ABSTRACT
As the most recent evidence of eukaryotic cell complexity, genome architecture has astounded the scientific community and prompted a variety of technical and cognitive challenges. Several technologies have emerged and evidenced the integration of chromatin packaging and topology, epigenetic processes, and transcription for the pertinent regulation of gene expression. In the present addendum we present and discuss some of our recent research, directed toward the holistic comprehension of the processes by which plants respond to environmental and developmental stimuli. We propose that the study of genome topology and genomic interactions is essential for the understanding of the molecular mechanisms behind a phenotype. Even though our knowledge and understanding of genome architecture and hierarchy has improved substantially in the last few years -in Arabidopsis and other eukaryotes -, there is still a long way ahead in this relatively new field of study. For this, it is necessary to take advantage of the high resolution of the emerging available techniques, and perform integrative approaches with which it will be possible to depict the role of chromatin architecture in the regulation of transcription and ultimately, physiological processes.
KEYWORDS: Chromatin architecture, genome topology, gene expression, genomic interactions, gene loops, lncRNAs
The physiological processes behind the plant responses to environmental and developmental stimuli have been exhaustively characterized. Thanks to this, nowadays there is a wide understanding in stress biology, hormonal signaling, and their ultimate impact on gene expression. Even though epigenetics and epigenomics have risen as an intriguing and exciting research field, giving insights into the molecular mechanisms behind gene expression, there are still plenty of interesting questions to be answered. Epigenomic and 3D-chromatin conformation approaches have emerged as tools to address these questions from an integrative perspective; tools to find the missing links between physiological, cellular and molecular processes that, despite being coordinated and corregulated, have been habitually independently addressed by the scientific community.
Some studies have characterized specific cases that illustrate the importance of the physical configuration of the chromatin fiber on the regulation of plant development, and responses to environmental stimuli.1 It is generally accepted that high levels of DNA compaction are correlated with low gene expression and vice versa, this phenomenon being mainly attributed to the covalent modifications of histone tails and DNA. However, it is becoming clearer that the panorama in the eukaryotic nucleus is significantly more complex, and that the interactions between distal and proximal genomic regions have a great impact over transcription.
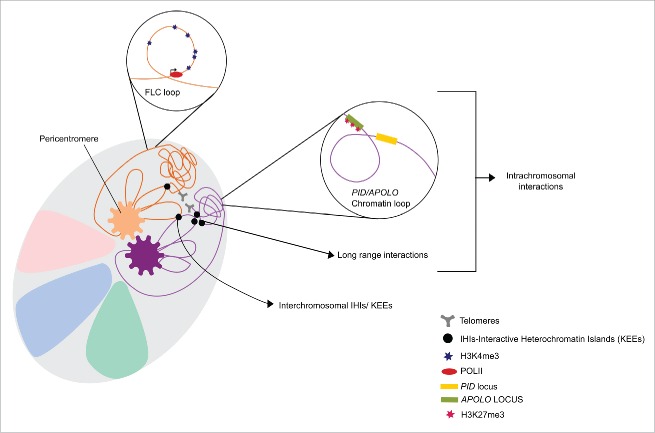
A Hi-C study depicted the general 3D-genome organization in Arabidopsis, finding a non-random distribution of diverse genomic regions. Furthermore, such conformation was proven to be different to the one described in animal nuclei -and characterized by a higher frequency of intrachromosomal interactions enriched with H3K27me3, H3.1 and H3.3 marks-.2 There does not seem to be abundant interactions between highly transcribed genes in Arabidopsis, being long-range interactions mainly between heterochromatic regions of plant chromosomes.3 Nevertheless, short-range interactions occur between regulatory elements and their gene-encoding targets in the same chromosome, in order to establish a dynamic transcriptional network based on physical proximity.4 Gene loops, a type of short-range interaction, have been proven to have an important role in the transcriptional regulation and memory in animals, yeast and plants.5-7 These consist in dynamic 3-dimensional local interactions, which structure is a determinant factor for the regulation of transcription (Fig. 1).6
Figure 1.
Schematic repersentation of chromatin organization in Arabidopsis thaliana. Chromosomes territories are repersented with different colors, being two of chromosomes showed in detailed with the most relevant interactions, observed in previous 3C-derived analyses. Pericentromeric regions are recognized for establishing strong intra-and inter-chromosomal intreraction between them. Intreractive Heterochromatic Island (IHIs) can be found in different region along the five chromosomes arms and coverge in the three dimensional space, where they from a KNOT, or a superstructure that consist of inter and intra-chromosomal interaction of both long and short-range. Regulatory elements and genes belonging to the same chromosomes are comonly connected by the formation of loops or higher order chromatin structures. For instance, FLC expression is activated by the formation a gene loop between its 3′ and 5′ flanking region and H3K4 trimethylation along the locus. Moreover, chromatin loops encompassing different genes have also been involved in gene expression fine-tuning; the ragulation of the PID gene by the formation of a loop between PID and APOLO loci is a clear example of these structures. Ploidy level of the cell and genemic position of the intrective region shown above are not considerd.
In one of our studies we described an example of these latter interactions, where BAF60, a subunit of the SWI/SNF chromatin remodeling complexes, regulates cell division and differentiation during development in Arabidopsis plantlets. The mechanism by which this occurs is through the targeting of IPT3 and IPT7, genes involved in cytokinin synthesis. BAF60 binds to these loci and to the cell cycle negative regulator KRP7, preventing the formation of gene loops in each locus, and repressing their expression. Such repression leads to a decrease in cytokinin levels and has a positive effect in the progression of the cell cycle and root growth.8
Interestingly, BAF60 also prevents the formation of a gene loop in FLC (FLOWERING LOCUS C), a very well described flowering repressor in Arabidopsis.9,10 Under long day conditions BAF60 binds FLC and promotes the addition of H3K27me3, which, together with the loop repression, leads to the downregulation of this gene. In the absence of FLC the molecular events related to flowering are positively regulated and the development of sexual organs occurs (Fig. 1).11,1
In a more recent study we described the role of long nonconding intergenic RNAs (lncRNAs) on genome topology and gene expression.12 With this study we were able to evidence the plasticity of chromatin conformation and gene expression, as a result of the integration of hormone signaling, DNA methylation, noncoding transcription, and histone modifications. Thus, we elucidated some of the mechanisms by which the expression of the PINOID (PID) gene, an important regulator of the spatial localization of auxin transporters, is regulated. Robust evidences have shown that PINOID expression relies on APOLO, a lncRNA capable of triggering several downstream molecular pathways involved in the determination of chromatin topology.12 A Chromosome Conformation Capture (3C) assay showed that the promoter region of PID interacts with the APOLO locus through the formation of an LHP1-mediated -and auxin sensitive- loop. Auxin signaling induces the activity of several DNA demethylases, leading to a reduction of DNA methylation along APOLO. Such changes result in the opening of the loop encompassing the PID promoter region, thereby exposing the locus to the transcriptional machinery. PolII divergent transcription starts at both, PID and APOLO loci, a phenomenon coupled with the increase in the H3K9 acetylation at the 5′ region of PID (Fig. 1).
Interestingly, it is the accumulation of APOLO transcripts what leads to the recruitment of LHP1, a PRC (Polycomb Repressive Complex) protein that participates in the subsequent reestablishment of the loop. In parallel, POLIV/V transcription triggers the RNA dependent DNA Methylation (RdDM) pathway and the recruitment of PRC1 and PRC2 is in charge of the deposition of H3K27 trimethylation of APOLO. Recently, we demonstrated that LHP1 controls the spreading of H3K27me3 toward the 3′of its genomic targets, suggesting that this protein may be contributing to loop formation.13 One of the most notorious and exciting characteristics of LHP1 is its potential to co-regulate the expression of distant genes through the establishment of LHP1-dependent physical interactions. Even though LHP1 can control genome topology and hence expression patterns of several genes,13 there is still a need for identifying the precise mechanisms by which this occurs.
Hence, we propose the existence of at least several hundreds of this type of loops and genomic interactions in the eukaryotic cells, which may be influencing the transcription of diverse genomic regions.14,15 The discovery of genome "interactomes" and their role in the tuning of on gene expression have become the foundations of genome topology research. These basic structures have encouraged the scientific community to elucidate their complexity and hierarchical organization. Our understanding of the complexity and nature of genome topology and conformation has continuously increased in the last years, as the technical robustness and resolution of the available and new techniques improve.16 However, there are still many questions to be addressed, and their answer will probably need from an integrative molecular biology. With these approaches we will be able to discover some of the missing shackles in the well defined, and intensely studied, relation between genotype and phenotype, in plants and other eukaryotes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Reference
- 1.Rodriguez-Granados NY, Ramirez-Prado JS, Veluchamy A, Latrasse D, Raynaud C, Crespi M, Ariel F, Benhamed M. Put your 3D glasses on: plant chromatin is on show. J Exp Bot 2016; 67:3205-21; PMID:27129951; http://dx.doi.org/ 10.1093/jxb/erw168 [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Liu C, Roqueiro D, Grimm D, Schwab R, Becker C, Lanz C, Weigel D. Genome-wide analysis of local chromatin packing in Arabidopsis thaliana. Genome Res 2015; 25(2), 246-56; PMID:25367294; http://dx.doi.org/ 10.1101/gr.170332.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Wang C, Wang G, Becker C, Zaidem M, Weigel D. Genome-wide analysis of chromatin packing in Arabidopsis thaliana at single- gene resolution. Genome Res 2016; 26(8):1057-68; PMID:27225844; http://dx.doi.org/ 10.1101/gr.186668.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louwers M, Bader R, Haring M, van Driel R, de Laat W, Stam M. Tissue- and expression level-specific chromatin looping at maize b1 epialleles. Plant Cell 2009; 21(3):832-42; PMID:19336692; http://dx.doi.org/ 10.1105/tpc.108.064329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh BN, Laine J, Krishnamurthy S, Hampsey M. A physiological role for gene loops in yeast. Genes Dev 2009; 23(22):2604-9; PMID:19933150; http://doi.org/ 10.1101/gad.1823609.Genes [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampsey M, Singh BN, Ansari A, Laine J, Krishnamurthy S. Control of eukaryotic gene expression: gene loops and transcriptional memory. Adv Enzyme Regul 2011; 4(164):118-25; PMID:21036187; http://doi.org/ 10.1126/scisignal.2001449.Engineering [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oti M, Falck J, Huynen MA, Zhou H. CTCF-mediated chromatin loops enclose inducible gene regulatory domains. BMC Genomics 2016; 17(1):252; PMID:27004515; http://dx.doi.org/ 10.1186/s12864-016-2516-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jegu T, Domenichini S, Blein T, Ariel F, Christ A, Kim SK, Crespi M, Boutet-Mercey S, Mouille G, Bourge M, et al.. A SWI/SNF chromatin remodelling protein controls cytokinin production through the regulation of chromatin architecture. PLoS One 2015; 10(10):1-18; PMID:26457678; http://dx.doi.org/ 10.1371/journal.pone.0138276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaels SD, Amasino RM. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 2001; 13(4):935-41; PMID:11283346; http://dx.doi.org/ 10.1105/tpc.13.4.935; http://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 2006; 20(7):898-912; PMID:16600915; http://dx.doi.org/ 10.1101/gad.373506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jégu T, Latrasse D, Delarue M, Hirt H, Domenichini S, Ariel F, Crespi M, Bergounioux C, Raynaud C,Benhamed M. The BAF60 subunit of the SWI/SNF chromatin-remodeling complex directly controls the formation of a gene loop at FLOWERING LOCUS C in Arabidopsis. The Plant Cell 2014; 26(2):538-51; PMID:24510722; http://dx.doi.org/ 10.1105/tpc.113.114454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariel F, Jegu T, Latrasse D, Romero-Barrios N, Christ A, Benhamed M, Crespi M. Noncoding transcription by alternative rna polymerases dynamically regulates an auxin-driven chromatin loop. Molecular Cell 2014; 55(3):383-96; PMID:2501801; http://dx.doi.org/ 10.1016/j.molcel.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Veluchamy A, Jegu T, Ariel F, Latrasse D, Gayathri Mariappan K, Kim SK, Crespi M, Hirt H, Bergounioux C, Raynaud C, et al.. LHP1 regulates H3K27me3 spreading and shapes the three-dimensional conformation of the Arabidopsis genome. PLoS One 2016; 11(7):e0158936; PMID:27410265; http://dx.doi.org/ 10.1371/journal.pone.0158936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grob S, Schmid MW, Grossniklaus U. Hi-C Analysis in Arabidopsis Identifies the KNOT, a Structure with Similarities to the flamenco Locus of Drosophila. Molecular Cell 2014; 55(5):678-93; PMID:25132176; http://dx.doi.org/ 10.1016/j.molcel.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 15.Grob S, Schmid MW, Luedtke NW, Wicker T, Grossniklaus U. Characterization of chromosomal architecture in Arabidopsis by chromosome conformation capture. Genome Biol 2013; 14(11):R129; PMID:24267747; http://dx.doi.org/ 10.1186/gb-2013-14-11-r129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Weigel D. Chromatin in 3D: progress and prospects for plants. Genome Biol 2015; 16(1):1; PMID:25583448; http://dx.doi.org/ 10.1186/s13059-014-0572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]