Abstract
Background
The purpose of this study was to examine whether very short duration, very high intensity sprint interval training (SIT) leads to loss of body fat mass in association with improvements to VO2max and fatty acid oxidation, and to assess the extent of sex dimorphism in these physiological responses.
Methods
A total of 24 men and 17 women (mean (SEM) age: 39 (±2) years; body mass index 24.6 (0.6)) completed measurements of the maximal rate of oxygen uptake (VO2max) and fatty acid oxidation (FATmax). Body fat and lean mass were measured by dual emission x-ray absorptiometry, and fasting blood lipid, glucose and insulin profiles were assessed before and after training. SIT consisted of 4×20 s sprints on a cycle ergometer at approximately 175% VO2max, three times per week for 12 weeks.
Results
Fat mass decreased by 1.0 kg, although men lost statistically significantly more fat than women both when expressed in Kg and as % body fat. VO2max increased by around 9%, but women improved VO2max significantly more than men. FATmax improved by around 13%, but fasting plasma glucose, insulin, total triglyceride, total cholesterol and high-density lipoprotein (HDL) did not change after training, while low-density lipoprotein decreased by 8% (p=0.028) and the HDL:Total Cholesterol ratio improved by 6%. There were no sex differences in these metabolic responses to training.
Conclusions
These results show lower body fat %, and higher rates of fatty acid oxidation and VO2max after 12 weeks of training for just 4 min per week. Notably, women improved VO2max more than men, while men lost more fat than women.
Keywords: Body composition, Sprint, Weight loss, Skeletal muscle, Fat
Summary.
In previous research, sprint interval training (SIT) has been shown to increase concentrations of enzymes of fatty acid metabolism in skeletal muscles of young adults.
In the present study, adults from the general population performed SIT of 4 min per week for 12 weeks.
The men and women improved maximal rate of oxygen uptake (VO2max) by an average of 9%, rates of fatty acid oxidation during exercise by 13% and decreased body fat by 1 kg.
Men and women responded differently to training, with women increasing VO2max more than men, and men losing more body fat than women.
Introduction
Aerobic-type training of between 225 and 420 min/week is recommended to those who wish to lose fat mass by increasing physical activity levels,1 but very high intensity, short duration training might also be effective.2 3 ‘Sprint interval training’ (SIT) sessions require participants to perform repeated short duration (often approximately 30–60 s) bouts at very high or maximal power output, each separated by a recovery period of very low intensity exercise.4 5 This exercise differs from conventional aerobic training that typically involves prolonged and continuous exercise, which during cycling is equivalent to around 25% of the peak leg power output6 that is met principally by the aerobic energy systems.7 8
Despite the intensity, duration and energetic differences between short duration, high-intensity activities and endurance activities lasting around 60 min per session, they promote similar physiological adaptations.9 This includes improvements to the maximal rate of oxygen uptake (VO2max)9 10 occurring in association with improvements to skeletal muscle capillarisation,11 12 enzymes of fat metabolism2 5 13–18 and improved insulin sensitivity,4 5 19 20 which contribute to better health-status and physical performance.
There are also reports that very high intensity, short duration training can lead to loss of total body fat mass.2 21–23 These studies used weekly durations of around 9 min2 21 22 and 30 min23 without controlling food intake. Whether even shorter duration exercise will be effective at reducing fat mass in men and women remains unknown.
Compared with men, women have lower relative muscle mass and higher relative fat mass,24 and slower skeletal muscle contractile properties;25 26 they oxidise fatty acids at higher relative exercise intensity27 and have less reliance on muscle glycogen stores following bouts of repeated sprinting.28 Therefore, men and women might not necessarily show similar adaptations to fatty acid metabolism and body composition after SIT, but such sex differences remain unknown because the overwhelming majority of research into SIT has included only young men (usually university students) who trained for between 2 and 6 weeks.
The aim of this study was to examine the hypothesis that very short duration, very high-intensity sprinting exercise (on cycle ergometers) could lead to loss of body fat mass as well as improvements to rates of fat oxidation and VO2max after 12 weeks in men and women recruited from the general population. In the light of the sex differences in body composition and muscle metabolism, we also hypothesised that men and women would differ in their physiological responses to SIT.
Design
Ethical approval and participant recruitment
Volunteers provided written informed consent prior to participation. Those with a history of cardiovascular, neuromuscular or metabolic disease were excluded as well as people who had suffered a leg fracture within the past 2 years. A total of 41 participants completed the 12-week SIT intervention and post-training measurements. Participant characteristics are shown in table 1.
Table 1.
Body composition in men and women before and after 12 weeks of SIT
| Men (pre) | Men (post) | Women (pre) | Women (post) | Training effect | Sex effect | Sex×training | |
|---|---|---|---|---|---|---|---|
| Age (years) | 38 (2.7) | 41 (3.2) | |||||
| Height (cm) | 177 (1.6) | 165 (1.2) | |||||
| Total body mass (kg) | 80.0 (2.2) | 79.2 (2.1) | 62.5 (2.6) | 62.1 (2.5) | 0.070 | <0.001 | 0.542 |
| Body mass index (kg/m2) | 26.3 (0.8) | 26.0 (0.7) | 22.2 (0.7) | 22.1 (0.6) | 0.081 | 0.001 | 0.554 |
| Total body fat (kg) | 17.9 (1.3) | 16.6 (1.3) | 18.6 (1.4) | 18.0 (1.2) | <0.001 | 0.556 | 0.052 |
| Total Body Fat (%) | 22.7 (1.7) | 21.2 (1.4) | 31.2 (1.7) | 31.3 (1.6) | <0.001 | <0.001 | 0.015 |
| Trunk fat (kg) | 9.7 (0.8) | 9.0 (0.9) | 8.1 (0.8) | 8.0 (0.7) | 0.014 | 0.313 | 0.046 |
| Leg fat (kg) | 5.7 (0.4) | 5.4 (0.4) | 7.8 (0.6) | 7.0 (0.5) | <0.001 | 0.001 | 0.208 |
| Total body lean mass (kg) | 60.4 (1.6) | 61.1 (1.6) | 39.1 (0.9) | 39.2 (1.0) | 0.016 | <0.001 | 0.162 |
| Leg lean mass (kg) | 21.6 (0.7) | 21.0 (0.7) | 13.6 (0.3) | 13.0 (0.4) | 0.185 | <0.001 | 0.821 |
The bold font highlights statistically significant values (p<0.05). Data are shown as mean (SEM).
SIT, sprint interval training.
Measurements
Participants attended the research laboratory in the morning, following a 12 h overnight fast, and having avoided strenuous activity and alcohol in the 24 h prior to testing. After providing informed consent and resting for at least 15 min, a 10 mL blood sample was collected into an EDTA collection tube from a vein in the forearm and then a second sample during the same laboratory visit was collected 20 min after completion of the physiological testing. Blood was immediately centrifuged at 4°C, with the plasma separated and stored at −80°C until analysis.
Body fatness was assessed from a total-body dual-energy X-ray absorptiometry (DEXA) scan using a Lunar Prodigy Advance and off-line analysis (GE Medical; EnCore V.10.50.086). Total body fat, trunk fat and limb fat mass were analysed as previously reported.30 31
Maximal rates of oxygen uptake and fatty acid oxidation were measured during cycle ergometry (Jaeger Ergocycle, Germany) with VO2, VCO2 and heart rate (HR) measurements (Cortex Biophysik, Germany). Workload began at 50 W and a cadence of 70 rpm was maintained throughout. Workload was increased in increments of 50 W for men and 30 W for women every 3 min until the respiratory exchange ratio (calculated as VCO2/VO2) was higher than 1.0 for at least 1 min. From this point onwards, increments of 20 W were given every minute until volitional exhaustion. Off-line analysis was used to estimate rates of fatty acid oxidation, which were calculated from VO2 and VCO2 values collected during steady-state stages of the VO2max test using the formula described by Frayn,32 assuming urinary nitrogen was negligible:
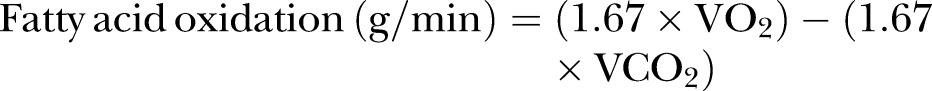 |
The maximal rate of fatty acid oxidation (FATmax) was estimated as the highest value occurring along the line of best fit of estimated rates of fatty acid oxidation (plotted from 50 W through to the point at which respiratory exchange ratio was higher than 1.0), adapted from Venables et al.27
Sprint interval training
Participants were asked to maintain their usual dietary and exercise habits throughout the intervention. SIT was completed on cycle ergometers (Cateye, Japan). The training consisted of a 2 min warm-up at a self-selected moderate intensity. This was followed by four bouts of 20 s ‘maximal effort’ sprints at a workload that was set at 175% of the workload attained in the VO2max test. Each of these intervals was separated by 2 min of very low intensity cycling (a workload of approximately 20% of that attained at VO2max). Thus, each training session lasted less than 10 min and only 80 s was completed at an intensity that would be expected to improve physical fitness.
The first training session for each participant was fully supervised in the research laboratory, and participants also received clear instructions on the use of the cycle ergometers and the training regimen. Participants trained three times per week for 12 weeks using the ergonomic cycles that we provided. The training workload was increased by 5% every 2 weeks. Gym staff were fully informed of the research and training protocols, they logged the training session and were available to offer advice to research participants if needed during training sessions. Participants maintained a training-log to record workloads during training sessions.
Fasting blood lipoprotein profile, insulin and glucose
Four fasting plasma samples were collected as described above: (1) at baseline in the fasted and rested state; (2) at baseline in the fasted state but 20 min after completion of the physiological testing session; (3) after the 12-week exercise intervention in the fasted and rested state; (4) after the 12-week exercise intervention in the fasted state but 20 min after completion of the physiological testing session.
Insulin sensitivity was estimated using the Homeostatic Model of Assessment (HOMA) as described by Matthews et al,33 which was calculated as:
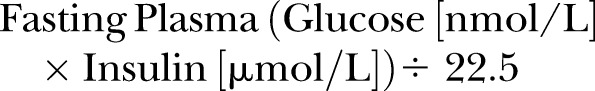 |
Biochemical markers were determined from fasting plasma samples using the RX Daytona auto analyser (Randox Laboratories, UK). High-density lipoprotein (HDL, low-density lipoprotein (LDL), total cholesterol (CHOD-PAP), triglycerides (GPO-PAP) and glucose (GOD-PAP) concentrations were determined in duplicate, and an average value calculated.
Statistical analysis
Data were analysed using SPSS (V.20 IBM). Repeated measures of ANOVA were used to assess adaptations to training and sex differences, using Greenhouse-Geisser correction when sphericity was violated. Pearson's Product Moment or Spearman's Correlations, as appropriate, were used to examine relationships between variables. Statistical significance was accepted at p<0.05. Data are presented as mean±SEM, unless otherwise stated.
Results
Body composition
Changes in body composition for men and women are shown in table 1. Body fatness (%) decreased after training by 1.2% (p<0.001), with men losing more total body (%) and trunk fat (kg) than women (table 1). Total body lean mass increased by 1.2% in men and 0.03% in women after training, but there was no statistically significant sex×training interaction (p=0.162), indicating that men and women showed similar changes to total lean mass after training (table 1).
Maximal oxygen uptake and fatty acid oxidation
Table 2 shows data for VO2max and FATmax in men and women. In the untrained state, men were able to utilise an absolute higher amount of fatty acids (in g/min) compared with women (p=0.027), and FATmax (g/min) occurred at a higher absolute workload (W) (p<0.001). Lean mass (kg) was correlated with FATmax (g/min) at baseline (figure 1) (r=0.366; p=0.019), however, the training-related change in total body lean mass (kg) was not statistically significantly correlated to training-related change in FATmax (g/min) (r=0.100, p=0.536) or when normalised to body mass (mg/kg/min) (r=0.065, p=0.688).
Table 2.
Maximal oxygen uptake and rates of fat oxidation measured during exercise in men and women before and after 12 weeks of SIT
| Men (pre) | Men (post) | Women (pre) | Women (post) | Training effect | Sex effect | Sex×training | |
|---|---|---|---|---|---|---|---|
| VO2 max (L/min) | 3.49 (0.13) | 3.68 (0.15) | 1.99 (0.11) | 2.37 (0.12) | 0.001 | <0.001 | 0.049 |
| VO2 max (mL/kg/min) | 42.91 (1.84) | 45.49 (1.48) | 33.56 (2.29) | 39.84 (2.44) | <0.001 | 0.005 | 0.009 |
| HRmax (bpm) | 177 (3) | 178 (3) | 175 (4) | 174 (4) | 0.645 | 0.977 | 0.268 |
| RERmax | 1.21 (0.01) | 1.18 (0.02) | 1.20 (0.02) | 1.21 (0.02) | 0.430 | 0.763 | 0.214 |
| FATmax (g/min) | 0.41 (0.03) | 0.49 (0.04) | 0.31 (0.03) | 0.34 (0.02) | 0.032 | 0.004 | 0.465 |
| FATmax (mg/kg/min) | 5.12 (0.39) | 6.04 (0.40) | 5.14 (0.52) | 5.77 (0.41) | 0.033 | 0.815 | 0.690 |
| FATmax (mg/kg lean mass/min) | 6.90 (0.50) | 8.10 (0.62) | 7.90 (0.76) | 8.87 (0.65) | 0.041 | 0.272 | 0.824 |
| Workload at FATmax (W) | 111.6 (9.81) | 112.80 (7.56) | 62.94 (5.43) | 62.73 (6.76) | 0.961 | <0.001 | 0.834 |
| FATmax (as %VO2max) | 51.43 (1.88) | 51.83 (1.65) | 60.26 (3.61) | 53.25 (2.18) | 0.229 | 0.066 | 0.047 |
| Heart Rate at FATmax (bpm) | 123 (5) | 115 (3) | 120 (4) | 112 (4) | 0.038 | 0.601 | 0.465 |
The bold font highlights statistically significant values (p<0.05). Data are shown as mean (SEM). All measurements were recorded during an incremental cycling test.
FATmax, maximal rate of fat oxidation; HRmax, maximal heart rate; RERmax, maximal respiratory exchange ratio; SIT, sprint interval training; VO2max, maximal rate of oxygen uptake.
Figure 1.
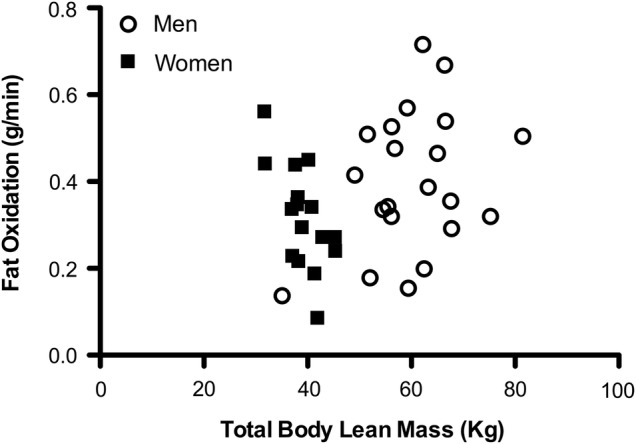
The relationship between total body lean mass and rates of fatty acid oxidation. In the untrained men and women, total body lean mass (kg) was correlated with rates of fatty acid oxidation (FATmax (g/min)) measured during exercise (r=0.366; p=0.019).
When normalised to total body mass and to lean mass (kg), FATmax did not differ between men and women (p=0.985 and 0.287, respectively). FATmax (g/min) occurred at a lower %VO2max in men than in women (p=0.023).
After 12 weeks of SIT, men and women increased VO2max (mL/kg/min), with the percentage gains being 18.7% in women and 6.0% in men (sex×training interaction: p=0.009). Men and women showed similar gains in FATmax after training (table 2). The sex×training interaction revealed that FATmax occurred at a statistically significantly lower %VO2max in women after training but slightly higher %VO2max in men after training (table 2).
Fasting plasma glucose, insulin and lipids
There were no statistically significant training effects for fasting plasma glucose (p=0.496), insulin (p=0.708), HOMA (p=0.426), total triglyceride (p=0.702), total cholesterol (p=0.129) and HDL (p=0.332). Training decreased LDL by 8% (p=0.028) and the HDL:Total Cholesterol ratio improved by 6% (p=0.005). There were no statistically significant sex×training interaction effects (all p>0.05; table 3).
Table 3.
Glucose and insulin concentrations in serum of men and women before and after 12 weeks of SIT
| Men (Pre) | Men (Post) | Women (Pre) | Women (Post) | Training effect | Sex effect | Sex×training | |
|---|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 5.10 (0.16) | 5.06 (0.05) | 4.99 (0.11) | 4.99 (0.05) | 0.496 | 0.634 | 0.663 |
| Insulin (μU/mL) | 3.50 (0.06) | 3.48 (0.05) | 3.35 (0.08) | 3.44 (0.08) | 0.708 | 0.995 | 0.241 |
| HOMA (%S) | 76 (2) | 77 (1) | 71 (2) | 75 (2) | 0.426 | 0.880 | 0.897 |
| Triglycerides (mmol/L) | 1.14 (0.27) | 0.83 (0.07) | 0.85 (0.06) | 0.84 (0.08) | 0.702 | 0.899 | 0.661 |
| Total Cholesterol (mmol/L) | 4.97 (0.24) | 5.18 (0.20) | 4.68 (0.20) | 5.09 (0.19) | 0.129 | 0.307 | 0.456 |
| High-density Lipoprotein (mmol/L) | 1.39 (0.06) | 1.81 (0.14) | 1.43 (0.07) | 1.83 (0.14) | 0.332 | 0.008 | 0.811 |
| Low-density Lipoprotein (mmol/L) | 2.75 (0.18) | 2.55 (0.20) | 2.51 (0.13) | 2.42 (0.21) | 0.042 | 0.562 | 0.523 |
| Total Cholesterol:HDL ratio | 3.60 (0.16) | 3.01 (0.21) | 3.29 (0.09) | 2.91 (0.21) | 0.010 | 0.039 | 0.161 |
The bold font highlights statistically significant values (p<0.05). Data are shown as mean (SEM).
HOMA, homeostatic model of assessment; SIT, sprint interval training.
The responses to the acute mixed-exercise session were analysed by comparing the rested samples with those taken 20 min after exercise both at baseline and after the 12-week SIT. Total triglycerides, total cholesterol and LDL did not change in concentration between time points 1 and 2, or 3 and 4 (all p>0.05). HDL increased by 4% from time point 1 to 2 (p=0.001), but did not differ between 3 and 4 (p=0.318). Total cholesterol:HDL ratio decreased by 2% from time point 1 to 2 (p=0.002) and by 1% between time points 3 and 4 (p=0.013). There were no statistically significant sex×time point interactions, indicating similar responses for men and women.
Discussion
The present study showed that the SIT programme of just 4 min/week for 12 weeks decreased total body fat mass in men but not in women, while women improved VO2max more than men. Improvements to the rates of fatty acid oxidation during submaximal exercise after the 12-week training programme were similar in men and women. These results highlight statistically significant sex-differences in physiological responses to very high-intensity training in the participants of this study who were recruited from the general population.
Body fatness
Body fat levels were within normal ranges for participants’ ages at baseline34 and lower body fat than their BMI related cut-offs.35 Just 80 s of very intense sprint exercise per session, equal to 48 min exercise over 12 weeks, resulted in statistically significant reductions to body fat mass. As far as we are aware, the training duration of 4 min/week is the shortest reported to effectively cause fat loss without additionally restricting food intake. These results build on previous high-intensity training studies that also reported fat loss,2 21–23 although they utilised lower intensity and longer duration intervals than our training programme did.
The fat loss was principally due to changes seen in men, since women did not change their total body composition despite losing approximately 800 g fat from their legs. Our study is the first to show sex-differences in the changes to body composition after SIT and are in line with previous work indicating that men generally lose more fat than women after endurance training.36 The reasons for the sex differences in training responses of body fatness and metabolism are not clear.37 There is evidence that sex-differences in body fatness are linked to physiological actions of sex hormones,38 but when energy balance was more closely regulated throughout an endurance training programme, overweight and obese men and women had similar fat loss.39 The sex differences in fat loss might also be related to the sex differences in relative muscle mass,24 skeletal muscle contractile25 26 and metabolic characteristics.27 28
SIT sessions have very low energy consumption, of around 200 kJ/week, compared with endurance exercise of around 2000 kJ/week.13 The direct energy expenditure therefore cannot explain the fat loss occurring after 12 weeks of SIT. Other contributing factors might include an increase in post exercise energy expenditure or overall shift towards greater fatty acid oxidation during habitual activities throughout the day, as occurs after endurance training40: the results showing increased fatty acid oxidation during low and moderate intensity activity are consistent with this. It is also possible that metabolic rate and lipid oxidation remain elevated after each training session, and there are reports that such effects are pronounced in men and negligible in women,40 41 which might help to explain our observed loss of fat in men but not in women. Women may also have a lower energy expenditure during exercise, due to the lower absolute workloads, despite working at similar relative exercise intensities, allowing men to reduce fat mass more than women after training.42 Treuth et al43 found that resting metabolic rate remained around 15% higher in participants performing an acute session of SIT compared to those who completed endurance training. Higher intensity exercise is also associated with prolonged suppression of appetite and hunger.44 However, others have reported increased energy intake in participants completing high-intensity training compared to non-exercising controls and those performing lower intensity exercise.45 The combination of a shift towards greater fatty acid oxidation and elevated resting energy expenditure could lead to a negative calorie balance and thus loss of body fat.
Maximal rate of oxygen uptake:
Our results showed an overall 9% increase in VO2max after 12 weeks of SIT. This increase is similar to previous reports from younger participants after SIT9 and of similar magnitude to VO2max gains after conventional endurance training programmes in which training sessions lasted around 1 h.13 18 Men and women both improved VO2max, but the gains in women were greater than those in men. It is not clear why disparity between sexes would occur in VO2max SIT responses. Men have been shown to have higher gains in VO2max following conventional endurance exercise,46 but results from SIT studies are mixed. Scalzo et al47 showed young women had similar gains in VO2max to young men, but other studies reported that men did not increase VO2max48 while women showed large increases after SIT.49
VO2max is an indicator of overall cardiopulmonary fitness and is dependent on the transportation of oxygen through the respiratory, cardiovascular and muscle systems to supply oxygen to the mitochondria for oxidative metabolism. A higher relative amount of lean mass in men compared to women, coupled with a higher relative body fat mass in women compared to men, may go some way in explaining the differences between men and women in maximal oxygen consumption.50 However, the supply of oxygen to the working skeletal muscles is thought to be a limiting factor in VO2max,51 so the higher VO2max response in women might point to higher adaptations of oxygen supply than those in men following SIT, but more focused studies examining cardiac output, blood volume, haematocrit and blood flow distribution are needed to clarify this finding. Conversely, after regular endurance training, men had higher gains in VO2max compared with women.46 It is possible that the training volume (higher in endurance) and training intensity (higher in SIT) lead to disparate adaptations between men and women in the oxygen carrying capacity of blood (eg, total blood volume, haemoglobin or cardiac output) or local vasculature, but physiological mechanisms driving such responses are unclear.
Rates of fatty acid oxidation
In the untrained state, women have higher relative oxidative capacity than men,52 and higher relative rates of fatty acid oxidation during prolonged exercise,53 slower muscle phenotype with proportionally more type I fibres54 and lower mitochondrial fractional synthetic rate after training.55 Despite these sex differences in muscle metabolism and contractile characteristics, men and women in the present study showed similar gains in rates of fatty acid oxidation at all workloads from 30% to 60% VO2max after training (figure 2). This metabolic adaptation occurred independently of changes to VO2max and was not associated with the changes to body composition. Ours was the first study to look for sex differences in FATmax responses after SIT, but increased fat oxidation has previously been reported,2 3 16 and could be related to improved muscle oxidative capacity and enzymes of fatty acid oxidation.56–58 The underlying metabolic pathways coordinating these adaptations seem to be similar in SIT and conventional endurance exercise, with increased metabolic enzymes and capillarisation13 7 19 regulated in part by PGC-1α, AMPk and CAMk.13 20 Elevated post exercise oxygen consumption may reflect increased rates of fat utilisation after high-intensity exercise.59 60 The elevated rates of fatty acid oxidation are associated with improved muscle metabolism61 and, alongside changes to blood lipid profiles, cardiopulmonary fitness and body composition, it is a defining feature of health status.62
Figure 2.
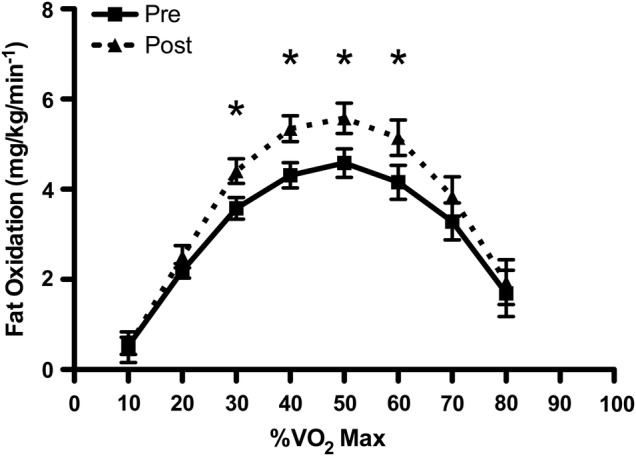
Maximal rates of fat oxidation in men and women across different submaximal exercise intensities in the untrained state and after 12 weeks of SIT. *Indicates statistically significant increase from baseline (p<0.05). SIT, sprint interval training.
Fasting plasma glucose, insulin and lipid profiles
Levels of circulating total triglycerides in fasting plasma samples remained unchanged after training, while LDL and the cholesterol:HDL ratio improved. A reduction of total circulating triglycerides has been associated with regular aerobic exercise,63–65 although it is not a consistent finding in high-intensity training programmes,66 67 as noted in a recent review.68 HDL increased after the first mixed exercise session, but did not change over the 12-week training. Fasting plasma glucose, insulin and the insulin sensitivity estimated using HOMA,33 did not change significantly with training. Nor was change in fasting plasma glucose and insulin reported in a study of 16 healthy men after 6 SIT sessions, although those participants did improve glucose tolerance when measured using an oral glucose tolerance test.4 Hood et al69 reported 35% improvement in HOMA in seven middle-aged men (n=4) and women (n=3) after six interval training sessions performed at lower intensity than that used in the present study. Richards et al70 used the hyperinsulinaemic euglycaemic glucose clamp to show improved insulin sensitivity in 12 participants after 6 SIT sessions, and Whyte et al16 showed improved insulin sensitivity in 10 young, sedentary men after 6 SIT sessions. These previous reports outlining positive effects of SIT on glucose metabolism contrast with those from the present study.
Limitations
The semisupervised design of the training programme gave exercise volunteers more control, and although this is the case in real-life situations, it may confer less commitment or obligation to training compared with typical fully supervised laboratory-based programmes. We were not able to control for physical activities outside of the training programme and dietary intake was not monitored throughout the training programme. Instead, participants were asked to maintain their usual patterns of food and drink consumption. Finally, we did not control for menstrual cycle variations.
Conclusion
A total of 4 min exercise per week over 12 weeks improved cardiorespiratory, metabolic and body composition profiles of men and women recruited from the general population. Sex differences were observed, with men losing more fat mass than women and women showing higher VO2max adaptations than men.
Acknowledgments
The investigators would like to thank the participants for their involvement in this study, and The Midland Hotel (operated by Q Hotels) and gym staff for the use of their training facilities.
Footnotes
Contributors: LB and JSM made contributions to the design, acquisition, analysis and interpretation of data; and drafted and critically revised the submitted work. MS made contribution to the conception and design of the study and interpretation of data; and critically revised the submitted work. SB made contribution to the acquisition, analysis and interpretation of data; and critically revised the submitted work. DL and MP made contributions to the acquisition of data; and critically revised the submitted work. CM made contribution to the design and interpretation of data; and critically revised the submitted work. GM made contribution to the acquisition of data; and critically revised the submitted work. MC made contribution to the design and interpretation of data; and critically revised the submitted work. WSG made contribution to the conception and design of the study; and critically revised the submitted work.
Funding: This study was funded by Manchester Metropolitan University. JMcP is supported by UK Medical Research Council (MR/K025252/1).
Competing interests: None declared.
Ethics approval: Manchester Metropolitan University Local Research Ethics Committee (application number: SE111216).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Swift DL, Johannsen NM, Lavie CJ et al. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis 2014;56:441–7. 10.1016/j.pcad.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trapp EG, Chisholm DJ, Freund J et al. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (Lond) 2008;32:684–91. 10.1038/sj.ijo.0803781 [DOI] [PubMed] [Google Scholar]
- 3.Shepherd SO, Cocks M, Tipton KD et al. Improvements in insulin sensitivity and whole-body fat oxidation after a period of high-intensity interval training. Br J Sports Med 2010;44:i11 10.1136/bjsm.2010.078972.32 [DOI] [Google Scholar]
- 4.Babraj JA, Vollaard NB, Keast C et al. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 2009;9:3 10.1186/1472-6823-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little JP, Gillen JB, Percival ME et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 2011;111:1554–60. 10.1152/japplphysiol.00921.2011 [DOI] [PubMed] [Google Scholar]
- 6.Zoladz JA, Rademaker AC, Sargeant AJ. Human muscle power generating capability during cycling at different pedalling rates. Exp Physiol 2000;85:117–24. 10.1111/j.1469-445X.2000.01840.x [DOI] [PubMed] [Google Scholar]
- 7.Talanian JL, Galloway SD, Heigenhauser GJ et al. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol 2007;102:1439–47. 10.1152/japplphysiol.01098.2006 [DOI] [PubMed] [Google Scholar]
- 8.Garber CE, Blissmer B, Deschenes MR et al. , American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 9.Gist NH, Fedewa MV, Dishman RK et al. Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med 2014;44:269–79. 10.1007/s40279-013-0115-0 [DOI] [PubMed] [Google Scholar]
- 10.Sloth M, Sloth D, Overgaard K et al. Effects of sprint interval training on VO2max and aerobic exercise performance: a systematic review and meta-analysis. Scand J Med Sci Sports 2013;23:e341–52. 10.1111/sms.12092 [DOI] [PubMed] [Google Scholar]
- 11.Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol (Lond) 2004;557:571–82. 10.1113/jphysiol.2003.057711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iaia FM, Hellsten Y, Nielsen JJ et al. Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J Appl Physiol 2009;106:73–80. 10.1152/japplphysiol.90676.2008 [DOI] [PubMed] [Google Scholar]
- 13.Burgomaster KA, Howarth KR, Phillips SM et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol (Lond) 2008;586:151–60. 10.1113/jphysiol.2007.142109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little JP, Safdar A, Bishop D et al. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2011;300:R1303–10. 10.1152/ajpregu.00538.2010 [DOI] [PubMed] [Google Scholar]
- 15.Perry CG, Heigenhauser GJ, Bonen A et al. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab 2008;33:1112–23. 10.1139/H08-097 [DOI] [PubMed] [Google Scholar]
- 16.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism 2010;59:1421–8. 10.1016/j.metabol.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Stensvold D, Tjønna AE, Skaug EA et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol 2010;108:804–10. 10.1152/japplphysiol.00996.2009 [DOI] [PubMed] [Google Scholar]
- 18.Tremblay A, Simoneau JA, Bouchard C. Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 1994;43:814–18. 10.1016/0026-0495(94)90259-3 [DOI] [PubMed] [Google Scholar]
- 19.Shepherd SO, Cocks M, Tipton KD et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J Physiol (Lond) 2013;591:657–75. 10.1113/jphysiol.2012.240952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibala MJ, Little JP, Macdonald MJ et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol (Lond) 2012;590:1077–84. 10.1113/jphysiol.2011.224725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macpherson RE, Hazell TJ, Olver TD et al. Run sprint interval training improves aerobic performance but not maximal cardiac output. Med Sci Sports Exerc 2011;43:115–22. 10.1249/MSS.0b013e3181e5eacd [DOI] [PubMed] [Google Scholar]
- 22.Hazell TJ, Hamilton CD, Olver TD et al. Running sprint interval training induces fat loss in women. Appl Physiol Nutr Metab 2014;39:944–50. 10.1139/apnm-2013-0503 [DOI] [PubMed] [Google Scholar]
- 23.Gillen JB, Percival ME, Ludzki A et al. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 2013;21:2249–55. 10.1002/oby.20379 [DOI] [PubMed] [Google Scholar]
- 24.Bijlsma AY, Meskers MC, Molendijk M et al. Diagnostic measures for sarcopenia and bone mineral density. Osteoporos Int 2013;24:2681–91. 10.1007/s00198-013-2376-8 [DOI] [PubMed] [Google Scholar]
- 25.Mcphee JS, Maden-Wilkinson TM, Narici MV et al. Knee extensor fatigue resistance of young and older men and women performing sustained and brief intermittent isometric contractions. Muscle Nerve 2014;50:393–400. 10.1002/mus.24174 [DOI] [PubMed] [Google Scholar]
- 26.Simoneau JA, Lortie G, Boulay MR et al. Skeletal muscle histochemical and biochemical characteristics in sedentary Male and female subjects. Can J Physiol Pharmacol 1985;63:30–5. 10.1139/y85-005 [DOI] [PubMed] [Google Scholar]
- 27.Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol 2005;98:160–7. 10.1152/japplphysiol.00662.2003 [DOI] [PubMed] [Google Scholar]
- 28.Esbjörnsson-Liljedahl M, Sundberg CJ, Norman B et al. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol 1999;87:1326–32. [DOI] [PubMed] [Google Scholar]
- 29.Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 30.McPhee JS, Hogrel JY, Maier AB et al. Physiological and functional evaluation of healthy young and older men and women: design of the European MyoAge study. Biogerontology 2013;14:325–37. 10.1007/s10522-013-9434-7 [DOI] [PubMed] [Google Scholar]
- 31.Maden-Wilkinson TM, Degens H, Jones DA et al. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 2013;13:320–8. [PubMed] [Google Scholar]
- 32.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 1983;55:628–34. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–19. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 34.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e7038 10.1371/journal.pone.0007038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo M, Faith MS, Pietrobelli A et al. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr 2012;95:594–602. 10.3945/ajcn.111.025171 [DOI] [PubMed] [Google Scholar]
- 36.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev 2005;33:169–74. 10.1097/00003677-200510000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Devries MC. Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 2016;101:243–9. 10.1113/EP085369 [DOI] [PubMed] [Google Scholar]
- 38.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 2008;40:648–54. 10.1249/MSS.0b013e31816212ff [DOI] [PubMed] [Google Scholar]
- 39.Caudwell P, Gibbons C, Hopkins M et al. No sex difference in body fat in response to supervised and measured exercise. Med Sci Sports Exerc 2013;45:351–8. 10.1249/MSS.0b013e31826ced79 [DOI] [PubMed] [Google Scholar]
- 40.Henderson GC, Alderman BL. Determinants of resting lipid oxidation in response to a prior bout of endurance exercise. J Appl Physiol 2014;116:95–103. 10.1152/japplphysiol.00956.2013 [DOI] [PubMed] [Google Scholar]
- 41.Henderson GC. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front Endocrinol (Lausanne) 2014;5:162 10.3389/fendo.2014.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donnelly JE, Hill JO, Jacobsen DJ et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med 2003;163:1343–50. 10.1001/archinte.163.11.1343 [DOI] [PubMed] [Google Scholar]
- 43.Treuth MS, Hunter GR, Williams M. Effects of exercise intensity on 24-h energy expenditure and substrate oxidation. Med Sci Sports Exerc 1996;28:1138–43. 10.1097/00005768-199609000-00009 [DOI] [PubMed] [Google Scholar]
- 44.Thompson DA, Wolfe LA, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Med Sci Sports Exerc 1988;20:222–7. 10.1249/00005768-198806000-00002 [DOI] [PubMed] [Google Scholar]
- 45.Pomerleau M, Imbeault P, Parker T et al. Effects of exercise intensity on food intake and appetite in women. Am J Clin Nutr 2004;80:1230–6. [DOI] [PubMed] [Google Scholar]
- 46.Bouchard C, An P, Rice T et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 1999;87:1003–8. [DOI] [PubMed] [Google Scholar]
- 47.Scalzo RL, Peltonen GL, Binns SE et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 2014;28:2705–14. 10.1096/fj.13-246595 [DOI] [PubMed] [Google Scholar]
- 48.Allemeier CA, Fry AC, Johnson P et al. Effects of sprint cycle training on human skeletal muscle. J Appl Physiol 1994;77:2385–90. [DOI] [PubMed] [Google Scholar]
- 49.Talanian JL, Holloway GP, Snook LA et al. Exercise training increases sarcolemmal and mitochondrial fatty acid transport proteins in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;299:E180–8. 10.1152/ajpendo.00073.2010 [DOI] [PubMed] [Google Scholar]
- 50.Ogawa T, Spina RJ, Martin WH et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 1992;86:494–503. 10.1161/01.CIR.86.2.494 [DOI] [PubMed] [Google Scholar]
- 51.Saltin B, Calbet JA. Point: in health and in a normoxic environment, VO2 max is limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol 2006;100:744–5. 10.1152/japplphysiol.01395.2005 [DOI] [PubMed] [Google Scholar]
- 52.Maughan RJ, Harmon M, Leiper JB et al. Endurance capacity of untrained males and females in isometric and dynamic muscular contractions. Eur J Appl Physiol Occup Physiol 1986;55:395–400. 10.1007/BF00422739 [DOI] [PubMed] [Google Scholar]
- 53.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 2001;280:E898–907. [DOI] [PubMed] [Google Scholar]
- 54.Simoneau JA, Lortie G, Boulay MR et al. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol 1985;54:250–3. 10.1007/BF00426141 [DOI] [PubMed] [Google Scholar]
- 55.Karakelides H, Irving BA, Short KR et al. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010;59:89–97. 10.2337/db09-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 1984;56:831–8. [DOI] [PubMed] [Google Scholar]
- 57.Hoppeler H, Howald H, Conley K et al. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 1985;59:320–7. [DOI] [PubMed] [Google Scholar]
- 58.Gollnick PD, Armstrong RB, Saltin B et al. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 1973;34:107–11. [DOI] [PubMed] [Google Scholar]
- 59.Børsheim E, Bahr R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med 2003;33:1037–60. 10.2165/00007256-200333140-00002 [DOI] [PubMed] [Google Scholar]
- 60.LaForgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci 2006;24:1247–64. 10.1080/02640410600552064 [DOI] [PubMed] [Google Scholar]
- 61.Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review. Part I: fatty acid mobilization and muscle metabolism. Int J Sports Med 1998;19:231–44. 10.1055/s-2007-971911 [DOI] [PubMed] [Google Scholar]
- 62.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–82. [DOI] [PubMed] [Google Scholar]
- 63.Plaisance EP, Mestek ML, Mahurin AJ et al. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr 2008;88:30–7. [DOI] [PubMed] [Google Scholar]
- 64.Durstine JL, Grandjean PW, Davis PG et al. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 2001;31:1033–62. 10.2165/00007256-200131150-00002 [DOI] [PubMed] [Google Scholar]
- 65.Crouse SF, O'Brien BC, Grandjean PW et al. Effects of training and a single session of exercise on lipids and apolipoproteins in hypercholesterolemic men. J Appl Physiol 1997;83:2019–28. [DOI] [PubMed] [Google Scholar]
- 66.Tsekouras YE, Magkos F, Kellas Y et al. High-intensity interval aerobic training reduces hepatic very low-density lipoprotein-triglyceride secretion rate in men. Am J Physiol Endocrinol Metab 2008;295:E851–8. 10.1152/ajpendo.90545.2008 [DOI] [PubMed] [Google Scholar]
- 67.Bellou E, Magkos F, Kouka T et al. Effect of high-intensity interval exercise on basal triglyceride metabolism in non-obese men. Appl Physiol Nutr Metab 2013;38:823–9. 10.1139/apnm-2012-0468 [DOI] [PubMed] [Google Scholar]
- 68.Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 2012;42:489–509. 10.2165/11630910-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 69.Hood MS, Little JP, Tarnopolsky MA et al. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc 2011;43:1849–56. 10.1249/MSS.0b013e3182199834 [DOI] [PubMed] [Google Scholar]
- 70.Richards JC, Johnson TK, Kuzma JN et al. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to beta-adrenergic stimulation. J Physiol (Lond) 2010;588:2961–72. 10.1113/jphysiol.2010.189886 [DOI] [PMC free article] [PubMed] [Google Scholar]


