Abstract
Background
Information regarding the safety and efficacy of artemisinin combination treatments for malaria in pregnant women is limited, particularly among women who live in sub-Saharan Africa.
Methods
We conducted a multicenter, randomized, open-label trial of treatments for malaria in pregnant women in four African countries. A total of 3428 pregnant women in the second or third trimester who had falciparum malaria (at any parasite density and regardless of symptoms) were treated with artemether-lumefantrine, amodiaquine-artesunate, mefloquine-artesunate, or dihydroartemisinin-piperaquine. The primary end points were the polymerase-chain-reaction (PCR)-adjusted cure rates (i.e., cure of the original infection; new infections during follow-up were not considered to be treatment failures) at day 63 and safety outcomes.
Results
The PCR-adjusted cure rates in the per-protocol analysis were 94.8% in the artemether-lumefantrine group, 98.5% in the amodiaquine-artesunate group, 99.2% in the dihydroartemisinin-piperaquine group, and 96.8% in the mefloquine-artesunate group; the PCR-adjusted cure rates in the intention-to-treat analysis were 94.2%, 96.9%, 98.0%, and 95.5%, respectively. There was no significant difference among the amodiaquine-artesunate group, dihydroartemisinin-piperaquine group, and the mefloquine-artesunate group. The cure rate in the artemether-lumefantrine group was significantly lower than that in the other three groups, although the absolute difference was within the 5-percentage-point margin for equivalence. The unadjusted cure rates, used as a measure of the post-treatment prophylactic effect, were significantly lower in the artemether-lumefantrine group (52.5%) than in groups that received amodiaquine-artesunate (82.3%), dihydroartemisinin-piperaquine (86.9%), or mefloquine-artesunate (73.8%). No significant difference in the rate of serious adverse events and in birth outcomes was found among the treatment groups. Drug-related adverse events such as asthenia, poor appetite, dizziness, nausea, and vomiting occurred significantly more frequently in the mefloquine-artesunate group (50.6%) and the amodiaquine-artesunate group (48.5%) than in the dihydroartemisinin-piperaquine group (20.6%) and the artemether-lumefantrine group (11.5%) (P<0.001 for comparison among the four groups).
Conclusions
Artemether-lumefantrine was associated with the fewest adverse effects and with acceptable cure rates but provided the shortest posttreatment prophylaxis, whereas dihydroartemisinin-piperaquine had the best efficacy and an acceptable safety profile. (Funded by the European and Developing Countries Clinical Trials Partnership and others; ClinicalTrials.gov number, NCT00852423.)
Introduction
Malaria during pregnancy is a major public health problem in countries where the disease is endemic.1 In areas in which the intensity of transmission is moderate to high and does not vary substantially from year to year, most malaria infections during pregnancy remain asymptomatic but increase the risk of maternal anemia and low birth weight, the latter of which is associated with increased infant mortality.2 In areas where the intensity of transmission is low and varies substantially between years, symptomatic malaria and severe disease can develop in pregnant women, with an associated increased risk of fetal loss and maternal death.2 Considering the harmful effects of malaria during pregnancy, it is extremely important to treat the disease adequately with efficacious medicines. However, little information is available regarding the pharmacokinetics, safety, and efficacy of new antimalarial agents in pregnant women3–5 because pregnant women are systematically excluded from regulatory trials.
For women in the second or third trimester of pregnancy, World Health Organization (WHO) guidelines recommend a 3-day course with either an artemisinin-based combination therapy that is known to be effective in the country or region or clindamycin plus a 7-day course of either artesunate or quinine.6 Although the experience regarding the use of artemisinin-based combination therapies in pregnancy is increasing,6 this information is still limited, particularly in sub-Saharan Africa.
Methods
Trial design
We conducted this randomized, open-label trial from June 2010 through August 2013 at seven sites in four sub-Saharan African countries: Burkina Faso (two sites), Ghana (three), Malawi (one), and Zambia (one). The trial protocol, which is available with the full text of this article at NEJM. org, has been described in detail elsewhere.7 In brief, pregnant women in the second or third trimester who had a Plasmodium falciparum monoinfection of any density, regardless of symptoms, a hemoglobin level of 7 g per deciliter or more, and no other serious illness were recruited into the trial and randomly assigned to one of the following four treatments: artemether-lumefantrine, amodiaquine- artesunate, mefloquine-artesunate, or dihydroartemisinin- piperaquine.
Sigma-Tau Industrie Farmaceutiche Riunite donated dihydroartemisinin-piperaquine, Novartis donated artemether-lumefantrine, and Sanofi-Aventis donated artesunate-amodiaquine. The Drugs for Neglected Diseases Initiative facilitated the negotiation for the procurement of artesunate-mefloquine from Farmanguinhos, which donated the treatment. The donors did not have any role in reviewing the protocol or the manuscript, although the protocol synopsis was provided to the manufacturers.
The trial was set up in a pragmatic approach with three treatment groups per country with the use of a balanced, incomplete block design, which allowed for the maximized use of resources and took into account the policies regarding antimalarial treatment in the respective countries (Table S1 in the Supplementary Appendix, available at NEJM.org). All doses of the study drugs were given under direct observation on days 0, 1, and 2 and according to the recommendations of the manufacturer (Table S2 in the Supplementary Appendix). At recruitment, the gestational age was estimated with the use of the symphysiofundal height, and the fetal viability was assessed by means of ultrasonography with a portable multipurpose machine.
Follow-up
After completion of the 3-day treatment, patients were asked to return to the clinic (in Ghana, patients were visited at home) for follow-up visits on days 3 and 7 and then once every week until day 63. At each visit, a medical history was obtained, and information was collected regarding current signs and symptoms, including the start and end date, the severity (mild, moderate, severe, or life-threatening), and the perceived relationship to the study treatment (definitely unrelated, unlikely to be related, possibly related, probably related, or definitely related), as well as the outcome of any adverse events. A blood sample was obtained for malaria smears and dried blood spots for later genotyping, for full blood counts (on days 7, 14, 28, and 63 only), and for measurement of the total bilirubin, alanine aminotransferase, and creatinine levels (on days 7 and 14 only). Rescue treatment for recurrent infection was given according to national guidelines.
At the end of the active follow-up period, women were asked to attend the antenatal clinic monthly or when they felt unhealthy, until delivery. After delivery, the newborn was examined for congenital malformations and weighed, and the gestational age was estimated with the use of the total Ballard score (range, −10 [20 weeks of gestation] to 50 [44 weeks of gestation]).8 A placental-biopsy specimen was obtained as soon as possible after delivery and was preserved in 10% neutral buffered formalin. The biopsy specimens were processed and embedded in paraffin wax by means of standard techniques and were kept at 4°C. Paraffin sections that were 4 mm thick were stained with hematoxylin and eosin and read at the Barcelona Center for International Health Research.7
Laboratory procedures
Giemsa-stained thick and thin blood films were read independently by two readers. Blood smears with discordant results (differences between the two microscopists with regard to the diagnosis of the species, positivity, or parasite density of >50%) were reexamined by a third, independent microscopist, and parasite density was calculated by averaging the two closest counts. We estimated parasite density by counting the number of asexual parasites per 200 white cells, assuming a white-cell count of 8000 per cubic millimeter. The total bilirubin, alanine aminotransferase, and creatinine levels were measured with the use of the Flexor Junior biochemistry analyzer. The full blood count was obtained with the use of the Sysmex XT-2000i hematology analyzer. The hemoglobin level was measured with the use of the HemoCue system. For polymerase-chain-reaction (PCR) analysis, blood samples were collected on filter papers (Whatman 3MM) that were subsequently transported to the Institute of Tropical Medicine, Antwerp, Belgium, where centralized genotyping (of the glutamate-rich protein [GLURP] and surface proteins of the P. falciparum merozoite [MSP2 and MSP1]) was performed to distinguish reinfection from recrudescence.9 Samples that did not produce a result were classified as indeterminate.
Trial end points
The primary end points of the trial were the PCR-adjusted cure rates at day 63 (Table S3 in the Supplementary Appendix) and the safety outcomes7 (Table S4 in the Supplementary Appendix). In the estimation of the PCR-adjusted cure rate, only recurrent infections that were shown by means of genotyping to be the same infections as those before treatment (i.e., recrudescent infections) were considered to be treatment failures; conversely, for the estimation of the PCR-unadjusted cure rate, all recurrent infections were considered to be treatment failures.
Treatment failures were classified as either early or late treatment failures, with the latter category comprising late clinical failures and late parasitologic failures. Early treatment failure was defined as one of the following: the development of danger signs or severe malaria or worsening of clinical conditions on day 0, 1, 2, or 3 in the presence of parasitemia; parasitemia on day 3 that was the same as or greater than the count on day 0; or parasitemia on day 3 and fever (axillary temperature, ≥37.5°C). Late clinical failure was defined as the either the development of danger signs or severe malaria or worsening of clinical conditions on any day after day 3 in the presence of parasitemia, without the patient having previously met any of the criteria for early treatment failure, or the presence of parasitemia and fever on any day after day 3, without the patient having previously met the criteria for early treatment failure. Late parasitologic failure was defined as the presence of parasitemia after day 3 and an axillary temperature of less than 37.5°C, without the patient having previously met any of the criteria of early treatment failure or late parasitologic failure. An adequate clinical and parasitologic response was defined as the absence of parasitemia at the end of follow-up (day 63), regardless of the axillary temperature, without the patient having previously met any of the criteria of early treatment failure or late treatment failure.
Adverse events and serious adverse events were recorded and monitored throughout the trial by an independent data and safety monitoring board. The relationship between treatment and adverse events or serious adverse events was determined by the local investigator on the basis of clinical judgment, possible alternative causes (e.g., concomitant therapy), time of occurrence relative to the study treatment, and available information on the study treatment. The data and safety monitoring board reviewed listings of serious adverse events regularly. Secondary end points were PCR-unadjusted cure rates (Table S3 in the Supplementary Appendix) at day 63, time to treatment failure (PCR-adjusted and PCR-unadjusted) (Fig. S1 in the Supplementary Appendix), asexual parasite clearance,10 gametocytemia (prevalence and density), and changes in the hemoglobin level.
Trial oversight
The contributions of the authors are listed in Table S5 in the Supplementary Appendix. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The trial was approved by the ethics committee at the Antwerp University Hospital, the relevant national or local ethics committees, and the national drug regulatory authorities (Table S6 in the Supplementary Appendix). All the study participants provided written informed consent. If the woman was illiterate, she would provide a fingerprint and a witness would write the name of the patient onto the form and sign and date it.
Statistical analysis
The trial was designed to determine whether all four treatments had similar PCR-adjusted cure rates (difference, <5 percentage points), with 95% power for each of the six pairwise comparisons and 80% power for the combined hypothesis that all the treatments would be equivalent.7 No multiplicity adjustment for the primary analysis was performed because the four treatments would be declared similar only if all six pairwise comparisons were shown to be within the 5-percentage-point margin. For this joint decision rule, no alpha-level correction was needed.11
Data were captured in an electronic case-report form that was developed with the use of MACRO software (Infermed). A statistical analysis plan was developed before the database lock. For the primary end point, three analysis populations were used: a per-protocol population, an intention-to-treat population that excluded patients who were lost to follow-up or withdrew and those with missing or indeterminate results on PCR assay, and an intention-to-treat population that included multiple imputations of data from women who were lost to follow-up or withdrew and from those who had missing or indeterminate results on PCR assay. The perprotocol analysis was considered to be the primary analysis approach. Persons with major protocol violations, defined as a violation of the inclusion or exclusion criteria, receipt of a treatment different from the randomly assigned one, missing at least a full day of treatment, intake of other drugs with antimalarial activity, and missing day 63 blood smears, were excluded from the per-protocol analysis. All the secondary end points were analyzed with the use of an available-data approach.
We tested the primary hypothesis by calculating the 95% confidence interval for the difference in cure rates. If the difference in true (PCR-adjusted) cure rates was less than 5 percentage points, the treatments were considered to be therapeutically equivalent. This margin was chosen on the basis of the WHO recommendation that a new recommended antimalarial treatment that is adopted as policy should have an average cure rate of 95% or more as assessed in clinical trials. The confidence interval was calculated from a generalized linear model with adjustment for differences among the four countries. A number of sensitivity analyses were performed, including an analysis with multiple imputation of missing outcomes, a pairwise comparison that was limited to trial sites where a head-to-head comparison of treatments was performed, an analysis with adjustment for parasite density, gestational age, and gravidity at trial entry, and an analysis of the time to treatment failure with the use of Cox regression models. For the safety analysis, all the women who received at least one dose of the study treatment were included. Details of the subgroup analyses are provided in the Supplementary Appendix.
Results
Participants
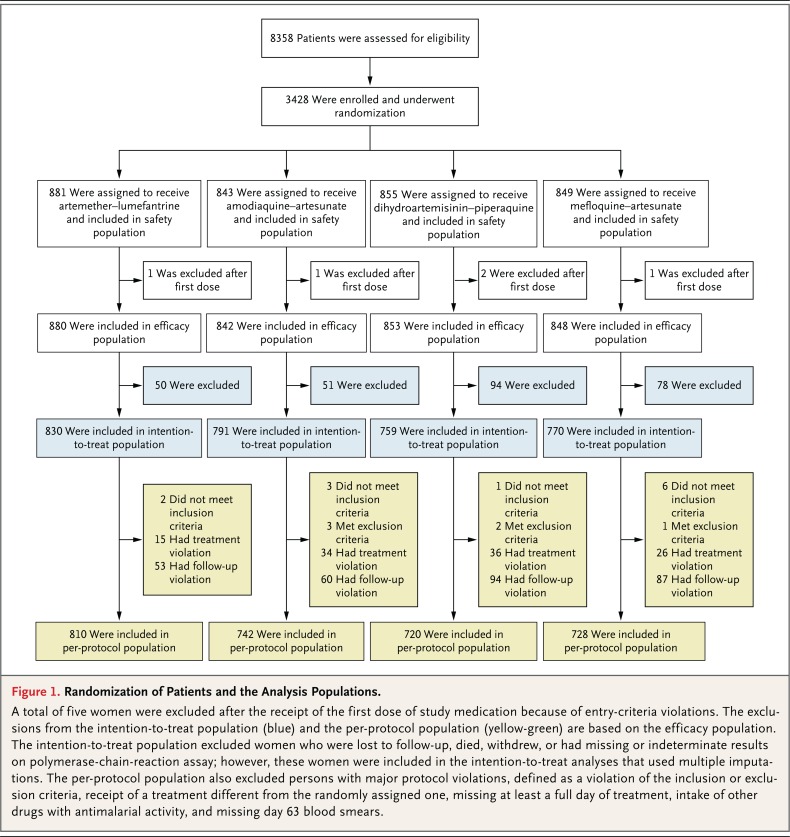
A total of 3428 pregnant women who had P. falciparum infection were randomly assigned to receive one of four treatment: artemether-lumefantrine (881 women), amodiaquine-artesunate (843), dihydroartemisinin-piperaquine (855), or mefloquine-artesunate (849) (Fig. 1). The trial sites in Burkina Faso recruited 870 women, those in Ghana 788, the one in Malawi 870, and the one in Zambia 900. Five women were withdrawn by the investigators immediately after starting the treatment because of protocol deviations (3 did not have malaria, 1 was not pregnant, and 1 had been enrolled previously), although they were included in the safety analysis.
Figure 1.
Randomization of Patients and the Analysis Populations.
A total of five women were excluded after the receipt of the first dose of study medication because of entry-criteria violations. The exclusions from the intention-to-treat population (blue) and the per-protocol population (yellow-green) are based on the efficacy population. The intention-to-treat population excluded women who were lost to follow-up, died, withdrew, or had missing or indeterminate results on polymerase-chain-reaction assay; however, these women were included in the intention-to-treat analyses that used multiple imputations. The per-protocol population also excluded persons with major protocol violations, defined as a violation of the inclusion or exclusion criteria, receipt of a treatment different from the randomly assigned one, missing at least a full day of treatment, intake of other drugs with antimalarial activity, and missing day 63 blood smears.
The intention-to-treat analysis included 3150 women (830 women in the artemether-lumefantrine group, 791 in the amodiaquine-artesunate group, 759 in the dihydroartemisinin-piperaquine group, and 770 in the mefloquine-artesunate group). The per-protocol analysis included 3000 women (810 women in the artemether- lumefantrine group, 742 in the amodiaquine-artesunate group, 720 in the dihydroartemisinin-piperaquine group, and 728 in the mefloquine-artesunate group) (Fig. 1). Approximately half the exclusions from the per-protocol analysis (227 of 423 women [53.7%]) were due to loss to follow-up, withdrawal, or death.
The characteristics of the participants at baseline were similar among the treatment groups (Table 1). Most women were included during the second trimester of pregnancy, and primigravidae represented approximately one third of the trial population. Parasite density was more than 2000 per cubic milliliter in approximately one third of the women, and few women (6.1%) had fever at the time of recruitment.
Table 1.
Characteristics of the Participants at Baseline.*
| Characteristic | Artemether- Lumefantrine (N = 880) |
Amodiaquine- Artesunate (N = 842) |
Dihydroartemisinin- Piperaquine (N = 853) |
Mefloquine- Artesunate (N = 848) |
| Country (no.) | ||||
| Burkina Faso | 290 | 291 | 0 | 288 |
| Ghana | 0 | 261 | 265 | 260 |
| Malawi | 290 | 290 | 288 | 0 |
| Zambia | 300 | 0 | 300 | 300 |
| Age (yr) | 22.6±5.6 | 23.4±5.9 | 22.3±5.4 | 23.5±5.9 |
| Symptomatic malaria (%)† | 37.2 | 34.9 | 37.4 | 43.8 |
| Fever (%) | 6.5 | 6.8 | 3.2 | 8.0 |
| Parasite density >2000/mm3 (%) | 30.6 | 25.3 | 29.1 | 32.1 |
| ≥3 symptoms (%)‡ | 7.2 | 9.3 | 11.8 | 14.3 |
| Gametocytes present (%) | 2.4 | 2.9 | 2.5 | 0.7 |
| Parasite density (per mm3) | ||||
| Median | 800 | 569 | 680 | 840 |
| Interquartile range | 213–2880 | 165–2025 | 200–2760 | 218–3040 |
| Hemoglobin (g/dl) | ||||
| Median | 10.2 | 10.1 | 10.1 | 10.1 |
| Interquartile range | 9.2–11.0 | 9.1–11.0 | 9.1–11.0 | 9.1–10.9 |
| Gravidity (%) | ||||
| 1 | 36.3 | 37.4 | 40.0 | 32.7 |
| 2 | 23.1 | 22.2 | 25.3 | 23.7 |
| ≥3 | 40.6 | 40.4 | 34.7 | 43.6 |
| Trimester of gestation (%)§ | ||||
| Second | 71.8 | 75.0 | 68.5 | 65.8 |
| Third | 28.2 | 24.9 | 31.5 | 34.2 |
| Bed net used before trial entry (%) | 34.4 | 34.6 | 27.9 | 37.5 |
| Insecticide-treated bed net used before trial entry (%)¶ |
24.8 | 23.9 | 17.1 | 27.9 |
| Use of intermittent preventive treatment before day 0 (%) |
9.9 | 10.9 | 13.7 | 16.4 |
Plus-minus values are means ±SD. There were no significant differences at baseline among the treatment groups.
Symptomatic malaria was defined as any of the following: fever (axillary temperature, ≥37.5°C) at baseline with parasitemia of any density; a parasite count of more than 2000 per cubic millimeter, regardless of symptoms; or at least three of the following symptoms — fever in the previous 24 hours, weakness or fatigue, muscle or joint aches, headache, or convulsion — with parasitemia of any density.
Symptoms included fever in the previous 24 hours, weakness or fatigue, muscle or joint aches, or headache.
One woman in the amodiaquine-artesunate group was included in the trial during the first trimester of pregnancy.
Women were provided with an insecticide-treated bed net at the start of the trial.
Treatment efficacy
The large majority of treatment failures were late parasitologic failures, with fewer women having a late clinical failure (Table 2). Most cases of late treatment failures were classified as new infections.
Table 2.
Efficacy Outcomes and Treatment Success Rates.*
| Variable | Artemether- Lumefantrine (N = 880) |
Amodiaquine- Artesunate (N = 842) |
Dihydroartemisinin- Piperaquine (N = 853) |
Mefloquine- Artesunate (N = 848) |
| Efficacy outcome — no. (%)† | ||||
| Early treatment failure | 0 | 2 (0.2) | 1 (0.1) | 0 |
| Development of danger signs or severe malaria |
0 | 0 | 1 (0.1) | 0 |
| Rescue treatment on any of days 0–3 | 0 | 1 (0.1) | 0 | 0 |
| Parasitemia on day 3 ≥ day 0 | 0 | 1 (0.1) | 0 | 0 |
| Late clinical failure | 26 (3.0) | 13 (1.5) | 6 (0.7) | 28 (3.3) |
| Recrudescence | 4 (0.5) | 2 (0.2) | 1 (0.1) | 8 (0.9) |
| New infection | 21 (2.4) | 10 (1.2) | 5 (0.6) | 18 (2.1) |
| Indeterminate or sample unavailable | 1 (0.1) | 1 (0.1) | 0 | 2 (0.2) |
| Late parasitologic failure | 362 (41.1) | 123 (14.6) | 91 (10.7) | 176 (20.8) |
| Recrudescence | 37 (4.2) | 9 (1.1) | 5 (0.6) | 17 (2.0) |
| New infection | 303 (34.4) | 100 (11.9) | 71 (8.3) | 144 (17.0) |
| Indeterminate or sample unavailable | 22 (2.5) | 14 (1.7) | 15 (1.8) | 15 (1.8) |
| Adequate clinical and parasitologic response | 436 (49.5) | 642 (76.2) | 653 (76.6) | 557 (65.7) |
| Response could not be determined | 56 (6.4) | 62 (7.4) | 102 (12.0) | 87 (10.3) |
| Received rescue treatment but had no infection |
6 (0.7) | 11 (1.3) | 8 (0.9) | 9 (1.1) |
| Died‡ | 0 | 0 | 0 | 1 (0.1) |
| Lost to follow-up or withdrew | 50 (5.7) | 51 (6.1) | 94 (11.0) | 77 (9.1) |
| Treatment success§ | ||||
| Per-protocol analysis | ||||
| PCR-adjusted | ||||
| No. of patients | 789 | 729 | 707 | 716 |
| Rate — % (95% CI) | 94.8 (93.0–96.1) | 98.5 (97.3–99.2) | 99.2 (98.2–99.6) | 96.8 (95.2–97.9) |
| PCR-unadjusted | ||||
| No. of patients | 810 | 742 | 720 | 728 |
| Rate — % (95% CI) | 52.5 (49.0–55.9) | 82.3 (79.4–84.9) | 86.9 (84.3–89.2) | 73.8 (70.4–76.8) |
| Intention-to-treat analysis | ||||
| PCR-adjusted | ||||
| No. of patients | 807 | 776 | 744 | 753 |
| Rate — % (95% CI) | 94.2 (92.3–954.6) | 96.9 (95.4–97.9) | 98.0 (96.7–98.8) | 95.5 (93.8–96.8) |
| PCR-unadjusted | ||||
| No. of patients | 830 | 791 | 759 | 770 |
| Rate — % (95% CI) | 52.5 (49.1–55.9) | 81.2 (78.3–83.7) | 86.0 (83.4–88.3) | 72.3 (69.1–73.4) |
CI denotes confidence interval.
Early treatment failure was defined as one of the following: the development of danger signs or severe malaria or worsening of clinical conditions on day 0, 1, 2, or 3 in the presence of parasitemia; parasitemia on day 3 that was the same as or greater than the count on day 0; or parasitemia on day 3 and fever (axillary temperature, −37.5°C). Late clinical failure was defined as the either the development of danger signs or severe malaria or worsening of clinical conditions on any day after day 3 in the presence of parasitemia, without the patient having previously met any of the criteria for early treatment failure, or the presence of parasitemia and fever on any day after day 3, without the patient having previously met the criteria for early treatment failure. Late parasitologic failure was defined as the presence of parasitemia after day 3 and an axillary temperature of less than 37.5°C, without the patient having previously met any of the criteria of early treatment failure or late clinical failure. An adequate clinical and parasitologic response was defined as the absence of parasitemia at the end of the follow-up period (day 63), regardless of the axillary temperature, without the patient having previously met any of the criteria of early treatment failure or late clinical or parasitologic failure. In the polymerase-chain-reaction (PCR)-adjusted estimates, patients with late asexual-parasite reappearance (with or without fever) were considered to have had an adequate clinical and parasitologic response if the PCR analysis showed a new infection rather than a recrudescence.
The one death that occurred during the trial was not considered by the investigators to be related to malaria or to treatment (probably due to meningitis).
In the estimation of the PCR-adjusted cure rate, only recurrent infections that were shown to be the same as those before treatment were considered to be treatment failures. Conversely, for the estimation of the PCR-unadjusted cure rate, all recurrent infections were considered to be treatment failures. If the difference in the true (PCR-adjusted) cure rates was less than 5 percentage points, the treatments were considered to be therapeutically equivalent. Treatment success rates according to country are provided in Tables S7 (per-protocol analysis) and S8 (intention-to-treat analysis) in the Supplementary Appendix.
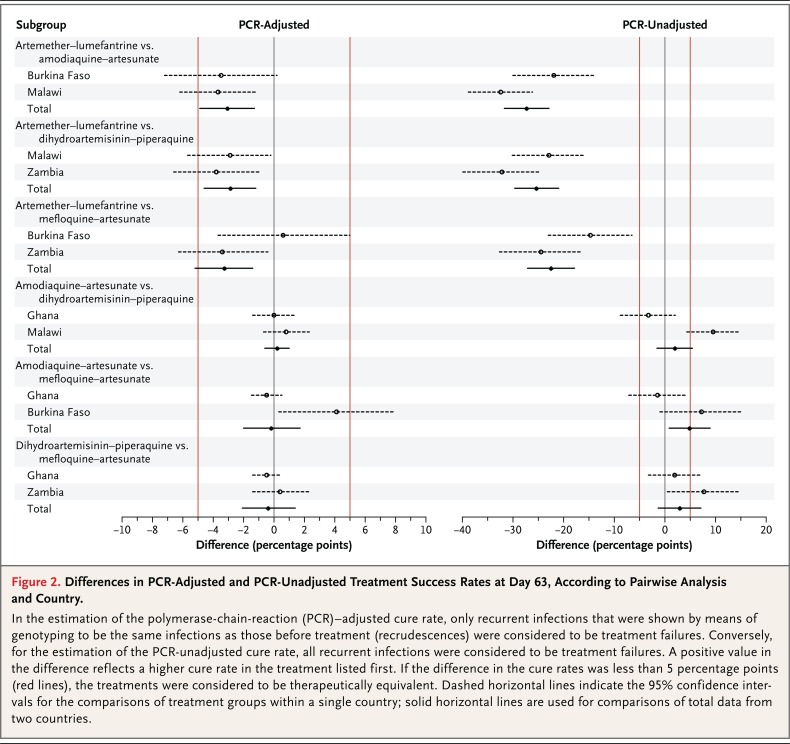
According to the per-protocol analysis, the overall PCR-adjusted cure rate at day 63 was 94.8% (95% confidence interval [CI], 93.0 to 96.1; 748 of 789 women) in the artemether-lumefantrine group, 98.5% (95% CI, 97.3 to 99.2; 718 of 729 women) in the amodiaquine-artesunate group, 99.2% (95% CI, 98.2 to 99.6; 701 of 707 women) in the dihydroartemisinin-piperaquine group, and 96.8% (95% CI, 95.2 to 97.9; 693 of 716 women) in the mefloquine-artesunate group (Table 2, and Fig. S1 in the Supplementary Appendix). There was no significant difference among the amodiaquine-artesunate group, the dihydroartemisinin-piperaquine group, and the mefloquine-artesunate group. The cure rate in the artemether-lumefantrine group was significantly lower than the rate in the other three treatment groups (P<0.001), although the difference was within the prespecified margin of 5 percentage points (Fig. 2).
Figure 2.
Differences in PCR-Adjusted and PCR-Unadjusted Treatment Success Rates at Day 63, According to Pairwise Analysis and Country.
In the estimation of the polymerase-chain-reaction (PCR)-adjusted cure rate, only recurrent infections that were shown by means of genotyping to be the same infections as those before treatment (recrudescences) were considered to be treatment failures. Conversely, for the estimation of the PCR-unadjusted cure rate, all recurrent infections were considered to be treatment failures. A positive value in the difference reflects a higher cure rate in the treatment listed first. If the difference in the cure rates was less than 5 percentage points (red lines), the treatments were considered to be therapeutically equivalent. Dashed horizontal lines indicate the 95% confidence intervals for the comparisons of treatment groups within a single country; solid horizontal lines are used for comparisons of total data from two countries.
The unadjusted cure rates (Table S3 in the Supplementary Appendix) were significantly lower in the artemether-lumefantrine group (52.5%; 425 of 810 women) than in the amodiaquine-artesunate group (82.3%; 611 of 742), the dihydroartemisinin-piperaquine group (86.9%; 626 of 720), and the mefloquine-artesunate group (73.8%; 537 of 728) (P<0.001) (Table 2). Country-specific results are provided in Table S7 in the Supplementary Appendix. The intention-to-treat analyses and analyses with multiple imputations of unavailable outcomes further supported the efficacy results (Table S8 in the Supplementary Appendix). Results from the sensitivity analyses were generally consistent with those from the primary analyses.
At day 2 after the initiation of treatment, nearly all the women (>99.5%) had a negative blood smear. However, parasite clearance was slower among the women treated with artemether-lumefantrine than among women treated with the other therapies; at day 1 after the start of treatment, 24.8% of the women (217 of 875 women) in the artemether-lumefantrine group still had detectable parasitemia, as compared with 6.9% (57 of 828) in the amodiaquine-artesunate group, 8.0% (67 of 837) in the dihydroartemisinin-piperaquine group, and 13.5% (113 of 837) in the mefloquine-artesunate group (P<0.001).
Gametocyte prevalence at enrollment was low (Table 1), with a median density between 11 and 40 gametocytes per cubic millimeter. Gametocyte carriage remained low throughout follow-up, with no significant difference among the treatment groups. Similarly, changes in the hemoglobin level did not differ significantly among the treatment groups throughout follow-up (Fig. S2 in the Supplementary Appendix).
The prevalence of placental malaria infection at delivery was similar among the treatment groups (P = 0.47). The mean birth weight of the babies, after adjustment according to country, was similar among the treatment groups. The mean (±SD) birth weight was 2854±449 g in the artemether-lumefantrine group, 2880±452 g in the amodiaquine-artesunate group, 2901±454 g in the dihydroartemisinin-piperaquine group, and 2875±433 g in the mefloquine-artesunate group (P = 0.40). Similarly, the percentage of babies with low birth weight did not vary significantly among the treatment groups (17.2% in the artemether-lumefantrine group, 15.5% in the amodiaquine-artesunate group, 14.1% in the dihydroartemisinin-piperaquine group, and 15.2% in the mefloquine-artesunate group; P = 0.32).
Safety
A total of 72 women had serious adverse events during the 63-day follow-up, including 1 woman in the mefloquine-artesunate group who died approximately 1 month after treatment, probably from meningitis (Table S9 in the Supplementary Appendix). There were 10 serious adverse events that were assessed by the site investigator as being probably related to the study medication, including 5 in the amodiaquine-artesunate group (anemia in 2 women, upper abdominal pain in 1, and malaise in 2), 4 in the mefloquine-artesunate group (abdominal pain in 1, vomiting in 2, and malaise in 1), and 1 in the dihydroartemisinin-piperaquine group (a possible adverse drug reaction with headache and general weakness 2 days after the completion of treatment; the woman recovered completely). No significant difference in the occurrence of serious adverse events was found among the treatment groups.
Women treated with mefloquine-artesunate and those treated with amodiaquine-artesunate had a significantly higher incidence of any adverse event (84.9% [722 of 850 women] and 79.0% [665 of 842], respectively) than did those in the artemether-lumefantrine group (72.8%; 641 of 881) and those in the dihydroartemisinin-piperaquine group (70.4%; 602 of 855) (P<0.001 for the comparison among the four groups) (Table 3). Adverse events that were related to the treatment, as determined by the investigators, occurred significantly more frequently in the mefloquine- artesunate group (in 50.6% of women; 430 of 850 women) and the amodiaquine-artesunate group (in 48.5%; 408 of 842) than in the dihydroartemisinin-piperaquine group (in 20.6%; 176 of 855) and the artemether-lumefantrine group (in 11.5%; 101 of 881) (P<0.001 for the comparison among the four groups). This result was due mainly to the higher occurrence of asthenia, poor appetite, dizziness, nausea, and vomiting among women treated with mefloquine-artesunate or amodiaquine-artesunate than among those treated with dihydroartemisinin-piperaquine or artemether-lumefantrine (Table 3).
Table 3.
Safety Outcomes.
| Event | Artemether- Lumefantrine (N = 881) |
Amodiaquine- Artesunate (N = 842) |
Dihydroartemisinin- Piperaquine (N = 855) |
Mefloquine- Artesunate (N = 850) |
| percent of patients | ||||
| Any serious adverse event during 63 days of follow-up |
0.7 | 2.6 | 2.1 | 2.9 |
| Mild | 0 | 0.2 | 0.2 | 0.5 |
| Moderate | 0.2 | 1.3 | 1.2 | 1.5 |
| Severe | 0.2 | 0.7 | 0.5 | 0.5 |
| Life-threatening | 0.2 | 0.4 | 0.2 | 0.5 |
| Specific serious adverse event during 63 days of follow-up |
||||
| Blood disorder | 0 | 0.7 | 0.2 | 0 |
| Moderate | 0 | 0.2 | 0 | 0 |
| Severe | 0 | 0.4 | 0.2 | 0 |
| Life-threatening | 0 | 0.1 | 0 | 0 |
| Moderate abdominal pain | 0 | 0.2 | 0 | 0.1 |
| Severe diarrhea | 0.1 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0.2 |
| Moderate | 0 | 0 | 0 | 0.1 |
| Severe | 0 | 0 | 0 | 0.1 |
| Malaise | 0 | 0.2 | 0 | 0.1 |
| Mild | 0 | 0.2 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0.1 |
| Moderate adverse drug reaction | 0 | 0 | 0.1 | 0 |
| Infection | 0.5 | 1.0 | 1.1 | 1.5 |
| Mild | 0 | 0 | 0.2 | 0.2 |
| Moderate | 0.2 | 0.8 | 0.8 | 0.9 |
| Severe | 0 | 0.1 | 0 | 0.2 |
| Life-threatening | 0.2 | 0 | 0 | 0.1 |
| Complications of pregnancy and delivery | 0.1 | 0.5 | 0.5 | 0.7 |
| Mild | 0 | 0.1 | 0 | 0 |
| Moderate | 0 | 0 | 0.1 | 0.2 |
| Severe | 0.1 | 0.1 | 0.1 | 0.1 |
| Life-threatening | 0 | 0.2 | 0.2 | 0.4 |
| Mild asthma | 0 | 0 | 0 | 0.1 |
| Any adverse event during 63 days of follow-up | 72.8 | 79.0 | 70.4 | 84.9 |
| Mild | 54.4 | 56.7 | 59.1 | 68.8 |
| Moderate | 18.2 | 21.5 | 11.1 | 15.2 |
| Severe | 0.1 | 0.7 | 0.2 | 0.8 |
| Life-threatening | 0.1 | 0.1 | 0 | 0.1 |
| Any event during first 7 days | 24.3 | 59.5 | 34.2 | 60.7 |
| Any drug-related event | 11.5 | 48.5 | 20.6 | 50.6 |
| Mild | 10.1 | 37.1 | 18.4 | 41.9 |
| Moderate | 1.2 | 11.2 | 2.1 | 8.2 |
| Severe | 0.1 | 0.2 | 0.1 | 0.5 |
| Specific drug-related adverse event* | ||||
| Abdominal pain | 2.7 | 7.1 | 2.1 | 5.3 |
| Mild | 2.2 | 6.3 | 2.0 | 4.2 |
| Moderate | 0.6 | 0.8 | 0.1 | 1.1 |
| Asthenia | 1.8 | 26.6 | 6.8 | 14.2 |
| Mild | 1.6 | 16.7 | 6.0 | 10.4 |
| Moderate | 0.2 | 9.9 | 0.8 | 3.5 |
| Severe | 0 | 0 | 0 | 0.4 |
| Decreased appetite | 0.3 | 8.2 | 2.1 | 7.1 |
| Mild | 0.3 | 7.1 | 2.0 | 6.6 |
| Moderate | 0 | 1.1 | 0.1 | 0.5 |
| Dizziness | 1.2 | 23.5 | 1.6 | 30.6 |
| Mild | 1.2 | 16.2 | 1.4 | 24.2 |
| Moderate | 0 | 7.2 | 0.2 | 6.2 |
| Severe | 0 | 0.1 | 0 | 0.1 |
| Headache | 4.3 | 6.3 | 5.1 | 7.5 |
| Mild | 3.7 | 4.8 | 4.4 | 6.6 |
| Moderate | 0.6 | 1.5 | 0.7 | 0.9 |
| Musculoskeletal pain | 0.8 | 7.2 | 2.6 | 4.5 |
| Mild | 0.8 | 5.8 | 2.3 | 3.2 |
| Moderate | 0 | 1.4 | 0.2 | 1.2 |
| Severe | 0 | 0 | 0 | 0.1 |
| Nausea | 0.9 | 11.5 | 4.0 | 13.9 |
| Mild | 0.9 | 10.6 | 3.7 | 12.6 |
| Moderate | 0 | 1.0 | 0.2 | 1.3 |
| Vomiting | 0.9 | 15.9 | 5.7 | 18.9 |
| Mild | 0.8 | 12.5 | 5.1 | 13.5 |
| Moderate | 0.1 | 3.4 | 0.6 | 5.4 |
| Abnormality in vital sign during treatment† | ||||
| Pulse rate <60 beats/min | 1.1 | 2.1 | 1.6 | 1.5 |
| Diastolic blood pressure <50 mm Hg | 8.4 | 15.1 | 7.7 | 4.7 |
| Systolic blood pressure <90 mm Hg | 5.1 | 12.2 | 6.3 | 4.1 |
The specific drug-related adverse events reported here were those that occurred in more than 5% of the patients in at least one treatment group.
Abnormality in vital signs during treatment was assessed during days 1, 2, and 3; the percentage of patients shown is the percentage of those with an abnormality on any of these days.
Behavioral changes were observed in 4 women, of whom 2 were in the amodiaquine-artesunate group (changes noted on day 2 and day 3 after treatment), 1 was in the mefloquine- artesunate group (changes noted on day 2 after treatment), and 1 was in the artemether-lumefantrine group (changes noted on day 60 after treatment); the two behavioral changes in the amodiaquine-artesunate group were considered by the site investigator to be possibly related to treatment. All the women recovered completely. A woman treated with amodiaquine-artesunate reported hallucinations on day 3 after treatment; these were considered by the investigator to be possibly related to treatment. The woman recovered completely. Significantly more women in the amodiaquine-artesunate group than in the other three groups reported insomnia: 4.0% (34 of 842 women) in the amodiaquine-artesunate group versus 2.5% (21 of 850) in the mefloquine-artesunate group, 1.6% (14 of 855) in the dihydroartemisinin-piperaquine group, and 0.3% (3 of 881) in the artemether-lumefantrine group (P = 0.04).
The pulse rate and blood pressure tended to be lower among women treated with amodiaquine-artesunate than among those in the other three groups (Figs. S3 and S4 in the Supplementary Appendix). The percentage of women with a diastolic blood pressure of less than 50 mm Hg and a systolic blood pressure of less than 90 mm Hg was higher in the amodiaquine-artesunate group than in the other groups (P<0.001). Similarly, the percentage of women with a pulse rate of less than 60 beats per minute appeared to be higher in the amodiaquine-artesunate group than in the other groups, but this difference was not significant (P = 0.40). Hypotension or a low diastolic blood pressure as an adverse event (i.e., considered by the local investigator to be clinically significant) occurred more frequently in the amodiaquine-artesunate group (1.5%) than in the other treatment groups (range, 0.6 to 0.8%). There were no significant differences in the laboratory safety values among the treatment groups.
Outcome of pregnancy
There were 13 miscarriages (1 miscarriage in the artemether- lumefantrine group and 4 in each of the other three groups). There were 78 stillbirths overall, with 16 stillbirths occurring in 856 births (1.9%) in the artemether-lumefantrine group, 17 in 815 (2.1%) in the amodiaquine-artesunate group, 22 in 818 (2.7%) in the dihydroartemisinin-piperaquine group, and 23 in 821 (2.8%) in the mefloquine-artesunate group. The proportion of live births did not differ significantly among the treatment groups (P = 0.85). The percentage of preterm babies, as determined by the total Ballard score, was 10.2% in the artemether-lumefantrine group, 3.4% in the amodiaquine-artesunate group, 9.5% in the dihydroartemisinin-piperaquine group, and 7.7% in the mefloquine-artesunate group (P = 0.64). A total of 44 congenital malformations were observed, with 17 occurring in 832 newborns (2.0%) in theartemether-lumefantrine group, 8 in 776 (1%) in the amodiaquine-artesunate group, 6 in 767 (0.8%) in the dihydroartemisinin-piperaquine group, and 13 in 780 (1.7%) in the mefloquine-artesunate group.
Discussion
The PCR-adjusted cure rates were in the range of 94.8 to 99.2% for all four artemisinin-based combination therapies, and the differences among them were within the prespecified equivalence margin of 5 percentage points. The high success rates are remarkable given the long follow-up period, which was 3 weeks longer than the 6 weeks recommended by the WHO. Nevertheless, the cure rates in the artemether-lumefantrine group were significantly lower than those in the groups that received the other artemisinin-based combination therapies, which had similar high efficacy. In a previous trial in Uganda, the efficacy of artemether-lumefantrine (until day 42) during pregnancy was 99.3%.12 The longer follow-up until day 63 in our trial cannot explain the lower cure rates in our trial than in the Uganda trial, because most treatment failures occurred between day 28 and day 42 (Fig. S1 in the Supplementary Appendix). The efficacy of artemether-lumefantrine was low (82%) among pregnant women at the Thai-Burmese border,13 a finding that was attributed to low drug concentrations and low antimalarial immunity.14,15 In Uganda, the plasma concentration of lumefantrine was 27% lower in pregnant women than in nonpregnant women,16 which suggests that the high efficacy of artemether-lumefantrine was probably due to the higher background immunity in Uganda than in Thailand. In our trial, artemether-lumefantrine was tested in three countries with a high risk of malaria infection (owing to the high intensity of malaria transmission), so the background immunity among recruited pregnant women was probably high.
Patients treated with artemether-lumefantrine had the highest rate of reinfection and the shortest time to reinfection. The duration of post-treatment prophylaxis is an important factor in the choice of antimalarial drugs, especially in areas with a high risk of infection. Lumefantrine has the shortest elimination half-life,17 followed by mefloquine,18 amodiaquine,19 and then piperaquine.20
The efficacy of artemether-lumefantrine was relatively low in Burkina Faso (93.2%). This was the same trial site at which the efficacy of artemether-lumefantrine among children with malaria was the lowest (90.2%) among trial sites in sub-Saharan Africa.21 The sites in Burkina Faso also had the highest intensity of transmission, as suggested by the high rates of reinfection observed in all the treatment groups (Table S7 in the Supplementary Appendix). This high intensity of transmission may influence the interpretation of the genotyping results, with the risk that new infections may be misclassified as recrudescences.22 In our trial, capillary electrophoresis, a technique that can minimize misclassification,23 was used for MSP2 genotyping. In addition, transmission intensity may influence the individual treatment cure rates but not the risk difference between treatments.23
There was no significant difference in birth outcomes among the treatment groups. The mean birth weight as well as the proportion of miscarriages, stillbirths, preterm deliveries, and congenital malformations were similar among the groups.
Fewer adverse events were seen in the artemether-lumefantrine group and the dihydroartemisinin-piperaquine group than in the other two groups.15,20,24 Approximately half the adverse events in the amodiaquine-artesunate group and mefloquine-artesunate group were considered by the investigator to be related to treatment. Asthenia was the most common event in the amodiaquine-artesunate group, followed by dizziness; both these events may be related to low blood pressure and pulse rate. Asthenia was more common in the amodiaquine-artesunate group than in the other three groups. Nausea or vomiting was also relatively common. General weakness, vomiting, dizziness, and nausea were the most commonly reported adverse events among pregnant women in Ghana who were treated with amodiaquine.25,26 Dizziness was the most frequent treatmentrelated adverse event in the mefloquine-artesunate group, followed by vomiting, nausea, and asthenia. The association between mefloquine and dizziness has already been reported; more than 30% of pregnant women who were treated with mefloquine monotherapy at a dose of 15 mg per kilogram of body weight reported dizziness, but the occurrence decreased after subsequent doses.27 Because this trial was an open-label trial, determination of the cause of adverse events may have been influenced by the knowledge of the treatment given.28 Three women had behavior changes soon after the onset of treatment, one in the mefloquine-artesunate group and two in the amodiaquine-artesunate group. Mefloquine use has been associated with neuropsychiatric adverse events,29 a phenomenon that has also been described for 4-aminoquinolines.6
In conclusion, artemether-lumefantrine was associated with the fewest adverse effects and with acceptable cure rates but provided the shortest post-treatment prophylaxis. Dihydroartemisinin-piperaquine had the best efficacy and an acceptable safety profile.
Footnotes
This article originally appeared in the New England Journal of Medicine
Citation: PREGACT Study Group, Pekyi D, Ampromfi AA, Tinto H, Traoré-Coulibaly M, Tahita MC, Valéa I, Mwapasa V, Kalilani-Phiri L, Kalanda G, Madanitsa M, Ravinetto R, Mutabingwa T, Gbekor P, Tagbor H, Antwi G, Menten J, De Crop M, Claeys Y, Schurmans C, Van Overmeir C, Thriemer K, Van Geertruyden JP, D'Alessandro U, Nambozi M, Mulenga M, Hachizovu S, Kabuya JB, Mulenga J. Four artemisinin-based treatments in African pregnant women with malaria. N Engl J Med. 2016 Mar 10;374(10):913–27
doi: 10.1056/NEJMoa1508606
(Published 10 March 2016)
© 2016 Massachusetts Medical Society
Republished with permission from the Massachusetts Medical Society
Supported by the European and Developing Countries Clinical Trials Partnership, the Malaria in Pregnancy Consortium (which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine), the Belgian Development Cooperation Agency, the Liverpool School of Tropical Medicine, the Medical Research Council UK, the Netherlands Organization for Scientific Research, and Sanofi-Aventis.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dr. D'Alessandro reports receiving grant support from Sigma-Tau Industrie Farmaceutiche Riunite. No other potential conflict of interest was reported.
The members of the writing group (D. Pekyi, A.A. Ampromfi, H. Tinto, M. Traoré-Coulibaly, M.C. Tahita, I. Valéa, V. Mwapasa, L. Kalilani-Phiri, G. Kalanda, M. Madanitsa, R. Ravinetto, T. Mutabingwa, P. Gbekor, H. Tagbor, G. Antwi, J. Menten, M. De Crop, Y. Claeys, C. Schurmans, C. Van Overmeir, K. Thriemer, J.-P. Van Geertruyden, U. D'Alessandro, M. Nambozi, M. Mulenga, S. Hachizovu, J.-B.B. Kabuya, and J. Mulenga) assume responsibility for the overall content and integrity of the article. The full names, degrees, and affiliations of the members of the writing group are listed in the Appendix.
We thank the members of the data and safety monitoring board (Michael Boele van Hensbroek [chair], Emmanuel Lesaffre, Kalifa Bojang, and Robert Busingye); Diana Arango, Christophe Burm, Jozefien Buyze, Annette Erhart, Greta Gondol, Pieter Guetens, Tom Koyen, Evi Pockele, Harry van Loen, Danielle Van Melle, and Jef Verellen, of the Institute of Tropical Medicine, Antwerp, Belgium, for their contribution to the success of this trial; Jaume Ordi, of the Barcelona Center for International Health Research, for reading the placenta-biopsy specimens; Bruno Gryseels, director of the Institute of Tropical Medicine, Antwerp, for continuous support throughout the trial; the field teams at each site where the trial was implemented; and the pregnant women who participated in this trial.
References
- 1.Takem EN, D'Alessandro U. Malaria in pregnancy. Mediterr J Hematol Infect Dis. 2013;5(1):e2013010. doi: 10.4084/MJHID.2013.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2013;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.D'Alessandro U. Existing antimalarial agents and malaria-treatment strategies. Expert Opin Pharmacother. 2009;10:1291–1306. doi: 10.1517/14656560902942319. [DOI] [PubMed] [Google Scholar]
- 4.Lutje V, Gerritsen A, Siegfried N. Randomized controlled trials of malaria intervention trials in Africa, 1948 to 2007: a descriptive analysis. Malar J. 2011;10:61. doi: 10.1186/1475-2875-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orton LC, Omari AAA. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database Syst Rev. 2008;4:CD004912. doi: 10.1002/14651858.CD004912.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. ( http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf) [PubMed] [Google Scholar]
- 7.Nambozi M, Mulenga M, Halidou T, et al. Safe and efficacious artemisininbased combination treatments for African pregnant women with malaria: a multicentre randomized control trial. Reprod Health. 2015;12:5. doi: 10.1186/1742-4755-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 9.Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization; 2008. [Google Scholar]
- 10.WorldWide Antimalarial Resistance Network (WWARN), author Clinical module: data management and statistical analysis plan. 2012. ( http://www.wwarn.org/sites/default/files/ClinicalDMSAP.pdf)
- 11.Dmitrienko A, D'Agostino RB. Traditional multiplicity adjustment methods in clinical trials. Stat Med. 2013;32:5172–5218. doi: 10.1002/sim.5990. [DOI] [PubMed] [Google Scholar]
- 12.Piola P, Nabasumba C, Turyakira E, et al. Efficacy and safety of artemetherlumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis. 2010;10:762–769. doi: 10.1016/S1473-3099(10)70202-4. [DOI] [PubMed] [Google Scholar]
- 13.McGready R, White NJ, Nosten F. Parasitological efficacy of antimalarials in the treatment and prevention of falciparum malaria in pregnancy 1998 to 2009: a systematic review. BJOG. 2011;118:123–135. doi: 10.1111/j.1471-0528.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 14.McGready R, Tan SO, Ashley EA, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;5(12):e253. doi: 10.1371/journal.pmed.0050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manyando C, Kayentao K, D'Alessandro U, Okafor HU, Juma E, Hamed K. A systematic review of the safety and efficacy of artemetherlumefantrine against uncomplicated Plasmodium falciparum malaria during pregnancy. Malar J. 2012;11:141. doi: 10.1186/1475-2875-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloprogge F, Piola P, Dhorda M, et al. Population pharmacokinetics of lumefantrine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. CPT Pharmacometrics Syst Pharmacol. 2013;2:e83. doi: 10.1038/psp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–416. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valea I, Tinto H, Traore-Coulibaly M, et al. Pharmacokinetics of co-formulated mefloquine and artesunate in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum infection in Burkina Faso. J Antimicrob Chemother. 2014;69:2499–2507. doi: 10.1093/jac/dku154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rijken MJ, McGready R, Jullien V, et al. Pharmacokinetics of amodiaquine and desethylamodiaquine in pregnant and postpartum women with Plasmodium vivax malaria. Antimicrob Agents Chemother. 2011;55:4338–4342. doi: 10.1128/AAC.00154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoglund RM, Adam I, Hanpithakpong W, et al. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J. 2012;11:398. doi: 10.1186/1475-2875-11-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Four Artemisinin-Based Combinations (4ABC) Study Group, author. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8(11):e1001119. doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhouse B, Dokomajilar C, Hubbard A, Rosenthal PJ, Dorsey G. Impact of transmission intensity on the accuracy of genotyping to distinguish recrudescence from new infection in antimalarial clinical trials. Antimicrob Agents Chemother. 2007;51:3096–3103. doi: 10.1128/AAC.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta V, Dorsey G, Hubbard AE, Rosenthal PJ, Greenhouse B. Gel versus capillary electrophoresis genotyping for categorizing treatment outcomes in two anti-malarial trials in Uganda. Malar J. 2010;9:19. doi: 10.1186/1475-2875-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam I, Tarning J, Lindegardh N, Mahgoub H, McGready R, Nosten F. Pharmacokinetics of piperaquine in pregnant women in Sudan with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2012;87:35–40. doi: 10.4269/ajtmh.2012.11-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagbor H, Bruce J, Browne E, Randal A, Greenwood B, Chandramohan D. Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine-pyrimethamine used alone or in combination for malaria treatment in pregnancy: a randomised trial. Lancet. 2006;368:1349–1356. doi: 10.1016/S0140-6736(06)69559-7. [DOI] [PubMed] [Google Scholar]
- 26.Clerk CA, Bruce J, Affipunguh PK, et al. A randomized, controlled trial of intermittent preventive treatment with sulfadoxinepyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis. 2008;198:1202–1211. doi: 10.1086/591944. [DOI] [PubMed] [Google Scholar]
- 27.González R, Mombo-Ngoma G, Ouédraogo S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIVnegative women: a multicentre randomized controlled trial. PLoS Med. 2014;11(9):e1001733. doi: 10.1371/journal.pmed.1001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González R, Hellgren U, Greenwood B, Menéndez C. Mefloquine safety and tolerability in pregnancy: a systematic literature review. Malar J. 2014;13:75. doi: 10.1186/1475-2875-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Riemsdijk MM, Sturkenboom MC, Ditters JM, et al. Low body mass index is associated with an increased risk of neuropsychiatric adverse events and concentration impairment in women on mefloquine. Br J Clin Pharmacol. 2004;57:506–512. doi: 10.1046/j.1365-2125.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]