Abstract
Insulin like growth factor binding protein two (IGFBP-2) is important for acquisition of normal bone mass in mice; however, the mechanism by which IGFBP-2 functions is not defined. These studies investigated the role of IGFBP-2 in stimulating osteoblast differentiation. MC-3T3 preosteoblasts expressed IGFBP-2, and IGFBP-2 knockdown resulted in a substantial delay in osteoblast differentiation, reduced osteocalcin expression and Alizarin red staining. These findings were replicated in primary calvarial osteoblasts obtained from IGFBP-2 −/− mice and addition of IGFBP-2 rescued the differentiation program. In contrast, overexpression of IGFBP-2 accelerated the time course of differentiation as well as increasing the total number of differentiating cells. By day 6 IGFBP-2 overexpressing cells expressed twice as much osteocalcin as control cultures and this difference persisted. To determine the mechanism by which IGFBP-2 functions, the interaction between IGFBP-2 and receptor tyrosine phosphatase β (RPTPβ) was examined. Disruption of this interaction inhibited the ability of IGFBP-2 to stimulate AKT activation and osteoblast differentiation. Knockdown of RPTPβ enhanced osteoblast differentiation whereas overexpression of RPTPβ was inhibitory. Adding back IGFBP-2 to RPTPβ overexpressing cells was able to rescue cell differentiation via enhancement of AKT activation. To determine the region of IGFBP-2 that mediated this effect an IGFBP-2 mutant that contained substitutions of key amino acids in the heparin binding domain-1 (HBD-1) was prepared. This mutant had a major reduction in its ability to stimulate differentiation of calvarial osteoblasts from IGFBP-2 −/− mice. Addition of a synthetic peptide that contained the HBD-1 sequence to calvarial osteoblasts from IGFBP-2 −/− mice rescued differentiation and osteocalcin expression. In summary, the results clearly demonstrate that IGFBP-2 stimulates osteoblast differentiation and that this effect is mediated through its heparin binding domain-1 interacting with RPTPβ. The results suggest that stimulation of differentiation is an important mechanism by which IGFBP-2 regulates the acquisition of normal bone mass in mice.
Keywords: IGFBP-2, Osteoblast differentiation, pAKT, PTEN, RPTPβ
Introduction
IGFBP-2 is a member of a family of six IGF binding proteins. Although a major function of this class of proteins is to transport the IGFs through the circulation and extracellular fluids, thereby restricting their access to receptors, each form of binding protein has been found to have distinct actions.(1) Initial studies showed that IGFBP-2 can enhance the effect of IGF-II to stimulate alkaline phosphatase in bone cell cultures.(2) When IGFBP-2 gene expression was deleted in mice, tibial bone volume was reduced and both micro CT and pQCT analysis showed diminished trabecular number and volume.(3) A subsequent study defined a 13 amino acid region of IGFBP-2 (termed HBD-1) that was required for biologic activity. Substitution of key residues within this region resulted in loss of the ability of IGFBP-2 to stimulate osteoblast proliferation in vitro.(4) Importantly when a synthetic peptide containing the HBD-1 sequence was injected into the IGFBP-2 −/− mice, micro CT analysis showed that trabecular volume and density could be rescued.(4) Furthermore this peptide was shown to stimulate osteoblast proliferation in vivo.
Prior studies have suggested that IGF-I and IGFBP-2 play a role in osteoblast differentiation. Cell type specific deletion of IGF-I in osteoblasts resulted in decreased femoral BMD and decreased bone formation rate.(5) Some studies have suggested a correlation between IGFBP-2 and osteoblast differentiation. During induction of differentiation in the osteoblast sheets, analysis of gene expression profiles showed that IGFBP-2 is one of the genes that showed the greater increase.(6) PTH increases IGFBP-2 expression in differentiated osteoblasts.(7) Finally mesenchymal stromal cells can be made to further differentiate into osteoblasts with dexamethasone and this requires the interaction of the α5 integrin and IGFBP-2.(8)
Although recent studies have documented the importance of the HBD-1 domain of IGFBP-2 for osteoblast growth, the relative importance of this domain for osteoblast differentiation has not been determined. A recent study demonstrated that the HBD-1 region bound directly to a cell surface receptor termed receptor tyrosine phosphatase β and that RPTP-β was expressed by MC-3T3 cells.(9) It further demonstrated that IGFBP-2 binding to this receptor induced RPTPβ dimerization which inhibited its phosphatase activity. Since the primary substrate of this phosphatase was shown to be PTEN, subsequent analysis showed that engagement of this receptor on osteoblast surfaces resulted in enhanced tyrosine phosphorylation of PTEN which inhibited its activity. This was associated with increased AKT activation. These data imply that IGFBP-2 may be functioning directly to augment constitutive AKT activation in osteoblasts. Since AKT activation has been linked to osteoblast differentiation(10), the current studies were undertaken to determine if IGFBP-2 could directly stimulate osteoblast differentiation, if it was functioning through interaction with RPTPβ and if this interaction was mediated through the HBD-1 domain.
Materials and Methods
Human IGF-I was a gift from Genentech (South San Francisco, CA). Immobilon-P membrane, LY294002 and PD98059 were purchased from EMDmillipore Corp. (Billerica, MA). α-MEM, streptomycin and penicillin were purchased from Life Technologies (Grand Island, NY). Anti-RPTPβ antibody was purchased from BD Bioscience (San Diego, CA). Antibodies against phospho-AKT (S473), pErk1/2, cleaved caspase-3 and PTEN were purchased from Cell Signaling Technology Inc. (Beverly, MA). Anti-phospho-tyrosine (PY99), osteocalcin, β-actin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PQ401 was purchased from TOCRIS bioscience (Bristol, United Kindom). IGFBP-2 antiserum was prepared as previously described.(11) The horseradish peroxidase-conjugated mouse anti-rabbit, goat anti-mouse, and mouse anti-rabbit light chain-specific antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were obtained from Sigma unless otherwise stated. The synthetic peptide containing the linker located heparin-binding domain of IGFBP-2 (188KHLSLEEPKKLRP200) (referred to as HBD-1 peptide) and a scrambled HBD peptide (CKPLRLSKEEHPLK) (referred to as HBD control peptide), were synthesized by the Protein Chemistry Core Facility at the University of North Carolina at Chapel Hill. Purity and the sequences were confirmed by mass spectrometry.
Mice
Generation of the original mixed background strain B6;129-Igfbp2<tm1Jep>, which we refer to as Igfbp2−/− mice, has been described previously.(3) The original mice were backcrossed onto C57BL/6J background for 10 generations. Igfbp2+/+ mice were C57BL/6J controls. All of the experimental studies were performed with male mice. All of the animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill.
Cell culture
MC-3T3 E1 clone 4 (CL4) cells were obtained from ATCC (Manassas, VA). Cells were cultured in α-MEM (glucose 1000mg/L) containing 10% fetal bovine serum (Thermo Fishers Scientific, Pittsburgh, PA). After confluency, culture medium was changed to differentiation medium (DM) which contained 10% fetal bovine serum plus 50 ug/ml ascorbic acid and 4 mM β-glycerol phosphate. Fresh DM was applied every 72 hr. IGFBP-2 (1 ug/ml), HBD-1 (1ug/ml or as stated), control peptide (1ug/ml) or HBD mutant IGFBP-2 (1 ug/ml) were added to the differentiation medium, and replaced every 72 hr.
Neonatal calvarial osteoblasts were isolated from 3–5-day-old mice. Briefly, calvariae were digested five times with collagenase type 2 (250 unit/ml) and trypsin (0.05%) plus EDTA (0.02%) in the PBS. The cells released from digests 2–5 were collected as primary calvarial osteoblasts and maintained in DMEM (glucose 1000 mg/L) supplemented with 10% FBS and nonessential amino acids.
Construction of cDNAs and establishment of MC3T3 cells expressing wild type IGFBP-2, RPTPβ and LacZ
Mouse IGFBP-2 cDNA was amplified from mouse pCMV-SPORT6 (ATCC, Manassas, VA) using a 5′ primer sequence corresponding to nucleotides 89 to 110 of mouse IGFBP-2 (5′-ATGCTGCCGAGATTGGGCGGCC-3′) and a 3′ primer sequence complementary to nucleotides 981 to 1003 (5′-GGGCCCATGCCCAAAGTGTGCAG-3′). After DNA sequencing to confirm that the correct sequence had been amplified, the PCR product was subcloned into pENTR/D-TOPO vector and subsequently transferred into the pLenti6-V5 DEST expression vector using the LR Clonase reaction and following the manufacturer's instructions (Life Technologies, Grand Island, NY). Constructions of RPTPβ and LacZ have been described previously.(9) The constructs contained the correct sequences was verified by DNA sequencing. 293FT cells (Life Technologies, Grand Island, NY) were prepared for generation of virus stocks and CL4 expressing IGFBP-2, RPTPβ and LacZ were established using procedures that have been described previously.(12)
Construction of cDNAs and establishment of IGFBP-2 Si, RPTPβ Si and LacZ Si cells
Based on Life Technologies’ website design tools, a sequence containing 21 oligonucleotides (GGAAAGAGACCAACACTGAGC) was used to construct the shRNA template plasmid to inhibit the translation of mouse IGFBP-2 mRNA. GCCAATGCATACAGCAGTAAT was used to construct the shRNA template to knock down mouse RPTPβ. The oligonucleotides were synthesized by Nucleic Acids Core Facility at UNC, annealed and ligated into BLOCK-iT™ U6 RNAi Entry Vector (Cat# K4945-00, Life Technologies, Grand Island, NY) following manufacturer’s instructions. The complete sequence was verified by DNA sequencing. The expression vector was generated using the Gateway LR recombination reaction between the Entry Vector and BLOCK-iT™ Lentiviral RNAi Gateway® Vector (Cat# K4943-00, Life Technologies, Grand Island, NY). A sequence targeting LacZ was used as a control. After confirmation of the sequence, plasmid DNA was prepared using a Plasmid Midi Kit (Promega, Madison, WI). 293FT cells (Life Technologies, Grand Island, NY) were transfected and used to prepare for generation of virus stocks.(12) CL4 cells expressing small hairpin RNA sequence targeting IGFBP-2 (IGFBP-2 Si), RPTPβ (RPTPβ Si) and corresponding control CL4 expressing small hairpin RNA sequence targeting LacZ (LacZ Si) were established using procedures described previously.(12)
Immunoprecipitation and Immunoblotting
The cell monolayers were lysed in a modified radioimmunoprecipitation assay (RIPA) buffer as previously described.(12) Immunoprecipitation was performed by incubating 0.5 mg of cell lysate protein with 1 ug of each of the following antibodies: anti-IGFBP-2 and PY99 at 4°C overnight. Immunoblotting was performed as previously described(12) using a dilution 1:1000 for anti-pAKT (Ser473), PTEN and β-actin antibodies, a dilution 1:500 for anti-RPTPβ antibody, a dilution 1:200 for anti-osteocalcin antibody and a dilution 1:10000 for anti-IGFBP-2 antibody. The proteins were visualized using enhanced chemiluminescence (Thermo Fisher Scientific, Rockford, IL). Total cellular protein in the lysates was determined using BCA (Thermo Fisher Scientific, Rockford, IL).
Alizarin Red staining
Cells were washed with PBS twice before were fixed with 10% formalin. After 10 min fixation, 1% Alizarin Red (pH 4.2) was applied and incubated for another 10 min before it was removed. Cells were washed with ddH2O twice and drying. Images were captured using Leica M420 Microscope.
RNA isolation and quantitative real-time PCR
Total RNA was prepared using RNeasy plus mini kit (Qiagen, Valencia, CA, USA) for cellular extracts. cDNA was then generated from 500 ng of RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) per the manufacturer’s instructions. Quantitative real-time expression analysis was run on the CFX384 Real-time System using the iQ SYBR Green Supermix and C1000 thermal Cycler (Bio-Rad, Hercules, CA, USA). Relative expression of mRNA was determined after normalization to Hprt levels using the ΔCt method. Primers were designed, sequenced and validated to be 95% to 100% efficient by Primer Design Ltd (Southampton, UK). All primer sequences are listed in Supplementary Table 1.
Cell proliferation and apoptosis assay
Calvarial osteoblasts isolated from IGFBP-2 −/− mice were seeded in 6 well plate. After reaching confluency the culture medium was changed to DM or DM plus HBD-1 or DM plus IGFBP-2. Control cells and IGFBP-2 overexpressing cells were plated in 24 well plates using the same plating density. After reaching confluency the culture medium was changed to DM. Fresh DM was applied every 72 hr. After cells were exposed to DM for indicated days, the cells were released with 0.05% Trypsin-EDTA and counted.
To quantify apoptosis, calvarial osteoblasts isolated from IGFBP-2 −/− mice were exposed to DM alone or DM plus the different concentrations of HBD-1 peptide for 21 days. Cell lysates were harvested as described previously and immunoblotted using an anti-cleaved caspase-3 antibody.
Statistical analysis
Densitometry results are expressed as the mean ± standard deviation (SD). All experiments were replicated at least three times to assure reproducibility. The results were analyzed for statistically significant differences using Student’s t-test or analysis of variance followed by Bonferroni multiple comparison post hoc test. Statistical significance was set at p<0.05.
Results
IGFBP-2 stimulates osteoblast differentiation
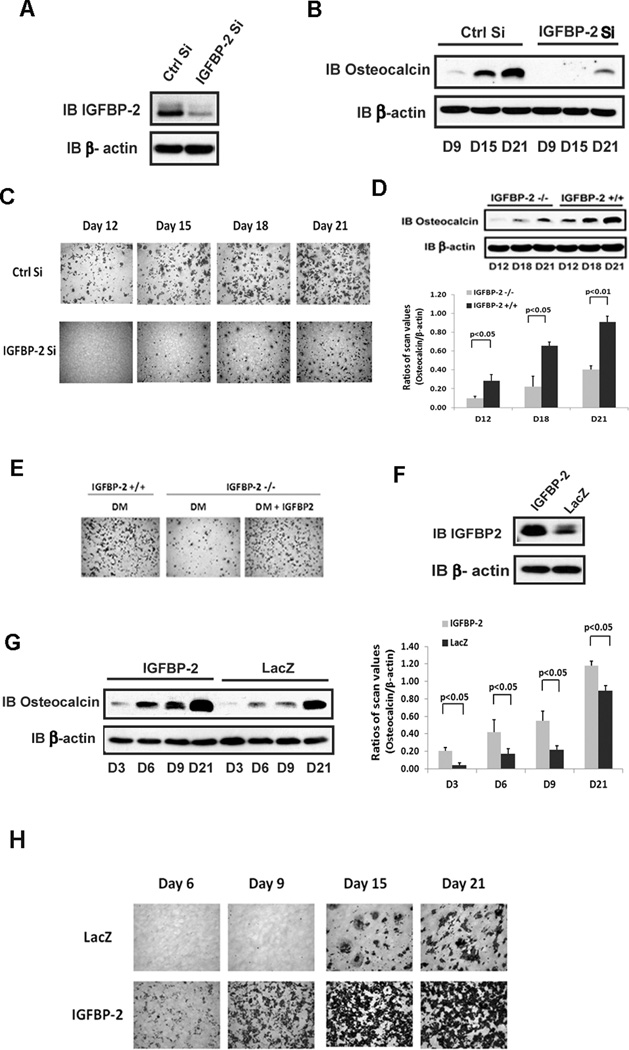
Since we had shown that IGFBP-2 enhances AKT activation in osteoblasts,(9) we determined if IGFBP-2 regulates osteoblast differentiation. MC-3T3 cells have been shown to secrete IGFBP-2 and its secretion increases significantly between day 6 and 9 following the addition of differentiation medium. RNAi was used to determine the significance of these changes and if inhibiting IGFBP-2 synthesis would alter differentiation (Fig 1A). Compared to control cultures the differentiation of MC-3T3 cells in which IGFBP-2 synthesis had been inhibited was significantly attenuated. Both osteocalcin expression (Fig 1B) and the number of alizarin red positive cells were reduced (Fig 1C).
Figure 1. IGFBP-2 stimulates osteoblast differentiation.
(A) Equal amounts of cell lysate from MC-3T3 cells expressing a shRNA sequence targeting LacZ (Ctrl Si) or IGFBP-2 (IGFBP-2 Si) were immunoblotted with indicated antibody. β-actin was immunoblotted as a loading control. (B) Cell lysate from Ctrl Si or IGFBP-2 Si cells on the indicated day after differentiation medium (DM) exposure were immunoblotted with the indicated antibody. (C) Ctrl Si or IGFBP-2 Si expressing cells were stained by Alizarin Red following the procedure described in “Materials and Methods” on indicated day after DM exposure. (D) Cell lysates from IGFBP-2−/− or IGFBP-2 +/+ derived calvarial osteoblasts prepared on indicated day after DM exposure were immunoblotted with indicated antibody. The bar graph shows the ratio of scanning densitometry units of osteocalcin/β-actin obtained from three individual experiments. (E) Calvarial osteoblasts isolated from IGFBP-2 +/+ or IGFBP-2 −/− mice were exposed to DM alone or DM plus IGFBP-2 (1 ug/ml) and stained with Alizarin Red on day 21. (F) Lysates from cells expressing LacZ or IGFBP-2 were immunoblotted with indicated antibody. (G) Lysates from cells expressing LacZ or IGFBP-2 on indicated day after DM exposure were immunoblotted with indicated antibody. β-actin was immunoblotted as a loading control. The bar graphs show the ratio of scanning densitometry units of osteocalcin/β-actin obtained from three individual experiments. (H) Cells expressing LacZ or IGFBP-2 were stained with Alizarin Red on indicated day after DM exposure.
To confirm the importance of IGFBP-2 for differentiation of preosteoblasts, calvarial pre-osteoblasts, isolated from IGFBP-2 −/− mice were analyzed. These cells showed impaired osteocalcin expression and differentiation compared to cells from control littermates (Fig 1D) (e.g., a 2.3 ± 0.1 fold greater level of osteocalcin in control cells on day 21, compared to IGFBP-2 −/− cells, p<0.01). The time course of differentiation was prolonged in cultures from the IGFBP-2 −/− mice and only 1.8 ± 0.2% cells had completed differentiation by day 21 (Fig 1E). The addition of IGFBP-2 to these cultures restored differentiation (Fig 1E). In contrast, overexpression of IGFBP-2 in MC-3T3 cells significantly enhanced the osteocalcin expression (Fig 1G) (e.g., a 2.5 ± 0.1 fold greater level of osteocalcin on day 6 compared to control cultures, p<0.05). In addition, in IGFBP-2 overexpressing cells osteocalcin was detected on day 3 whereas it was detected on day 6 following the addition of differentiation medium in control cells (Fig 1G). Major differences in osteocalcin expression were detected at each time point and persisted up to day 21. In addition alizarin red positive cells were detected on day 6 in IGFBP-2 overexpressing cells whereas they were not detected until day 15 in control cells (Fig 1H). To confirm these results, we analyzed the expression of several genes that are important for osteoblast differentiation. As shown in supplemental figures 1 and 2, osteocalcin, alkaline phosphatase and Wnt10b were induced significantly in the IGFBP-2 overexpressing cells compared to control cells on days 6 and 9, thereby reflecting the acceleration of differentiation. Osteopontin was significantly increased at day 9. Runx2 and osterix whose expression peaks early in differentiation declined between days 3 and 9 in the control cells and were significantly decreased in the IGFBP-2 overexpression cells whereas following IGFBP-2 knockdown, their expression was increased compared to control cultures, suggesting that differentiation is delayed (Supplemental Fig 1 and 2).
Overexpression of IGFBP-2 reduced the amount of serum supplementation that was necessary to induce differentiation. For example the addition of 5% serum containing differentiation medium to IGFBP-2 transfected cells induced a similar level of cell differentiation compared to control cells exposed to 10% serum (Supplemental Fig 3A). IGF-I stimulated differentiation of control and IGFBP-2 overexpressing cells but the percentage of cells that differentiated remained significantly greater in the IGFBP-2 overexpressing cells (Supplemental Figure 3B and C). These results strongly suggest that IGFBP-2 is able to stimulate preosteoblast differentiation and that it is one of the factors that is present in 10% FBS that induces these changes.
AKT and PI-3 kinase activation are required for osteoblast differentiation
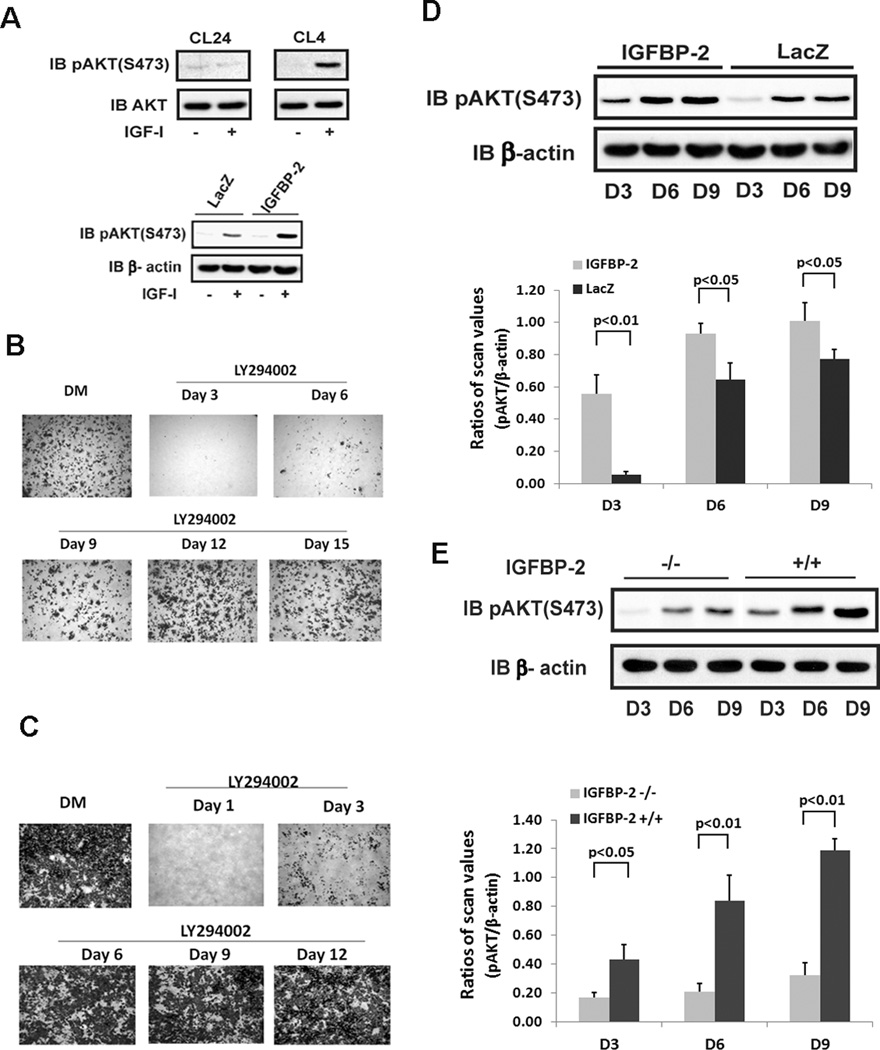
Several sub clones of MC-3T3 cells were originally derived from mouse calvarial osteoblasts.(13) Among them: clone 4 cells (CL4 cells) were found to differentiate in the appropriate medium. Since previous studies have shown the importance of AKT activation for osteoblast differentiation(14), we determined the IGF-I-stimulated AKT activation response during differentiation. The results showed that AKT Ser473 phosphorylation was stimulated by IGF-I during differentiation phase in MC-3T3, CL4 cells (Fig 2A). A clone of MC-3T3 cells derived from the same parental cell line (CL24) is unable to differentiate. When these cells were analyzed neither basal nor IGF-I-stimulated AKT Ser473 phosphorylation could be detected after several days in differentiation medium (Fig 2A). Importantly, overexpression of IGFBP-2 enhanced IGF-I stimulated AKT activation (Fig 2A)
Figure 2. AKT activation is required for osteoblast differentiation.
(A) Lysates obtained from MC-3T3 cells, clone 24 (CL24) or clone 4 (CL4) on day 6 after DM exposure were immunoblotted with indicated antibody. Lysates from quiescent cells expressing LacZ or IGFBP-2 on day 6 after DM exposure were immunoblotted with the indicated antibody. Wild type MC-3T3 cells (B) or IGFBP-2 overexpressing cells (C) were stained by Alizarin Red on day 21 after differentiation medium (DM) alone or DM plus LY294002 which was added on the indicated day. The medium was changed every 72hr. (D) Lysates obtained from cells overexpressing IGFBP-2 or LacZ after indicated day of DM exposure were immunblotted with indicated antibody. The bar graph shows the ratio of scanning densitometry units of pAKT/β-actin obtained from three individual experiments. (E) Lysates from IGFBP-2−/− or IGFBP-2 +/+ derived calvarial osteoblasts obtained on indicated day after DM exposure were immunoblotted with indicated antibody. The bar graph shows the ratio of scanning densitometry units of pAKT/β-actin obtained from three individual experiments.
In order to determine the time point in the differentiation cycle wherein PI-3 kinase activation was required to induce preosteoblast differentiation, the PI-3 kinase inhibitor, LY 294002, was utilized. The results show that inhibition of PI-3 kinase completely prevented differentiation when the inhibitor was applied on day 3 following the addition of differentiation medium (Fig 2B). It also significantly suppressed differentiation when it was applied on day 6. However if it was applied after day six the inhibitory effect was minimal at day 9 and completely lost by day 12 or later (Fig 2B). When this experiment was repeated using cells overexpressing IGFBP-2, differentiation was detectible and was attenuated when the inhibitor was added on day 1 or day 3 but it was not altered if the inhibitor was added at day 6 or later (Fig 2C).
Since inhibition of PI-3 kinase activation prevented differentiation, we examined the time course of AKT phosphorylation at different time points during differentiation in control and IGFBP-2 overexpressing cells. AKT activation was minimal at day 3 in control cells but it was increased significantly in the overexpressing cells (Fig. 2D). (e.g., to a level that was 7.5 fold greater at day 3, p<0.001). These results are consistent with the differences in osteocalcin expression shown in Fig 1G. When AKT activation was analyzed in primary osteoblasts that were obtained from IGFBP-2 −/− mice there was a significant reduction in constitutive pAKT expression compared to cells from control +/+ animals on days 3, 6 and 9 (Fig 2E) (e.g., to a level that was 3.1 ± 0.4 fold less than osteoblasts from +/+ mice at day 9, p<0.01). Importantly the differences in constitutive AKT activation correlated with those detected in osteocalcin expression (Fig 1D).
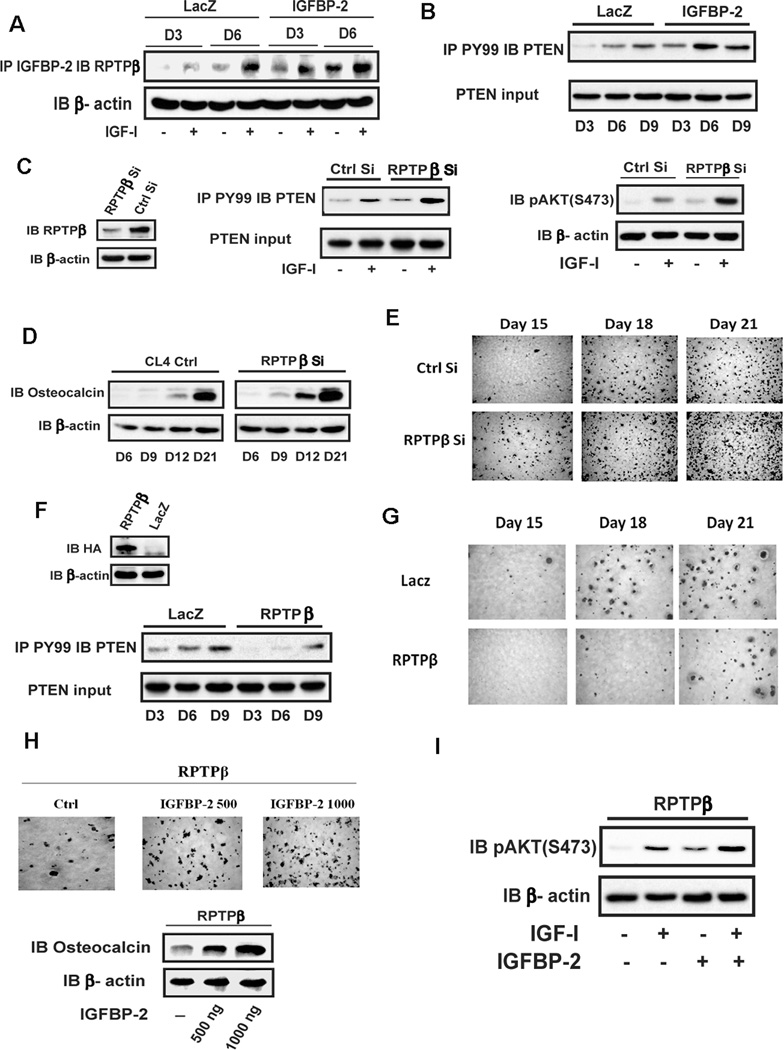
IGFBP-2 enhances AKT activation via suppressing RPTPβ dephosphorylation of PTEN
Our previous study in smooth muscle cells showed that IGFBP-2 enhances IGF-I-stimulated AKT activation via direct binding of IGFBP-2 to RPTPβ which catalyzes its polymerization and thereby inhibits its ability to dephosphorylate PTEN.(9) That study also showed that MC-3T3 cells expressed RTPTβ and that IGFBP-2 exposure increased PTEN tyrosine phosphorylation. Since tyrosine phosphorylation of PTEN attenuates its ability to inhibit AKT activation, this results in an enhancement of constitutive and IGF-I-stimulated AKT phosphorylation. RPTPβ expression increases during osteoblast differentiation(15), therefore we hypothesized that IGFBP-2 enhanced AKT activation during osteoblast differentiation through this same mechanism. To test this hypothesis we first examined whether IGFBP-2 overexpression and IGF-I addition stimulated IGFBP-2/RPTPβ association during differentiation. The results showed that the formation of the IGFBP-2/RPTPβ complex was detected on day 6 in control cultures (Fig 3A). This is consistent with the level of constitutive AKT activation (Fig 2D). Following IGF-I stimulation, complex formation increased in control cells and, in cells overexpressing IGFBP-2, there was an increase in basal and IGF-I stimulated IGFBP-2/RPTPβ association on days 3 and 6 compared to control cells (Fig 3A) (e.g., 3.6 ± 0.9 fold and 2.6 ± 0.1 fold increases in IGF-I stimulated complex formation in IGFBP-2 overexpressing cells compared to LacZ cells on days 3 and 6, p<0.05, respectively). Correspondingly PTEN tyrosine phosphorylation was increased in the IGFBP-2 overexpressing cells compared to control cells on days 3 and 6 (Fig 3B). To directly determine the role of RPTPβ on osteoblast differentiation, we manipulated the RPTPβ level in MC-3T3 cells. Knockdown of RPTPβ during the differentiation phase enhanced basal and IGF-I stimulated PTEN tyrosine phosphorylation as well as AKT activation (Fig 3C) (e.g., a 2.3 ± 0.2 fold greater level of pAKT expression in RPTPβ Si cells compared to control, p<0.05). Further analysis showed that osteocalcin expression was enhanced on days 9, 12, and 21 (Fig 3D) as well as cell differentiation on days 15, 18 and 21 compared to control cultures (Fig 3E). In contrast overexpression of RPTPβ inhibited PTEN tyrosine phosphorylation (Fig 3F) and impaired osteoblast differentiation (Fig 3G).
Figure 3. IGFBP-2 enhances AKT activation via suppressing RPTPβ dephosphorylation of PTEN.
(A) Cell lysates from quiescent LacZ or IGFBP-2 overexpressing cells were immunoprecipitated with an anti-IGFBP-2 antibody and immunoblotted with an anti-RPTPβ antibody. β-actin was immunoblotted as a loading control. (B) Lysates from LacZ or IGFBP-2 overexpressing cells on indicated day after differentiation medium (DM) exposure were immunoprecipated with an anti-PY99 antibody and immunoblotted with an anti-PTEN antibody. PTEN was immunoblotted as an input control. (C, D) Lysates from MT-3C3 cells expressing shRNA sequence targeting LacZ (Ctrl Si) or RPTPβ (RPTPβ Si) were immunoblotted with the indicated antibodies. (E) Cells expressing Ctrl Si or RPTPβ Si were stained by Alizarin Red on indicated day after DM exposure. (F) Lysates from LacZ or RPTPβ overexpressing cells were immunoblotted with anti-HA and β-actin antibodies. The same cell lysates were immunoprecipitated with an anti-PY99 antibody and immuoblotted with an anti-PTEN antibody. PTEN was immunoblotted as an input control. (G) Cells expressing LacZ or RPTPβ were stained by Alizarin Red on indicated day after DM exposure. (H) Cells expressing RPTPβ were stained by Alizarin Red on day 21 after DM alone or DM plus IGFBP-2 exposure. Lysates from the same RPTPβ overexpressing cultures were immunoblotted with anti-osteocalcin and β-actin antibodies. (I) Lysates from quiescent RPTPβ overexpressing cells obtained on day 6 after IGF-I alone or IGFBP-2 alone (1ug/ml) or IGF-I plus IGFBP-2 (1 ug/ml) were immunoblotted with anti-pAKT and β-actin antibodies.
We have shown previously that IGFBP-2 binding to RPTPβ induces polymerization which inhibits its phosphatase activity. When IGFBP-2 was added back to the RPTPβ overexpressing cells there was enhanced osteocalcin expression at day 12 and this also rescued differentiation (Fig 3H). Correspondingly the addition of IGFBP-2 enhanced basal (e.g., a 3.9 ± 0.4 fold greater compared to control, p<0.01) and IGF-I-stimulated AKT phosphorylation (e.g., a 1.7 ± 0.2 fold greater compared to no IGFBP-2 treatment, p<0.05) (Fig 3I). These results clearly show that IGFBP-2 regulation of RPTPβ activity plays an important role in preosteoblast differentiation and that RPTPβ regulates osteoblast differentiation through modulation of PTEN tyrosine phosphorylation.
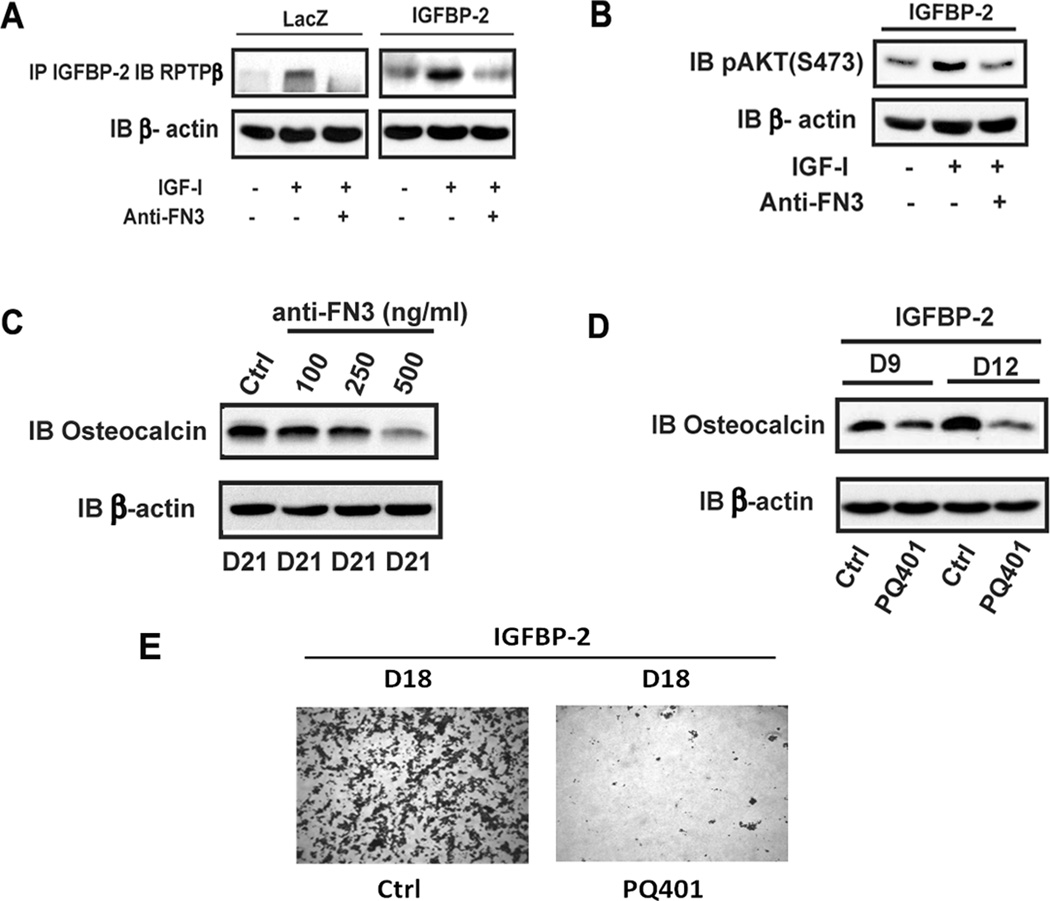
Disruption of IGFBP-2/RPTPβ interaction impairs IGF-I-stimulated AKT activation and osteoblast differentiation
To confirm the importance of the IGFBP-2/RPTPβ interaction, MC-3T3 cells overexpressing IGFBP-2 were analyzed. Following the addition of IGF-I there was a significant increase in IGFBP-2/RPTPβ association (Fig 4A) (e.g., a 2.5 ± 0.2 fold greater level of complex formation in IGFBP-2 overexpressing cells compared to control cells p< 0.05). To inhibit this interaction we utilized an anti-RPTPβ blocking antibody and determined its effect on the IGFBP-2/RPTPβ interaction, downstream signaling and differentiation. The antibody inhibited IGF-I stimulated IGFBP-2/RPTPβ association in control and IGFBP-2 overexpressing cells (Fig 4A). Disruption of their interaction was functionally significant since it inhibited AKT activation (Fig 4B) and osteocalcin expression (Fig 4C) (e.g., 74 ± 8% reduction in osteocalcin with 500ng/ml, p<0.01). Since IGF-I stimulates IGFBP-2/RPTPβ association that is critical for AKT activation(9) and osteoblast differentiation, we determined whether the requirement for IGF-I was changed when IGFBP-2 was overexpressed. To block IGF-I signaling, PQ401, a specific inhibitor of IGF-I receptor tyrosine kinase was used. The results show that PQ401 treatment significantly impaired osteocalcin expression and cell differentiation in IGFBP-2 overexpressing cells (Fig 4D and E).
Figure 4. Disruption of IGFBP-2/RPTPβ interaction impairs IGF-I-stimulated AKT activation and osteoblast differentiation.
(A) Lysates from quiescent LacZ or IGFBP-2 overexpressing cells obtained on day 6 after differentiation medium (DM) exposure treated with or without IGF-I alone (10 min) or IGF-I following a 4 hr exposure to anti-fibronectin domain (FN3) antibody were immunoprecipitated using an anti-IGFBP-2 antibody and immunoblotted with an anti-RPTPβ antibody (B) Lysates from quiescent IGFBP-2 overexpressing cells that received the same treatments as in panel A were obtained on Day 6 after DM exposure and were immunoblotted with indicated antibody. (C) Lysates from LacZ overexpressing cells obtained on day 21 after DM exposure following incubation with the indicated concentration of anti-fibronectin antibody (FN3) were immunoblotted with anti-osteocalcin and anti-β-actin antibodies. (D) Lysates from IGFBP-2 overexpressing cells obtained on day 9 and 12 after DM exposure following incubation with PQ401 (10 uM) were immunoblotted with anti-osteocalcin and anti-β-actin antibodies. (E) Cells expressing IGFBP-2 that had been incubated with or without PQ401 were stained with Alizarin Red on day 18 after DM exposure.
HBD-1 peptide mediates the IGFBP-2 effect on osteoblast differentiation
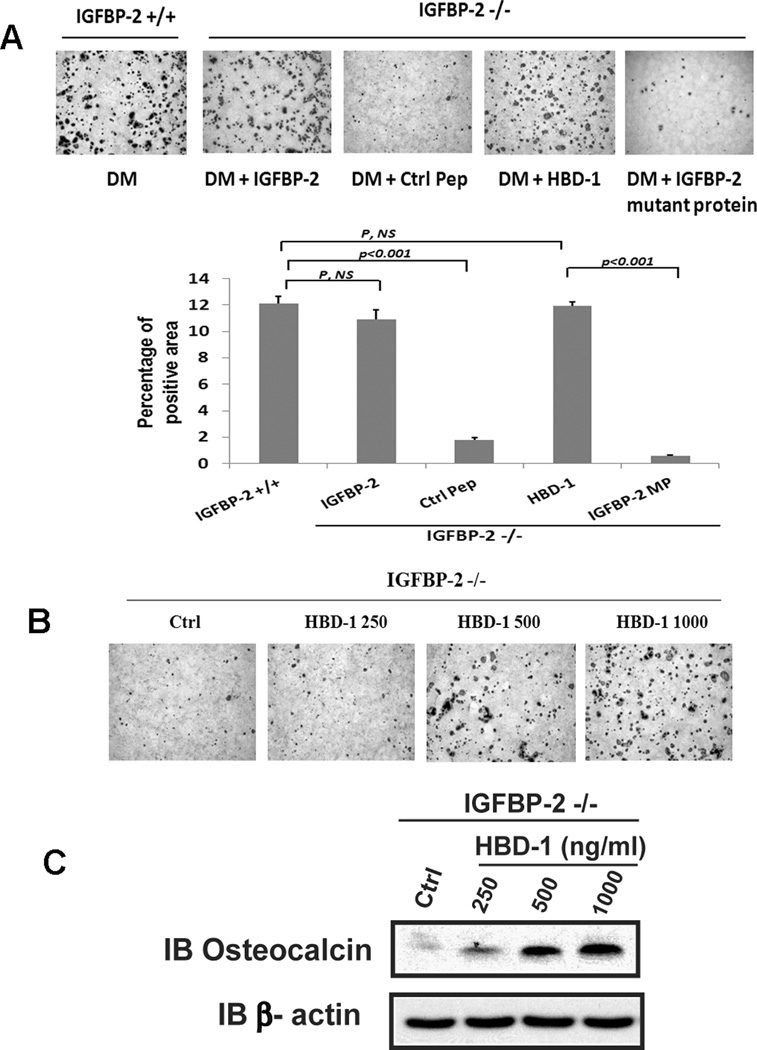
The HBD-1 domain of IGFBP-2 mediates its stimulatory effect on osteoblast proliferation.(4) To investigate the importance of the HBD-1 domain for osteoblast differentiation, we utilized an IGFBP-2 mutant in which the charged amino acids within the HBD-1 sequence were changed to alanine and an 13 amino acid synthetic peptide that contained this sequence and examined their abilities to alter the differentiation of calvarial preosteoblasts isolated from IGFBP-2 null mice. The results show that unlike wild type IGFBP-2 when the HBD-1 mutant form of IGFBP-2 was added, its ability to stimulate preosteoblast differentiation was significantly impaired (Fig 5A). To further investigate the function of HBD-1 domain, an HBD-1 peptide was added to differentiation medium. The peptide was able to rescue IGFBP-2 −/− cell differentiation (Fig 5A). When the results were quantified the differences were significant (Fig 5A). When increasing concentrations of the HBD-1 peptide were added a substantial increase in the percentage of cells that differentiated was noted at 500 ng/ml and it increased further with 1000 ng/ml (Fig 5B). When osteocalcin expression was analyzed this effect was confirmed and there was an incremental increase between 250 and 1000 ng/ml (Fig 5C). To determine whether HBD-1-enhanced cell differentiation was due to change of cell survival, we measured the cleaved caspase-3, an indicator for cell apoptosis. The results showed that the HBD-1 peptide had no effect on osteoblast apoptosis (Supplemental Figure 4A).
Figure 5. The heparin binding domain-1 (HBD-1) mediates the IGFBP-2 effect on osteoblast differentiation.
(A) Calvarial osteoblasts isolated from IGFBP-2 +/+ or IGFBP-2 −/− mice were exposed to differentiation medium (DM) alone, DM plus IGFBP-2 (1 ug/ml), DM plus control peptide (Ctrl Pep, 1 ug/ml), DM plus HBD-1 (1 ug/ml) or DM plus the HBD-1 IGFBP-2 mutant protein (IGFBP-2 MP, 1 ug/ml) then stained with Alizarin Red on day 21. The bar graph shows the percentage of stained area that was quantified using NIH Image J (1.47n). (B, C) Calvarial osteoblasts isolated from IGFBP-2 −/− mice were exposed to DM alone (Ctrl) or DM plus the indicated concentration of HBD-1 peptide for 21 days and stained with Alizarin Red (B). Cell lysates were immunoblotted with anti-osteocalcin and β-actin antibodies (C).
Discussion
Although IGFBP-2 functions with IGF-II to increase bone mass(16), and IGFBP-2 knockout mice have decreased cortical and trabecular bone(3–4), the specific role of IGFBP-2 in modifying osteoblast differentiation has not been reported. Prior studies showed that IGFBP-2 enhances the effect of both IGF-I and IGF-II in stimulating bone accretion in vivo.(4,16) Subsequently we showed that a peptide containing the HBD-1 sequence rescues the normal bone phenotype in IGFBP-2 −/− mice and that this peptide stimulated osteoblast proliferation.(4) These studies extend those findings to demonstrate that the HBD-1 peptide as well as intact IGFBP-2 stimulates osteoblast differentiation. The results clearly demonstrate that overexpression of IGFBP-2 results in acceleration of the differentiation program as well as increasing the total number of cells reaching the stage of mature osteoblast formation. Proteins that are markers of differentiation, such as osteocalcin, are increased in response to IGFBP-2 and they are expressed earlier in the differentiation program following IGFBP-2 stimulation. The results show that the expression of Wnt10b, alkaline phosphatase and osteopontin were increased in a similar manner. The effect of IGFBP-2 is mediated through the cell surface receptor RPTPβ since addition of an antibody which inhibited its binding to this receptor significantly attenuated its ability to stimulate signaling events that are linked to osteoblast differentiation. Moreover, knockdown of this receptor inhibited the ability of IGFBP-2 to stimulate differentiation.
That the HBD-1 domain was important for signaling within the intact protein was confirmed using site directed mutagenesis. Specifically addition a mutant with an altered HBD-1 sequence resulted in attenuated differentiation. Both the time course and the absolute number of cells as well as expression of osteocalcin were deceased. Our prior studies showed that an 13 amino acid peptide containing the HBD-1 sequence stimulated trabecular bone formation in IGFBP-2 −/− mice.(4) Keipe et al(17) demonstrated that a 117 amino acid carboxy terminal fragment of IGFBP-2 that would have contained the HBD-1 sequence exerted a strong mitogenic effect on growth plate chondrocytes and the effect was equal to intact IGFBP-2. These studies extend those observations to show that a peptide encompassing the sequence of HBD-1 is sufficient to stimulate osteoblast differentiation through its interaction with RPTPβ.
Our prior study showed that RPTPβ was present on the surface of MC-3T3 cells and that IGFBP-2 could interact with this protein to alter PTEN tyrosine phosphorylation.(9) IGFBP-2 stimulated RPTPβ polymerization thereby attenuating its phosphatase activity. Since PTEN is a RPTPβ substrate, this resulted in increased tyrosine phosphorylation of PTEN which attenuated PTEN enzymatic activity thereby leading to increased AKT phosphorylation. These studies extend those observations showing that enhancement of constitutive AKT phosphorylation occurs concomitantly with earlier differentiation in cells that overexpress IGFBP-2 and that these responses are attenuated when IGFBP-2 expression is diminished. That these changes are mediated through RPTPβ was proven by demonstrating that inhibition of IGFBP-2 binding to RPTPβ could block enhanced AKT activation and differentiation, and that knocking down of RPTPβ resulted in escape from its ability to inhibit AKT activation as well as a reduction in cell responsiveness to intact IGFBP-2. The studies also demonstrated that constituently synthesized IGFBP-2 is important for osteoblast differentiation. Knockdown of constituently synthesized IGFBP-2 resulted in attenuation of differentiation and expression of osteocalcin as well as constitutive AKT phosphorylation. Addition of an antibody that inhibits the binding of IGFBP-2 to RPTPβ could attenuate these responses in non-transfected MC-3T3 cells. Therefore, these results further support the conclusion that IGFBP-2 functions by attenuating RPTPβ mediating PTEN dephosphorylation and stimulating AKT activation, leading to enhanced osteoblast differentiation. The importance of AKT activation for differentiation was also shown by inhibiting PI-3 kinase, which is upstream of AKT. The addition of a PI-3 kinase inhibitor, after 3 day exposure to differentiation medium, completely prevented osteoblast differentiation. However, to obtain similar level of inhibition in IGFBP-2 overexpressing cells the inhibitor needed to be added at an earlier time point.
Since previous studies have also shown that suppression of MAP kinase activation stimulated osteoblast differentiation,(14,18–19) we also analyzed MAP kinase activation using a similar experimental paradigm. Consistent with prior reports, our results showed that inhibition of MAP kinase activation significantly stimulated osteoblast differentiation, however, this stimulation was only detected when inhibitor was added at the early stage of cell differentiation, such as on day 3 and day 6 (Supplemental Fig 4B). Importantly, over-expression of IGFBP-2 did not significantly alter MAP kinase activation, compared to control cells (Supplemental Fig 4C), indicating that MAP kinase pathway did not play an important role in mediating the stimulatory effect of IGFBP-2 on osteoblast differentiation. Consistently, exogenous addition of a peptide containing HBD-1 sequence or IGFBP-2 or overexpression of IGFBP-2 had no significant effect on cell proliferation in the differentiation medium (Supplemental Fig 4D and E).
Other studies have suggested that expression of IGFBP-2 correlates with changes in osteoblast differentiation although they have not shown the direct causal links reported herein. Specifically Kawase et al induced chondrocytes sheets to differentiate into osteoblasts and showed increased secretion of both IGF-I and IGFBP-2 that occurred during deposition of osteoid and mineralized tissue.(6) Similarly, Hamidouche et al. demonstrated that induction of osteoblast differentiation from mesenchymal stromal cells was accompanied by an increase in the synthesis of IGF-II, IGFBP-2 and the α5 integrin subunit.(8) Both IGF-II and IGFBP-2 were shown to increase the expression of phenotypic markers as well as the in vitro osteogenic capacity of the cells. They also demonstrated that downregulation of the α5 subunit decreased IGF-II and IGFBP-2 expression and that their expression was dependent on constituitive integrin α5 activation suggesting a link between increased IGFBP-2 synthesis and differentiation. Lee et al showed that treatment of mesenchymal stem cells during osteoblast induction with parathyroid hormone resulted in increased expression of IGF-I, IGF-II and IGFBP-2 whereas PTH treatment of cord blood derived the stem cells that did not differentiate into osteoblasts did not show these changes.(7) These findings have been extended to human osteoblasts wherein it was demonstrated that IGFBP-2 and IGF-I or II expression are upregulated during in vitro induction of differentiation and that this correlated negatively with proliferation.(20) Palermo et al showed that IGF-II stimulated IGFBP-2 synthesis in tibial osteoblast cultures during differentiation and that IGFBP-2 was the most abundant form of IGFBP that was induced.(2) Thraikill et al reported that MC-3T3 cells increased IGFBP-2 expression between days 10 and 14 of differentiation: concomitant with the onset of osteocalcin expression.(21) Several factors that have been shown to stimulate IGFBP-2 expression by MC-3T3 cells specifically phorbol esters(22) and FGF(23). A more recent study by Yerges et al demonstrated that IGFBP-2 SNPs were associated with lumbar volumetric bone mineral density in humans and that only 7 genes were found to be this tightly associated.(24)
Other cell types have also been analyzed to determine the role of IGFBP-2 in differentiation. In hematopoetic stem cells IGFBP-2 supports stem cell expansion but no specific mechanism by which it stimulates differentiation in this cell type has been defined.(25–26) Knockdown of IGFBP-2 in zebrafish embryos resulted in disruption of cardiac development and impaired differentiation of cardiomyocytes.(27) Additionally there were vessel sprouting defects which suggested a role in angiogenesis and endothelial cell differentiation. Our studies have demonstrated that IGFBP-2 expression is required for osteoclast differentiation.(28) Cells derived from IGFBP-2 −/− mice showed minimal osteoclast differentiation which could be rescued with exogenous addition of IGFBP-2. The defect appeared to be an inability to form mature osteoclasts that retain full bone resorbing activity. The role of IGFBP-2 in differentiation has been intensively studied in skeletal myoblasts wherein it has been demonstrated that the addition of differentiation medium to myoblasts in culture results in a major increase in expression of IGFBP-2 and inhibition of IGFBP-2 using neutralizing antibodies inhibits myoblast differentiation.(29) Therefore it appears that IGFBP-2 coordinately regulates the ability of IGF-I and IGF-II stimulate differentiation in several cell types.
Numerous studies have shown a positive effect of IGF-I on bone formation in vivo and on osteoblast differentiation in vitro. Addition of IGF-I to culture medium with BMP-2 enhanced osteoblast differentiation and this effect was believed to be mediated through AKT.(30) Yeh et al. demonstrated that BMP-2 and IGF-I induced a synergistic increase in osteoblast differentiation and that this response could be inhibited by a protein kinase D inhibitor.(31) IGF-I also mediates chondrocyte differentiation and mature chondrocytes can differentiate into osteoblasts therefore this indirectly alters osteoblast differentiation.(32) Similarly IGF-II has been shown to enhance osteogenic differentiation and it directly potentiates the effects of BMP 9 on alkaline phosphatase activity.(33) These effects are inhibited by PI-3 kinase inhibitors suggesting the AKT pathway that is the primary mediator. Matrix IGF-I has been shown to maintain bone mass by enhancing osteoblast differentiation. This effect required concomitant injection of IGFBP-3 which improved bone matrix localization.(10) Our current study also showed that blockage of IGF-I signaling significantly impaired osteoblast differentiation even though IGFBP-2 was overexpressed. We conclude that IGF-I and IGFBP-2 function coordinately to stimulate differentiation and both peptides are required for an optimal stimulation.
Transgenic mice that overexpress IGF-I in osteoblasts have increased trabecular bone and increased bone formation.(34) Conditional IGF-I receptor null mice showed decreased osteoblast number and reduced trabecular volume and impaired differentiation and calcification(35) and locally produced skeletal IGF-I plays an important role in trabecular bone integrity.(36) Since IGFBP-2 stimulates trabecular bone formation and osteoblast differentiation, our results suggest that the two proteins are functioning coordinately. This conclusion is supported by the observation that PTH is a potent stimulant of not only IGF-I synthesis in bone but it also induces IGFBP-2.
In conclusion, the results of our studies suggest that IGFBP-2 functions to enhance the ability of IGF-I to stimulate osteoblast differentiation and that this effect is specific for IGFBP-2. Since IGF-I can stimulate both osteoblast proliferation and differentiation, our findings suggests that IGFBP-2 may function directly to coordinate these responses and independently of its transport capacity for the IGFs.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Laura Lindsey, University of North Carolina at Chapel Hill, for her help in preparing the manuscript. This work was supported by a grant (AR-06114) from the National Institute of Health.
Footnotes
Disclosures
The authors state that they have no conflicts of interest.
Author’s roles: Study design: GX, CR and DC; Data collection: GX, CW and VD; Data analysis and interpretation: GX, CR and DC. Drafting and reviewing manuscript: GX, CR and DC.
References
- 1.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23(6):824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 2.Palermo C, Manduca P, Gazzerro E, Foppiani L, Segat D, Barreca A. Potentiating role of IGFBP-2 on IGF-II-stimulated alkaline phosphatase activity in differentiating osteoblasts. Am J Physiol Endocrinol Metab. 2004;286(4):E648–E657. doi: 10.1152/ajpendo.00049.2003. [DOI] [PubMed] [Google Scholar]
- 3.DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149(5):2051–2061. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai M, Breggia AC, DeMambro VE, Shen X, Canalis E, Bouxsein ML, Beamer WG, Clemmons DR, Rosen CJ. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286(16):14670–14680. doi: 10.1074/jbc.M110.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007;30(3):354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawase T, Okuda K, Kogami H, Nakayama H, Nagata M, Nakata K, Yoshie H. Characterization of human cultured periosteal sheets expressing bone-forming potential: in vitro and in vivo animal studies. J Tissue Eng Regen Med. 2009;3(3):218–229. doi: 10.1002/term.156. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Hwang KJ, Kim MY, Lim YJ, Seol IJ, Jin HJ, Jang YK, Choi SJ, Oh W, Cho YH, Lee YH. Human parathyroid hormone increases the mRNA expression of the IGF system and hematopoietic growth factors in osteoblasts, but does not influence expression in mesenchymal stem cells. J Pediatr Hematol Oncol. 2012;34(7):491–496. doi: 10.1097/MPH.0b013e318266c0ef. [DOI] [PubMed] [Google Scholar]
- 8.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Marie PJ. Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. 2010;11:44. doi: 10.1186/1471-2121-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase beta and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32(20):4116–4130. doi: 10.1128/MCB.01011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohick WS, Clemmons DR. Regulation of insulin-like growth factor binding protein synthesis and secretion in a bovine epithelial cell line. Endocrinology. 1991;129(3):1347–1354. doi: 10.1210/endo-129-3-1347. [DOI] [PubMed] [Google Scholar]
- 12.Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22(9):2162–2175. doi: 10.1210/me.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14(6):893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 14.Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol. 2008;215(2):442–451. doi: 10.1002/jcp.21323. [DOI] [PubMed] [Google Scholar]
- 15.Schinke T, Gebauer M, Schilling AF, Lamprianou S, Priemel M, Mueldner C, Neunaber C, Streichert T, Ignatius A, Harroch S, Amling M. The protein tyrosine phosphatase Rptpzeta is expressed in differentiated osteoblasts and affects bone formation in mice. Bone. 2008;42(3):524–534. doi: 10.1016/j.bone.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard FJ, Doran PM, Khosla S. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002;12(3):178–183. doi: 10.1016/s1096-6374(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 17.Kiepe D, Van Der Pas A, Ciarmatori S, Standker L, Schutt B, Hoeflich A, Hugel U, Oh J, Tonshoff B. Defined carboxy-terminal fragments of insulin-like growth factor (IGF) binding protein-2 exert similar mitogenic activity on cultured rat growth plate chondrocytes as IGF-I. Endocrinology. 2008;149(10):4901–4911. doi: 10.1210/en.2007-1395. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi C, Myoui A, Hashimoto N, Kuriyama K, Yoshioka K, Yoshikawa H, Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J Bone Miner Res. 2002;17(10):1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama K, Tamura Y, Suzawa M, Harada S, Fukumoto S, Kato M, Miyazono K, Rodan GA, Takeuchi Y, Fujita T. Receptor tyrosine kinases inhibit bone morphogenetic protein-Smad responsive promoter activity and differentiation of murine MC3T3-E1 osteoblast-like cells. J Bone Miner Res. 2003;18(5):827–835. doi: 10.1359/jbmr.2003.18.5.827. [DOI] [PubMed] [Google Scholar]
- 20.Viereck V, Siggelkow H, Pannem R, Braulke T, Scharf JG, Kubler B. Alteration of the insulin-like growth factor axis during in vitro differentiation of the human osteosarcoma cell line HOS 58. J Cell Biochem. 2007;102(1):28–40. doi: 10.1002/jcb.21274. [DOI] [PubMed] [Google Scholar]
- 21.Thrailkill KM, Siddhanti SR, Fowlkes JL, Quarles LD. Differentiation of MC3T3-E1 osteoblasts is associated with temporal changes in the expression of IGF-I and IGFBPs. Bone. 1995;17(3):307–313. doi: 10.1016/8756-3282(95)00223-z. [DOI] [PubMed] [Google Scholar]
- 22.Hakeda Y, Yoshizawa K, Hurley M, Kawaguchi H, Tezuka K, Tanaka K, Satoh T, Kumegawa M. Stimulatory effect of a phorbol ester on expression of insulin-like growth factor (IGF) binding protein-2 and level of IGF-I receptors in mouse osteoblastic MC3T3-E1 cells. J Cell Physiol. 1994;158(3):444–450. doi: 10.1002/jcp.1041580308. [DOI] [PubMed] [Google Scholar]
- 23.Hurley MM, Abreu C, Hakeda Y. Basic fibroblast growth factor regulates IGF-I binding proteins in the clonal osteoblastic cell line MC3T3-E1. J Bone Miner Res. 1995;10(2):222–230. doi: 10.1002/jbmr.5650100208. [DOI] [PubMed] [Google Scholar]
- 24.Yerges LM, Klei L, Cauley JA, Roeder K, Kammerer CM, Ensrud KE, Nestlerode CS, Lewis C, Lang TF, Barrett-Connor E, Moffett SP, Hoffman AR, Ferrell RE, Orwoll ES, Zmuda JM. Candidate gene analysis of femoral neck trabecular and cortical volumetric bone mineral density in older men. J Bone Miner Res. 2010;25(2):330–338. doi: 10.1359/jbmr.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh H, Zheng J, Umikawa M, Zhang C, Silvany R, Iizuka S, Holzenberger M, Zhang W, Zhang CC. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood. 2011;118(12):3236–3243. doi: 10.1182/blood-2011-01-331876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celebi B, Mantovani D, Pineault N. Insulin-like growth factor binding protein-2 and neurotrophin 3 synergize together to promote the expansion of hematopoietic cells ex vivo. Cytokine. 2012;58(3):327–331. doi: 10.1016/j.cyto.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Wood AW, Schlueter PJ, Duan C. Targeted knockdown of insulin-like growth factor binding protein-2 disrupts cardiovascular development in zebrafish embryos. Mol Endocrinol. 2005;19(4):1024–1034. doi: 10.1210/me.2004-0392. [DOI] [PubMed] [Google Scholar]
- 28.DeMambro VE, Maile L, Wai C, Kawai M, Cascella T, Rosen CJ, Clemmons D. Insulin-like growth factor-binding protein-2 is required for osteoclast differentiation. J Bone Miner Res. 2012;27(2):390–400. doi: 10.1002/jbmr.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharples AP, Al-Shanti N, Hughes DC, Lewis MP, Stewart CE. The role of insulin-like-growth factor binding protein 2 (IGFBP2) and phosphatase and tensin homologue (PTEN) in the regulation of myoblast differentiation and hypertrophy. Growth Horm IGF Res. 2013;23(3):53–61. doi: 10.1016/j.ghir.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122(Pt 5):716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh LC, Ma X, Matheny RW, Adamo ML, Lee JC. Protein kinase D mediates the synergistic effects of BMP-7 and IGF-I on osteoblastic cell differentiation. Growth Factors. 2010;28(5):318–328. doi: 10.3109/08977191003766874. [DOI] [PubMed] [Google Scholar]
- 32.Longobardi L, Granero-Molto F, O'Rear L, Myers TJ, Li T, Kregor PJ, Spagnoli A. Subcellular localization of IRS-1 in IGF-I-mediated chondrogenic proliferation, differentiation and hypertrophy of bone marrow mesenchymal stem cells. Growth Factors. 2009;27(5):309–320. doi: 10.1080/08977190903138874. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, Luo J, Tang M, Wietholt C, Luo X, Bi Y, Su Y, Liu B, Kim SH, He CJ, Hu Y, Shen J, Rastegar F, Huang E, Gao Y, Gao JL, Zhou JZ, Reid RR, Luu HH, Haydon RC, He TC, Deng ZL. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25(11):2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141(7):2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 36.Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301(6):E1191–E1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.