyses are based on the efficacy cohort, which comprised 4417 infants — 2974 in the pooled vaccine group and 1443 in the placebo group.
P values were calculated with the use of a two-sided Fisher's exact test. P values of less than 0.05 were considered to indicate a statistically significant difference.
Safety
At least one serious adverse event occurred during the study period in 319 of the 3298 infants in the pooled vaccine group (9.7%; 95% CI, 8.7 to 10.7) and in 189 of the 1641 infants in the placebo group (11.5%; 95% CI, 10.0 to 13.2) (Table 7 in the Supplementary Appendix). During the entire study period, 83 deaths occurred among infants in the pooled vaccine group (2.5%; 95% CI, 2.0 to 3.1) and 43 deaths occurred among those in the placebo group (2.6%; 95% CI, 1.9 to 3.5). A single case of intussusception occurred 11 weeks after the third dose of rotavirus vaccine in a 6-month-old child who was assigned to the three-dose vaccine group. The child underwent bowel resection and recovered fully. Three adverse events were judged by the investigators to be related to vaccination — two cases of sepsis and one of otitis media.
Immunogenicity
At 1 month after the last dose of vaccine was administered, the seroconversion rates for antirotavirus IgA in South Africa were 57.1% (95% CI, 44.7 to 68.9) in the two-dose group and 66.7% (95% CI, 54.0 to 77.8) in the three-dose group. The seroconversion rates in Malawi were 47.2% (95% CI, 30.4 to 64.5) in the two-dose group and 57.1% (95% CI, 42.2 to 71.2) in the three-dose group. In the placebo group, the seropositivity rates for antirotavirus IgA at 1 month after the last dose were 16.7% (95% CI, 14.2 to 19.5) in South Africa and 40.4% (95% CI, 34.9 to 46.1) in Malawi.
Discussion
This study shows that a live, oral rotavirus vaccine significantly reduces the episodes of severe rotavirus gastroenteritis in African children during the first year of life. The attack rate for severe rotavirus gastroenteritis was higher in these populations than has been reported in other studies of rotavirus vaccines.4,5 Because of this high incidence of severe disease, a vaccine efficacy of 61.2% resulted in a substantial vaccine-attributable reduction in severe rotavirus gastroenteritis (reduction of 5.0 cases per 100 infant-years). In addition, the rotavirus vaccine was associated with a reduction in all-cause severe gastroenteritis of 30.2%. This reduction in the incidence of the disease occurred in a trial that was designed to simulate real-world conditions of use; thus, the rotavirus vaccine is expected to deliver a considerable public health benefit when it is introduced into similar settings.
The overall efficacy of the rotavirus vaccine in preventing episodes of severe rotavirus gastroenteritis (61.2%) was lower than that observed in European studies and Latin American studies (96.4% and 84.8%, respectively), which included some low- to middle-income countries.4–6,15 This finding is consistent with findings from other studies of live oral vaccines, such as the oral poliovirus vaccine,16 the cholera vaccine,17 oral typhoid vaccines,18 and earlier rotavirus vaccines,19 none of which were as immunogenic or effective in populations in developing countries as they were in populations in industrialized countries. Several mechanisms have been proposed to explain why live oral rotavirus vaccines may not be as efficacious in populations of infants from low-income countries.20 Possible reasons include host characteristics, such as poor nutritional status and enteric coinfections; levels of antirotavirus antibodies in breast milk; and interference by maternal antibody or by coadministration of the oral poliovirus vaccine, which may reduce rotavirus antibody levels.21,22 The potential role of these and other factors will be important to elucidate, in order to further improve the performance of these vaccines in populations where they are most needed.
The efficacy of the rotavirus vaccine in Malawian infants in this study was lower than that in their South African counterparts (49.4% vs. 76.9%). In addition to potential differences mentioned above, rotavirus circulation differs in the two countries (a winter-spring peak in South Africa8,9 as compared with year-round circulation in Malawi10), and that difference was reflected in the study enrollment strategies (preceding the rotavirus season in South Africa as compared with year-round in Malawi). The rotavirus seropositivity rate among placebo recipients 1 month after the last dose had been given was greater among Malawian infants than among South African infants (40.4% vs. 16.7%), suggesting that Malawian infants have high exposure to wild-type rotavirus infection in the first 5 months of life. Since infection with wildtype rotavirus confers protection against the development of severe rotavirus disease later in infancy,23 the greater exposure of the infants in the placebo group in Malawi to rotavirus infection before their entry into the follow-up period may have lowered the estimate of vaccine efficacy in Malawi. Despite the lower point estimate for efficacy, the number of severe cases of rotavirus gastroenteritis prevented was greater in Malawi than in South Africa (6.7 vs. 4.2 cases prevented per 100 infant-years), owing to the higher incidence of severe rotavirus gastroenteritis in Malawi.
In this study, the diversity of the circulating strains was striking. In Malawi, only 12.9% of the rotavirus strains were G1P[8] — the strain on which this vaccine is based, and the most commonly occurring strain globally.24,25 A substantial proportion of G2, G8, G9, and G12 strains were isolated during the course of this study. Rotavirus types G2, G8, and G9 have circulated for several years in both countries, whereas rotavirus type G12 has been reported more recently.26,27 It is unlikely, however, that these differences in strains contributed to the lower vaccine efficacy, since the efficacy against G1 and non-G1 severe rotavirus gastroenteritis was similar. These data are consistent with an integrated analysis of previous efficacy trials of the rotavirus vaccine, which indicates that the rotavirus vaccine provides protection against severe rotavirus gastroenteritis caused by G1 and non-G1 strains.28 The ability of a rotavirus vaccine to protect against a wide panel of strains is important in Africa, where the diversity of rotavirus strains is substantial.24,25,29
We did not detect significant differences in vaccine immunogenicity or efficacy between the cohort receiving two vaccine doses and the cohort receiving three doses, although this study was not powered to detect such differences. There was a slight but nonsignificant trend toward higher seroconversion rates and vaccine efficacy with the three-dose schedule. It should be noted that in the two-dose schedule used in this study, the doses of vaccine were administered at the second and third childhood vaccine visit, when the infants were an average of 11 and 16 weeks of age, respectively. Outside the setting of a clinical trial, a two-dose schedule in which the rotavirus vaccine is administered at the second and third childhood vaccination visits is not practical, since it is recommended that the first dose of rotavirus vaccine be delivered before the infant is 12 weeks of age, owing to lingering concerns stemming from the age-dependent risk of intussusception associated with a previous rotavirus vaccine.1 Since children in developing countries frequently present late for vaccination visits,30 this age restriction would deny many children the opportunity to receive the rotavirus vaccine if it were recommended at a later visit. Although a two-dose schedule in which the vaccine is administered at the first and second childhood vaccination visits is a more practical option, such a schedule was not tested in this study. Administering rotavirus vaccines at younger ages could lower the immunogenicity of the vaccines, because of the potential for greater interference of maternal antibody20 and enhanced replication of the oral poliovirus vaccine.22,31,32 It will be important to undertake studies to assess the effectiveness of two doses of rotavirus vaccine administered at earlier ages than those at which they were administered in this study.
The WHO's Global Advisory Committee on Vaccine Safety (GACVS) has reviewed the safety of the currently licensed rotavirus vaccines33,34 and has concluded that in clinical trials of these vaccines, no association was seen between receipt of the vaccines and an increased risk of intussusception. Further, postmarketing surveillance data show that an intussusception risk that is of a similar magnitude to that which had been associated with a previous rotavirus vaccine, Rotashield (Wyeth-Lederle), is unlikely.34,35 The single case of intussusception in this trial was not temporally related to rotavirus vaccination, and the rate of serious adverse events did not differ between infants who received the vaccine and those who received the placebo. Continuing surveillance of the safety of the vaccines as they are introduced into more countries will be important.
On the basis of this study and other supporting data, SAGE recently recommended that rotavirus vaccination of infants be included in all national immunization programs, in conjunction with other proven interventions for diarrheal disease.36 Appropriate financing mechanisms that will allow ministries of health in Africa to bring this potentially lifesaving vaccine to the children in greatest need are urgently needed.
Footnotes
This article originally appeared in the New England Journal of Medicine
Citation: Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, Han HH, Neuzil KM. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010 Jan 28;362(4):289–98.
doi: 10.1056/NEJMoa0904797
(Published 28 January 2010)
© 2010 Massachusetts Medical Society
Republished with permission from the Massachusetts Medical Society
Supported by a grant (064722) from the Wellcome Trust and by independent grants from the Nutricia Research Foundation and the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences.
No potential conflict of interest relevant to this article was reported.
The clinical trials were funded and coordinated by GlaxoSmithKline and the PATH Rotavirus Vaccine Program, a collaboration with the WHO and the Centers for Disease Control and Prevention, with support from the Global Alliance for Vaccines and Immunization (GAVI).
Dr. Madhi reports receiving lecture fees from GlaxoSmithKline and consulting fees from Merck; Dr. Cunliffe, receiving lecture fees and grant support from Sanofi Pasteur and GlaxoSmithKline; Dr. Gillard, owning shares in GlaxoSmithKline; and Dr. Han, owning shares in GlaxoSmithKline.
No other potential conflict of interest relevant to this article was reported.
Drs. Madhi and Cunliffe contributed equally to this article.
We thank the volunteers and their families; the members of the Clinical Trial Study Team: from Malawi — coinvestigators: Dr. S. Todd, Dr. N. Bostock; laboratory management: M. Goodall, J. Fulakesa; coordinator: A. Turner and the investigator team; from South Africa — coinvestigators: Dr. T.J. Lerumo (principal investigator, Stanza Bopape Clinic), Dr. P.R. Madiba, Dr. V.O. Seopela, Dr. N.M. Mahlase (principal investigator, Soshanguve Clinic), Dr. R.A.P. Selepe, Dr. M. Nchabeleng (principal investigator, Soshanguve Block L Clinic), Dr. M.R. Lekalakala, Dr. T. Van Der Westhuizen (principal investigator, Mamelodi West Clinic), Dr. T. Vally, Dr. T.P. Skosana (principal investigator, Maibuye Clinic), Dr. M.R. Kenoshi, Dr. B. Maroane (Diepkloof And Eldorado Clinics), Dr. C. Cutland, Dr. M. Groome, Dr. V. Gosai, Dr. E.V. Aghachi (Bertoni), Dr. F. Kiggundu (Lethlabile Clinic), Dr. N. Nyalunga, Dr. C. Werner (principal investigator, Oukasie Clinic), Dr. F. Scholtz, Dr. T.J. Botha (principal investigator, Karenpark Clinic), Dr. M. Venter; Rota Consortium — Dr. S. Aspinall (National Coordinator), P. Bos (Representative, Diarrhoeal Pathogens Research Unit), Prof. J. Hugo (Representative, Madibeng Centre For Research), S. Qolohle (Project Manager), D. Traynor (Operations Manager); Site Managers — A. Venter, I. Groenewold, Dr. T. Sithebe, M. Sauerman; from GlaxoSmithKline — A. Bouckenooghe, A. Delem, and L. Wannerud for their contribution to study design and S. Damaso, C. Bicer, and P.V. Suryakiran for statistical analysis; Y. Guerra and the safety team for management of safety information and C. Bougelet and team for laboratory testing; A. Dujardin, A. Ay, S. Vansteenkiste, and B. Anspach for global study management; local study monitoring teams and clinical operation teams from South Africa and Malawi; the data management team; G. Subramanyam, who contributed to technical writing aspects, and N. Van Driessche for editorial assistance.
References
- 1.World Health Organization, author. Rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285–296. [PubMed] [Google Scholar]
- 2.Assessing vaccine-preventable diseases burden and immunization impact. Geneva: World Health Organization; [December 31, 2009]. http://www.who.int/immunization_monitoring/burden/en/ [Google Scholar]
- 3.Global networks for surveillance of rotavirus gastroenteritis, 2001–2008. Wkly Epidemiol Rec. 2008;83:421–428. [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 6.Araujo EC, Clemens SA, Oliveira CS, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. J Pediatr (Rio J) 2007;83:217–224. doi: 10.2223/JPED.1600. [DOI] [PubMed] [Google Scholar]
- 7.Meeting of the Immunization Strategic Advisory Group of Experts, November 2005 — recommendations and conclusions. Wkly Epidemiol Rec. 2006;81:1–12. [Google Scholar]
- 8.Steele AD, Alexander JJ, Hay IT. Rotavirus- associated gastroenteritis in black infants in South Africa. J Clin Microbiol. 1986;23:992–994. doi: 10.1128/jcm.23.5.992-994.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele AD, Peenze I, de Beer MC, et al. Anticipating rotavirus vaccines: epidemiology and surveillance of rotavirus in South Africa. Vaccine. 2003;21:354–360. doi: 10.1016/s0264-410x(02)00615-1. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe NA, Ngwira BM, Dove W, et al. Epidemiology of rotavirus infections in children in Blantyre, Malawi, 1997–2007. J Infect Dis. doi: 10.1086/653577. (in press) [DOI] [PubMed] [Google Scholar]
- 11.O'Ryan M. Rotarix (RIX4414): an oral human rotavirus vaccine. Expert Rev Vaccines. 2007;6:11–19. doi: 10.1586/14760584.6.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Pang XL, Joensuu J, Hoshino Y, Kapikian AZ, Vesikari T. Rotaviruses detected by reverse transcription polymerase chain reaction in acute gastroenteritis during a trial of rhesus-human reassortant rotavirus tetravalent vaccine: implications for vaccine efficacy analysis. J Clin Virol. 1999;13:9–16. doi: 10.1016/s1386-6532(98)00013-4. [DOI] [PubMed] [Google Scholar]
- 13.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrheal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 14.Ward RL, Bernstein DI, Shukla R, et al. Effects of antibodies to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 16.John TJ. Antibody response of infants in tropics to five doses of oral polio vaccine. Br Med J. 1976;1:812. doi: 10.1136/bmj.1.6013.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suharyoo, Simanjutak CH, Witham N, et al. Safety and immunogenicity of a single-dose live oral cholera vaccine CVD103-HgR in 5–9-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 18.Simanjuntak CH, Paleologo FP, Punjabi NH, et al. Oral immunization against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- 19.Georges-Courbot MC, Monges J, Siopathis MR, et al. Evaluation of the efficacy of a low-passage bovine rotavirus (strain WC3) vaccine in children in Central Africa. Res Virol. 1991;142:405–411. doi: 10.1016/0923-2516(91)90008-q. [DOI] [PubMed] [Google Scholar]
- 20.Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, Steele AD. Rotavirus vaccines: targeting the developing world. J Infect Dis. 2005;192(Suppl 1):S160–S166. doi: 10.1086/431504. [DOI] [PubMed] [Google Scholar]
- 21.Tregnaghi M, Lopez P, De Leon T, et al. Oral human rotavirus vaccine RIX4414 (Rotarix) co-administered with routine EPI vaccinations including oral polio vaccine (OPV) is highly efficacious in Latin-America; Presented at the 13th International Congress on Infectious Diseases; June 19–22, 2008; Kuala Lumpur, Malaysia. [Google Scholar]
- 22.Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 23.Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 24.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 25.Gentsch JR, Laird AR, Bielfelt B, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192(Suppl):S146–S159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 26.Cunliffe NA, Ngwira BM, Dove W, et al. Serotype G12 rotaviruses, Lilongwe, Malawi. Emerg Infect Dis. 2009;15:87–90. doi: 10.3201/eid1501.080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page NA, de Beer MC, Seheri LM, Dewar JB, Steele AD. The detection and molecular characterization of human G12 genotypes in South Africa. J Med Virol. 2009;81:106–113. doi: 10.1002/jmv.21362. [DOI] [PubMed] [Google Scholar]
- 28.De Vos B, Han HH, Bouckenooghe A, et al. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J. 2009;28:261–266. doi: 10.1097/INF.0b013e3181907177. [DOI] [PubMed] [Google Scholar]
- 29.Steele AD, Ivanoff B. Rotavirus strains circulating in Africa during 1996–1999: emergence of G9 strains and P[6] strains. Vaccine. 2003;21:361–367. doi: 10.1016/s0264-410x(02)00616-3. [DOI] [PubMed] [Google Scholar]
- 30.Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 31.Steele AD, Tumbo J, Reynders J, et al. Comparison of 2 different regimens (two doses versus three doses) in terms of reactogenicity and immunogenicity of the live attenuated human rotavirus vaccine RIX4414 in South African infants; Presented at the 11th Asian Conference on Diarrheal Disease and Nutrition; March 8–10, 2006; Bangkok, Thailand. [Google Scholar]
- 32.Anh DD, Thiem VD, Hutagalung Y, et al. Immunogenicity, reactogenicity and safety of the oral live attenuated human rotavirus vaccine (RIX4414) oral suspension (liquid formulation) in Vietnames infants; Presented at the 13th International Congress on Infectious Diseases; June 19–22, 2008; Kuala Lumpur, Malaysia. [Google Scholar]
- 33.Global Advisory Committee on Vaccine Safety, 2–3 December 2005. Wkly Epidemiol Rec. 2006;81:13–20. [Google Scholar]
- 34.Global Advisory Committee on Vaccine Safety, 17–18 December 2008. Wkly Epidemiol Rec. 2009;84:37–40. [PubMed] [Google Scholar]
- 35.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–5672. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 36.Meeting of the Immunization Strategic Advisory Group of Experts, April 2009 — conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–236. [PubMed] [Google Scholar]