Abstract
Objective
In resistance arteries, there is an emerging view that smooth muscle CaV3.2 channels restrain arterial constriction through a feedback response involving the large-conductance Ca2+-activated K+ channel (BKCa). Here, we used wild-type and CaV3.2 knockout (CaV3.2−/−) mice to definitively test whether CaV3.2 moderates myogenic tone in mesenteric arteries via the CaV3.2-ryanodine receptor-BKCa axis and whether this regulatory mechanism influences blood pressure regulation.
Approach and Results
Using pressurized vessel myography, CaV3.2−/− mesenteric arteries displayed enhanced myogenic constriction to pressure but similar K+-induced vasoconstriction compared with wild-type C57BL/6 arteries. Electrophysiological and myography experiments subsequently confirmed the inability of micromolar Ni2+, a CaV3.2 blocker, to either constrict arteries or suppress T-type currents in CaV3.2−/− smooth muscle cells. The frequency of BKCa-induced spontaneous transient outward K+ currents dropped in wild-type but not in knockout arterial smooth muscle cells upon the pharmacological suppression of CaV3.2 channel. Line scan analysis performed on en face arteries loaded with Fluo-4 revealed the presence of Ca2+ sparks in all arteries, with the subsequent application of Ni2+ only affecting wild-type arteries. Although CaV3.2 channel moderated myogenic constriction of resistance arteries, the blood pressure measurements of CaV3.2−/− and wild-type animals were similar.
Conclusions
Overall, our findings establish a negative feedback mechanism of the myogenic response in which CaV3.2 channel modulates downstream ryanodine receptor-BKCa to hyperpolarize and relax arteries.
Keywords: arteries, calcium-activated potassium channels, calcium channels, calcium signaling, ryanodine receptors, T-type calcium channels, vascular smooth muscle
The cardiovascular system comprises a muscular pump and a distribution network of arteries, veins, and capillaries. Within this integrated system, resistance arteries control the magnitude and distribution of tissue perfusion and respond to vasoactive stimuli, including mechanical forces, neurotransmitters, and metabolites.1–5 Bayliss first described the inherent ability of resistance arteries to constrict to elevated pressure,6 and studies have shown that the so-called myogenic response is intimately tied to depolarization and the activation of smooth muscle L-type Ca2+ channels. It is often presumed that CaV1.2 is the only Ca2+ channel of functional significance because dihydropyridines, L-type blockers, prominently attenuate myogenic tone.3 This traditional perspective has begun to change with the identification of arterial T-type Ca2+ channels, including CaV3.1 and CaV3.2 subtypes.7,8 Recent findings suggest that the former (ie, CaV3.1) modestly facilitates myogenic constriction at hyperpolarized voltages, whereas the latter (ie, CaV3.2) facilitates a negative feedback response restraining arterial constriction.9,10
Our recent observations have tied the paradoxical ability of rat cerebral arterial CaV3.2 channel to limit myogenic tone to the triggering of ryanodine receptors (RyR) on the sarcoplasmic reticulum. The RyR-mediated generation of Ca2+ sparks subsequently activates the large conductance Ca2+-activated K+ channels (BKCa), eliciting a hyperpolarization to counteract pressure-induced constriction.10 Furthermore, the CaV3.2 conductance in the human cerebral circulation seems to mediate a similar physiological role.10 Although the concept of a voltage-gated Ca2+ channel counterbalancing vasoconstriction is novel and intriguing, it is one delimited by 2 primary concerns. First, current work is heavily reliant on the presumed selectivity of Ni2+ to block CaV3.2 channels.11 Second, there is a lack of corroborative observations, outside the cerebral circulation, in vascular beds known to acutely and sustainably regulate systemic blood pressure.
Here, we used wild-type and CaV3.2 knockout (CaV3.2−/−) mice to definitively test whether CaV3.2 channel moderates myogenic tone in mesenteric arteries via the CaV3.2-RyR-BKCa axis and, more generally, whether this regulatory mechanism influences blood pressure regulation. Experiments ranged from cells to whole animals and encompassed the integrative use of myography, electrophysiology, Ca2+ imaging, and intravascular catheterization. Arteries displayed enhanced myogenic tone when CaV3.2 channels were genetically ablated or pharmacologically suppressed using Ni2+. Subsequent analyses indicated that Ni2+ inhibited BKCa currents and Ca2+ sparks in wild-type but not CaV3.2−/− arteries. Although CaV3.2 channel moderated myogenic constriction, the blood pressure measurements of both animal types were similar. In conclusion, this study establishes a negative feedback response in which CaV3.2 channel modulates downstream activity of the RyR-BKCa complex to hyperpolarize and relax resistance arteries.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Genetic Ablation of CaV3.2 Enhances Arterial Myogenic Tone
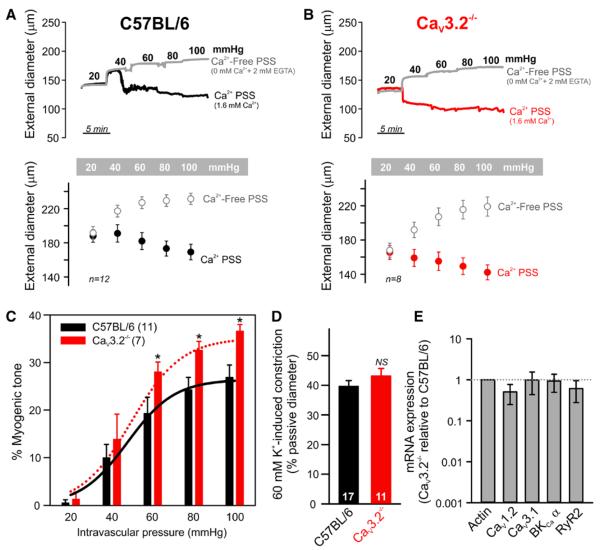
Our earlier reports revealed the involvement of CaV3.2 channels in negative feedback control of rat cerebral arterial tone10; thus, channel deletion should enhance myogenic tone. Figure 1A and 1B demonstrates that C57BL/6 and CaV3.2−/− arteries were myogenically active and constricted as intravascular pressure increased from 20 to 100 mm Hg. Ca2+-free saline (0 mmol/L Ca2++2 mmol/L EGTA) reversed myogenic constriction and evoked passive arterial dilation. Expressing data as a percentage myogenic tone (Figure 1C) revealed the predicted enhancement in CaV3.2−/− arteries (at 60 mm Hg: CaV3.2−/−, 28%±2%; C57BL/6, 19%±3%). Although myogenic tone was significantly different, 60 mmol/L K+-induced vasoconstriction was similar in C57BL/6 (40%±2%) and CaV3.2−/− (43%±2%) pressurized arteries (Figure 1D). Note, the basal diameter of CaV3.2−/− arteries was smaller than that of C57BL/6, a finding consistent with variable myogenic tone. Interpretational cautious is, however, warranted because CaV3.2−/− mice display a lighter body mass than wild-type animals.12 Quantitative PCR showed that mRNA expression of CaV1.2, CaV3.1, BKCa-α, or RyR2 was comparable in wild-type and CaV3.2−/− arteries (Figure 1E).
Figure 1.
CaV3.2−/− arteries display enhanced myogenic tone. A, Representative traces and summary data demonstrate that C57BL/6 mesenteric arteries constricted to elevations in intravascular pressure from 20 to 100 mm Hg in Ca2+ containing physiological saline solution (PSS). Maximum arterial diameters were obtained by replacing Ca2+ PSS with PSS with zero Ca2++2 mmol/L EGTA (n=12). B, Traces and averaged data of the myogenic response in CaV3.2−/− mesenteric arteries (n=8). C, Percentage myogenic tone was significantly higher in CaV3.2−/− (n=7) than in wild-type C57BL/6 (n=11) arteries (unpaired t test, *P≤0.05). D, Vasoconstriction evoked by 60 mmol/L K+ was similar in C57BL/6 and CaV3.2−/− pressurized (15 mm Hg) arteries (n=11–17, unpaired t test; NS denotes not significant). E, Messenger RNA of key genes (CaV1.2, CaV3.1, BKCa-α, and RyR2) were not different in C57BL/6 and CaV3.2−/− mesenteric arteries. Relative expression was calculated using 3 independent quantitative PCR reactions.
Micromolar Ni2+ Selectively Blocks CaV3.2 Currents
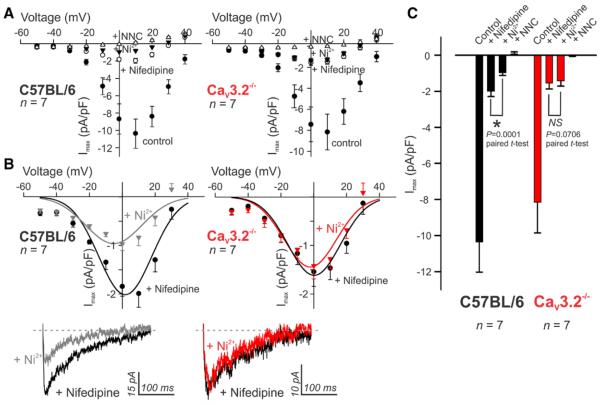
Mesenteric arterial smooth muscle cells from C57BL/6 mice express 3 subtypes of voltage-gated Ca2+ channels (CaV1.2, CaV3.1, and CaV3.2)9, and thus, total inward current is representative of this ensemble of channels. To distinguish between the subcomponents, we used patch clamp electrophysiology in combination with defined pharmacology.10 First, nifedipine (200 nmol/L) was applied to block L-type CaV1.2 channels and reveal a current predominated by T-type conductances (Figure 2A). Subsequent application of 50 μmol/L Ni2+, which is presumed to be a selective CaV3.2 blocker,10–14 reduced the nifedipine-insensitive T-type currents in C57BL/6 but not in CaV3.2−/− smooth muscle cells (Figure 2B and 2C). The absence of an effect of Ni2+ in CaV3.2−/− myocytes is consistent with this pharmacological selectivity. Note that the broad-spectrum T-type blocker (NNC 55–0396, 1 μmol/L) subsequently abolished the residual current15 in both C57BL/6 and CaV3.2−/− myocytes because of the suppression of remaining CaV3.1 current (Figure 2C). Importantly, voltage dependence profiles demonstrated that the T-type current is available for activation at physiological membrane potentials (Figure I in the online-only Data Supplement). As noted previously, CaV3.1 mRNA levels were similar among the 2 groups of animals, whereas CaV1.2 was modestly but insignificantly lower in CaV3.2−/− arteries (Figure 1E).
Figure 2.
Micromolar Ni2+ suppresses CaV3.2 current. A, Current–voltage (I-V) plots illustrate total inward currents in wild-type C57BL/6 and CaV3.2−/− smooth muscle cells and the effects of CaV1.2 and CaV3.2 blockade using nifedipine (200 nmol/L) and Ni2+ (50 μmol/L), respectively. All recordings used 10 mmol/L Ba2+ as the charge carrier. B and C, Representative traces and summary data illustrate the effect of Ni2+ on nifedipine insensitive T-type currents. Representative traces were evoked using a prepulse (−90 mV) followed by a test pulse (0 mV). Ni2+ significantly suppressed T-type currents in C57BL/6 but not CaV3.2−/− smooth muscle cells (n=7 each; *P≤0.05).
CaV3.2 Activity Regulates BKCa-Mediated STOCs
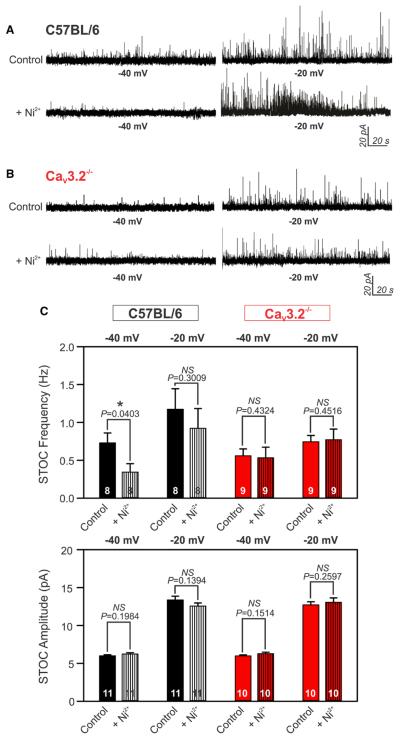
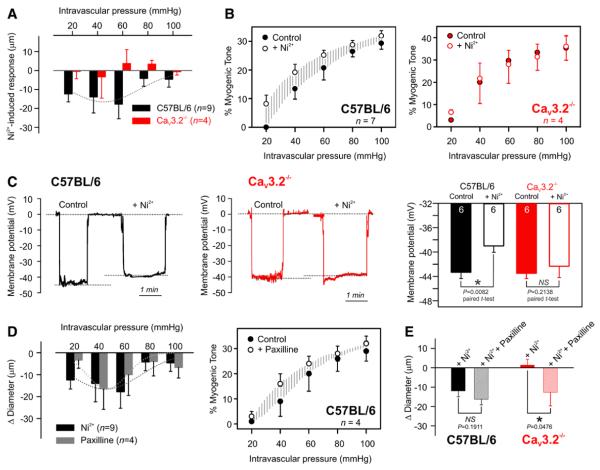
Given the enhancement of myogenic constriction in CaV3.2−/− arteries, we next tested whether this T-type channel modifies myogenic reactivity through a negative feedback response that involves downstream BKCa.10,12 We used perforated patch clamp electrophysiology to monitor BKCa-mediated spontaneous transient outward K+ currents (STOCs) in arterial smooth muscle cells from wild-type and knockout animals. In C57BL/6 cells held at the physiological voltage of −40 mV, Ni2+ significantly suppressed STOC frequency. In contrast, Ni2+ had no effect on STOCs when C57BL/6 cells were voltage-clamped at more depolarized potentials (−20 mV), a finding consistent with the voltage profile of CaV3.2 channel (Figure 3A). In CaV3.2−/− cells, Ni2+ had no effect at either −40 or −20 mV (Figure 3B). All STOCs were fully abolished by the application of the BKCa inhibitor paxilline (1 μmol/L;Figure II in the online-only Data Supplement). Message expression of the BKCa pore-forming subunit (BKCa-α) was similar in C57BL/6 and CaV3.2−/− arteries (Figure 1E). Further, Ni2+ had no effect on STOC amplitude under different experimental conditions (Figure 3C). Note, basal STOCs tended to fire at lower frequencies in CaV3.2−/− myocytes compared with C57BL/6 counterparts. Statistical analysis was not performed across groups as cells that did not fire sufficient STOCs were eliminated a priori from experimentation.
Figure 3.
CaV3.2 channel modulates BKCa-mediated spontaneous transient outward K+ currents (STOCs). A and B, Representative traces of STOCs recorded at −40 and −20 mV in wild-type C57BL/6 (A) or CaV3.2−/− (B) arterial smooth muscle cells. Application of 50 μmol/L Ni2+ suppressed STOC frequency only at −40 mV in C57BL/6 cells with no noticeable effect at other conditions. C, Averaged bar graphs illustrate the effect of Ni2+ on STOC frequency (Hz) and amplitude (pA) at −40 or −20 mV in C57BL6 or CaV3.2−/− arterial myocytes (n=8–11, *P≤0.05, paired t test).
CaV3.2 Channel Controls Ca2+ Spark Generation
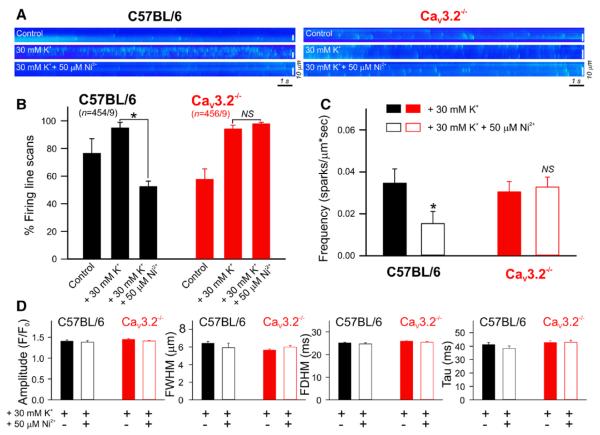
Given the ability of CaV3.2 channel to modulate BKCa current (Figure 3) and the reported correlation between RyR-mediated spark generation and BKCa activation,10,16 we next explored the CaV3.2-Ca2+ spark relationship using Ca2+ imaging and line scan analysis of mouse mesenteric arteries (Figure 4). In C57BL/6 arteries loaded with Fluo-4, Ca2+ sparks were observed in 76% of the 454 line scans performed under control conditions. Depolarizing arteries (30 mmol/L K+) increased spark activity (95%), whereas the subsequent addition of Ni2+ significantly reduced firing (52%; Figure 4A and 4B). In comparison, 30 mmol/L K+ increased spark firing in Cav3.2−/− arteries from 58% to 94% (456 line scans) but Ni2+ failed to attenuate sparks (98%; Figure 4A and 4B). In depolarized wild-type C57BL/6 arteries, Ca2+ spark frequency was calculated to be 0.0347 and 0.0153 sparks/μm s in the absence and presence of Ni2+, respectively; spark frequency in knockout tissues was distinctively insensitive to the application of Ni2+ (Figure 4C). The genetic absence of CaV3.2 channels was notably associated with lower percentage firing (CaV3.2−/−, 58%; C57BL/6, 76%) and lower basal Ca2+ spark frequency when compared with wild-type arteries (CaV3.2−/−, 0.0065±0.0014; C57BL/6, 0.0169±0.005 sparks/μm s). The amplitudes and spatiotemporal characteristics of Ca2+ sparks displayed no significant changes before and after the application of Ni2+ on C57BL/6 and CaV3.2−/− arteries (Figure 4D).
Figure 4.
CaV3.2 channel triggers Ca2+ spark generation. A, Representative line scan images (1.89 ms/line) of Fluo-4 loaded wild-type C57BL/6 or CaV3.2−/− mesenteric arteries showing Ca2+ sparks recorded before and after the sequential application of 30 mmol/L K+ and 50 μmol/L Ni2+. The latter suppressed Ca2+ spark generation in C57BL/6 but not CaV3.2−/− line scans. B, Percentage firing of Ca2+ sparks under control conditions and after the application of 30 mmol/L K+ followed by Ni2+. Line scans were recorded in C57BL/6 (n=454 line scans/9 animals) or CaV3.2−/− (red, n=456 line scans/9 animals) mesenteric arteries (*P≤0.05, paired t test). C, Bar graph of Ca2+ spark frequency (sparks/μm s) before and after the application of Ni2+. CaV3.2 suppression significantly attenuated spark frequency in C57BL/6 (n=9, 454 line scans in total, *P≤0.05) but not CaV3.2−/− (n=9, 456 line scans in total) arteries. D, Bar graphs of averaged Ca2+ spark amplitude (F/F0); full width at half maximum (FWHM, in μm); full duration at half maximum (FDHM, in ms); and time constants of decay (Tau, in ms) before and after Ni2+.
CaV3.2 Activity Restrains Myogenic Constriction by Altering BKCa Feedback
The application of Ni2+ (50 μmol/L) onto C57BL/6 arteries evoked vasoconstriction at intravascular pressure values between 20 and 60 mm Hg, and this vasomotor effect diminished at higher pressures; CaV3.2−/− arteries lacked a similar response (Figure 5A). The percentage of myogenic tone in wild-type C57BL/6 arteries increased after the application of Ni2+ (at 60 mm Hg: control 20%±4% versus Ni2+ 25%±1%), but was not altered in CaV3.2−/− arteries (control 30%±5% versus Ni2+ 28%±8%; Figure 5B). Coinciding with vasomotor data, membrane potential measurements showed that Ni2+ only depolarized C57BL/6 but not CaV3.2−/− pressurized arteries (60 mm Hg; Figure 5C). In C57BL/6 arteries, the BKCa blocker (paxilline, 1 μmol/L) evoked vasoconstriction and enhanced myogenic tone similar to that of Ni2+ (Figure 5D), an observation consistent with a common signaling axis between CaV3.2 and BKCa channels. When Ni2+ and paxilline were sequentially added to the same wild-type artery, Ni2+ evoked vasoconstriction, whereas subsequent paxilline had no additional effect. Similar experiments using CaV3.2−/− arteries demonstrated a lack of vasomotor responses to Ni2+ but preserved responsiveness to paxilline (Figure 5E).
Figure 5.
CaV3.2 suppression constricts/depolarizes wild-type arteries via altering the vasomotor BKCa response. A, Averaged data demonstrate that the application of 50 μmol/L Ni2+ constricted pressurized C57BL/6 arteries (n=9) at pressure values 20 to 60 mm Hg with no significant effect on CaV3.2−/− arteries (n=4). B, CaV3.2 blockade enhanced percentage myogenic tone in wild-type C57BL/6 arteries, but displayed no detectable change in CaV3.2−/− arteries. C, Representative traces and summary data of membrane potential (mV) in C57BL/6 and CaV3.2−/− pressurized arteries (60 mm Hg) before and after the application of Ni2+ (n=6 each; paired t test; *P≤0.05). D, Application of Ni2+ (50 μmol/L, n=9, as in A) or paxilline (1 μmol/L, n=4) on pressurized C57BL/6 arteries elicited similar constrictor responses. Percentage myogenic tone increased in response to BKCa blockade in C57BL/6 arteries (n=4). E, Changes in arterial diameter in response to Ni2+ followed by paxilline in pressurized C57BL/6 or CaV3.2−/− arteries (at 60 mm Hg, n=5–7, *P≤0.05).
CaV3.2−/− Mice Display Normal Blood Pressure Responses
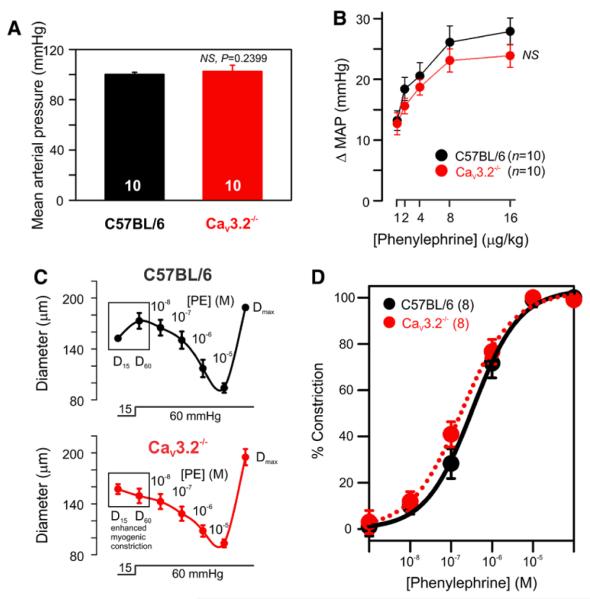
Pharmacological and genetic approaches suggested that CaV3.2 channel counterbalances myogenic constriction (Figure 1 and 5) and could as such influence blood pressure regulation. To explore this possibility, we catheterized carotid arteries of C57BL/6 and CaV3.2−/− mice to monitor blood pressure under resting conditions and in response to a vasopressor challenge. As depicted (Figure 6A), basal mean arterial pressure was similar in wild-type and knockout mice (C57BL/6, 100±2 mm Hg; CaV3.2−/−, 103±5 mm Hg), a finding consistent with earlier reports in conscious animals.17,18 Given that CaV3.2 channels seem to be involved in a feedback mechanism, we next assessed whether this conductance can alter mean arterial pressure responsiveness to a vasopressor challenge. The intravenous administration of phenylephrine (α1-adrenoceptor agonist, 1–16 μg/kg body weight) evoked dose-dependent rises in mean arterial pressure, and these transient responses were similar among the 2 groups (Figure 6B). Analogous to in vivo experiments, phenylephrine (0.01–10 μmol/L)-induced vasoconstriction was similar in C57BL/6 and CaV3.2−/− mesenteric arteries (Figure 6C and 6D).
Figure 6.
CaV3.2−/− mice display normal resting blood pressure and responsiveness to phenylephrine. A, Resting mean arterial pressure of anesthetized C57BL/6 or CaV3.2−/− mice (n=10 each). B, Changes in mean arterial pressure (ΔMAP, mm Hg) in response to intravenous phenylephrine (1–16 μg/kg body weight) in C57BL/6 and CaV3.2−/− mice (n=10 each). C, Diameter responses of C57BL/6 and CaV3.2−/− mesenteric arteries to increased pressure from 15 to 60 mm Hg followed by cumulative application of phenylephrine (PE; 0.01–10 μmol/L). Maximum diameters (Dmax) were finally achieved by perfusion of Ca2+ free PSS. D, Phenylephrine concentration response curve shows no significant differences between wild-type C57BL/6 and CaV3.2−/− arteries (n=8 each).
Discussion
This study used wild-type and CaV3.2 knockout mice to examine the purported contribution of CaV3.2 channels to a negative feedback response that counterbalances arterial tone development. Using mesenteric arteries, functional experiments illustrated that the genetic ablation or pharmacological suppression of CaV3.2 channel selectively enhanced myogenic constriction. Subsequent electrophysiological recordings revealed that CaV3.2 channel modulates downstream BKCa-mediated STOCs. Ca2+ imaging further demonstrated that Ca2+ spark generation is an intermediary step in the CaV3.2-BKCa functional axis. Finally, although CaV3.2 moderated myogenic tone, this regulatory mechanism did not influence resting blood pressure or vasopressor-induced responses. In summary, findings from this study establish a model by which CaV3.2 channel restrains myogenic constriction by driving a process where Ca2+ influx triggers Ca2+ sparks and downstream activation of BKCa currents (Figure III in the online-only Data Supplement).
Resistance arteries control tissue perfusion and respond to a range of vasoactive stimuli.1–5 The integral ability of resistance arteries to respond to perturbations in arterial pressure, known as the myogenic response,6 is intrinsic to vascular smooth muscle and plays an essential role in maintaining blood flow and capillary pressure to tissues, such as the brain and the heart.19 This response is mechanistically linked to a rise in cytosolic [Ca2+]i driven by a depolarization that activates L-type CaV1.2 channels. The traditional perspective that CaV1.2 is the only voltage-gated Ca2+ channel of functional significance3 has shifted with the identification of 2 T-type Ca2+ channels,7,8 the first being CaV3.1, which modestly facilitates myogenic tone at hyperpolarized voltages.9 In contrast, CaV3.2 channels have been reported to drive a paradoxical feedback response that limits arterial constriction.10,12 The feedback response begins with Ca2+ influx through CaV3.2 channels triggering ryanodine receptors to initiate Ca2+ sparks. These discrete sarcoplasmic reticulum–driven events in turn activate BKCa channels to hyperpolarize and dilate resistance arteries.10 Although an intriguing concept, data interpretation is singularly dependent on the presumed selectivity of Ni2+ as a pharmacological CaV3.2 blocker.11 Current knowledge is also restricted to the cerebral circulation, and experiments have not extended to other vascular beds essential to blood pressure regulation.
One means to better probe the functional properties of arterial CaV3.2 channel is to use an animal model in which the channel has been genetically ablated.12 In this regard, we performed pressure myography on arteries from CaV3.2 knockout (CaV3.2−/−) mice to study the vascular phenotype. Consistent with the view that CaV3.2 channel mediates feedback vasodilation, we observed enhanced myogenic constriction in mesenteric arteries from CaV3.2−/− mice. This finding was somewhat akin to the functional observations in coronary arteries12 and the enhancement of rat cerebral arterial myogenic tone noted in the presence of Ni2+.10 Our present findings overcome concerns raised by past studies that off-target effects of Ni2+ could theoretically have accounted for the vasomotor responses.20–22 In particular, we were unable to elicit Ni2+-sensitive current in CaV3.2−/− arterial myocytes, and Ni2+ also failed to constrict/depolarize arteries from knockout mice.
The unexpected ability of CaV3.2 channel to mediate a negative feedback response has been previously tied to downstream modulation of BKCa channels through intermediary activation of ryanodine receptors.10 In arterial smooth muscle, BKCa is ubiquitously expressed and known to moderate membrane depolarization and arterial constriction.23 To activate arterial BKCa, vasoactive stimuli must induce depolarization and elicit a discrete micromolar rise in [Ca2+]i in the subsarcolemma taking the form of a Ca2+ spark.24 Here, we present multiple lines of evidence implicating BKCa as the final downstream effector of CaV3.2 channel. First, electrophysiology revealed that BKCa-mediated STOCs were sensitive to Ni2+ in wild-type but not in CaV3.2−/− arterial myocytes. Second, pressure myography illustrated that adding Ni2+ or paxilline (BKCa inhibitor) to the superfusate comparably augmented myogenic tone. Intriguingly, applying paxilline to wild-type arteries pre-treated with Ni2+ had no additive effect, consistent with CaV3.2 and BKCa channels being linked through a common sequential pathway.
It has been long established that RyR activation is responsible for the initiation of BKCa-mediated STOCs in arterial smooth muscle,16,24 and this recognized relationship led us to examine the nature of Ca2+ spark generation in mouse mesenteric arteries. Indeed, inhibitors of RyR (eg, ryanodine) has been shown to suppress the generation of STOCs in vascular smooth muscle irrespective of their origin.24 Here and in consistency with CaV3.2 channel driving Ca2+ sparks and subsequently STOC generation, we used Ca2+ imaging and line scan analysis and observed that spark frequency decreased in wild-type arteries when CaV3.2 channels were inhibited with Ni2+. We also found that firing of Ca2+ sparks was unaffected by this divalent cation in CaV3.2−/− arteries and that basal Ca2+ spark frequency was lower than those of wild-type arteries. These findings align well with recent work from the cerebral circulation where a variety of functional, structural, electro-physiological, and computational observations draw a critical relationship between CaV3.2 and RyR and then BKCa (Figure III in the online-only Data Supplement).10 Intriguingly, the preceding work is distinct from neuronal studies where T-type conductances have been suggested to directly activate Ca2+-activated K+ channels independent of RyR.25–28
With CaV3.2 channels playing an intimate role in limiting myogenic constriction, it is logical to argue that the loss of this conductance would alter peripheral resistance and systemic blood pressure regulation. We tested this supposition by catheterizing anesthetized mice and assessing blood pressure at rest and in response to a vasopressor challenge. Resting blood pressure was similar among wild-type and knockout mice, a finding consistent with earlier reports which used different monitoring approaches (eg, catheterization, tail cuff) in conscious animals over longer time spans (days to weeks).17,18 Given the presumed role of CaV3.2 in feedback (rather than active vasodilation), we challenged blood pressure regulation using intravenous phenylephrine. This multidose challenge evoked comparable rises in blood pressure among the 2 animal groups; likewise, mesenteric arteries isolated from wild-type and CaV3.2−/− mice constricted similarly to this α1-adrenergic agonist. These in vivo and in vitro results interestingly seem to rule out a direct modulatory role for vascular CaV3.2 channels in agonist-induced vasoconstriction.
It is not clear at present why blood pressure responses fail to change. Perhaps, the loss of smooth muscle feedback in CaV3.2−/− arteries is compensated by enhanced dilatory feedback from the endothelium. Other functional compensation may have occurred in this global knockout independent of vascular smooth muscle29; in this context, establishing a smooth muscle–specific CaV3.2 knockout model would be valuable. It should also be recognized that for the proposed CaV3.2/RyR/BKCa feedback mechanism to effectively drive a blood pressure response, it should be ideally present in a full range of vascular beds. Recent work documenting heterogeneity of BKCa activity,30 because of differences in channel activity and Ca2+ spark generation, challenges this key assumption. Further investigation is warranted to address this intriguing anomaly.
In conclusion, this study showed that arterial CaV3.2 channels retain a unique ability to counterbalance myogenic constriction. This negative feedback response entails a modulatory paradigm in which Ca2+ flux through CaV3.2 channel triggers Ca2+ spark generation and then activates BKCa channels to hyperpolarize and relax arteries (Figure III in the online-only Data Supplement). This novel functional axis, recently described in the human cerebral circulation,31 challenges the traditional view that voltage-gated Ca2+ channels solely facilitate arterial tone development.
Supplementary Material
Significance.
Vascular smooth muscle cells express T-type Ca2+ channels along with L-type channels. Although the latter have been long implicated in arterial excitation–contraction coupling, studies have only recently begun to assess the role of T-type channels. Using an animal model in which CaV3.2, a T-type channel, was genetically deleted, we tested its role in arterial tone development. We demonstrate that resistance arteries from knockout animals paradoxically display enhanced responsiveness to arterial pressure. This enhancement was mechanistically attributed to the ability of CaV3.2 to modulate downstream K+ channels, which hyperpolarize and relax arteries. This novel data challenge the traditional view that voltage-gated Ca2+ channels are singularly involved in the genesis of arterial constriction.
Acknowledgments
Sources of Funding
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR, MOP-69088 to D.G. Welsh). D.G. Welsh is the recipient of the Rorabeck Chair in Neuroscience and Vascular Biology (University of Western Ontario). O.F. Harraz is a Vanier Scholar and was supported by salary studentships from Alberta Innovates Health Solutions (AIHS) and Achievers in Medical Sciences. A. Zechariah was supported by AIHS and Eyes High postdoctoral fellowships. Imaging was performed in the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core that is supported by the National Science Foundation (MRI-DBI 0923559 to S.M. Wilson) and the Loma Linda University School of Medicine. The work was also supported in part by USPHS (United States Public Health Service) Grant (HD069746 to S.M. Wilson).
Nonstandard Abbreviations and Acronyms
- BKCa
large-conductance Ca2+-activated K+ channel
- CaV3.2−/−
CaV3.2-deficient (knockout) mice
- RyR
ryanodine receptor
- STOC
spontaneous transient outward K+ current
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.115.305736/-/DC1.
References
- 1.Brayden JE, Bevan JA. Neurogenic muscarinic vasodilation in the cat. An example of endothelial cell-independent cholinergic relaxation. Circ Res. 1985;56:205–211. doi: 10.1161/01.res.56.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Roldan JL, Bevan JA. Flow-induced constriction and dilation of cerebral resistance arteries. Circ Res. 1990;66:1445–1448. doi: 10.1161/01.res.66.5.1445. [DOI] [PubMed] [Google Scholar]
- 3.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Si ML, Lee TJ. Alpha7-nicotinic acetylcholine receptors on cerebral perivascular sympathetic nerves mediate choline-induced nitrergic neurogenic vasodilation. Circ Res. 2002;91:62–69. doi: 10.1161/01.res.0000024417.79275.23. [DOI] [PubMed] [Google Scholar]
- 5.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 6.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab. 2010;30:1226–1239. doi: 10.1038/jcbfm.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development. Am J Physiol Heart Circ Physiol. 2013;304:H58–H71. doi: 10.1152/ajpheart.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein-Rathlou NH, Jensen LJ. Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated CaV 3.1 T-type Ca(2+) channel. Acta Physiol (Oxf) 2013;207:709–720. doi: 10.1111/apha.12066. [DOI] [PubMed] [Google Scholar]
- 10.Harraz OF, Abd El, Rahman RR, Bigdely-Shamloo K, et al. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res. 2014;115:650–661. doi: 10.1161/CIRCRESAHA.114.304056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 13.Zamponi GW, Bourinet E, Snutch TP. Nickel block of a family of neuronal calcium channels: subtype- and subunit-dependent action at multiple sites. J Membr Biol. 1996;151:77–90. doi: 10.1007/s002329900059. [DOI] [PubMed] [Google Scholar]
- 14.Harraz OF, Welsh DG. Protein kinase A regulation of T-type Ca2+ channels in rat cerebral arterial smooth muscle. J Cell Sci. 2013;126(Pt 13):2944–2954. doi: 10.1242/jcs.128363. [DOI] [PubMed] [Google Scholar]
- 15.Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res. 2013;98:449–457. doi: 10.1093/cvr/cvt043. [DOI] [PubMed] [Google Scholar]
- 16.Pérez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res. 2009;104:522–530. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- 18.Thuesen AD, Andersen H, Cardel M, Toft A, Walter S, Marcussen N, Jensen BL, Bie P, Hansen PB. Differential effect of T-type voltage-gated Ca2+ channel disruption on renal plasma flow and glomerular filtration rate in vivo. Am J Physiol Renal Physiol. 2014;307:F445–F452. doi: 10.1152/ajprenal.00016.2014. [DOI] [PubMed] [Google Scholar]
- 19.Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys. 2011;510:160–173. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Cheng YM, Fedida D, Kehl SJ. Kinetic analysis of the effects of H+ or Ni2+ on Kv1.5 current shows that both ions enhance slow inactivation and induce resting inactivation. J Physiol. 2010;588(Pt 16):3011–3030. doi: 10.1113/jphysiol.2010.191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ene FA, Kalmbach A, Kandler K. Metabotropic glutamate receptors in the lateral superior olive activate TRP-like channels: age- and experience-dependent regulation. J Neurophysiol. 2007;97:3365–3375. doi: 10.1152/jn.00686.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luebbert M, Radtke D, Wodarski R, Damann N, Hatt H, Wetzel CH. Direct activation of transient receptor potential V1 by nickel ions. Pflugers Arch. 2010;459:737–750. doi: 10.1007/s00424-009-0782-8. [DOI] [PubMed] [Google Scholar]
- 23.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 24.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 25.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. doi: 20026345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cueni L, Canepari M, Luján R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Lüthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 27.Engbers JD, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, McKay BE, Kruskic M, Zamponi GW, Turner RW. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2012;109:2601–2606. doi: 10.1073/pnas.1115024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One. 2013;8:e61844. doi: 10.1371/journal.pone.0061844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuru H, Tanimitsu N, Hirai T. Role of perivascular sympathetic nerves and regional differences in the features of sympathetic innervation of the vascular system. Jpn J Pharmacol. 2002;88:9–13. doi: 10.1254/jjp.88.9. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol. 2009;587(Pt 12):3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harraz OF, Visser F, Brett SE, Goldman D, Zechariah A, Hashad AM, Menon BK, Watson T, Starreveld Y, Welsh DG. CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries. J Gen Physiol. 2015;145:405–418. doi: 10.1085/jgp.201511361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.