Abstract
The treatment of metastatic renal cell carcinoma (mRCC) is rapidly changing. During first-line treatment with targeted therapy, patients ultimately develop resistance to therapy and the disease progresses. Recently, cabozantinib has demonstrated a better response rate, progression-free survival and overall survival compared with everolimus after failure of prior targeted therapy in patients with advanced or metastatic renal cell carcinoma (RCC). Cabozantinib is a small-molecule tyrosine kinase inhibitor (TKI). It exerts inhibition of MET, vascular endothelial growth factor receptor type 2, AXL, and many other receptor tyrosine kinases that are also implicated in tumor pathobiology, including RET, KIT, and FLT3. MET drives tumor survival, invasion, angiogenesis, and metastasis through several downstream signaling pathways. AXL has recently been described as an essential mediator of cancer metastasis that mediates crosstalk and resistance to TKIs. MET and AXL are thought to be anti-vascular endothelial growth factor receptor (VEGF) resistance pathways and thus cabozantinib represents a logical choice after progression on initial VEGF therapy. Subgroup analyses examining those with good performance status or visceral and bone metastases indicate that the hazard ratios may be better when using cabozantinib versus everolimus. However, there were no clear statistically significant differences between any subgroups.
Keywords: cabozantinib, renal cell carcinoma, vascular endothelial growth factor receptor 2
Introduction
Worldwide, kidney cancer is the 14th leading cause of all malignant neoplasms in both sexes (2.4%) and has a 5-year prevalence of 4.4% [GLOBOCAN, 2012]. The most common of its variants are clear-cell renal cell carcinoma (RCC) and papillary and chromophobe RCC, which together comprise 90% of all cases of RCC [Muglia et al. 2015]. Up to one-third of patients present with an initial diagnosis of metastatic disease and one third of patients with initial localized disease exhibit metastatic relapse after surgical removal of the tumor [Kroeger et al. 2014].
One of the most common mutations in RCC involves the inactivation of the Von-Hippel-Lindau tumor suppressor gene (VHL), which is located at the short arm of the third chromosome. Nearly 60–80% of cases of sporadic RCC harbor a mutated VHL gene [Baldewijns et al. 2010]. In its usual functional form and under normoxic circumstances, the VHL complex normally targets hypoxia-inducible transcription factors (HIFs), resulting in ubiquitin-mediated proteolysis through hydroxylation. Since HIF monitors and controls many critical downstream targets through hypoxia-inducible genes like vascular endothelial growth factor (VEGF), epidermal growth factor, transforming growth factor α, platelet-derived growth factor (PDGF), erythropoietin, and glucose transporter 1, it has an important role in angiogenesis [Klatte et al. 2008]. In conditions of hypoxia, unhydroxylated HIF is upregulated and borders degradation with the help of VHL complex. In case the VHL complex is malfunctioning due to factors such as genetic mutation, the same sequence of events takes place: HIF aggregates, shifts to the nucleus and results in the transcription of angiogenic factors and tumorigenesis. RCC is a highly vascular tumor and has a high expression of VEGF, VEGF receptor (VEGFR), PDGF receptor, as well as basic fibroblast growth factor [Pfaffenroth et al. 2008]. Some of the other oncogenic pathways include the activation of the mammalian target of rapamycin (mTOR) pathway which is a repetitive event in cancer. The mTOR protein is an intracellular serine or threonine kinase which takes part in the moderation of cell growth, proliferation, survival, as well as metabolism. Multiple growth factors, nutrients, hormones, and mitogens lead to stimulation of mTOR and thus result in protein synthesis, degradation or angiogenesis. All of these variations lead to RCC progression and metastasis [Voss et al. 2011].
Currently, patients diagnosed with advanced or metastatic RCC (mRCC) are provided with a choice of active treatments that can improve symptoms and reduce the disease burden (Table 1). The logic used for the majority of the first-line therapies is fundamentally related to targeting angiogenic factors and their receptors, both of which are required by the tumor for growth and metastasis. However, most patients treated with first-line treatment will later develop resistance to this through complex mechanisms and will need second-line treatment [Capitanio et al. 2016].
Table 1.
Main outcomes for first and second-line therapies in metastatic renal cell carcinoma.
| Molecule | Phase | Control arm | PFS (months) | OS (months) | ORR (%) | Reference |
|---|---|---|---|---|---|---|
| First-line targeted therapies | ||||||
| High-dose IL-2 | III | Subcutaneous IL-2 + IFNα | 3.1 versus 3.1 HR not reported (ns) |
17 versus 13 (ns) | 23.2 versus 9.9 | [McDermott et al. 2005] |
| Sunitinib | III | IFNα | 11 versus 5 HR 0.53 |
26.4 versus 21.8 HR 0.82 |
47 versus 12 | [Motzer et al. 2009] |
| Pazopanib | III noninferiority | Sunitinib | 8.4 versus 9.5 HR 1.04 |
28.3 versus 29.1 HR 0.92 |
31 versus 25 | [Motzer et al. 2013b] |
| IFNα + bevacizumab | III | IFNα + placebo | 10.2 versus 5.4 HR 0.63 |
23.3 versus 21.3 (ns) | 30.6 versus 12.4 | [Escudier et al. 2007] |
| Temsirolimus (only in poor-risk patients) | III | IFNα | 5.5 versus 3.1 HR not reported |
10.9 versus 7.3 HR 0.73 |
8.6 versus 4.8 | [Hudes et al. 2007] |
| Second-line targeted therapies | ||||||
| Nivolumab | III | Everolimus | 4.6 versus 4.4 HR 0.88 (ns) |
25 versus 19.6 HR 0.73 |
25 versus 5 | [Motzer et al. 2015a] |
| Cabozantinib | III | Everolimus | 7.4 versus 3.9 HR 0.51 |
21.4 versus 16.5 HR 0.66 |
17 versus 3 | [Choueiri et al. 2016] |
| Axitinib | III | Sorafenib | 6.7 versus 4.7 HR 0.66 |
20.1 versus 19.2 HR 0.96 (ns) |
23 versus 12 | [Motzer et al. 2013a] |
| Everolimus | III | Placebo | 4.9 versus 1.9 HR 0.33 |
14.8 versus 14.4 HR 0.87 (ns) |
1.8 versus 0 | [Motzer et al. 2008] |
| Lenvatinib plus everolimus | II | Lenvatinib | 14.6 versus 7.4 HR 0.66 (ns) |
25.5 versus 19.1 HR 0.68 (ns) |
43 versus 27 (ns) | [Motzer et al. 2015b] |
| Everolimus | 14.6 versus 5.5 HR 0.40 |
25.5 versus 15.4 HR 0.51 |
43 versus 6 | |||
HR, hazard ratio; IFNα, interferon α; IL-2, interleukin 2; ns, not significant; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Second-line therapies include axitinib [Rini et al. 2011] and everolimus [Motzer et al. 2008]. In September 2015, two clinical trials reported that nivolumab [Motzer et al. 2015a] and cabozantinib [Choueiri et al. 2015] have an advantage over everolimus for patients with mRCC who have previously received VEGF inhibitors. Second-line options are thus expanding, and the current debate is about which one is preferred, which contrasts with previous years when options were scarce.
The aim of this review is to discuss the use of cabozantinib in the treatment of advanced RCC or mRCC, the available data from clinical trials, and the clinical experience from treating patients with advanced RCC or mRCC.
Cabozantinib: rationale for use in advanced RCC or mRCC and mechanism of action
Importance of mesenchymal-epithelial transition (MET) and anexelekto (AXL) in renal cell carcinoma
As a consequence of VHL inactivation in RCC, MET and AXL are upregulated. MET [also known as hepatocyte growth factor (HGF) receptor] has been identified as a proto-oncogene, and its activation is a common feature of human tumors. MET drives tumor survival, invasion, angiogenesis, and metastasis through several other signaling pathways such as phosphoinositide 3 kinase/Akt, Src, signal transducer and activation of transcription 3, and Ras/MEK [Gherardi et al. 2012]. In general, the MET pathway can be activated through paracrine or autocrine HGF, amplification of the MET gene locus, and activation of mutations in the kinase domain of the MET receptor [Comoglio et al. 2008]. Greater MET expression is associated with poor prognosis and poor pathological features in RCC, such as a higher Fuhrman grade and more advanced disease stage. MET expression is greatest in the papillary and sarcomatoid subtypes of RCC [Gibney et al. 2013]. VHL-deficient RCC cells have been shown to be more sensitive to MET targeting than their counterparts in which VHL function has been restored [Bommi-Reddy et al. 2008].
AXL has recently been described as an essential mediator of cancer metastasis that mediates crosstalk and resistance to tyrosine kinase inhibitors (TKIs) [Linger et al. 2010]. HIF-1 and HIF-2 directly activate the expression of AXL. Growth arrest-specific 6 (GAS6)–AXL signaling forms a complex with the SRC proto-oncogene nonreceptor tyrosine kinase and activates MET receptor in an HGF-independent manner to optimize RCC migration and invasion [Rankin et al. 2014].
Overexpression of MET and AXL confer more aggressive disease and worse survival for patients with RCC [Gibney et al. 2013]. MET upregulation occurs in response to antiangiogenic therapy, which promotes migration to tissue with greater oxygenation and subsequently invasion [Bottaro and Liotta, 2003]. Increased expression of MET and AXL promotes prometastatic behavior and angiogenesis, and is implicated in acquired resistance to VEGR TKIs used in the treatment of advanced RCC or mRCC.
Cabozantinib: an oral bioavailable multiple TKI
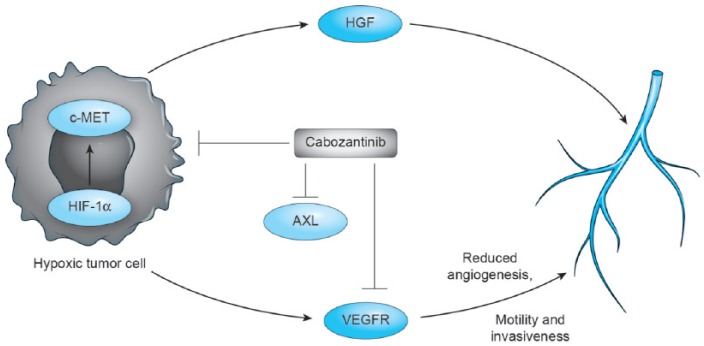
Cabozantinib is an orally administered drug that is classified as a small-molecule TKI. It exhibits balanced inhibition of MET, VEGFR type 2 (VEGFR2), and several other receptor tyrosine kinases that are also implicated in tumor pathobiology, including RET, KIT, AXL, FLT3 and tunica interna endothelial cell kinase 2 (TIE-2). The previous mechanism of action makes it a novel TKI different to the already available treatments for mRCC. Cabozantinib is a reversible competitive inhibitor of adenosine triphosphate of both c-MET and VEGFR2 tyrosine kinase domains with high affinity [Grullich, 2014]. Patients pretreated with VEGF TKIs exhibited MET upregulation, which logically leads to a strategy of therapeutic MET inhibition via cabozantinib (Figure 1) [Sennino et al. 2012].
Figure 1.
Mechanism of action of cabozantinib. Cabozantinib exhibits balanced inhibition of MET, vascular endothelial growth factor receptor type 2 (VEGFR), and several other receptor tyrosine kinases that are also implicated in tumor pathobiology, including RET, KIT, AXL, and FLT3. HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor 1α.
Cabozantinib also demonstrated activity in VEGFR TKI resistant cell lines. For example, one model used a loss-of-function approach (inhibition of the MET pathway in resistant tumors) and a gain-of-function approach (upregulation of HGF in sensitive tumors), indicating a role for HGF in tumor resistance to sunitinib. These results suggest that the HGF–MET pathway acts as an alternative angiogenic pathway in sunitinib-resistant tumors [Shojaei et al. 2010]. In another study, tissue microarrays containing sunitinib-treated and -untreated RCC tissues were stained with antibodies to MET and AXL. Inhibition of AXL and MET activation using cabozantinib impaired chronic sunitinib treatment-induced prometastatic behavior in cell culture and rescued acquired resistance to sunitinib in xenograft models [Zhou et al. 2016].
In 2012, cabozantinib was approved for the treatment of unresectable, locally advanced, or metastatic medullary thyroid cancer [Elisei et al. 2013]. Now in 2016, cabozantinib has been approved by the US Food and Drug Administration (FDA) for the treatment of advanced RCC in patients who have received prior antiangiogenic therapy [FDA, 2016].
Clinical trials
Phase I in solid tumors
Many solid tumors were evaluated in a phase I trial. Eighty-five patients were enrolled, including 37 with medullary thyroid carcinoma (MTC). The maximum tolerated dose was 175 mg daily. Dose-limiting toxicities were grade 3 palmar plantar erythrodysesthesia, mucositis, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lipase elevations, and grade 2 mucositis that resulted in dose interruption and reduction. Ten (29%) of 35 patients with MTC with measurable disease had a confirmed partial response. Overall, 18 patients experienced tumor shrinkage of 30% or more, including 17 (49%) of 35 patients with MTC with measurable disease. Additionally, 15 (41%) of 37 patients with MTC had stable disease (SD) for at least 6 months, resulting in SD for 6 months or longer, or confirmed partial response in 68% of patients with MTC [Kurzrock et al. 2011].
Cabozantinib trials in advanced RCC or mRCC
Phase I clinical trial
The results from a phase I clinical trial in RCC were published in 2014. This trial was a single-arm, open-label study that evaluated the safety and tolerability of cabozantinib in 25 pretreated patients with clear-cell, advanced, or metastatic RCC. Twenty patients were classified as showing intermediate prognosis as per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria. Patients began daily oral dosing of 140 mg cabozantinib on day 2. From day 24 onward, patients continued to receive, at the discretion of the investigator, daily cabozantinib until progressive disease or unacceptable adverse events (AEs), or patient withdrawal of consent. Most patients had previously received two agents, and most had received at least one VEGF pathway-inhibiting therapy. Partial response was reported in seven patients (28%). The median progression-free survival (PFS) was 12.9 months, and the median overall survival (OS) was 15 months. Clinical activity against metastatic lesions in bone was observed in two patients, who reported less use of narcotics because of the bone lesions. The most common all-grade AEs included fatigue (80%), diarrhea (64%), nausea (44%), proteinuria (36%), appetite decrease (36%), palmar–plantar erythrodysesthesia (36%), and vomiting (36%). The most common grade 3–4 AEs included hypophosphatemia (40%), hyponatremia (20%), fatigue (20%), diarrhea (12%), lipase increase (12%), and proteinuria (8%). Based on the tolerability and clinical activity associated with doses of cabozantinib less than 140 mg in this trial, 60 mg daily was selected as the starting dose in subsequent trials [Choueiri et al. 2014].
METEOR clinical trial
Based on the results of the previous phase I clinical trial of cabozantinib in advanced RCC, a randomized, open-label, phase III trial was performed to evaluate the efficacy of second-line cabozantinib versus everolimus in patients with advanced RCC or mRCC whose disease progressed after VEGFR-targeted therapy. Eligible patients had advanced RCC or mRCC with a clear-cell component and measurable disease, and also a Karnofsky performance status score of at least 70%. Patients must have received prior treatment with at least one VEGFR-targeting TKI and must have had radiographic progression during treatment or within 6 months after the most recent dose of the VEGFR inhibitor. Patients with known brain metastases that were adequately treated and stable were eligible.
A total of 658 patients were randomized to receive either cabozantinib at a dose of 60 mg daily or everolimus at a dose of 10 mg daily. Baseline clinical characteristics were balanced between both groups, with 69% with an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0, 44% with a favorable Memorial Sloan Kettering Cancer Center prognostic risk category, 27% who had received two or more prior VEGFR TKIs, and 83% who had previous nephrectomy. Five percent of patients randomized to the cabozantinib arm and 6% of patients randomized to the everolimus arm had received nivolumab before enrollment. This clinical trial reported a median PFS of 7.4 months with cabozantinib and 3.8 months with everolimus [hazard ratio (HR) 0.58; 95% confidence interval (CI) 0.45–0.75; p < 0.001), which represented a 42% reduction in the risk of disease progression in favor of cabozantinib. The objective response rates (ORRs) were 21% with cabozantinib and 5% with everolimus (p < 0.001). A planned interim analysis showed that OS was longer with cabozantinib than with everolimus (HR for death 0.67; 95% CI 0.51–0.89; p = 0.005), but this did not cross the significance boundary for the interim analysis. An updated analysis presented at annual meeting of the American Society of Clinical Oncology (ASCO) 2016 and published in Lancet Oncology indicated that the OS benefit has been confirmed as statistically significant. The median OS was 21.4 months for cabozantinib versus 16.5 months for everolimus, with a 33% reduction in the rate of death (HR 0.66; 95% CI 0.53–0.83; p = 0.0003) [Choueiri et al. 2016].
The toxicity profile of cabozantinib was similar to that for previous experiences using other VEGFR TKIs for treatment of advanced RCC or mRCC. The incidence of AEs of grade 3 or 4 was 68% with cabozantinib and 58% with everolimus. The most common grade 3 or 4 AEs were hypertension (15%), diarrhea (11%), and fatigue (9%) for cabozantinib, and anemia (16%), fatigue (7%), and hyperglycemia (5%) for everolimus (Table 2). AEs were managed with dose reduction; the doses were reduced in 60% of the patients who received cabozantinib and in 25% of those who received everolimus. The most common AEs leading to dose reduction for cabozantinib were diarrhea (16%), palmar–plantar erythrodysesthesia syndrome (11%), and fatigue (10%). Discontinuation of the study treatment because of AEs occurred in 9% of patients who received cabozantinib and in 10% of those who received everolimus. Grade 5 events considered to be treatment related occurred in one patient in the cabozantinib group (death not otherwise specified) [Choueiri et al. 2015].
Table 2.
Grade 3–4 all-cause adverse events of cabozantinib and everolimus as reported for METEOR.
| Event | Cabozantinib (n = 331) | Everolimus (n = 322) |
|---|---|---|
| Hypertension | 49 (15%) | 10 (3%) |
| Diarrhea | 38 (11%) | 7 (2%) |
| Fatigue | 30 (9%) | 22 (7%) |
| Palmar–plantar erythrodysesthesia syndrome | 28 (8%) | 3 (<1%) |
| Asthenia | 14 (4%) | 7 (2%) |
| Nausea | 13 (4%) | 1 (<1%) |
| Total grade 3–4 all-cause adverse events | 226 (68%) | 187 (58%) |
Subgroup analysis of the METEOR trial found that PFS HRs were similar for subgroups defined by prior number of TKIs [HR = 0.56 (one prior) and 0.67 (at least two prior)], and showed a trend in better PFS on cabozantinib over everolimus in patients with fewer prognostic risk factors (HR = 0.54 for no factors, 0.56 for one factor, 0.84 for at least two factors) or with metastases in various organs (HR 0.47 for lung metastases, 0.53 for liver metastases, 0.33 for bone metastases, and 0.26 for both visceral and bone metastases). Patients with an ECOG score of 0 had a median PFS of 9.1 months compared with patients with an ECOG score of 1, who had a median PFS of 5.6 months with cabozantinib [Escudier et al. 2016]. The favorable HR for patients with bone metastases, which are traditionally associated with a poorer prognosis [McKay et al. 2014], warrant further investigation into the mechanisms underlying the activity of cabozantinib in the bone [Choueiri et al. 2016].
Selection of cabozantinib as second-line treatment for advanced RCC or mRCC
Given the results of the METEOR [Choueiri et al. 2015] and CHECKMATE 025 [Motzer et al. 2015a] trials, where both cabozantinib and nivolumab surpassed everolimus as a second-line option in advanced RCC or mRCC, it is obvious that now we have two more options for second-line therapy.
Nivolumab is a novel antibody that blocks the interaction between programmed death receptor-1 (PD-1) and its ligand programmed death receptor ligand-1 (PD-L1) which prevent T-cell activation. Because RCC can overexpress PD-1 and thus prevent T-cell activation, PD-1 inhibitors such as nivolumab can prevent the inhibition of the immune system and thus allow it to mount a response to RCC. In the phase III trial CheckMate 025, patients with advanced clear-cell RCC who had been previously treated with one or more lines of therapy (excluding mTOR) were randomly assigned to receive nivolumab or everolimus. The median OS was 5.4 months longer with nivolumab compared with everolimus (25.0 versus 19.6 months). The HR for death (from any cause) with nivolumab versus everolimus was 0.73 (p = 0.002). The ORR for nivolumab was 25% versus 5% for everolimus (95% CI 3.68–9.72; p < 0.001). No benefit was observed in PFS. The median PFS was 4.6 months (95% CI 3.7–5.4) in the nivolumab group and 4.4 months (95% CI 3.7–5.5) in the everolimus group (HR 0.88; 95% CI 0.75–1.03; p = 0.11) The most common treatment-related AEs among patients who received nivolumab were fatigue (33%), nausea (14%), and pruritus (14%). The most common grade 3–4 event with nivolumab was fatigue (2%) [Motzer et al. 2015a].
Other options for second-line therapy include previously approved agents for which these new trials of nivolumab and cabozantinib did not compare against such as axitinib. Axitinib is an orally administered, selective TKI of VEGFR1, VEGFR2, and VEGFR3. As a second-generation VEGFR inhibitor, axitinib offers increased potency and specificity for the VEGFR compared with earlier VEGFR inhibitors that demonstrated multikinase activity with off-target effects [Narayan et al. 2016]. A multicenter, randomized phase III study (AXIS) compared axitinib versus sorafenib as second-line therapy after one prior systemic therapy. The median PFS was 6.7 months for axitinib versus 4.7 months for sorafenib (HR 0.665; p < 0.0001), and the response rate was 19% for axitinib versus 9% for sorafenib (p = 0.0001). AEs of all grades that occurred more frequently with axitinib were hypertension, fatigue, dysphonia, and hypothyroidism. AEs that occurred more frequently with sorafenib were hand–foot syndrome, rash, alopecia, and anemia [Rini et al. 2011]. In the recently reported updated results of the same trial, the median OS was 20.1 months (95% CI 16.7–23.4) with axitinib and 19.2 months (17.5–22.3) with sorafenib (HR 0.969; 95% CI 0.800–1.174; one-sided p = 0.3744). The median investigator-assessed PFS was 8.3 months (95% CI 6.7–9.2) with axitinib and 5.7 months (4.7–6.5) with sorafenib (HR 0.656; 95% CI 0.552–0.779; one-sided p < 0.0001) [Motzer et al. 2013a].
Currently, nivolumab use in the second line is widespread ever since its FDA approval in November 2015. Now, cabozantinib has been approved for use as well [FDA, 2016]. In view of the data for second-line therapy in patients with advanced RCC or mRCC, cabozantinib is the only therapy in this setting that offers a benefit in PFS, response rate (RR), and OS. It is probable that the selection of the best second-line therapy for patients will be based on the performance status; for example, cabozantinib will be preferred for younger and healthier patients who are more tolerant of its side effects. Because cabozantinib is an oral option, it may be preferred over intravenous nivolumab every 2 weeks for patients from rural areas or those that prefer fewer cancer center visits for infusions. Nivolumab is also contraindicated in patients with autoimmune diseases as these can be reactivated. Axitinib is still an option as an active treatment, but it did not demonstrate an OS benefit in the second-line setting, possibly because of post-trial treatment. While we await results of more clinical trials and subpopulation analysis, physicians should rely on their clinical judgment for determining the best option in the second-line setting (Table 3).
Table 3.
Treatment algorithm for metastatic renal cell carcinoma (mRCC).
| IMDC stratification | First line | Second line (choose any) | Third line | |
|---|---|---|---|---|
| mRCC | Favorable / | Pazopanib Sunitinib IFNα + bevacizumab High-dose IL-2 |
Cabozantinib Nivolumab Axitinib (PFS benefit only) |
If not used previously: Cabozantinib Nivolumab PFS benefit only: Axitinib Everolimus Sorafenib |
| Intermediate | ||||
| Poor (can choose any first-line option) | Temsirolimus (only in poor-risk patients) |
IFN, interferon; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IL-2, interleukin 2; OS, overall survival.
Bone metastases
The available data show hints that there may be specific subpopulations of patients with advanced RCC or mRCC who are more likely to benefit from cabozantinib [Ciccarese et al. 2015]. Bone metastases depend on complex interactions between tumor cells, osteoblasts, and osteoclasts, and they can occur in almost 35% of patients with advanced RCC or mRCC [Woodward et al. 2011]. Bone metastases can be a difficult problem in advanced RCC or mRCC because of the risk of pathological fractures, hypercalcemia, spinal cord compression, and the need for narcotics to control pain. There is evidence that bone metastases are not well controlled and do not respond well to VEGF inhibitors [Plimack et al. 2009]. MET is expressed by both osteoclasts and osteoblasts, and its signaling is important for their activity and bone growth. HGF can substitute for macrophage colony-stimulating factor to promote survival and induce differentiation of CD14+ monocytes to osteoclasts [Adamopoulos et al. 2006]. HGF can also be secreted by osteoblasts and osteoclasts [Taichman et al. 2001]. HGF produced by osteoblasts induces migration of cancer cells from sinusoidal capillaries to the bone marrow space, where it stimulates growth of cancer cells in the bone microenvironment. HGF induces osteoblasts to enter the cell cycle and to express bone morphogenetic protein 2, which is required for bone formation and repair [Tsai et al. 2012]. Thus, osteoblasts appear to promote bone metastasis of some cancers via HGF–MET signaling. Agents targeting only one of these pathways have not shown similar effects [Lee et al. 2013].
The VEGF pathway is also important to both osteoblasts and osteoclasts, and is involved in their activity and survival. Both cell types express VEGFR and are involved in mineralization of normal bone [Marini et al. 2012]. In one study, murine osteoblasts were shown to express VEGFR1 and VEGFR2 during differentiation, as well as the ligand VEGF-A, which is maximally expressed during mineralization [Deckers et al. 2000]. Thus, similar to HGF–MET signaling, VEGF–VEGFR signaling mediates both autocrine and paracrine effects of the activities and survival of osteoblasts and osteoclasts. Although the precise interactions between vascular cells and bone-forming cells are unclear, VEGFR signaling may mediate the close association between angiogenesis and bone formation in fracture healing and may play a similar role in the pathogenesis of bone metastasis [Lee et al. 2013]. Given this evidence, inhibition of both MET and VEGRF may be an effective strategy for treating this specific subpopulation of patients with advanced RCC or mRCC and bone metastases.
Cabozantinib in the third-line setting
Cabozantinib should be considered as an option in the third-line setting after treatment with TKIs. Retrospective data showed that in patients previously treated with nivolumab, TKIs remain active in subsequent lines of treatment, which supports the use of cabozantinib after immune checkpoint inhibitors [Albiges et al. 2015; Malouf et al. 2016].
Future trends
The potential of cabozantinib will continue to be explored in patients with advanced RCC or mRCC in studies such as a phase II trial of cabozantinib versus sunitinib in the first-line setting for advanced RCC or mRCC [ClinicalTrials.gov identifier: NCT01835158], which recently had a press release declaring a statistically significant improvement in PFS for cabozantinib compared with sunitinib in patients with advanced intermediate- or poor-risk RCC [Business Wire, 2016]. Immunotherapy has led to a breakthrough in the treatment of malignant diseases including mRCC. Cabozantinib in combination with nivolumab with or without ipilimumab is currently being studied as part of a phase I trial in patients with metastatic genitourinary tumors, including RCC [ClinicalTrials.gov identifier: NCT02496208].
Currently we lack biomarkers in advanced RCC or mRCC to select the best treatment option for patients and we base our treatment selection on a patient’s relative contraindications to certain therapy, the results of clinical trials, and subgroup analyses to guide the therapy decision. Of note, MET expression was investigated by immunohistochemistry as a potentially predictive biomarker for cabozantinib in the METEOR study population. However, the results suggest that the MET expression level might not affect treatment outcomes with cabozantinib in this patient population, which might reflect the broader target profile of cabozantinib [Choueiri et al. 2016]. Next-generation sequencing (NGS) may help us identify mutations to assist in biomarker discovery. NGS is revolutionizing knowledge of human genome diversity at both the germline level (population) and the somatic level (tumors) [Real et al. 2014]. In RCC, signatures have been identified that are independently associated with outcome, including those revealing metabolic remodeling of tumors and having potential as therapeutic targets [Cancer Genome Atlas Research Network, 2013; Sato et al. 2013].
Conclusion
Cabozantinib is an oral TKI therapeutic agent that targets VEGFR2, MET, and AXL. Resistance to first-line therapy and MET upregulation occur as a result of VEGFR-targeted treatment. Preclinical studies have shown that one way to overcome this problem is by using cabozantinib to suppress MET and AXL. In a recent randomized phase III trial, cabozantinib was superior to everolimus in the second-line setting for treating advanced RCC or mRCC in terms of RR, PFS, and OS. The side effects include high blood pressure, diarrhea, and fatigue, which are commonly seen with other VEGFR TKI therapies and are easily managed. Preclinical studies, reports from prostate cancer trials, and subgroup analysis of the METEOR trial have shown that patients with good performance status and bone and visceral metastases benefit the most from this drug. Currently, cabozantinib is a second-line drug for treating patients with advanced RCC or mRCC that offers benefits in terms of OS, PFS, and RR. Cabozantinib is now being studied in the first-line setting for patients with advanced RCC or mRCC, and those with metastatic genitourinary tumors, including RCC, in a phase I trial in combination with other immunotherapy agents such as ipilimumab and nivolumab.
Acknowledgments
The authors acknowledge ‘Consejo Nacional de Ciencia y Tecnologia’, ‘Sociedad Mexicana de Oncologia’ and ‘Instituto Cientifico Pfizer’ for their support of Dr Ruiz-Morales’ genitourinary tumors fellowship at the University of Calgary, Tom Baker Cancer Centre.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Jose Manuel Ruiz-Morales has received travel, accommodation and expenses from Janssen-Cilag, MSD Oncology, Bristol-Myers Squibb, and Pfizer. Daniel Y.C. Heng has consultancies with Bayer, Bristol-Myers Squibb, Exelixis, Pfizer, and Novartis.
Contributor Information
Jose Manuel Ruiz-Morales, Tom Baker Cancer Center, Alberta Health Services Cancer Care, University of Calgary, Calgary, Alberta, Canada; Hospital Medica Sur, Mexico City, Mexico.
Daniel Y.C. Heng, Tom Baker Cancer Center, Alberta Health Services Cancer Care, University of Calgary, 1331 29th St NW, Calgary, Alberta, Canada T2N 4N2.
References
- Adamopoulos I., Xia Z., Lau Y., Athanasou N. (2006) Hepatocyte growth factor can substitute for M-CSF to support osteoclastogenesis. Biochem Biophys Res Commun 350: 478–483. [DOI] [PubMed] [Google Scholar]
- Albiges L., Fay A., Xie W., Krajewski K., McDermott D., Heng D., et al. (2015) Efficacy of targeted therapies after PD-1/PD-L1 blockade in metastatic renal cell carcinoma. Eur J Cancer 51: 2580–2586. [DOI] [PubMed] [Google Scholar]
- Baldewijns M., Van Vlodrop I., Vermeulen P., Soetekouw P., Van Engeland M., De Bruine A. (2010) VHL and HIF signalling in renal cell carcinogenesis. J Pathol 221: 125–138. [DOI] [PubMed] [Google Scholar]
- Bommi-Reddy A., Almeciga I., Sawyer J., Geisen C., Li W., Harlow E., et al. (2008) Kinase requirements in human cells: III. Altered kinase requirements in Vhl-/- cancer cells detected in a pilot synthetic lethal screen. Proc Natl Acad Sci USA 105: 16484–16489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro D., Liotta L. (2003) Cancer: out of air is not out of action. Nature 423: 593–595. [DOI] [PubMed] [Google Scholar]
- Business Wire (2016) Exelixis Announces Results from Randomized Phase 2 Trial CABOSUN Demonstrate Cabozantinib Significantly Improved Progression-free Survival Versus Sunitinib in Previously Untreated Advanced Renal Cell Carcinoma. New York, NY: Business Wire. [Google Scholar]
- Cancer Genome Atlas Research Network (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio U., Montorsi F. (2016) Renal cancer. Lancet 387: 894–906. [DOI] [PubMed] [Google Scholar]
- Ciccarese C., Massari F., Tortora G. (2015) Cabozantinib in advanced renal cell carcinoma: is it a METEOR? Eur Urol 69: 969–970. [DOI] [PubMed] [Google Scholar]
- Choueiri T., Escudier B., Powles T., Mainwaring P., Rini B., Donskov F., et al. (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri T., Escudier B., Powles T., Tannir N., Mainwaring P., Rini B., et al. (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17: 917–927. [DOI] [PubMed] [Google Scholar]
- Choueiri T., Pal S., McDermott D., Morrissey S., Ferguson K., Holland J., et al. (2014) A phase I study of cabozantinib (Xl184) in patients with renal cell cancer. Ann Oncol 25: 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P., Giordano S., Trusolino L. (2008) Drug development of met inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 7: 504–516. [DOI] [PubMed] [Google Scholar]
- Deckers M., Karperien M., Van Der Bent C., Yamashita T., Papapoulos S., Lowik C. (2000) Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 141: 1667–1674. [DOI] [PubMed] [Google Scholar]
- Elisei R., Schlumberger M., Muller S., Schoffski P., Brose M., Shah M., et al. (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31: 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B., Motzer R., Powles T., Tannir N, Davis I, Donskov F., et al. (2016) Subgroup analyses of meteor, a randomized phase 3 trial of cabozantinib versus everolimus in patients (pts) with advanced renal cell carcinoma (RCC). J Clin Oncol 34(Suppl. 2S; abstr 499). [Google Scholar]
- Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., et al. (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: 2103–2111. [DOI] [PubMed] [Google Scholar]
- FDA (2016) FDA approves cabozantinib for renal cell carcinoma. http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm497483.htm (accessed 20 June 2016).
- Gherardi E., Birchmeier W., Birchmeier C., Vande Woude G. (2012) Targeting met in cancer: rationale and progress. Nat Rev Cancer 12: 89–103. [DOI] [PubMed] [Google Scholar]
- Gibney G., Aziz S., Camp R., Conrad P., Schwartz B., Chen C., et al. (2013) C-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 24: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOBOCAN (2012) Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. France: International Agency for Research on Cancer; Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx [Google Scholar]
- Grullich C. (2014) Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res 201: 207–214. [DOI] [PubMed] [Google Scholar]
- Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281. [DOI] [PubMed] [Google Scholar]
- Klatte T., Pantuck A. (2008) Molecular biology of renal cortical tumors. Urol Clin North Am 35: 573–580, vi. [DOI] [PubMed] [Google Scholar]
- Kroeger N., Choueiri T., Lee J., Bjarnason G., Knox J., Mackenzie M., et al. (2014) Survival outcome and treatment response of patients with late relapse from renal cell carcinoma in the era of targeted therapy. Eur Urol 65: 1086–1092. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Sherman S., Ball D., Forastiere A., Cohen R., Mehra R., et al. (2011) Activity of Xl184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29: 2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Smith M. (2013) Targeting MET and vascular endothelial growth factor receptor signaling in castration-resistant prostate cancer. Cancer J 19: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger R., Keating A., Earp H., Graham D. (2010) Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets 14: 1073–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf G., Flippot R., Khayat D. (2016) Therapeutic strategies for patients with metastatic renal cell carcinoma in whom first-line vascular endothelial growth factor receptor-directed therapies fail. J Oncol Pract 12: 412–420. [DOI] [PubMed] [Google Scholar]
- Marini M., Sarchielli E., Toce M., Acocella A., Bertolai R., Ciulli C., et al. (2012) Expression and localization of Vegf receptors in human fetal skeletal tissues. Histol Histopathol 27: 1579–1587. [DOI] [PubMed] [Google Scholar]
- McDermott D., Regan M., Clark J., Flaherty L., Weiss G., Logan T., et al. (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23: 133–141. [DOI] [PubMed] [Google Scholar]
- McKay R., Kroeger N., Xie W., Lee J., Knox J., Bjarnason G., et al. (2014) Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol 65: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R., Escudier B., McDermott D., George S., Hammers H., Srinivas S., et al. (2015a) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R., Escudier B., Oudard S., Hutson T., Porta C., Bracarda S., et al. (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372: 449–456. [DOI] [PubMed] [Google Scholar]
- Motzer R., Escudier B., Tomczak P., Hutson T., Michaelson M., Negrier S., et al. (2013a) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14: 552–562. [DOI] [PubMed] [Google Scholar]
- Motzer R., Hutson T., Cella D., Reeves J., Hawkins R., Guo J., et al. (2013b) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369: 722–731. [DOI] [PubMed] [Google Scholar]
- Motzer R., Hutson T., Glen H., Michaelson M., Molina A., Eisen T., et al. (2015b) Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 16: 1473–1482. [DOI] [PubMed] [Google Scholar]
- Motzer R., Hutson T., Tomczak P., Michaelson M., Bukowski R., Oudard S., et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia V., Prando A. (2015) Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 48: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan V., Haas N. (2016) Axitinib in the treatment of renal cell carcinoma: patient selection and perspectives. Int J Nephrol Renovasc Dis 9: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffenroth E., Linehan W. (2008) Genetic basis for kidney cancer: opportunity for disease-specific approaches to therapy. Expert Opin Biol Ther 8: 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimack E., Tannir N., Lin E., Bekele B., Jonasch E. (2009) Patterns of disease progression in metastatic renal cell carcinoma patients treated with antivascular agents and interferon: impact of therapy on recurrence patterns and outcome measures. Cancer 115: 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin E., Fuh K., Castellini L., Viswanathan K., Finger E., Diep A., et al. (2014) Direct regulation of Gas6/Axl signaling by Hif promotes renal metastasis through Src and Met. Proc Natl Acad Sci USA 111: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real F., Boutros P., Malats N. (2014) Next-generation sequencing of urologic cancers: next is now. Eur Urol 66: 4–7. [DOI] [PubMed] [Google Scholar]
- Rini B., Escudier B., Tomczak P., Kaprin A., Szczylik C., Hutson T., et al. (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (axis): a randomised phase 3 trial. Lancet 378: 1931–1939. [DOI] [PubMed] [Google Scholar]
- Sato Y., Yoshizato T., Shiraishi Y., Maekawa S., Okuno Y., Kamura T., et al. (2013) Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 45: 860–867. [DOI] [PubMed] [Google Scholar]
- Sennino B., Mcdonald D. (2012) Controlling escape from angiogenesis inhibitors. Nat Rev Cancer 12: 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F., Lee J., Simmons B., Wong A., Esparza C., Plumlee P., et al. (2010) HGF/c-MET acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 70: 10090–10100. [DOI] [PubMed] [Google Scholar]
- Taichman R., Reilly M., Verma R., Ehrenman K., Emerson S. (2001) Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. Br J Haematol 112: 438–448. [DOI] [PubMed] [Google Scholar]
- Tsai S, Huang Y., Yang W., Tang C. (2012) Hepatocyte growth factor-induced BMP-2 expression is mediated by c-MET receptor, FAK, JNK, Runx2, and p300 pathways in human osteoblasts. Int Immunopharmacol 13: 156–162. [DOI] [PubMed] [Google Scholar]
- Voss M., Molina A., Motzer R. (2011) MTOR inhibitors in advanced renal cell carcinoma. Hematol Oncol Clin N Am 25: 835–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward E., Jagdev S., McParland L., Clark K., Gregory W., Newsham A., et al. (2011) Skeletal complications and survival in renal cancer patients with bone metastases. Bone 48: 160–166. [DOI] [PubMed] [Google Scholar]
- Zhou L., Liu X., Sun M., Zhang X., German P., Bai S., et al. (2016) Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 35: 2687–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]