Abstract
Background
The overall aim of this study was to determine to what extent objectively measured physical activity in a school-based sample aged 11–13 years predicted incident cases of spinal pain (neck pain, mid back pain or low back pain) over the following 2 years.
Methods
Data were collected at baseline (2010) and 2 years later in a school-based prospective cohort study. Spinal pain was assessed via an e-survey that the participants completed during school time. Participants who, at baseline, reported never having had spinal pain were included in the study. An incident case of spinal pain was defined as a report of pain in at least one spinal area at follow-up. Physical activity was measured objectively using the Actigraph GT3X Triaxial Activity Monitor for 1 week.
Results
Objectively measured sedentary activity, moderate-to-vigorous physical activity and vigorous physical activity were generally not predictive of the 2-year incidence of spinal pain. However, 10% of participants with the highest proportion of the day spent in vigorous physical activity were at increased risk of reporting spinal pain at follow-up with a relative risk (RR) of 1.44 (95% CI 1.09 to 1.91). For the overall physical activity, the RR was 1.03 (95% CI 1.01 to 1.05) for reporting spinal pain at follow-up.
Conclusions
In general, physical activity did not affect the risk of spinal pain during follow-up, but the 10% most active adolescents were at increased risk of developing spinal pain. Thus, vigorous physical activity appears to be a risk factor for spinal pain in adolescents.
Keywords: Accelerometer, Spine, Physical activity, Adolescent
What are the new findings?
A group of very physically active adolescents at age 11–13 years is at increased risk for developing spinal pain over the following 2 years.
Moderate-to-vigorous physical activity at age 11–13 years does not predict incident cases of spinal pain over the following 2 years.
Sedentary activity at age 11–13 years does not predict incident cases of spinal pain over the following 2 years.
Introduction
Low back pain is now the leading cause of years lived with disability globally1 and societal burdens are high and increasing.2 We know that spinal pain starts early in life and is common in adolescence,3 where it negatively affects participation in sport and physical activity.4 The presence of spinal pain in adolescence is strongly associated with spinal pain in adulthood,5 6 and therefore decreasing individual disability and overall societal burden caused by spine pain may be dependent on preventive measures in the younger population. Thus, longitudinal studies aimed at assessing modifiable risk factors for the development of incident cases of spinal pain at this age are needed.
Physical activity could be one potentially modifiable risk factor for spinal pain in adolescents. It has an undisputed beneficial impact on musculoskeletal health, but there is conflicting evidence for an association between physical activity and back pain.7 One of the reasons for this conflict is the challenge of obtaining good measures of physical activity in children and adolescents because of inconsistencies between self-reported and objective measures, as inactive children tend to overestimate their time being physically active.8 Moreover, we know that children's natural movement pattern with its short bouts of high physical activity, usually lasting <15 s, differs from that of adults.9 As a result, it will be crucial to choose an assessment method of physical activity that detects and measures these short bursts.
The aim of this study was to determine to what extent objectively measured physical activity in a school-based sample aged 11–13 years predicted incident cases of spinal pain over the following 2 years. The specific objectives were:
To determine to what extent various levels of physical activity at age 11–13 years were predictive for incident cases of spinal pain over the following 2 years;
To determine to what extent overall physical activity at age 11–13 years was predictive for incident cases of spinal pain over the following 2 years.
Material and methods
Participants
We conducted a 2-year prospective cohort study including 11–13-year-old participants who, at baseline, reported never having had spinal pain. The definition was based on three identical questions asked for the three spinal regions (neck, mid back and low back) separately. The first question was ‘Have you ever had neck pain?’ with the response options ‘often’, ‘sometimes’, ‘once or twice’ and ‘never’. This was repeated for the mid back and low back. A diagram with the spinal areas clearly shaded and labelled was shown alongside the questions. The inclusion criterion was self-report of ‘never’ in all three spinal regions at baseline. Participants who (1) were lost to follow-up; (2) did not wear the accelerometer; (3) had <3 days with valid accelerometer data; or (4) had missing data in physical measurements were excluded.
This study was nested within the SPACE study,10 which was a school-based cluster-designed randomised controlled trial aimed at investigating how physical environment combined with organisational initiatives could promote engagement in physical activity in children aged 11–13 years. It involved 14 schools in the Region of Southern Denmark. All 1348 fifth and sixth grade students at these schools were invited to participate. Participation did not require parental consent, but the parents were informed that they could decline their child's participation at any time. The interventions had no effects in the SPACE study,11 and therefore we have treated the study sample as a cohort study.
According to Danish laws, a study that does not contain invasive tests or interventions aimed at individuals does not require ethics approval,12 but the Regional Ethics Committee for Southern Denmark was advised about the study and data collection. Approval from the Danish Data Protection Agency was obtained (#2010-41-5147).
Data collection
Baseline data were collected from April to June 2010 and follow-up data from April to June 2012. The questionnaires (e-survey) were completed individually with teachers observing in order to ensure that there was no interaction between participants. The questionnaires were completed both at baseline and follow-up. Accelerometers were handed out in the classroom with instructions on how to use them. All anthropometrical assessments and physical fitness tests were performed during school time in a sports hall close to the school that the participant attended. Assessors who were members of the research staff and who were trained in the test procedures provided instruction in the use of the accelerometer and performed all physical measurements. The accelerometer data and physical measurements were collected at baseline only. Detailed information on the SPACE protocol can be found elsewhere.10
Accelerometer data
Physical activity was measured using the Actigraph GT3X Triaxial Activity Monitor.13 Participants were asked to wear the accelerometer on their waist for seven consecutive days, except during activities in water. In order to increase compliance, participants and their parents received an SMS text reminder every morning.
The Actigraph registers and records accelerations ranging from 0.05G to 2.5G in the vertical, horizontal and transverse axes. Currently, there are no available calibration studies for the Actigraph Triaxial Activity Monitor, but the vertical dimension of the instrument has been compared with uniaxial accelerometers. Only the vertical axis was used in the analyses. However, activity in all three axes was used to calculate the non-wear time because the triaxial accelerometer records more activity in the anteroposterior direction during sedentary activities and is therefore more sensitive to differentiating between non-wear time and sedentary activity.14
To ensure valid measures, a minimum wear-time of the accelerometer was set to 10 hours a day between 6:00 and midnight. This was required for a period of at least 3 days, which has been shown to give a reliable estimate of physical activity in children aged 7 years.15 In preliminary analyses, there were no statistical differences in the overall physical activity between weekdays and weekend days, and therefore the required days needed neither to be consecutive nor include both weekdays and weekend days. Non-wear time was defined as no activity measured by the accelerometer for at least 60 consecutive minutes as recommended by Toftager et al14 and was excluded from the data. Activity was summarised for every 10 s (epoch length) with counts per minute (cpm) as output. We categorised the total time spent at different activity levels using cut-points recommended by Evenson et al:16 sedentary was between 0 and 100 cpm; light was between 101 and 2295 cpm; moderate was between 2296 and 4011 cpm; and vigorous was between 4012 and 50 000 cpm. More than 50 000 cpm was considered non-physiological and replaced by 0. Processing of the accelerometer raw data was undertaken using the software Propero Actigraph Data Analyzer V.1.1.2 (RICH, University of Southern Denmark, Denmark).
Variables
Spinal pain (outcome)
The same questions used to define the cohort as described above in the participant section were used to define the outcome. An incident case was defined as a report of pain ‘often’, ‘sometimes’ or ‘once or twice’ in at least one of the questions addressed to neck pain, mid back pain or low back pain at follow-up. The questions were developed and tested for feasibility, content validity and item agreement between questionnaire scores and interview findings in 9–11-year-olds.17
Objective measure of physical activity (exposure)
We calculated the proportion of the day spent for different activity levels (ie, sedentary, moderate and vigorous combined, and vigorous alone) by dividing the total time spent at the specific level by the total wear time across all valid accelerometer days. Then, we dichotomiszed the activity at the 90th, 75th and 50th centiles in order to explore cut-points for potential risk. For the 90th centile cut-point, we compared the top 10% with the remaining 90%.
Overall physical activity was defined by taking the mean cpm divided by 100 across all valid accelerometer days. The variable was investigated for outliers, defined as three SDs above and below the mean. In cases with outliers, raw data were visually inspected for abnormal activity patterns. No suspicious movement patterns were registered, and therefore all data were included.
Potential confounders
Sex, height (cm), weight (kg), body mass index (BMI) (kg/m2), waist-to-height-ratio, participation in contact and collision sports, 10×5 m shuttle run test (sec), handgrip strength test (kg) and Andersen test (m) were included as potential confounders in the analysis. A more detailed description of these is presented in online supplementary appendix 1. Psychological factors were included in the questionnaires but not included as potential confounders because of insufficient numbers of participants who felt low, were irritable/in a bad mood, felt nervous or had sleeping difficulties.
bmjsem-2015-000097supp.pdf (93KB, pdf)
Other variables
We adjusted for the mean cpm for time spent in other activity levels. For example, we adjusted for non-sedentary activity, that is, the mean cpm of light activity and above (101–50 000 cpm) in sedentary models. The rationale for this was that adolescents may have tolerated sedentary activity better if they had higher exposure levels of physical activity the rest of the time and vice versa.
Statistical analyses
Attrition bias was investigated by comparing who responded to the follow-up questionnaire against those who did not with regard to sex and BMI. In order to investigate selection bias, we compared the excluded participants to the final sample with regard to sex, but due to missing assessments of height and weight, a comparison with regard to BMI was not possible.
Descriptive statistics with frequencies were calculated for the categorical and ordinal variables. Mean, SD, median with 95% CIs, and range were calculated for interval and ratio variables.
To determine if physical activity predicted the development of spinal pain, a generalised Poisson regression model for data with underdispersion was fitted.18 This was because the variance (0.22) was smaller than the mean (0.67). The unadjusted and adjusted relative risks (RRs) for the incidence of spinal pain were calculated for each physical activity exposure variable. Each potential confounder was tested by including it in the unadjusted model and determining if the β coefficient of the physical activity exposure variable changed by more than 10%.19 This approach was preferred rather than forcing all confounders into the model because the theoretical basis for choice of variables is weak. All confounders that met the above criteria were entered into the final model for each physical activity exposure variable. Multicollinearity was checked and if variance inflation factors of each variable exceeded 10, the variable was transformed by squaring the variable. Model assumptions were checked.
Sensitivity analyses were performed with a 30 s epoch because choice of epoch length is debatable and because a longer epoch length might lead to an underestimation of moderate-to-vigorous physical activity.20 All analyses were performed using STATA V.11.2 (Stata Corporation, College Station, Texas, USA).
Results
Participants
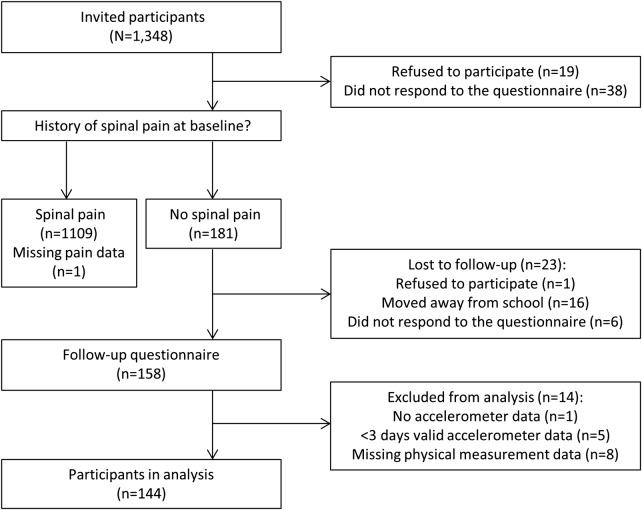
Of 1348 invited participants, 1291 (95.8%) completed the baseline questionnaire, of whom 181 were free of spinal pain at baseline and reported no previous episodes. Two years later, 158 (87.3%) of these completed the follow-up questionnaire and 144 (79.6%) were included in the analysis with 97 reporting spinal pain (figure 1). The majority of participants (84 (58.3%)) were boys. There were no differences between the responders and non-responders to the follow-up questionnaire with regard to sex and BMI. There was no difference in the sex of the participants who were included and who were not included in the analysis.
Figure 1.
Flow chart of the participants.
Physical activity and the incidence of spinal pain
Descriptive statistics of the accelerometer data and the covariates are shown in tables 1 and 2.
Table 1.
Descriptive statistics of the accelerometer data at baseline
| Mean | SD | Median (95% CI) | Range | 90th centile* | 75th centile* | 50th centile* | |
|---|---|---|---|---|---|---|---|
| Number of days wearing an accelerometer | 6.1 | 1.2 | 7 (6 to 7) | 3–7 | |||
| Mean wear time per day (hr) | 14.0 | 0.8 | 14.0 (13.9 to 14.2) | 11.3–16.0 | |||
| Overall physical activity, mean cpm/100 | 6.1 | 3.0 | 5.5 (4.9 to 6.1) | 2.4–29.2 | |||
| Per cent of the day spent at different levels of physical activity | |||||||
| Sedentary (0–100 cpm) | 66.1 | 6.0 | 65.8 (64.7 to 67.9) | 48.7–77.6 | 74.3 | 70.8 | 65.9 |
| Light (101–2295 cpm) | 26.1 | 4.0 | 25.7 (25.1 to 26.7) | 17.4–36.3 | |||
| Moderate (2296–4011 cpm) | 4.6 | 1.6 | 4.5 (4.0 to 5.0) | 1.7–10.1 | 11.9† | 9.9† | 7.2† |
| Vigorous (4012–50 000 cpm) | 3.2 | 2.2 | 2.7 (2.5 to 3.0) | 0.5–18.8 | 5.7 | 4.3 | 2.7 |
| Mean cpm for the time of the day spent in | |||||||
| Sedentary and light physical activity | 222 | 46 | 222 (208 to 229) | 130–385 | |||
| Sedentary, light and moderate physical activity | 356 | 87 | 353 (334 to 375) | 185–641 | |||
| Light, moderate and vigorous physical activity | 1747 | 678 | 1611 (1535 to 1676) | 1017–6442 | |||
*The proportion of the day spent at different levels of physical activity was dichotomised at these percentiles in the analyses.
†Presented for moderate and vigorous physical activity combined.
Cpm, counts per minute.
Table 2.
Descriptive statistics of the potential confounders at baseline
| n (%) | Mean | SD | Median (95% CI) | Range | |
|---|---|---|---|---|---|
| Sex (boys) | 84 (58.3) | ||||
| Anthropometry | |||||
| Weight (kg) | 45.4 | 9.6 | 43.5 (42.2 to 46.5) | 27.2–90.5 | |
| Height (cm) | 156.2 | 8.9 | 156.5 (154.4 to 158.9) | 134.2–181.6 | |
| BMI (overweight/obese) | 15 (10.4) | ||||
| Waist-to-height ratio | 0.44 | 0.04 | 0.43 (0.42 to 0.44) | 0.36–0.60 | |
| Contact/collision sport | 96 (66.7) | ||||
| Physical fitness | |||||
| 10×5 m shuttle run test (s) | 21.5 | 1.7 | 21.5 (21.1 to 21.7) | 17.6–25.1 | |
| Handgrip strength test (kg) | 23.5 | 5.3 | 22.7 (21.5 to 23.4) | 13.1–42.1 | |
| Andersen test (m) | 1021 | 9.4 | 1030 (1014 to 1040) | 800–1235 | |
BMI, body mass index.
The proportion of the day spent at different physical activity levels did not predict the incidence of spinal pain (table 3). However, in the 90th centile cut-point analysis, the participants in the highest proportion of the day spent in vigorous physical activity were at an increased risk for reporting an episode of spinal pain at follow-up (crude RR=1.26; 95% CI 1.00 to 1.58). This relationship did not change after adjusting for the mean of activity spent in other activity levels (RR=1.35; 95% CI 1.06 to 1.70), nor when adjusted with other potential confounders (RR=1.44; 95% CI 1.09 to 1.91) (table 3).
Table 3.
Bivariate and multivariable analyses of different levels of physical activity as predictor of the 2-year incidence of spinal pain
| Spinal pain (SP) status at follow-up |
||||||
|---|---|---|---|---|---|---|
| Proportion of the day spent at different levels of physical activity |
No SP (n=47) n (%) |
SP (n=97) n (%) |
Crude RR (95% CI) |
Adjusted PA* RR (95% CI) |
Adjusted full model† RR (95% CI) |
|
| Sedentary activity, dichotomised at the | ||||||
| 90th centile | Below | 41 (87.2) | 88 (90.7) | Ref. | Ref. | Ref. |
| Above | 6 (12.8) | 9 (9.3) | 0.83 (0.54 to 1.29) | 0.86 (0.55 to 1.32) | 0.82 (0.52 to 1.28)‡ | |
| 75th centile | Below | 36 (76.6) | 72 (74.2) | Ref. | Ref. | Ref. |
| Above | 11 (23.4) | 25 (25.8) | 1.04 (0.70 to 1.55) | 1.02 (0.79 to 1.33) | 1.02 (0.78 to 1.32)§ | |
| 50th centile | Below | 20 (42.6) | 52 (53.6) | Ref. | Ref. | Ref. |
| Above | 27 (57.5) | 45 (46.4) | 0.81 (0.65 to 1.02) | 0.84 (0.66 to 1.06) | 0.83 (0.66 to 1.04)¶ | |
| Moderate-to-vigorous physical activity, dichotomised at the | ||||||
| 90th centile | Below | 42 (89.4) | 87 (89.7) | Ref. | Ref. | Ref. |
| Above | 5 (10.6) | 10 (10.3) | 0.93 (0.64 to 1.37) | 0.97 (0.65 to 1.44) | 1.04 (0.66 to 1.63)** | |
| 75th centile | Below | 37 (78.7) | 71 (73.2) | Ref. | Ref. | Ref. |
| Above | 10 (21.3) | 26 (26.8) | 1.04 (0.81 to 1.32) | 1.11 (0.84 to 1.47) | 1.22 (0.89 to 1.67)§ | |
| 50th centile | Below | 25 (53.2) | 47 (48.5) | Ref. | Ref. | Ref. |
| Above | 22 (46.8) | 50 (51.6) | 1.00 (0.80 to 1.25) | 1.07 (0.82 to 1.41) | 1.18 (0.87 to 1.59)§ | |
| Vigorous physical activity, dichotomised at the | ||||||
| 90th centile | Below | 45 (95.7) | 84 (86.6) | Ref. | Ref. | Ref. |
| Above | 2 (4.3) | 13 (13.4) | 1.26 (1.00 to 1.58) | 1.35 (1.06 to 1.70) | 1.44 (1.09 to 1.91)†† | |
| 75th centile | Below | 38 (80.9) | 70 (72.2) | Ref. | Ref. | Ref. |
| Above | 9 (19.2) | 27 (27.8) | 1.09 (0.86 to 1.38) | 1.17 (0.90 to 1.52) | 1.31 (0.97 to 1.78)‡‡ | |
| 50th centile | Below$ | 25 (53.2) | 47 (48.5) | Ref. | Ref. | Ref. |
| Above | 22 (46.8) | 50 (51.6) | 1.00 (0.80 to 1.25) | 1.05 (0.80 to 1.38) | 1.15 (0.88 to 1.50)§ | |
Statistically significant at 95% level RRs in bold.
*Adjusted for the counts per minute (cpm) for the time spent at other activity levels, that is, for sedentary activity: adjustment for cpm for the time spent in light, moderate and vigorous activity.
†Adjusted for all variables that changed the regression coefficient of the exposure variable in the bivariate analysis by more than 10%.
‡Adjusted for: sex, height, BMI, Handgrip strength test, Andersen test.
§Adjusted for: sex, weight2, height, BMI, waist-to-height ratio, contact/collision sport, 10×5 m shuttle run test, Handgrip strength test, Andersen test.
¶Adjusted for: sex, Handgrip strength test.
**Adjusted for: sex, height, BMI, waist-to-height ratio, 10×5 m shuttle run test, Handgrip strength test, Andersen test.
††Adjusted for: sex, BMI, 10×5 m shuttle run test, Andersen test.
‡‡Adjusted for: sex, weight, height, BMI, waist-to-height ratio, contact/collision sport, 10×5 m shuttle run test, Andersen test.
BMI, body mass index; Cpm, counts per minute; PA, physical activity; RR, relative risk.
For the overall physical activity, the RR in the multivariable analysis was 1.03 (95% CI 1.01 to 1.05) for reporting spinal pain at follow-up. All results were reached after using 10 iterations because convergence was not achieved in the models initially.
Sensitivity analyses
Sensitivity analyses using 30 s as epoch length compared with 10 s epoch resulted in small differences in the unadjusted and adjusted RRs. The differences were too small to alter our CIs in the primary analyses.
Discussion
Objectively measured physical activity in 11–13-year-old adolescents was generally not predictive of the 2-year incidence of spinal pain. The proportion of time spent in daily sedentary activities did not predict spinal pain. However, when examining vigorous activity by the 90% cut-point, participants who were in the higher category had an increased risk of having reported an episode of spinal pain at follow-up compared with the participants in the lower category. The marginal statistically significant risk related to the overall physical activity seems to be driven by this group.
Comparison with other studies
Our results are similar to a longitudinal study of pain-free children that found that children who participated in sporting activities for more than 6 hours a week (self-reported) were at an increased risk for developing back pain 1 year later.21 Children not participating in organised sporting activities are unlikely to reach the high levels of vigorous physical activity as measured in our study. Thus, sporting activities would be a proxy measure for vigorous activities.
In contradiction to our study, Wedderkopp et al22 found that high levels of objectively measured physical activity in children aged 8–10 years was protective for back pain 3 years later. The authors processed the accelerometer data differently from what we did. They used a longer epoch length of 1 min compared against our 10 s epoch length. The longer epoch length may have resulted in an underestimation of the time spent in moderate-to-vigorous physical activity. In addition, their non-wear time was defined at 10 min rather than 60 min. This could have led to inappropriately labelling sedentary time as non-wear time and thereby explain their higher estimate of overall physical activity (707 in boys and 582 in girls). Their inclusion criteria also differed as 30% of the participants reported pain at baseline and therefore are not comparable to ours as our participants reported no spinal pain at baseline.
Strengths and limitations
The longitudinal design, the cohort being school-based and the participants being free of spinal pain at baseline are all strengths of this study. Furthermore, a validated questionnaire developed specifically for this age group was used to assess spinal pain.17 Using accelerometers to assess physical activity also strengthened this study because they have been shown to be superior to using self-report.8 23 Finally, we explored different levels of physical activity rather than using averages only, which was obviously important in order to identify the group at the highest risk for spinal pain.
We note several limitations of this study, noticeably the lack of psychological factors due to insufficient numbers of participants who were feeling low, were irritable/in a bad mood, felt nervous or who had sleeping difficulties at baseline. These factors are associated with spinal pain, but not with high levels of vigorous physical activity. Therefore, it is unlikely that the absence of these factors has influenced the conclusion that can be drawn for vigorous physical activity. Nevertheless, we cannot exclude that other psychological factors such as ambitions and feeling pressure from parents could have played a role in developing spinal pain. Also, the small sample size (n=144) increases the risk of type II error. Additionally, among the 10% with most time in vigorous physical activity, 13 developed spinal pain, which is a rather small number to base conclusions on. Nevertheless, we believe our conclusion is reasonable because of the increasing risk ratios for spinal pain from the top 50% to the top 10%, resembling a dose–response pattern for vigorous physical activity.
Limitations could also be addressed to processing of accelerometer data. There is a large variety of methods to process accelerometer data and currently there is no consensus regarding the optimal method.24 However, the definitions for a valid day, minimum number of wear days, and cut-points for the different activity levels used in this study are commonly used in other studies,24 and on the basis of our thorough inspection of the data prior to making the reduction decisions, we believe our choices are sensible. Finally, we may have misclassified some participants at baseline due to recall bias regarding previous episodes of spine pain.
Implications of results
The WHO has recommended that children and young people spend at least 1 hour each day in moderate to vigorous physical activity. However, this recommendation has not taken prevention of musculoskeletal pain into account. We emphasise that the WHO recommendation should be maintained, but we advocate that the quality and type of the physical activity should be considered in addition to duration if these are to be relevant for musculoskeletal health. Perhaps the spinal pain reported in our study was caused by overuse or injuries incurred during vigorous physical activity, and therefore increased focus on strategies that prevent injuries in the spine in organised sport, both inside and outside school, might be warranted. Since literature on the prevention of spinal pain is lacking, a parallel might be drawn with knee injury where neuromuscular exercises have been shown to decrease the incidence rate of anterior cruciate ligament injury.25
Future research
The impact of physical activity on spinal pain is still poorly understood. Longitudinal studies with larger sample sizes from the general population are needed to confirm the results of this study and to determine the contribution of psychological and social factors in the development of spinal pain. In addition, the reported pain in this study could be minor bangs and bruises only; future research should also investigate the long-term course of spinal pain in the most and least physically active adolescents. Furthermore, there needs to be greater consensus on how to process complex accelerometer data to improve the validity and interpretability, as well as comparability of results across studies.
Footnotes
Contributors: EA contributed towards conception and design, data collection, analysis and drafting the manuscript. EB, JH, PHF, CGM and MLF contributed towards conception and design, analysis and revision of the manuscript. LH contributed towards conception and design, data collection, analysis and revision of the manuscript. All authors have read and approved the manuscript.
Funding: The SPACE study was part of the Center for Intervention Research and was funded by TrygFonden. EA was funded by an International Stipend from the Danish Chiropractors Research Foundation, University of Southern Denmark and from the Norwegian Chiropractic Association. There are no conflicts of interests.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Vos T, Flaxman AD, Naghavi M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin BI, Deyo RA, Mirza SK et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299:656–64. 10.1001/jama.299.6.656 [DOI] [PubMed] [Google Scholar]
- 3.Jeffries LJ, Milanese SF, Grimmer-Somers KA. Epidemiology of adolescent spinal pain: a systematic overview of the research literature. Spine (Phila Pa 1976) 2007;32:2630–7. 10.1097/BRS.0b013e318158d70b [DOI] [PubMed] [Google Scholar]
- 4.Jones MA, Stratton G, Reilly T et al. A school-based survey of recurrent non-specific low-back pain prevalence and consequences in children. Health Educ Res 2004;19:284–9. 10.1093/her/cyg025 [DOI] [PubMed] [Google Scholar]
- 5.Harreby M, Neergaard K, Hesselsoe G et al. Are radiologic changes in the thoracic and lumbar spine of adolescents risk factors for low back pain in adults? A 25-year prospective cohort study of 640 school children. Spine (Phila Pa 1976) 1995;20:2298–302. 10.1097/00007632-199511000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Hestbaek L, Leboeuf-Yde C, Kyvik KO et al. The course of low back pain from adolescence to adulthood: eight-year follow-up of 9600 twins. Spine (Phila Pa 1976) 2006;31:468–72. 10.1097/01.brs.0000199958.04073.d9 [DOI] [PubMed] [Google Scholar]
- 7.Sitthipornvorakul E, Janwantanakul P, Purepong N et al. The association between physical activity and neck and low back pain: a systematic review. Eur Spine J 2011;20:677–89. 10.1007/s00586-010-1630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamo KB, Prince SA, Tricco AC et al. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: a systematic review. Int J Pediatr Obes 2009;4:2–27. 10.1080/17477160802315010 [DOI] [PubMed] [Google Scholar]
- 9.Bailey RC, Olson J, Pepper SL et al. The level and tempo of children's physical activities: an observational study. Med Sci Sports Exerc 1995;27:1033–41. 10.1249/00005768-199507000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Toftager M, Christiansen LB, Kristensen PL et al. SPACE for physical activity—a multicomponent intervention study: study design and baseline findings from a cluster randomized controlled trial. BMC Public Health 2011;11:777 10.1186/1471-2458-11-777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toftager M, Christiansen LB, Ersbøll AK et al. Intervention effects on adolescent physical activity in the multicomponent SPACE study: a cluster randomized controlled trial. PLoS ONE 2014;9:e99369 10.1371/journal.pone.0099369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines about Notification etc. of a Biomedical Research Project to the Committee System on Biomedical Research Ethics, No 9154, 5 May 2011. Secondary Guidelines about Notification etc. of a Biomedical Research Project to the Committee System on Biomedical Research Ethics, No 9154, 5 May 2011. http://www.cvk.sum.dk/English/guidelinesaboutnotification.aspx.
- 13.Actigraph Secondary Actigraph 2011. http://www.theactigraph.com/products/gt3x/.
- 14.Toftager M, Kristensen PL, Oliver M et al. Accelerometer data reduction in adolescents: effects on sample retention and bias. Int J Behav Nutr Phys Act 2013;10:140 10.1186/1479-5868-10-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich C, Geraci M, Griffiths L et al. Quality control methods in accelerometer data processing: defining minimum wear time. PLoS ONE 2013;8:e67206 10.1371/journal.pone.0067206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evenson KR, Catellier DJ, Gill K et al. Calibration of two objective measures of physical activity for children. J Sports Sci 2008;26:1557–65. 10.1080/02640410802334196 [DOI] [PubMed] [Google Scholar]
- 17.Lauridsen HH, Hestbaek L. Development of the young spine questionnaire. BMC Musculoskelet Disord 2013;14:185 10.1186/1471-2474-14-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris T, Yang Z, Hardin JW. Modeling underdispersed count data with generalized Poisson regression. Stata J 2012; 12:736–47. [Google Scholar]
- 19.Rothman KJ GS, Lash TL. Modern epidemiology. 3rd edn Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 20.Edwardson CL, Gorely T. Epoch length and its effect on physical activity intensity. Med Sci Sports Exerc 2010;42:928–34. 10.1249/MSS.0b013e3181c301f5 [DOI] [PubMed] [Google Scholar]
- 21.Jones GT, Watson KD, Silman AJ et al. Predictors of low back pain in British schoolchildren: a population-based prospective cohort study. Pediatrics 2003;111(4 Pt 1):822–8. 10.1542/peds.111.4.822 [DOI] [PubMed] [Google Scholar]
- 22.Wedderkopp N, Kjaer P, Hestbaek L et al. High-level physical activity in childhood seems to protect against low back pain in early adolescence. Spine J 2009;9:134–41. 10.1016/j.spinee.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Lubans DR, Hesketh K, Cliff DP et al. A systematic review of the validity and reliability of sedentary behaviour measures used with children and adolescents. Obes Rev 2011;12:781–99. 10.1111/j.1467-789X.2011.00896.x [DOI] [PubMed] [Google Scholar]
- 24.Cain KL, Sallis JF, Conway TL et al. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health 2013;10:437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagnier JJ, Morgenstern H, Chess L. Interventions designed to prevent anterior cruciate ligament injuries in adolescents and adults: a systematic review and meta-analysis. Am J Sports Med 2013;41:1952–62. 10.1177/0363546512458227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2015-000097supp.pdf (93KB, pdf)