Abstract
Background:
The number of children worldwide requiring palliative care services is increasing due to advances in medical care and technology. The use of outcome measures is important to improve the quality and effectiveness of care.
Aim:
To systematically identify health-related quality-of-life outcome measures that could be used in paediatric palliative care and examine their feasibility of use and psychometric properties.
Design:
A systematic literature review and analysis of psychometric properties.
Data sources:
PsychInfo, Medline and EMBASE were searched from 1 January 1990 to 10 December 2014. Hand searches of the reference list of included studies and relevant reviews were also performed.
Results:
From 3460 articles, 125 papers were selected for full-text assessment. A total of 41 articles met the eligibility criteria and examined the psychometric properties of 22 health-related quality-of-life measures. Evidence was limited as at least half of the information on psychometric properties per instrument was missing. Measurement error was not analysed in any of the included articles and responsiveness was only analysed in one study. The methodological quality of included studies varied greatly.
Conclusion:
There is currently no ‘ideal’ outcome assessment measure for use in paediatric palliative care. The domains of generic health-related quality-of-life measures are not relevant to all children receiving palliative care and some domains within disease-specific measures are only relevant for that specific population. Potential solutions include adapting an existing measure or developing more individualized patient-centred outcome and experience measures. Either way, it is important to continue work on outcome measurement in this field.
Keywords: Outcome assessment (healthcare), psychometrics, palliative care, child, quality of life, paediatrics
What is already known about the topic?
The use of outcome assessment tools is important to measure quality and effectiveness of care.
The population of children requiring paediatric palliative care services is diverse.
There are no outcome assessment tools validated specifically for use within paediatric palliative care.
What this paper adds?
This is the first review to systematically identify existing health-related quality-of-life outcome measures for use in paediatric palliative care.
The paper finds that there is currently no ‘ideal’ outcome assessment tool for use in paediatric palliative care.
Implications for practice, theory or policy
As with adults, both outcome and experience measures are important to achieve and maintain best care for children and young people.
Understanding a child or young person’s own goals for care and treatment – alongside more standardized outcomes – is likely to be most valuable.
Background
Palliative care for children begins at diagnosis and encompasses children with a variety of life-limiting and life-threatening conditions. Life-limiting conditions are diseases where there is no reasonable hope of cure and that will ultimately be fatal.1 Life-threatening conditions are those where curative treatment may be possible but can fail, for example, cancer. Worldwide, more children are living longer with such conditions due to advances in medical care. Paediatric palliative care (PPC) is about helping children and their families deal with their medical condition, while enabling them to live life to the fullest.2 Palliative care for children and young people (CYP) is an active and total approach to care, and begins at the point of diagnosis, throughout the child’s life, death and beyond.3 The scope of PPC is broad and PPC services care for CYP with a wide variety of illnesses, many of which are extremely rare.3,4 Children can be receiving care from these services for many years and therefore it is imperative to ensure that they are supported to live life to their fullest potential.
Health-related quality of life (HRQOL) has been described as a subjective, multidimensional and dynamic construct that comprises physical, psychological and social functioning.5 HRQOL measurement instruments must consist of physical, social and mental health dimensions as delineated by the World Health Organization (WHO).6 Given that one of the goals of PPC is to improve HRQOL, service providers, researchers, fundraisers and policy makers will want to measure HRQOL and determine the effectiveness of services in achieving this.
There are no paediatric HRQOL measures that have been successfully validated for use within PPC. One study did attempt to validate the well-used Pediatric Quality of Life 4.0 measure in children with a variety of life-limiting conditions. However, the study found that the instrument did not have valid psychometric properties for use within this population.7 Therefore, within PPC, two possibilities exist: devising a completely new HRQOL instrument, or revising and validating an existing one. A review of existing HRQOL measures is essential prior to deciding which course of action to take.
The aim of this systematic literature review is to examine the measurement properties of existing HRQOL instruments for use in those up to 18 years old. It will also assess the feasibility of the measures being used in the CYP palliative care population in terms of completion time, response options, recall period, format, domains and whether the measure is parent, professional or self-completed.
Methods
This systematic literature review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.8
Identification of studies
PsychInfo, Medline and EMBASE were searched from 1 January 1990 to 10 December 2014. Experts in the field were asked to suggest any further measures. The search terms used included keywords such as child, adolescent and teenager in combination with terms used to find studies on measurement properties of HRQOL measures.9,10 Language restriction to the English language was applied due to practical constraints within the research team. Reference lists of included articles were also searched for further publications. Box 1 shows the search strategy used.
Box 1.
Search strategy.
| 1. patient reported outcomes.ti,ab.(6383) 2. quality of life.ti.ab.(266914) 3. health status.ti,ab.(175146) 4. global health.ti.ab.(53851) 5. health related quality of life.ti.ab.(53363) 6. outcome measurement.ti.ab.(26871) 7. 1 or 2 or 3 or 4 or 5 or 6(491494) 8. classical test theory.ti.ab.(1460) 9. validity.ti.ab(205095) 10. reliability.ti.ab.(162542) 11. content validity.ti.ab.(16710) 12. confirmatory factor analysis.ti.ab.(17892) 13. exploratory factor analysis.ti.ab.(13537) 14. internal consistency.ti.ab.(39236) 15. test-retest.ti.ab.(30481) 16. psychometr*.ti.ab.(66358) 17. known group.ti.ab.(108113) |
18. Rasch.ti.ab.(5442) 19. DIF.ti.ab.(2784) 20. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (998874) 21. child*.ti.ab.(1286933) 22. neonat*.ti.ab.(185516) 23. adolescent*.ti.ab.(308550) 24. pediatric*.ti.ab.(213646) 25. paediatric*.ti.ab.(48497) 26. 23 or 24 or 25 or 26 or 27 (1715918) 27. 7 and 22 and 28 (7138) 28. 29 limited to Human and English Language and Age Groups All Child Age 0–18 years (4849) 29. Duplicates removed 1398 30. Total 3451 |
Inclusion/exclusion criteria
A study was included if it met the following inclusion criteria:
Full-text article;
Written in the English language;
Examining one or more measurement properties of an instrument that measured physical, mental and social aspects of HRQOL as delineated by WHO;6
The study population were under 18 years old;
The measure was generic or disease specific;
Disease-specific instruments had to assess HRQOL in an illness considered to be life-limiting or life-threatening;3,4
Studies of generic measures were included only if some or all of the population in the study had a life-limiting illness;
Included measures could be completed by the CYP, parent or clinician.
Study selection
The results of the search were thoroughly checked and full manuscripts of all studies whose title/abstract seemed to meet the selection criteria were retrieved. Independent reviewers (L.H.C. and G.L.) examined these full-text articles and made the final decision as to whether they were included.
Data extraction
The methodological quality of included studies was rated using the COnsensus based Standards for the selection of health Measurement INstruments (COSMIN) checklist.11 This checklist contains nine boxes, each dealing with one measurement property. There are 5–18 items per box (98 items in total) that can be used to assess whether a study on a specific measurement property meets the standard for good methodological quality. The checklist evaluates the following measurement properties: internal consistency, reliability, measurement error, content validity, construct validity (structural validity, hypothesis testing, cross-cultural validity), criterion validity and responsiveness. There are three additional boxes: one to assess the methodological quality of studies on interpretability, one to assess the generalizability of results and one that includes extra methodological standards for studies that use item response theory (IRT; 12 boxes in total). Each item is scored on a 4-point rating scale (poor, fair, good and excellent).12 An overall score for the methodological quality of a study is determined for each measurement property separately, by taking the lowest rating of any item in a box (worst score counts).11
Box 2 gives definitions of these measurement properties.
Box 2.
Definitions of measurement properties.
| Reliability |
| This term is used twice – as a term for the domain and also as the term for the measurement property.13,14 Defined as the degree to which measurement is free from measurement error. Includes internal consistency, reliability (test–retest, inter-rater, intra-rater) and measurement error.12,13,15 |
| Validity |
| The degree to which a tool measures the construct it claims to measure. Includes content validity, criterion validity and construct validity.13,15 |
| Responsiveness |
| The ability of an instrument to detect change over time in the construct being measured.16 |
| Interpretability |
| The degree to which qualitative meaning can be assigned to the quantitative scores or change in scores of an instrument. This is an important property of an instrument but not a measurement property.13 |
Synthesis of results
To summarize the evidence of measurement properties of each included instrument, the results were combined. The number and methodological quality of the studies were taken into account, along with the consistency of results. A method similar to that proposed by the Cochrane Back Review Group was used (Table 1).17
Table 1.
Levels of evidence for overall quality of measurement property.
| Level | Rating | Criteria |
|---|---|---|
| Strong | +++ or −−− | Consistent findings in multiple studies of good methodological quality OR in one study of excellent methodological quality |
| Moderate | ++ or −− | Consistent findings in multiple studies of fair methodological quality OR in one study of good methodological quality |
| Limited | + or − | One study of fair methodological quality |
| Conflicting | ± | Conflicting findings |
| Unknown | ? | Only studies of poor methodological quality |
Source: Tulder et al.17
+: positive results; −: negative results.
The overall rating of each measurement property is ‘positive’, ‘negative’ or ‘indeterminate’, accompanied by levels of evidence. These criteria were originally meant for systematic reviews of clinical trials but have been used in reviews on measurement properties.12 To assess whether results of measurement properties were positive, negative or indeterminate, criteria based on Terwee et al.18 were used (Table 2).
Table 2.
Quality criteria for measurement properties.
| Property | Rating | Quality criteria |
|---|---|---|
| Reliability | ||
| Internal consistency | + | (Sub)scale unidimensional AND Cronbach’s alpha(s) ⩾0.70 |
| ? | Dimensionality not known OR Cronbach’s alpha not determined | |
| − | (Sub)scale not unidimensional OR Cronbach’s alpha < 0.7 | |
| Measurement error | + | MIC > SDC OR MIC outside the LOA |
| ? | MIC not defined | |
| − | MIC ⩽ SDC OR MIC equals or inside LOA | |
| Reliability | + | ICC/weighted kappa ⩾ 0.70 OR Pearson’s r ⩾ 0.80 |
| ? | Neither ICC/weighted kappa nor Pearson’s r determined | |
| − | ICC/weighted kappa < 0.70 OR Pearson’s r < 0.80 | |
| Validity | ||
| Content validity | + | The target population considers all items in a questionnaire to be relevant AND considers the questionnaire to be complete |
| ? | No target population involvement | |
| − | The target population considers items in a questionnaire to be irrelevant OR considers the questionnaire to be incomplete | |
| Construct validity | ||
| Structural validity | + | Factors should explain at least 50% of the variance |
| ? | Explained variance not mentioned | |
| − | Factors explain <50% of the variance | |
| Hypothesis testing | + | (Correlation with an instrument measuring the same construct ⩾0.50 OR at least 75% of the results are in accordance with the hypotheses) AND correlation with related constructs is higher than with unrelated constructs |
| ? | Solely correlations determined with unrelated constructs | |
| − | Correlation with an instrument measuring the same construct <0.50 OR < 75% of the results are in accordance with the hypotheses OR correlation with related constructs is lower than with unrelated constructs | |
| Responsiveness | ||
| Responsiveness | + | (Correlation with an instrument measuring the same construct ⩾0.50 OR at least 75% of the results are in accordance with the hypotheses OR AUC ⩾0.70) AND correlation with related constructs is higher than with unrelated constructs |
| ? | Solely correlations determined with unrelated constructs | |
| − | Correlation with an instrument measuring the same construct <0.50 OR <75% of the results are in accordance with the hypotheses OR AUC <0.70 OR correlation with related constructs is lower than with unrelated constructs | |
Source: Terwee et al.18
MIC: minimal important change; SDC: smallest detectable change; LOA: limits of agreement; ICC: intraclass correlation coefficient; AUC: area under the curve; +: positive rating; ?: indeterminate; −: negative rating.
This assessment of measurement properties was then looked at alongside the feasibility of each measure being used in PPC in terms of completion time, response options, recall period, format and domains.
Research ethics committee/institutional review board (IRB) approvals were not required as this was a systematic review of pre-existing evidence.
Results
Paper selection
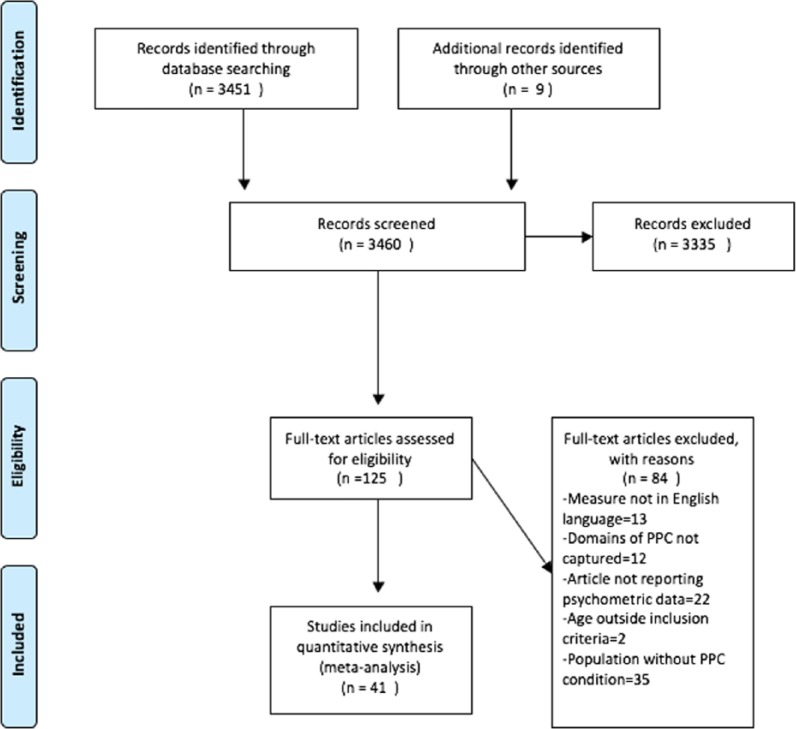
A total of 3451 articles were found using the search strategy and a further 9 were found via reference searching. A total of 125 of these were selected for full-text review based on title and abstract. A total of 41 were selected to be included in the review. Figure 1 shows a flow chart of the article selection process, and Table 3 shows the general characteristics of included studies.
Figure 1.
PRISMA flow chart.
Source: From Moher et al.8
Table 3.
Summary of included studies.
| Measure | Study | Report | Age (years) | No. of domains | No. of items | Recall period | Completion time (min) | Study population | Generic/disease specific | Country of origin |
|---|---|---|---|---|---|---|---|---|---|---|
| Child Health Questionnaire – Parent Form 50 | Drotar et al.19
Ferro et al.20 McCullough et al.21 Wake et al.22 |
Parent | 5–18 | 11 (physical functioning, role/social-physical, general health perceptions, bodily pain/discomfort, parental impact time, role/social-emotional/behavioural, self-esteem, mental health, general behaviour, family activities, family cohesion, change in health) | 50 | 4 weeks | Not reported | Chronic illness Epilepsy Cerebral palsy Cerebral palsy |
Generic | USA |
| ConQOL | Macran et al.23 | Self | 8–11 12–16 |
3 (symptoms, activities, relationships) 4 (symptoms, activities, relationships, control and coping) |
29 35 |
1 week | Not reported | Congenital heart disease | Disease-specific congenital heart disease | UK |
| CP-QOL | Waters et al.24 | Self Parent |
9–12 4–12 |
5 (social well-being and acceptance, functioning, participation and physical health, emotional well-being, pain and impact of disability) 7 (social well-being and acceptance, functioning, participation and physical health, emotional well-being, access to services, pain and impact of disability, family health) |
52 66 |
15–25 | Cerebral palsy | Disease-specific cerebral palsy | Australia | |
| CP-QOL Teen | Davis et al.25 | Self Parent |
13–18 | 7 (well-being and participation, communication and physical health, school well-being, social well-being, access to services, family health, feelings about functioning) | 70 87 |
Not reported | Cerebral palsy | Disease-specific cerebral palsy | Australia | |
| DISABKIDS | Petersen et al.26
Ravens-Sieberer et al.27/Schmidt et al.28(b) Simeoni et al.29 |
Self Parent |
4–16 | 6 (independence, emotion, social exclusion, social inclusion, physical, medication) | 37 | Not reported | Chronic illness | Condition generic | Multiple – Europe | |
| DISABKIDS Smiley (TAKE 6 scale) | Chaplin et al.30 | Assisted report Parent |
4–7 | 1 (HRQOL) | 6 | 2 | Chronic illness | Condition generic | Multiple – Europe | |
| Glasgow Epilepsy Outcome Scale for Young Persons (GEOSYP) | Townshend et al.31 | Self | 10–18 | 9 (peer acceptance, school/work, development of autonomy, future focus, epilepsy as part of me, medication issues, seizures, knowledge about epilepsy, sense of uncertainty) | 50 | 4 weeks | Not reported | Epilepsy | Disease-specific epilepsy | UK |
| Health-related quality of life in children with epilepsy | Ronen et al.32 | Self Parent |
8–15 years | 5 (interpersonal/social, future worries, present worries, intrapersonal/emotional, secrecy) | 25 | Current moment | Not reported | Epilepsy | Disease-specific epilepsy | Canada |
| KIDSCREEN-52 | Erhart et al.33 | Self Parent |
8–18 | 10 (physical well-being, psychological well-being, moods and emotions, self-perception, autonomy, parent relation and home life, social support and peers, school environment, social acceptance (bullying), financial resources) | 52 | 1 week | 15–20 | Mixed | Generic | Multiple – Europe wide |
| Memorial Symptom Assessment Scale (MSAS) 7–12 MSAS 10–18 |
Collins et al.34,35 | Self Self |
7–12 10–18 |
1 (symptom experience) 3 (physical, psychological, global distress) |
8 30 |
2 days 1 week |
6 11 |
Cancer Cancer |
Disease-specific cancer Disease-specific cancer |
UK Australia USA |
| Pediatric Cancer Quality of Life Inventory (PCQL-32) | Varni et al.36
Seid et al.37 |
Self/parent | 8–18 | 5 (disease/treatment, physical functioning, psychological functioning, social functioning, cognitive functioning) | 32 | 1 month | Not reported | Cancer | Disease-specific cancer | USA |
| Pediatric Cardiac Quality of Life Inventory | Marino et al.38,39
Wray et al.40 |
Self Parent Self Parent |
8–12 8–12 13–18 13–18 |
2 (disease impact, psychosocial impact) 2 (as above) 2 (as above) 2 (as above) |
23 23 29 29 |
Current moment | 10 | Cardiac disease | Disease-specific cardiac disease | USA UK testing |
| Pediatric Oncology Quality of Life Scale | Goodwin et al.41 | Parent | 0–18 | 3 (physical function and role restriction, emotional distress, reaction to current treatment) | 21 | 2 weeks | Not reported | Cancer | Disease-specific cancer | USA |
| PedsQL™ 4.0 Generic Core Scales | Varni et al.42–46
Amin et al.47 Huang et al.7 Varni et al.42,43,48 |
Self Parent |
5–18 2–18 |
5 (physical health, psychosocial health, emotional functioning, social functioning, school functioning) 5 (as child version) |
23 23 |
1 month 1 month |
4 <5 |
Cancer Mixed Mixed Mixed Mixed Cancer Palliative Cancer Mixed Mixed |
Generic | USA |
| PedsQL™ Brain Tumour Module | Palmer et al.49 | Self Parent |
5–18 2–18 |
6 (cognitive, pain, movement and balance, procedural anxiety, nausea, worry) 6 (as child version) |
24 24 |
7 days 7 days |
Not reported | Brain tumours | Disease-specific brain tumour | USA |
| PedsQL™ Cancer Module | Varni et al.50 | Self Parent |
5–18 2–18 |
8 (pain, nausea, procedural anxiety, treatment anxiety, worry, cognitive problems, perceived physical appearance, communication) 8 (as child version) |
27 27 |
7 days 7 days |
5 5 |
Cancer | Disease-specific cancer | USA |
| PedQL™ 3.0 Cardiac Module | Uzark et al.51 | Self Parent |
8–18 8–18 |
5 (symptoms, physical appearance, treatment anxiety, cognitive problems, communication) 5 (as child version) |
22 22 |
7 days | 10–15 10–15 |
Cardiac disease | Disease-specific cardiac disease | USA |
| PedsQL™ Cerebral Palsy Module | Varni et al.52 | Self Parent |
5–18 2–18 |
7 (daily activities, school activities, movement and balance, pain and hurt, fatigue, eating, speech and communication) 7 (as child version) |
35 35 |
1 month | Not reported | Cerebral palsy | Disease-specific cerebral palsy | USA |
| PedsQL™ Neuromuscular module | Iannaccone et al.53
Davis et al.54 Dunaway et al.55 |
Self Parent |
5–18 2–18 |
3 (about my neuromuscular disease, communication, family resources) 3 |
25 25 |
1 month | Not reported | DMD SMA SMA |
Disease-specific neuromuscular disease | USA |
| Quality of Life in Childhood Epilepsy Questionnaire | Sabaz et al.56,57 | Parent | 4–18 years | 16 (physical restrictions, energy/fatigue, depression, anxiety, control/helplessness, self-esteem, attention/concentration, memory, language, other cognitive processes, social interactions, stigma, behaviour, general health, quality of life) | 73 | 4 weeks | Not reported | Refractory epilepsy Epilepsy |
Disease-specific epilepsy | Australia USA version |
| Royal Marsden Hospital Paediatric Oncology Quality of Life Questionnaire | Watson et al.58 | Parent | 2–19 | 8 (functional status, global health, physical symptoms, emotional status, social functioning, cognitive functioning, behavioural problems, global quality of life) | 78 | 1 month | 15 | Cancer | Disease-specific cancer | UK Sweden |
CP-QOL: cerebral palsy quality of life; HRQOL: health-related quality of life; DMD: Duchenne muscular dystrophy; SMA: spinal muscular atrophy.
Mixture of healthy, chronically ill and acutely ill children.
Two papers reporting same data.
Summary of results
The 41 articles included evaluated 22 HRQOL measures for use with children aged 0–18 years. Two papers discussed the results from the same study so were only analysed once.19,20 All included measures were originally developed to be completed in paper format. Five of the included measures were generic and 17 were disease specific. Of the disease-specific measures, three are for use with children with cardiac disease, three for cerebral palsy, six for cancer, one for brain tumours, three for epilepsy and one for neuromuscular disease. Four measures are child completed, four parent completed, thirteen have both parent and child forms and one measure had both child and clinician forms. Completion time ranged from 2 to 25 min, with the number of items ranging from 6 to 87. Recall time ranged from the current moment to 1 month. Table 3 shows a summary of the included studies, where data are missing it is because they are not available.
None of the studies included analysed measurement error or cross-cultural validity. One study reported on responsiveness and one on criterion validity.31 The COSMIN panel define criterion validity as ‘the degree to which the scores of an instrument are an adequate reflection of a gold standard’.16 As outcome measures focus on perceptions that may by subjective, they usually lack a gold standard. The only exception to this would be if a shorter version of a measure was developed from an already validated longer one, where the longer version could be considered to be the gold standard. Therefore, the results of this analysis of criterion validity have not been included here.
The methodological quality of included studies is shown in Table 4 and ranged from poor to excellent. Table 5 shows the synthesis of results per outcome measure with levels of evidence of quality. This ranged from strong to unknown.
Table 4.
Methodological quality of included measures.
| Study | Internal consistency | Reliability | Measurement error | Content validity | Structural validity | Hypothesis testing | Responsiveness | Cross-cultural validity |
|---|---|---|---|---|---|---|---|---|
| CHQ-PF50 | ||||||||
| Drotar et al.19 | Fair | |||||||
| Ferro et al.20 | Good | |||||||
| McCullough et al.21 | Good | Good | ||||||
| Wake et al.22 | Poor | |||||||
| ConQOL | ||||||||
| Macran et al.23 | Poor | Fair | Excellent | Fair | ||||
| CP-QOL | ||||||||
| Waters et al.24 | Poor | Fair | Poor | Fair | ||||
| CP-QOL Teen | ||||||||
| Davis et al.25 | Poor | Fair | Poor | Good | ||||
| DISABKIDS | ||||||||
| Petersen et al.26 | Fair | Excellent | Fair | |||||
| Ravens-Sieberer et al.27/Schmidt et al.28 | Fair | Fair | Excellent | Fair | Fair | |||
| Simeoni et al.29 | Fair | Fair | Fair | Fair | ||||
| DISABKIDS Smiley | ||||||||
| Chaplin et al.30 | Poor | Excellent | Poor | |||||
| GEOSYP | ||||||||
| Townshend et al.31 | Poor | Poor | Fair | Fair | ||||
| HRQOL in children with epilepsy | ||||||||
| Ronen et al.32 | Fair | Fair | Fair | |||||
| KIDSCREEN-52 | ||||||||
| Erhart et al.33 | Fair | |||||||
| MSAS 7–12 | ||||||||
| Collins et al.34 | Fair | Fair | Fair | |||||
| MSAS 10–18 | ||||||||
| Collins et al.35 | Fair | Fair | Fair | Fair | Fair | |||
| PCQL-32 | ||||||||
| Varni et al.36 | Poor | Good | Fair | |||||
| Seid et al.37 | Poor | Good | ||||||
| Ped Cardiac QOL Inventory | ||||||||
| Marino et al.38 | Good | Good | Fair | |||||
| Marino et al.39 | Fair | Fair | ||||||
| Wray et al.40 | Fair | Fair | ||||||
| Ped Oncology QOL Scale | ||||||||
| Goodwin et al.42 | Fair | Poor | Good | Fair | Fair | |||
| PedsQL™ Generic Core Scale (child) | ||||||||
| Varni et al.42 | Fair | Fair | ||||||
| Varni et al.43 | Good | Good | ||||||
| Varni et al.44 | Poor | Fair | Good | |||||
| Varni et al.45 | Fair | |||||||
| Varni et al.46 (parent) | ||||||||
| Amin et al.47 | Poor | Fair | ||||||
| Huang et al.7 | Good | Excellent | Poor | |||||
| Varni et al.42 | Fair | Fair | ||||||
| Varni et al.43 | Good | Good | ||||||
| Varni et al.48 | Good | Good | ||||||
| PedsQL™ Brain Tumour Module | ||||||||
| Palmer et al.49 | Poor | Fair | ||||||
| PedsQL™ Cancer Module | ||||||||
| Varni et al.50 | Poor | Fair | ||||||
| PedsQL™ Cardiac Module | ||||||||
| Uzark et al.51 | Fair | Excellent | ||||||
| PedsQL™ Cerebral Palsy Module | ||||||||
| Varni et al.52 | Poor | Fair | ||||||
| PedsQL™ Neuromuscular Module | ||||||||
| Davis et al.54 | Poor | Poor | Poor | |||||
| Dunaway et al.55 | Poor | |||||||
| Iannaccone et al.53 | Poor | Fair | Good | Fair | ||||
| Quality of Life in Childhood Epilepsy Questionnaire | ||||||||
| Sabaz et al.56 | Poor | Fair | Poor | |||||
| Sabaz et al.57 | Poor | Poor | ||||||
| Royal Marsden Ped Oncology QOL Questionnaire | ||||||||
| Watson et al.58 | Poor | Fair | Poor | |||||
Two papers reporting on the same data.
Table 5.
Data synthesis.
| Questionnaire | Internal consistency | Reliability | Measurement error | Content validity | Structural validity | Hypothesis testing | Responsiveness | Cross-cultural validity |
|---|---|---|---|---|---|---|---|---|
| CHQ-PF50 | ± | ± | ||||||
| ConQOL | ? | + | +++ | ± | ||||
| CQOL | ? | ? | ? | |||||
| CP-QOL | ? | + | ? | + | ||||
| CP-QOL Teen | ? | + (child) − (parent) |
? | − | ||||
| DISABKIDS | ++ | + | +++ | ++ | ++ | |||
| DISABKIDS SMILEY | ? | +++ | ? | |||||
| GEOSYP | ? | ? | + | + | ||||
| HRQOL epilepsy | + | + | + | |||||
| KIDSCREEN-52 | + | |||||||
| MSAS 7–12 | + | + | + | |||||
| MSAS 10–18 | + | ± | + | + | + | |||
| PCQL-32 | ? | ++ | ++ | |||||
| Pediatric cardiac quality of life inventory | ++ | ++ | ++ | ++ | ||||
| Pediatric oncology quality of life scale | + | ? | ++ | + | + | |||
| PedsQL™ Generic | +++ | ++ | ++ | |||||
| PedsQL™ Brain tumour module | ? | + | ||||||
| PedsQL™ Cancer module | ? | + | ||||||
| PedsQL™ Cardiac module | + | +++ | ||||||
| PedsQL™ CP module | ? | + | ||||||
| PedsQL™ Neuromuscular module | ? | + (child) − (parent) |
++ | + | ||||
| Quality of Life in Childhood Epilepsy | ? | + | ? | |||||
| Royal Marsden Paediatric oncology quality of life questionnaire | ? | + | ? |
+++ or – – –: strong evidence for positive/negative results; ++ or – –: moderate evidence for negative/positive results; ±: conflicting evidence; ?: unknown due to poor methodological quality.
Internal consistency was tested in 34 of the included studies. However, 40% of these lacked a check of the uni-dimensionality of the scale, leading to a score of poor for methodological quality.59
Test–retest reliability testing was carried out in 14 of the studies. In all, 38% of studies had a sample size of at least 100 which is needed for an excellent quality score.11 Only one study described how missing items were handled. If not handled appropriately, this could lead to over or underestimation of reliability.
Content validity testing was carried out in 13 of the included studies. The main flaws in the methodology of content validity testing were inclusion of only small numbers in focus groups, pilot studies and cognitive testing and not involving children, parents and professionals in the process. Ideally, all should be included to make sure the items are relevant and ensure no important items are missing.
Structural validity can be assessed by factor analysis or IRT tests for dimensionality.12 Structural validity was assessed in 14 of the studies and generally there was appropriate use of confirmatory or exploratory factor analysis. In order to carry out structural validity testing, a sample size of 5–7 times the number of items (and greater than 100) is recommended.11 This was achieved in 93% of the studies. Lack of description of missing items and how they were handled let down 64% of the studies.
Only one study analysed responsiveness.58 No correlations between change scores were calculated in the included study; a paired t-test was carried out instead, thus the methodology for this was scored as poor. Sample size was also inadequate and there was no description of how missing items were handled.
Discussion
Main findings
To the authors’ knowledge, this is the first systematic review of outcome measures that could potentially be used in PPC. The aim of this review was to examine the feasibility of use of measures, as well as the methodological quality of analysis of measurement properties of included studies. The review identified 22 measures, 5 generic and 17 disease specific, which could potentially be useful. The disease-specific measures included those for use in children with cardiac disease,23,38–40,51 cerebral palsy,24,27,52 cancer,34–37,41,50,58 brain tumours,49 epilepsy31,32,56 and neuromuscular disease.53–55 All measures were initially developed in the English language. None of the measures were developed for use in CYP receiving palliative care. All were developed to be completed in paper format, predominantly by the CYP and/or their parent.
The PedsQL™ Generic Core Scale was the most widely analysed in terms of its measurement properties. It is unique because it contains a generic core scale and various disease-specific modules that can be administered alongside the core scale.
Quality of assessment of measurement properties
None of the studies on measurement properties in this review achieved a score of fair methodological quality or higher in all characteristics. Most of the studies show positive results (except parent test–retest reliability in two studies and hypothesis testing in one, see Table 5). Evidence is mainly limited and at least half the information on measurement properties per questionnaire is missing. The methodological quality of the included studies varied greatly and therefore results should be treated cautiously.
Internal consistency, reliability, content validity and hypothesis testing were widely assessed in the papers. Only one study analysed responsiveness.58 It is imperative that any measure used to assess HRQOL is responsive to change, particularly in PPC, where a child’s condition can change frequently and sometimes rapidly. Measurement error was not tested in any of the included studies. With the same data, both reliability and measurement error can be calculated.12 In all, 14 of the 22 included studies assessed reliability, thereby measurement error could easily have been reported.
Feasibility of use of included measures
In adult palliative care, there are concerns regarding the use and relevance of outcome measures.60 These concerns include the method of administration and whether the patient, carer or professional completes the measure.60 These concerns are probably just as applicable to PPC. Many children requiring palliative care services are non-verbal or too unwell to self-complete the tools and thereby rely on the reports of their carers and/or professionals. The method of administration of a measure is also important. Different modes of administration may be appropriate depending on the type and stage of a CYP illness. The PedsQL™ is the only measure included in this review that has been validated across different modes of administration.48 Multi-group confirmatory factor analysis was performed showing strong factorial invariance across three modes of administration groups (mail, in-person and telephone survey). With widespread mobile technology now available, new ways of collecting data, such as online or via an app, should be considered as these may be more acceptable to CYP and their carers, as well as being easier to access.
Within PPC, as in adult palliative care, there is a debate as to who should complete outcome measures. Most children with life-limiting and life-threatening illnesses are cared for at home by their parents, so a clinician completed measure is not always ideal. HRQOL is generally understood as a latent, not directly observable construct, and contains the perceptions and evaluation of one’s life from the subjective view of the individual, as well as the individual’s subjective well-being and affective mood.61 Wherever possible, the child’s self-report of HRQOL should be sought. Within this population, some children will be too young or too unwell to complete a measure and a parent/proxy completed measure will need to be used. A total of 19 of the 22 measures included in this review contain parent reports. Of those studies that looked at correlation between child and parent scores, three found moderate correlation between parent and child scores.37,38,49 One study showed poor correlation in the psychological and emotional subscales.52 These results support those of previous studies that show a higher correlation for observable constructs, such as physical aspects, and a lower correlation for non-observable constructs such as emotional problems between parents and children.62
Recall period in the included studies ranged from the current moment to 1 month. Research has shown that children as young as 8 years can use a 4-week recall period with accuracy.63 However, HRQOL measures with shorter recall periods are likely to elicit more accurate responses.64 Most of the disease-specific measures had shorter recall periods, which is more appropriate as there can be variation in symptoms over a longer period in many cases. Children with palliative care needs often have frequently changing symptoms which can affect their HRQOL so a measure with a shorter recall period may be more appropriate.
There were a variety of response options used in the included measures. The most common method was a Likert scale and response options ranged from 3 to 9 points. It has been recommended that fewer responses should be employed for younger children as they tend to choose responses at the extremes.63 There is also little evidence showing that young children can effectively respond to Likert scales.64 The completion time (when reported) for measures was between 2 and 25 minutes. Shorter measures are preferable in PPC as children will fatigue easily. Shorter parent-completed measures are also preferable as parents will already have the burden of caring for their sick child.
HRQOL instruments may be either generic or disease specific.7 Generic measures are useful for comparing general quality of life across different populations. These measures are used with healthy children so are more likely to have been validated based on large samples but may lack sensitivity in sick CYP. Disease-specific quality-of-life instruments, on the other hand, are used to compare quality of life within a given condition. Disease-specific measures are assumed to be more sensitive to the implications of different illnesses and may be more appropriate for evaluating interventions or different treatments within CYP with the same illness.62 The drawback of this is that it is not possible to compare HRQOL across groups of CYP with different illnesses, which is essential for a discipline as wide and varied as PPC. The measures included in this study contain varying numbers of domains but all covered the constructs of HRQOL (physical, emotional and social). Some of the domains included in the generic HRQOL measures may not be relevant for the PPC population. For example, domains such as school environment may be irrelevant for a child near the end of life. One of the included studies aimed to validate the PedsQL™ in children with life-limiting illnesses.7 Confirmatory factor analysis did not support the construct validity of the PedsQL™ in this group of children, implying that the hypothesized HRQOL structures between children with life-limiting illnesses and other populations may be different. Most of the generic measures included in this review do not capture the impact of life-limiting illness on daily functioning and well-being.
Implications for research
As discussed above, it is questionable whether any of the included generic measures, such as the PedsQL™ and Child Health Questionnaire, would be valid in the PPC population without adaptation, due to concerns regarding construct validity. The Memorial Symptom Assessment Scales (MSAS) for children could potentially be useful in PPC.34,35 Although they capture many of the domains of PPC, they would need testing for validity and reliability in the population. It is unlikely that without adaptation, they would be useful in a non-cancer population as there is a question about hair-loss, which is unique to this group of children. The methodological quality of studies on the MSAS was fair throughout. Other disease-specific measures included in this review may be useful in PPC. For example, the PedsQL™ Neuromuscular module was designed for use in children with spinal muscular atrophy and muscular dystrophy which are both life-limiting conditions.53,54,57 However, within the three studies included in this review, the majority of assessment of its psychometric properties was scored as fair or poor. It is unlikely that any of the included measures would have acceptable measurement properties in the entire range of children receiving PPC services, as the population is so diverse.
None of the measures included in this review meet all the requirements for use in the PPC population. The generic measures do not capture the full impact of living with a life-limiting illness and often have recall periods that could be considered too long in a child whose condition may be changing frequently. The disease-specific measures contain domains that are only relevant to CYP with specific illnesses so could not be used to compare children with different conditions. One potential solution to this is to revise an existing instrument. An alternative is to develop a completely new measure. It is questionable whether by using either method it will be possible to develop a HRQOL outcome measure for a population as diverse as PPC. Children have many different types of illnesses, some of which are extremely rare and each illness comes with its own set of physical, psychological and emotional needs. All items in a measure may not be equally useful for children with different life-limiting conditions. Findings from other studies have suggested that static models (all items are administered to all subjects) will increase measurement error and decrease precision.7 The use of IRT along with computerized adaptive testing (CAT) may better assess HRQOL for this population.7 Alternatively, using individualized measurement tools rather than standardized ones may be a solution.7 The Schedule for the Evaluation of Individual Quality of Life (SEIQoL) has been shown to be valid and reliable in a population of terminally ill adult cancer patients.65
Two relatively new concepts in healthcare, patient reported experience measures (PREMs) and patient-centred outcome measures (PCOMS), may also be beneficial to CYP and their families receiving palliative care services, but more research in this area is required. PCOMs involve putting patients and their families/carers at the heart of deciding which goals are most valuable for an individual, rather than clinicians deciding what is best.66 PREMs measure patient experience with the goal of improving services. It is desirable to combine measures of experience with measures of outcome to obtain a rounded view of the quality of care.67
Strengths and limitations
This review has several strengths. First of all, this is the first review the authors are aware of which examines the measurement properties and feasibility of using already developed outcome measures in the PPC population. The review was comprehensive, the search strategy found more than 3000 articles for potential inclusion and over 40 papers were systematically appraised and compared.
This review also has several limitations. First, it is never possible to be sure that all relevant studies have been identified. The COSMIN checklist is based on expert group opinion. The inter-rater agreement of the COSMIN checklist is adequate. The inter-rater reliability for many COSMIN items is poor, which has been suggested to be due to interpretation of checklist items.68 Selected articles were restricted to English language. Finally, it was sometimes not clear if certain criteria on the COSMIN checklist were not performed or not reported on. Therefore, it was not possible to distinguish between poor reporting and poor quality.
Conclusion
Although there is no ‘ideal’ HRQOL measure for use in PPC at the moment, it is important to continue developing and researching measures in this area.
Outcome measurement in PPC is rarely carried out and as of yet there are no specific HRQOL measures for use in this population. In light of new developments in the field of PREMS and PCOMs, it may be desirable to develop a combination of measures that are able to measure outcomes that are important to the individual child and family, as well as measuring their satisfaction of the experience of the services that deliver care. The purpose of measuring quality of life and outcomes in CYP receiving PPC is potentially fourfold: to improve clinical care, to audit and evaluate services, for research purposes and to inform commissioners and secure funding.60
Acknowledgments
This work was undertaken towards an MSc in Palliative Care at the Cicely Saunders Institute, King’s College London. This article presents independent research funded, in part, by the NIHR Collaboration for Leadership in Applied Health Research & Care (CLAHRC) Funding scheme, through CLAHRC South London. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health. CLAHRC South London is part of the National Institute for Health Research (NIHR) and is a partnership between King’s Health Partners, St. George’s, University London, and St George’s Healthcare NHS Trust.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Apart from NIHR CLAHRC support for the senior and administrative input as acknowledged above, this research received no additional funding from commercial, public or not-for-profit sectors.
References
- 1. Fraser L, Miller M, Hain R, et al. Rising national prevalence of life-limiting conditions in children in England. Pediatrics 2012; 129(4): e923–e929. [DOI] [PubMed] [Google Scholar]
- 2. Himelstein B. Palliative care for infants, children, adolescents, and their families. J Palliat Med 2006; 9(1): 163–181. [DOI] [PubMed] [Google Scholar]
- 3. Together for Short Lives. A core care pathway for children with life-limiting and life-threatening conditions, 3rd ed. Bristol: Together for Short Lives, 2013. [Google Scholar]
- 4. Hain R, Devins M, Hastings R, et al. Paediatric palliative care: development and pilot study of a ‘Directory’ of life-limiting conditions. BMC Palliat Care 2013; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor R, Gibson F, Franck L. A concept analysis of health-related quality of life in young people with chronic illness. J Clin Nurs 2008; 17(14): 1823–1833. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization (WHO). Constitution of the World Health Organization. Geneva: WHO, 1948. [Google Scholar]
- 7. Huang IC, Shenkman EA, Madden VL, et al. Measuring quality of life in pediatric palliative care: challenges and potential solutions. Palliat Med 2010; 24(2): 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 9. Brettle AJ, Long AF, Grant MJ, et al. Searching for information on outcomes: do you need to be comprehensive? Qual Health Care 1998; 7: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terwee CB, Jansma EP, Riphagen II, et al. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res 2009; 18: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Terwee CB, Mokkink LB, Knol DL, et al. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2011; 21: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schellingerhout JM, Verhagen AP, Heymans MW, et al. Measurement properties of disease-specific questionnaires in patients with neck pain: a systematic review. Qual Life Res 2012; 21: 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010; 63: 737–745. [DOI] [PubMed] [Google Scholar]
- 14. Higginson I, Harding R. Chapter 7: Outcome measurement. In: Addington-Hall J, Bruera E, Higginson I, et al. (eds) Research methods in palliative care. Oxford: Oxford University Press, 2007, pp. 99–113. [Google Scholar]
- 15. De Vet HCW, Terwee CB, Mokkink LB, et al. Chapter 9: reviews of measurement properties. In: De Vet HCW, Terwee CB, Mokkink LB, et al. (eds) Measurement in medicine. Cambridge: Cambridge University Press, 2011, pp. 275–314. [Google Scholar]
- 16. De Vet HCW, Terwee CB, Mokkink LB, et al. Chapter 6: validity.In: De Vet HCW, Terwee CB, Mokkink LB, et al. (eds) Measurement in medicine. Cambridge: Cambridge University Press, 2011, pp. 275–314. [Google Scholar]
- 17. Tulder V, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 2003; 28(12): 1290–1299. [DOI] [PubMed] [Google Scholar]
- 18. Terwee CB, Bot S, de Boer M, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60(1): 34–42. [DOI] [PubMed] [Google Scholar]
- 19. Drotar D, Schwartz L, Palermo TM, et al. Factor structure of the child health questionnaire-parent form in pediatric populations. J Pediatr Psychol 2006; 31(2): 127–138. [DOI] [PubMed] [Google Scholar]
- 20. Ferro MA, Landgraf JM, Speechley KN. Factor structure of the Child Health Questionnaire Parent Form-50 and predictors of health-related quality of life in children with epilepsy. Qual Life Res 2013; 22(8): 2201–2211. [DOI] [PubMed] [Google Scholar]
- 21. McCullough N, Parkes J, White-Koning M, et al. Reliability and validity of the Child Health Questionnaire PF-50 for European children with cerebral palsy. J Pediatr Psychol 2009; 34(1): 41–50. [DOI] [PubMed] [Google Scholar]
- 22. Wake M, Salmon L, Reddihough D. Health status of Australian children with mild to severe cerebral palsy: cross-sectional survey using the child health questionnaire. Dev Med Child Neurol 2003; 45(3): 194–199. [DOI] [PubMed] [Google Scholar]
- 23. Macran S, Birks Y, Parsons J, et al. The development of a new measure of quality of life for children with congenital cardiac disease. Cardiol Young 2006; 16(2): 165–172. [DOI] [PubMed] [Google Scholar]
- 24. Waters E, Salmon L, Wake M. The parent-form child health questionnaire in Australia: comparison of reliability, validity, structure, and norms. J Pediatr Psychol 2000; 25(6): 381–391. [DOI] [PubMed] [Google Scholar]
- 25. Davis E, Mackinnon A, Davern M, et al. Description and psychometric properties of the CP QOL-Teen: a quality of life questionnaire for adolescents with cerebral palsy. Res Dev Disabil 2013; 34(1): 344–352. [DOI] [PubMed] [Google Scholar]
- 26. Petersen C, Schmidt S, Power M, et al. Development and pilot-testing of a health-related quality of life chronic generic module for children and adolescents with chronic health conditions: a European perspective. Qual Life Res 2005; 14(4): 1065–1077. [DOI] [PubMed] [Google Scholar]
- 27. Ravens-Sieberer U, Schmidt S, Gosch A, et al. Measuring subjective health in children and adolescents: results of the European KIDSCREEN/DISABKIDS Project. GMS Psychosoc Med 2007; 4: Doc 08. [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt S, Debensason D, Muhlan H, et al. The DISABKIDS generic quality of life instrument showed cross-cultural validity. J Clin Epidemiol 2006; 59: 587–598. [DOI] [PubMed] [Google Scholar]
- 29. Simeoni M-C, Schmidt S, Muehlan H, et al. Field testing of a European quality of life instrument for children and adolescents with chronic conditions: the 37-item DISABKIDS Chronic Generic Module. Qual Life Res 2007; 16(5): 881–893. [DOI] [PubMed] [Google Scholar]
- 30. Chaplin J, Koopman H, Schmidt S. DISABKIDS smiley questionnaire: the TAKE 6 assisted health-related quality of life measure for 4 to 7-year-olds. Clin Psychol Psychother 2008; 15: 173–180. [DOI] [PubMed] [Google Scholar]
- 31. Townshend KH, Dorris L, McEwan MJ, et al. Development and validation of a measure of the impact of epilepsy on a young person’s quality of life: Glasgow epilepsy outcome scale for young persons (GEOS-YP). Epilepsy Behav 2008; 12(1): 115–123. [DOI] [PubMed] [Google Scholar]
- 32. Ronen GM, Streiner DL, Rosenbaum P. Health-related quality of life in children with epilepsy: development and validation of self-report and parent proxy measures. Epilepsia 2003; 44(4): 598–612. [DOI] [PubMed] [Google Scholar]
- 33. Erhart M, Ravens-Sieberer U, Dickinson HO, et al. Rasch measurement properties of the KIDSCREEN quality of life instrument in children with cerebral palsy and differential item functioning between children with and without cerebral palsy. Value Health 2009; 12(5): 782–792. [DOI] [PubMed] [Google Scholar]
- 34. Collins J, Devine T, Dick G, et al. The measurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7–12. J Pain Symptom Manage 2002; 23(1): 10–16. [DOI] [PubMed] [Google Scholar]
- 35. Collins J, Byrnes M, Dunkel I, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000; 19(5): 363–373. [DOI] [PubMed] [Google Scholar]
- 36. Varni J, Katz E, Seid M, et al. The pediatric cancer quality of life inventory-32 (PCQL-32): I. Reliability and validity. Cancer 1998; 82: 1184–1196. [DOI] [PubMed] [Google Scholar]
- 37. Seid M, Varni J, Rode C, et al. The Pediatric Cancer Quality of Life Inventory: a modular approach to measuring health-related quality of life in children with cancer. Int J Cancer 1999; 12: 71–76. [DOI] [PubMed] [Google Scholar]
- 38. Marino B, Shera D, Wernovsky G, et al. The development of the pediatric cardiac quality of life inventory: a quality of life measure for children and adolescents with heart disease. Qual Life Res 2008; 17: 613–626. [DOI] [PubMed] [Google Scholar]
- 39. Marino B, Tomlinson R, Wernovsky G, et al. Validation of the pediatric cardiac quality of life inventory. Pediatrics 2010; 126(3): 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wray J, Franklin R, Brown K, et al. Testing the pediatric cardiac quality of life inventory in the United Kingdom. Acta Paediatr 2013; 102(2): e68–e73. [DOI] [PubMed] [Google Scholar]
- 41. Goodwin D, Boggs S, Graham-Pole J. Development and validation of the pediatric oncology quality of life scale. Psychol Assess 1994; 6(4): 321–328. [Google Scholar]
- 42. Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Med Care 1999; 37(2): 126–139. [DOI] [PubMed] [Google Scholar]
- 43. Varni J, Burwinkle T, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3(6): 329–341. [DOI] [PubMed] [Google Scholar]
- 44. Varni J, Limbers C, Burwinkle T. How young can children reliably and validly self-report their health-related quality of life? An analysis of 8591 children across age subgroups with the PedsQL™ 4.0 generic core scales. Health Qual Life Outcomes 2007; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Varni J, Limbers C, Newman D. Factorial invariance of the PedsQL™ 4.0 generic core scales child self-report across gender: a multigroup confirmatory factor analysis with 11,356 children. Appl Res Qual Life 2008; 3: 137–148. [Google Scholar]
- 46. Varni J, Limbers C, Newman D. Using factor analysis to confirm the validity of children’s self-reported health-related quality of life across different modes of administration. Clin Trials 2009; 6: 185–195. [DOI] [PubMed] [Google Scholar]
- 47. Amin L, Rosenbaum P, Barr R, et al. Rasch analysis of the PedsQL: an increased understanding of the properties of a rating scale. J Clin Epidemiol 2012; 65: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 48. Varni J, Limbers C, Burwinkle T. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Palmer S, Meeske K, Katz E, et al. The PedsQL Brain Tumor Module: initial reliability and validity. Pediatr Blood Cancer 2007; 49: 287–293. [DOI] [PubMed] [Google Scholar]
- 50. Varni J, Burwinkle T, Katz E, et al. The PedsQL in pediatric cancer. Cancer 2002; 94(7): 2090–2106. [DOI] [PubMed] [Google Scholar]
- 51. Uzark K, Jones K, Burwinkle T, et al. The Pediatric Quality of Life Inventory™ in children with heart disease. Prog Pediatr Cardiol 2003; 18: 141–148. [Google Scholar]
- 52. Varni J, Burwinkle T, Berrin S, et al. The PedsQL in pediatric cerebral palsy: reliability, validity, and sensitivity of the Generic Core Scales and Cerebral Palsy Module. Dev Med Child Neurol 2006; 48: 442–449. [DOI] [PubMed] [Google Scholar]
- 53. Iannaccone S, Hynan L, Morton A, et al. The PedsQL in pediatric patients with Spinal Muscular Atrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Neuromuscular Module. Neuromuscul Disord 2009; 19: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davis E, Hynan L, Limbers C, et al. The PedsQL in pediatric patients with Duchenne muscular dystrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Neuromuscular Module and Generic Core Scales. J Clin Neuromuscul Dis 2010; 11(3): 97–109. [DOI] [PubMed] [Google Scholar]
- 55. Dunaway S, Montes J, Montgomery M, et al. Reliability of telephone administration of the PedsQL Generic Quality of Life Inventory and Neuromuscular Module in spinal muscular atrophy (SMA). Neuromuscul Disord 2010; 20(3): 162–165. [DOI] [PubMed] [Google Scholar]
- 56. Sabaz M, Cairns DR, Lawson JA, et al. Validation of a new quality of life measure for children with epilepsy. Epilepsia 2000; 41(6): 765–774. [DOI] [PubMed] [Google Scholar]
- 57. Sabaz M, Lawson JA, Cairns DR, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav 2003; 4(6): 680–691. [DOI] [PubMed] [Google Scholar]
- 58. Watson M, Edwards L, von Essen L, et al. Development of the Royal Marsden Hospital paediatric oncology quality of life questionnaire. Int J Cancer 1999; 12: 65–70. [PubMed] [Google Scholar]
- 59. Terwee CB, Schellingerhout JM, Verhagen AP, et al. Methodological quality of studies on the measurement properties of neck pain and disability questionnaires: a systematic review. J Manipulative Physiol Ther 2011; 34: 261–272. [DOI] [PubMed] [Google Scholar]
- 60. Hearn J, Higginson I. Outcome measures in palliative care for advanced cancer patients: a review. J Public Health Med 1997; 19(2): 193–199. [DOI] [PubMed] [Google Scholar]
- 61. Ravens-Sieberer U, Erhart M, Wille N, et al. Generic health-related quality-of-life assessment in children and adolescents. Methodological considerations. Pharmacoeconomics 2006; 24(12): 1199–1220. [DOI] [PubMed] [Google Scholar]
- 62. Eiser C. Children’s quality of life measures. Arch Dis Child 1997; 77(4): 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rebok G, Riley A, Forrest C, et al. Elementary school-aged children’s reports of their health: a cognitive interviewing study. Qual Life Res 2001; 10: 59–70. [DOI] [PubMed] [Google Scholar]
- 64. Quittner AL, Modi A, Cruz I. Systematic review of health-related quality of life measures for children with respiratory conditions. Paediatr Respir Rev 2008; 9: 220–232. [DOI] [PubMed] [Google Scholar]
- 65. Waldron D, O’Boyle C, Kearney M. Quality-of-life measurement in advanced cancer: assessing the individual. J Clin Oncol 1999; 17: 3603–3611. [DOI] [PubMed] [Google Scholar]
- 66. NHS England. Patient centred outcome measures, http://www.england.nhs.uk/2015/02/11/pcoms-cyp/ (2015, accessed 21 July 2015).
- 67. NHS Institute for Innovation and Improvement. Transforming patient experience: the essential guide, https://www.institute.nhs.uk/share_and_network/pen/how_to_measure_patient_experience.html (2006–2015, accessed 21 July 2015).
- 68. Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]