Abstract
Background
During a session of prolonged and exhaustive exercise, such as a marathon race, large quantities of free radicals are produced and can oxidise (ox) several molecules, such as low-density lipoprotein (LDL). To prevent oxidative damage, athletes present higher antioxidant levels. However, the effect of marathon running on the natural IgM or IgG anti-oxLDL autoantibodies is not understood. Thus, we investigated the effect of a marathon race on oxidative stress and the mechanisms of control of this stress.
Methods
Blood samples of 20 marathon runners were collected 24 hours before, immediately and 72 hours after a marathon race to evaluate: plasma lipid profile; serum levels of oxLDL and anti-oxLDL autoantibodies (IgM and IgG isotype) and total antioxidant capacity (TAC). Maximum oxygen uptake (VO2max) was also determined.
Results
Immediately after the race, oxLDL and TAC levels decreased in comparison to the basal levels; however, the IgM or IgG anti-oxLDL levels remain unchanged. Whereas no differences were observed in the IgM or IgG anti-oxLDL levels 72h after the marathon, the oxLDL and TAC levels returned to the basal values. Significant positive correlations were observed between oxLDL and LDL-cholesterol before, and 72h after the marathon. Significant negative correlations were observed between oxLDL and VO2max immediately after the marathon and 72 h later, as well as between oxLDL and TAC 72 h after the race.
Conclusions
Athletes with a higher VO2max and total antioxidant activity presented reduced LDL oxidation. The levels of IgM or IgG anti-oxLDL autoantibodies were not affected by running the marathon.
Keywords: coronary artery diseases, oxidized LDL, exhaustive exercise, total antioxidant capacity, autoantibodies
What are the new findings?
Marathon runners with higher VO2max showed reduction of oxLDL levels after a marathon race.
During and soon after the race, the antioxidative mechanisms, represented by TAC, could prevent the elevation of the oxLDL serum levels.
IgM or IgG isotype anti-oxLDL autoantibodies are not involved in the reduction of oxLDL levels observed immediately after a marathon race.
Introduction
Regular physical exercise contributes to reducing the prevalence of coronary artery disease (CAD).1 Aerobic exercises are associated with beneficial changes in the profile of circulating lipids and lipoproteins,2 body weight,3 blood pressure,4 insulin sensitivity5 and coagulation parameters.6 It has been reported in the literature that the benefits are proportional to the intensity of the exercise,7 8 which is determined according to the maximum oxygen uptake (VO2max) that represents the gold standard in exercise prescription. According to the American College of Sport Medicine, exercise performed at approximately 50–70% of the VO2max is classified as moderate exercise, and exercise performed between 70% and 85% of the VO2max is considered high-intensity exercise.9 The practice of moderate exercise training is associated with increased high-density lipoprotein (HDL) and a reduction in low-density lipoprotein (LDL), total cholesterol and triacylglycerol (TAG).7 10 Lipids are considered to be one of the most important fuel sources during moderate-intensity exercise.11 12 After a session of prolonged and exhaustive exercise, such as observed in a marathon, there are significant changes in lipid profile, particularly the susceptibility of LDL cholesterol (LDL-C) to oxidation.8 It is accepted that oxidised LDL (oxLDL) is an important risk factor for atherosclerosis.13
The oxidative hypothesis related to CAD development has been widely discussed, because many atherogenic effects have been attributed to oxidative stress.7 In spite of the large quantity of free radicals generated in response to increased oxygen consumption, especially during aerobic exercise, the organism is able to activate adequate antioxidant responses to prevent oxidative damage in tissues, especially in moderate intensity.14 15 However, in exhaustive exercise, the excessive production of free radicals could favour the oxidation of several molecules,16 including LDL-C,17 and cause tissue damage if the antioxidant mechanism is insufficient. Furthermore, it is important to emphasise that a marathon training schedule includes running long distances (between 15 to 25 km, which corresponds to a half-marathon), 2–3 times a week. According to Child et al18 and Briviba et al,19 athletes come into an oxidative stress state after a half-marathon. So, during the training, the athlete repeatedly induces an elevation on oxidative stress state. Marzatico et al20 showed that marathon runners had higher levels of malondialdehyde, conjugated dienes and superoxide dismutase, at rest, than did sedentary controls. Thus, presence of oxidative stress state in marathon runners is not an isolated fact, but a chronic feature.
In addition to antioxidant control, autoantibodies that recognise oxidised LDL (anti-oxLDL), which could be detected in plasma from healthy subjects as well, have been associated with the development of atherosclerosis that can trigger a CAD.21 22 Whereas the IgG subtype anti-oxLDL autoantibody is correlated with the development of atheromatosis,22 the IgM isotype demonstrates an atheroprotective function.23 Anti-oxLDL are autoantibodies or natural antibodies, usually defined as immunoglobulins, which are produced in the absence of any exogenous antigenic stimulation,24 by a subset of lymphocytes known as B1 or B CD5+ cells, which are highly reactive against autoantigens.25 These antibodies demonstrate several crucial functions not only as the first line of defence against pathogenic microorganisms by binding to a carbon group in the membrane of pathogens and inducing complement activation, but they also play a role in the recognition and removal of senescent cells, cell debris and other self-antigens.26
In this study, the effect of a marathon race on oxidative stress and the mechanisms of control of this stress were determined in athletes, before and after the race. We evaluated the plasma lipid profile and serum levels of oxLDL, total antioxidant capacity (TAC) and autoantibodies (IgM and IgG) specific to oxLDL in the marathon runners.
Material and methods
Study subjects
Twenty male recreational marathon runners living in the city of São Paulo were recruited for this study. The volunteers were invited for the study by email invitation sent for different associations or groups of runners. All the volunteers performed a training volume between 80 and 100 km/week with an average of 90–120 min/day, to prepare for the race. All volunteers of this study concluded the XVII International Marathon of São Paulo in 2012. The baseline characteristics and VO2max data of the volunteers are shown in table 1. All the individuals were aware of the possible risks involved in the study, and they provided their consent to the study protocol and signed a consent form. The study protocol and consent form were approved by the UNIFESP-EPM Ethics Committee (number 572/2011). The exclusion criteria included the use of lipid-lowering medications, smoking, use of alcohol or drugs, obesity and pathologies including systemic arterial hypertension, liver, renal, metabolic, inflammatory or neoplastic diseases.
Table 1.
Physical and laboratory (mean±SD) data of marathon runners at rest (baseline), immediately after a marathon race and again, 72 h later
| Marathon runners (n=20) |
|||
|---|---|---|---|
| Variables | Baseline | Immediately after | 72 h later |
| Age (year) | 35.7±9 | ||
| Body mass index (kg/m2) | 24.6±2.7 | ||
| VO2max (mL/min/kg) | 47.1±6.09 | ||
| Triacylglycerols (mg/dL) | 105.1±39.4 | 134.5±45.2* | 89.4±32.8# |
| Cholesterol (mg/dL) | |||
| Total | 219±38.8 | 214.6±44.8 | 194.1±48.9*# |
| HDL | 57.8±14.8 | 61.1±12.3 | 61.7±18.5 |
| LDL | 140±40.9 | 126.6±41.9* | 118.2±44.8* |
| Plasma volume§ | 100 | 98.8±2.7 | 100.3±2.0 |
*Statistical significant difference in comparison to baseline value.
#Statistical significant difference in comparison to immediately after value.
§Relative plasma volume is % plasma volume on baseline.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; VO2max, maximum oxygen uptake.
Collection of the samples
The blood samples were collected at rest (baseline—24), immediately after the marathon and again 72 h later. Most of the studies that aimed to evaluate the effect of the marathon race on oxidative stress showed results related to periods before a marathon, immediately after the marathon and 24 h later. Despite the fact that the alterations on oxidative stress induced by marathon race usually returns to basal levels 24 h after the race, there is a study showing that the repercussion of the marathon on oxidative stress may persist 24 h after the race.27 So we evaluated a late period of 72 h after the marathon to study if alterations induced by the marathon persist. Blood draw for all the individuals was made between 8:00 and 9:00 after a period of 12 h of fasting both at rest and 72 h after the marathon. The last training session was performed 24 h before the blood draw at rest. The athletes were oriented to not perform any training between the period immediately after the marathon and 72 h later. Moreover, all the marathon runners were oriented to ingest sufficient quantities of water before (400–500 mL) and during the race to avoid dehydration. The marathon runners ingested the water provided by the marathon organisers during the race according to their necessity. To assess whether the athletes were adequately hydrated during the race, not only did haematocrit percentages before (48.05±0.72) and immediately after (47.74±0.73) not show significance differences, but also, the relative changes in plasma volume,28 which were estimated as described by Strauss et al29 on the basis of haematocrit (%) and haemoglobin (g/dL), were not statistically different (table 1).
Plasma lipids
The plasma total cholesterol (measured using the cholesterol oxidase-phenol-aminophenazone (CHOD-PAP) method) was determined using commercial kits (Kovalente, São Gonçalo, Brazil). The results were analysed using an automated system (Dimension RxL Max Integrated Chemistry System, Siemens Healthcare Diagnostic, Inc, Deerfield, Illinois, USA). The high cholesterol (HDL-C) and TAG concentrations were determined using commercial kits and an automated analysis system (ADVIA 2400 Chemistry System, Siemens Healthcare Diagnostics, Inc, Deerfield, Illinois, USA). The LDL-C was estimated using the Friedewald formula.30
Measurement of oxLDL-specific IgM and IgG reactivity
Serum anti-oxLDL autoantibodies of the IgM and IgG isotypes were measured by ELISA using previously stored samples. OxLDL diluted (7.5 μg/mL) in phosphate-buffered saline (PBS, pH 7.3) was used to coat the 96-well high, binding microtitre plates (Corning Costar, Corning, New York, USA) overnight at 4oC, as described by Ketelhuth et al.21 After being washed with PBS, the plates were blocked with 1% gelatin (Sigma, St Louis, Missouri, USA) in PBS for 2 h at room temperature. The plates were washed with PBS, and the serum samples were diluted 1:400 in PBS for the IgM and IgG measurements. After 2 h of incubation at room temperature, the plates were washed with PBS containing 0.05% of Tween 20 (Merck, Germany) (PBS-T), and 100 μL of horseradish peroxidase-conjugated goat antihuman IgM or IgG (Sigma) diluted to 1:1000 in PBS was added to each well and kept for 2 h at room temperature. After washing with PBS-T, 100 μL of substrate (5.5 mg of o-phenylenediamine in 10 mL of citrate-phosphate buffer pH 4.5 plus 10 μL of 30% H2O2) was added to the wells, and the plates were kept in the dark for 10 min. The reaction was stopped by adding 50 μL of 4N H2SO4. Absorbance was read at 492 nm on a microplate reader (Labsystems Multiskan MS Plate Reader). A buffer blank and control (IgM (91.8 mg/dL) and IgG (1400 mg/dL)) were used to compensate for the intraplate variations. The index of reactivity of each sample was calculated as follows: (Sample Optical Density (OD)—Blank OD)/(Control IgG or IgM OD—Blank OD).
Measurement of the oxLDL and TAC
The concentration of the oxLDL and TAC in the serum previously at −80°C was determined by ELISA using the oxLDL Indirect Enzyme Immunoassay kit (USCN Life Science, Inc, Wuhan, China). The TAC was based on the Trolox equivalent antioxidant capacity assay, a colorimetric commercial kit (Cayman Chemical Corporation, Ann Arbour, Michigan, USA), in accordance with the manufacturer's instructions. The positive and negative controls were prepared as described by the manufacturer.
Statistical analysis
The participants age, body mass index, lipid profile and VO2max are shown as the mean and SD. The data regarding serum levels of autoantibodies (IgM and IgG isotypes) specific to the oxLDL, TAC and oxLDL are shown as the median, with the respective quartiles. The Friedman test and Müller-Dunn post-test were used to determine whether the difference between the baseline results and the results observed immediately after and 72 h after the marathon race were significant. Pearson's correlation analysis was used to identify a correlation between the oxLDL and VO2max, the oxLDL and LDL levels, or the oxLDL and TAC levels. The significance level was set to 5% (p<0.05).
Results
Changes in lipid profile of athletes after a marathon race
Immediately after the marathon race (table 1), the levels of total cholesterol and HDL-C did not show alterations in relation to the baseline values. The TAG levels were significantly increased, while the LDL-C levels showed a reduction in comparison to the baseline levels. Seventy-two hours after the marathon, levels of HDL-C did not show alterations in relation to the baseline or immediately after the competition, while the values of total cholesterol and LDL-C showed a significant reduction in comparison to the baseline levels, and the TAG levels returned to the baseline values.
Reduced levels of oxLDL and antioxidants after a marathon race
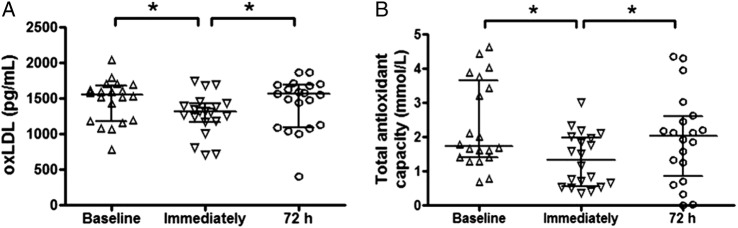
Immediately after the race, the serum concentration of oxLDL (A) and TAC (B) showed a significant reduction compared with the baseline levels, as seen in figure 1. Seventy-two hours after the race, oxLDL (A) and TAC (B) levels returned to the baseline values.
Figure 1.
Serum concentrations of (oxidised) low-density lipoprotein (ox)LDL (pg/mL—A) and total antioxidant capacity (mmol/L—B) in a group of marathon runners measured on three different occasions: at rest (baseline), immediately after a marathon and again, 72 h later. Data are presented as medians (IQR) with a significance level of *p<0.05.
Autoantibody response to oxLDL in marathon runners
Immediately after or 72 h after the marathon, the levels of the IgM (figure 2A) and IgG (figure 2B) isotype autoantibodies specific to oxLDL did not change in comparison to the baseline levels.
Figure 2.
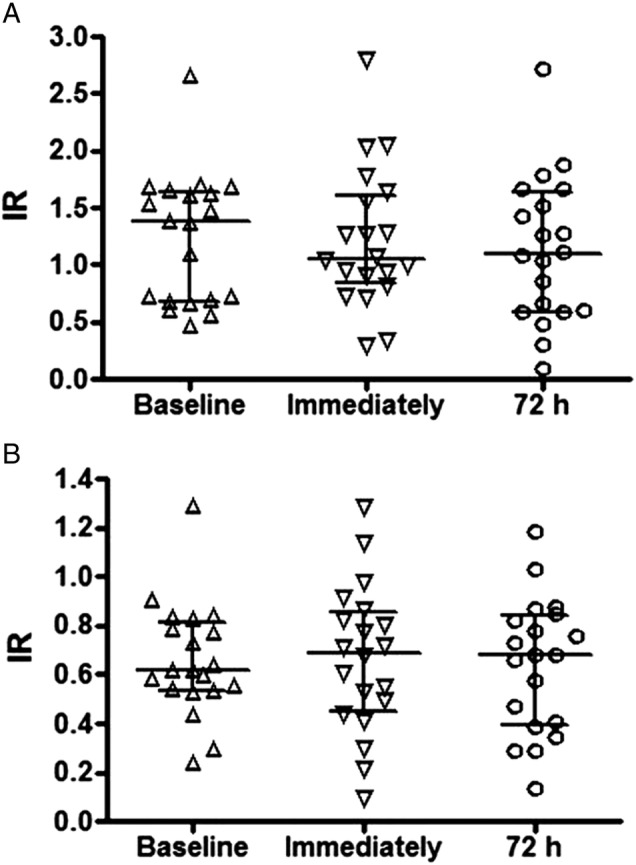
Index of reactivity of autoantibodies IgM (A) and IgG (B) specific to (oxidised) low-density lipoprotein (ox)LDL in a group of marathon runners measured on three different occasions: at rest (baseline), immediately after a marathon and again, 72 h later. Data are presented as medians (IQR) with a significance level of *p<0.05.
Higher VO2max is correlated with low oxLDL levels after a marathon race
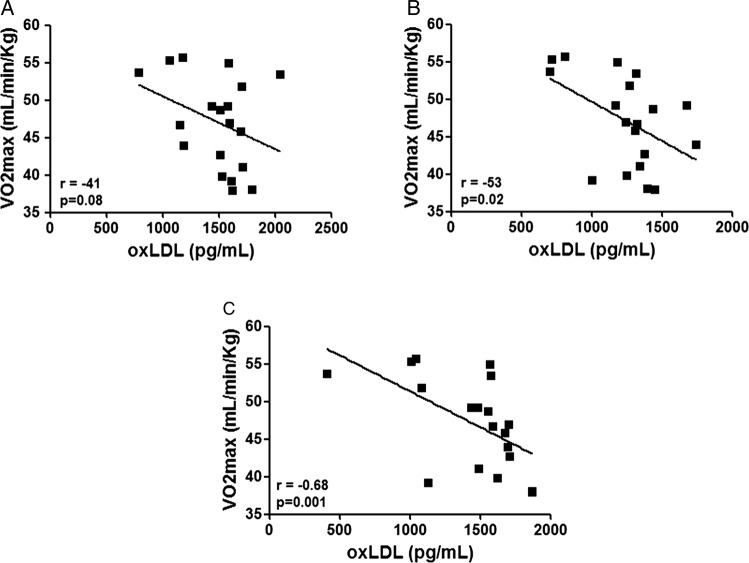
Figure 3 shows that no significant correlation was observed in the analysis between the VO2max values and oxLDL levels at rest (A). However, immediately after (B) and 72 h after (C) the race, a significant correlation was observed. The athletes with higher VO2max values presented lower oxLDL levels before and after the marathon race, whereas the athletes with reduced VO2max values showed higher oxLDL levels.
Figure 3.
Pearson's correlation analysis between (oxidised) low-density lipoprotein (ox)LDL (expressed in pg/mL) and VO2max (expressed in mL/min/kg) in a group of marathon runners measured at rest (baseline—A), immediately after a marathon (B) and again, 72 h later (C). Significance level: *p<0.05.
Lower levels of LDL-C 72 h after a marathon race is correlated with high oxLDL levels
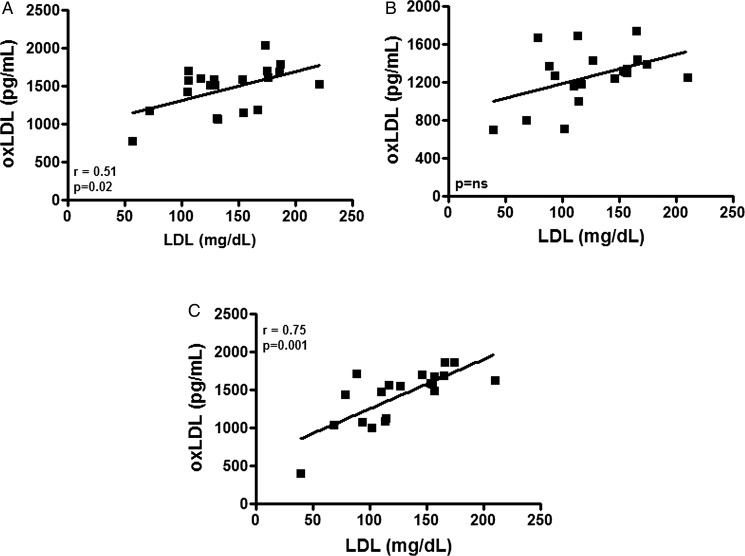
Figure 4A shows that a positive correlation was observed between the LDL-C and oxLDL levels at rest. This correlation ceased immediately after the marathon (figure 4B). Seventy-two hours after the marathon, the positive correlation returned; however, at this time, the statistical significance was higher than that in the baseline values (figure 4C).
Figure 4.
Pearson's correlation analysis between (oxidised) low-density lipoprotein (ox)LDL (expressed in pg/mL) and LDL cholesterol (mg/dL) in a group of marathon runners measured on three different occasions: at rest (baseline—A), immediately after a marathon (B) and again, 72 h later (C). Significance level: *p<0.05.
Higher levels of the serum antioxidant capacity 72 h after a marathon race is correlated with low oxLDL levels
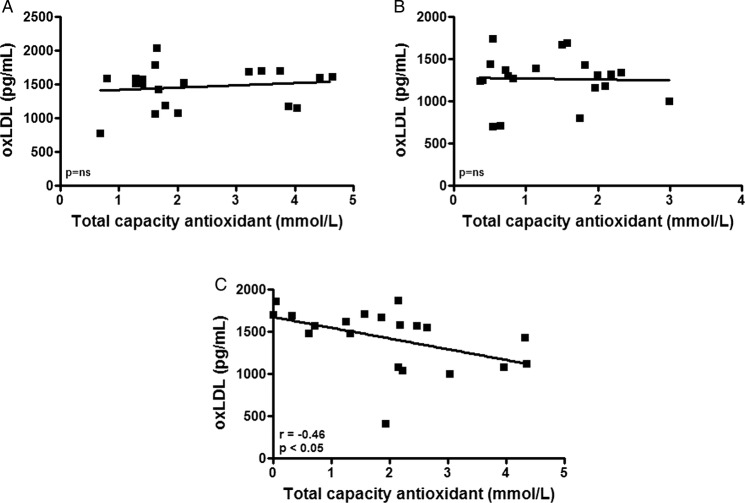
In figure 5, the analysis of the correlation between the oxLDL and the TAC levels showed no correlation at rest (A) or immediately after the marathon (B). However, there was a significant negative correlation 72 h after the race (C). We observed that the athletes with higher oxLDL levels presented lower TAC levels, and the marathon runners with reduced oxLDL levels showed higher TAC levels.
Figure 5.
Pearson's correlation analysis between (oxidised) low-density lipoprotein (ox)LDL (expressed in pg/mL) and total antioxidant capacity (—mmol/L) in a group of marathon runners measured at rest (baseline—A), immediately after a marathon (B) and again, 72 h later (C). Significance level: *p<0.05.
Discussion
In this study, we showed that after a marathon race, the HDL-C levels remain unchanged immediately after the race and 72 h later, although these levels could increase acutely during a session of exhaustive exercise.31 In a previous report, we showed a similar result, which corroborates our new observation of HDL-C variations during a marathon.32 In agreement with other studies, we observed a reduction of the LDL-C levels, a common occurrence presenting after exhaustive exercise such as an ultramarathon or bicycle marathon.28 33
Although the lipid profile remains as one of the most important factors involved in the development of CAD,34 in recent years, the oxidative hypothesis related to CAD development has received considerable attention among researchers due to the many proatherogenic effects attributed to oxidative stress.35 Thus, the study of the mechanisms associated with antioxidative activity in serum is very important.
Möhlenkamp et al36 demonstrated that marathon runners present the same index of coronary artery calcification as sedentary individuals, creating a new question for researchers involved in sports. The question presented is related to the fact that marathon runners have elevated VO2max levels and the consumption of oxygen during their athletic activity is increased. During training periods and competition, there is increased generation of free radicals, which could favour the oxidation of LDL-C and, as a deleterious consequence, increasing the probability of marathon runners to develop coronary atheromatosis. Although these facts could explain the findings reported by Möhlenkamp et al,36 according to the literature, until now, it is impossible to affirm it. So, to understand the effect of a marathon race on serum concentration of oxLDL, we analysed the kinetics of the concentration of the LDL-C and oxLDL during the three different occasions. As shown in table 1, the LDL-C levels displayed a continuing reduction after the marathon, whereas the oxLDL levels decreased immediately after the marathon and returned to the basal levels 72 h after the race. In relation to the reduction of oxLDL concentration observed immediately after the marathon, we could suppose that this reduction was related to the reduction of plasma LDL-C concentration. To test this hypothesis, we performed a correlation analysis between the concentration of LDL-C and oxLDL. We observed that there was a positive correlation at rest; however, this effect was lost immediately after the race. Seventy-two hours after the marathon, the positive correlation returned, with greater significance. Taken together, these results showed that the reduction of oxLDL is an event independent of the reduction of LDL-C observed immediately after the marathon race. Furthermore, in relation to the results observed 72 h after the marathon race, we found that whereas the levels of LDL-C are reduced 72 h after the race, these molecules are more oxidised. In agreement with our results, other studies have shown an enhanced depuration of LDL-C in individuals practising physical exercise37 38 and have demonstrated that exercise, particularly strenuous exercise, could trigger oxidative stress, resulting in an increased accumulation of secondary products of lipid peroxidation, decreased TAC and increased susceptibility of LDL-C to oxidation, in vitro. Elevated oxLDL levels might be associated with previous macrophage activation by oxLDL or a delay in depuration of oxLDL, perhaps because of a lack of increase of the CD36 receptor scavenger.39
Our novel findings could present interesting information regarding the mechanisms that athletes develop to protect against the harmful effects of oxidative stress. We observe that marathon runners with higher VO2max showed reduced oxLDL levels after a marathon race, raising the possibility that LDL oxidation in better-conditioned athletes is reduced. Antioxidant activity is active during a marathon race, except for that of the IgM or IgG isotype anti-oxLDL autoantibodies.
According to the literature, natural IgM (nIgM) isotype autoantibodies specific to oxLDL act as an atheroprotective factor,40–42 and it was demonstrated that lower levels of these antibodies are associated with atheromatosis. The importance of nIgM has been highlighted in recent years. Kyaw et al43 44 showed the action of B1 cells in atheroprotective activity by the production of nIgM. Elphick et al45 46 demonstrated, in an animal model, that training increases the production of nIgM. We observed that immediately after and up to 72 h after a marathon race, the levels of the IgM isotype anti-oxLDL autoantibodies remain unchanged, in contrast to the effect that would be expected if the autoantibody were acting to block the oxLDL. The levels of the IgG isotype autoantibodies specific to oxLDL did not show differences between the levels before and after the race. This autoantibody is implicated in the development of atheromatosis and, consequently, with CAD.47 48
Additionally, we observed other mechanisms of oxidative control during and soon after the race. In these periods, the antioxidative mechanisms were relevant to avoid the elevation of the serum oxidant levels. Immediately after the marathon, the levels of oxLDL and TAC showed a significant decrease compared with the baseline levels. Some studies have demonstrated that oxLDL levels after a marathon could remain unchanged49 or significantly decrease,50 as observed in this study. This finding suggests that the antioxidant molecules represented by TAC could buffer oxidative stress, preventing an increase of the oxLDL. Seventy-two hours after the race, the levels of oxLDL and TAC showed a tendency to return to the basal values.
Further studies evaluating a greater number of participants and the study of endothelia in athletes and sedentary individuals are necessary to definitively confirm the importance of this mechanism as a protective factor against the rise of oxidative stress and development of CAD in athletes who perform a session of exhaustive exercise, such as running a marathon race. Failure of these mechanisms, most likely acting in conjunction with other factors such as genetic propensity, could induce CAD in athletes.
Footnotes
Contributors: ALLB participated in the total production of the manuscript. APRS and MADPK participated in all analyses related to the measurement of the VO2max. FJOR and ABV participated in the analysis related to the measurement of the ox-LDL and anti-oxLDL autoantibodies (IgM and IgG). NG and DAG participated in the analysis related to the plasma lipid profile and haematocrit percentage. RLA and JMBdS participated in the analysis related to the measurement of the total antioxidant capacity (TAC). TCP-C and MV participated not only in the study planning, but also in manuscript preparation, especially the Discussion section.
Funding: This study was supported by the Fundação de Amparo à Pesquisa de São Paulo (FAPESP), São Paulo, Brazil (2010/50025-1).
Competing interests: None declared.
Ethics approval: UNIFESP-EPM Ethics Committee (number 572/2011).
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Marongiu E, Crisafulli A. Cardioprotection acquired through exercise: the role of ischemic preconditioning. Curr Cardiol Rev 2014;10:336–48. 10.2174/1573403X10666140404110229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis B, Moriguchi T, Sumpio B. Optimizing cardiovascular benefits of exercise: a review of rodent models. Int J Angiol 2013;22:13–22. 10.1055/s-0033-1333867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swift DL, Johannsen NM, Lavie CJ et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014;56:441–7. 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi A, Dikareva A, Bacon SL et al. The impact of physical activity on mortality in patients with high blood pressure: a systematic review. J Hypertens 2012;30:1277–88. 10.1097/HJH.0b013e3283544669 [DOI] [PubMed] [Google Scholar]
- 5.Buresh R. Exercise and glucose control. J Sports Med Phys Fitness 2014;54:373–82. [PubMed] [Google Scholar]
- 6.Lippi G, Maffulli N. Biological influence of physical exercise on hemostasis. Semin Thromb Hemost 2009;35:269–76. 10.1055/s-0029-1222605 [DOI] [PubMed] [Google Scholar]
- 7.Herzberg GR. Aerobic exercise, lipoproteins, and cardiovascular disease: benefits and possible risks. Can J Appl Physiol 2004;29:800–7. 10.1139/h04-052 [DOI] [PubMed] [Google Scholar]
- 8.Liu ML, Bergholm R, Makimattila S et al. A marathon run increases the susceptibility of LDL to oxidation in vitro and modifies plasma antioxidants. Am J Physiol 1999;276(6 Pt 1):E1083–91. [DOI] [PubMed] [Google Scholar]
- 9.Garber CE, Blissmer B, Deschenes MR et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 10.Tambalis K, Panagiotakos DB, Kavouras SA et al. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology 2009;60:614–32. 10.1177/0003319708324927 [DOI] [PubMed] [Google Scholar]
- 11.Petibois C, Paiva M, Cazorla G et al. Discriminant serum biochemical parameters in top class marathon performances. Jpn J Physiol 2002;52:181–90. 10.2170/jjphysiol.52.181 [DOI] [PubMed] [Google Scholar]
- 12.Petibois C, Cazorla G, Poortmans JR et al. Biochemical aspects of overtraining in endurance sports: a review. Sports Med 2002;32:867–78. 10.2165/00007256-200232130-00005 [DOI] [PubMed] [Google Scholar]
- 13.Maiolino G, Rossitto G, Caielli P et al. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm 2013;2013:714653 10.1155/2013/714653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 2008;44:126–31. 10.1016/j.freeradbiomed.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Radak Z, Zhao Z, Koltai E et al. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal 2013;18:1208–46. 10.1089/ars.2011.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vina J, Gomez-Cabrera MC, Lloret A et al. Free radicals in exhaustive physical exercise: mechanism of production, and protection by antioxidants. IUBMB Life 2000;50:271–7. 10.1080/15216540051080994 [DOI] [PubMed] [Google Scholar]
- 17.Kaikkonen J, Porkkala-Sarataho E, Tuomainen TP et al. Exhaustive exercise increases plasma/serum total oxidation resistance in moderately trained men and women, whereas their VLDL + LDL lipoprotein fraction is more susceptible to oxidation. Scand J Clin Lab Invest 2002;62:599–607. 10.1080/003655102764654330 [DOI] [PubMed] [Google Scholar]
- 18.Child RB, Wilkinson DM, Fallowfield JL et al. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med Sci Sports Exerc 1998;30:1603–7. 10.1097/00005768-199811000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Briviba K, Watzl B, Nickel K et al. A half-marathon and a marathon run induce oxidative DNA damage, reduce antioxidant capacity to protect DNA against damage and modify immune function in hobby runners. Redox Rep 2005;10:325–31. 10.1179/135100005X83716 [DOI] [PubMed] [Google Scholar]
- 20.Marzatico F, Pansarasa O, Bertorelli L et al. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J Sports Med Phys Fitness 1997;37:235–9. [PubMed] [Google Scholar]
- 21.Ketelhuth DF, Tonini GC, Carvalho MD et al. Autoantibody response to chromatographic fractions from oxidized LDL in unstable angina patients and healthy controls. Scand J Immunol 2008;68:456–62. 10.1111/j.1365-3083.2008.02154.x [DOI] [PubMed] [Google Scholar]
- 22.Matsuura E, Hughes GR, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmun Rev 2008;7:558–66. 10.1016/j.autrev.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 23.Garrido-Sanchez L, Chinchurreta P, Garcia-Fuentes E et al. A higher level of IgM anti-oxidized LDL antibodies is associated with a lower severity of coronary atherosclerosis in patients on statins. Int J Cardiol 2010;145:263–4. 10.1016/j.ijcard.2009.09.472 [DOI] [PubMed] [Google Scholar]
- 24.Binder CJ, Shaw PX, Chang MK et al. The role of natural antibodies in atherogenesis. J Lipid Res 2005;46:1353–63. 10.1194/jlr.R500005-JLR200 [DOI] [PubMed] [Google Scholar]
- 25.Bachi AL, Suguri VM, Ramos LR et al. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol 2013;3:10–16. 10.1016/j.rinim.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lleo A, Invernizzi P, Gao B et al. Definition of human autoimmunity—autoantibodies versus autoimmune disease. Autoimmun Rev 2010;9:A259–66. 10.1016/j.autrev.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 27.Machefer G, Groussard C, Rannou-Bekono F et al. Extreme running competition decreases blood antioxidant defense capacity. J Am Coll Nutr 2004;23:358–64. 10.1080/07315724.2004.10719379 [DOI] [PubMed] [Google Scholar]
- 28.Foger B, Wohlfarter T, Ritsch A et al. Kinetics of lipids, apolipoproteins, and cholesteryl ester transfer protein in plasma after a bicycle marathon. Metabolism 1994;43:633–9. 10.1016/0026-0495(94)90207-0 [DOI] [PubMed] [Google Scholar]
- 29.Strauss MB, Davis RK, Rosenbaum JD et al. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest 1951;30:862–8. 10.1172/JCI102501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 31.Skinner ER, Black D, Maughan RJ. Variability in the response of different male subjects to the effect of marathon running on the increase in plasma high density lipoprotein. Eur J Appl Physiol Occup Physiol 1985;54:488–93. 10.1007/BF00422957 [DOI] [PubMed] [Google Scholar]
- 32.Vaisberg M, Bachi AL, Latrilha C et al. Lipid transfer to HDL is higher in marathon runners than in sedentary subjects, but is acutely inhibited during the run. Lipids 2012;47:679–86. 10.1007/s11745-012-3685-y [DOI] [PubMed] [Google Scholar]
- 33.Wu HJ, Chen KT, Shee BW et al. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J Gastroenterol 2004;10:2711–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ugwuja E, Ogbonna N, Nwibo A et al. Overweight and obesity, lipid profile and atherogenic indices among civil servants in Abakaliki, South Eastern Nigeria. Ann Med Health Sci Res 2013;3:13–18. 10.4103/2141-9248.109462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parthasarathy S, Khan-Merchant N, Penumetcha M et al. Oxidative stress in cardiovascular disease. J Nucl Cardiol 2001;8:379–89. 10.1067/mnc.2001.114150 [DOI] [PubMed] [Google Scholar]
- 36.Möhlenkamp S, Lehmann N, Breuckmann F et al. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29:1903–10. 10.1093/eurheartj/ehn163 [DOI] [PubMed] [Google Scholar]
- 37.Ficker ES, Maranhao RC, Chacra AP et al. Exercise training accelerates the removal from plasma of LDL-like nanoemulsion in moderately hypercholesterolemic subjects. Atherosclerosis 2010;212:230–6. 10.1016/j.atherosclerosis.2010.04.030 [DOI] [PubMed] [Google Scholar]
- 38.Tozzi-Ciancarelli MG, Penco M, Di Massimo C. Influence of acute exercise on human platelet responsiveness: possible involvement of exercise-induced oxidative stress. Eur J Appl Physiol 2002;86:266–72. 10.1007/s00421-001-0542-8 [DOI] [PubMed] [Google Scholar]
- 39.Kennedy DJ, Chen Y, Huang W et al. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension 2013;61:216–24. 10.1161/HYPERTENSIONAHA.112.198770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cesena FH, Dimayuga PC, Yano J et al. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis 2012;220:59–65. 10.1016/j.atherosclerosis.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 41.de Faire U, Su J, Hua X et al. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun 2010;34:73–9. 10.1016/j.jaut.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 42.de Faire U, Frostegard J. Natural antibodies against phosphorylcholine in cardiovascular disease. Ann N Y Acad Sci 2009;1173:292–300. 10.1111/j.1749-6632.2009.04748.x [DOI] [PubMed] [Google Scholar]
- 43.Kyaw T, Tipping P, Bobik A et al. Protective role of natural IgM-producing B1a cells in atherosclerosis. Trends Cardiovasc Med 2012;22:48–53. 10.1016/j.tcm.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 44.Kyaw T, Tay C, Krishnamurthi S et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res 2011;109:830–40. 10.1161/CIRCRESAHA.111.248542 [DOI] [PubMed] [Google Scholar]
- 45.Elphick GF, Wieseler-Frank J, Greenwood BN et al. B-1 cell (CD5+/CD11b+) numbers and nIgM levels are elevated in physically active vs. sedentary rats. J Appl Physiol (1985) 2003;95:199–206. [DOI] [PubMed] [Google Scholar]
- 46.Elphick GF, Greenwood BN, Campisi J et al. Increased serum nIgM in voluntarily physically active rats: a potential role for B-1 cells. J Appl Physiol 2003;94:660–7. 10.1152/japplphysiol.00547.2002 [DOI] [PubMed] [Google Scholar]
- 47.Fernvik EC, Ketelhuth DF, Russo M et al. The autoantibody repertoire against copper- or macrophage-modified LDL differs in normolipidemics and hypercholesterolemic patients. J Clin Immunol 2004;24:170–6. 10.1023/B:JOCI.0000019782.67993.0b [DOI] [PubMed] [Google Scholar]
- 48.Izar MC, Fonseca HA, Pinheiro LF et al. Adaptive immunity is related to coronary artery disease severity after acute coronary syndrome in subjects with metabolic syndrome. Diab Vasc Dis Res 2013;10:32–9. 10.1177/1479164112443374 [DOI] [PubMed] [Google Scholar]
- 49.Nickel T, Emslander I, Sisic Z et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol 2012;112:1699–708. 10.1007/s00421-011-2140-8 [DOI] [PubMed] [Google Scholar]
- 50.Valimaki IA, Vuorimaa T, Ahotupa M et al. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int J Sports Med 2012;33:291–6. 10.1055/s-0031-1291223 [DOI] [PubMed] [Google Scholar]