ABSTRACT
Cardiac muscle contraction requires sarcoplasmic reticulum (SR) Ca2+ release mediated by the quaternary complex comprising the ryanodine receptor 2 (RyR2), calsequestrin 2 (CSQ2), junctin (encoded by ASPH) and triadin. Here, we demonstrate that a direct interaction exists between RyR2 and CSQ2. Topologically, CSQ2 binding occurs at the first luminal loop of RyR2. Co-expression of RyR2 and CSQ2 in a human cell line devoid of the other quaternary complex proteins results in altered Ca2+-release dynamics compared to cells expressing RyR2 only. These findings provide a new perspective for understanding the SR luminal Ca2+ sensor and its involvement in cardiac physiology and disease.
KEY WORDS: Ca2+ intracellular release, Calsequestrin, Excitation-contraction coupling, Luminal Ca2+ sensor, Protein–protein interaction, Ryanodine receptor, Sarcoplasmic reticulum
Summary: Direct physical and functional calsequestrin 2 association with the ryanodine receptor luminal domain is the minimal requirement to form the sarcoplasmic reticulum Ca2+ sensor, which governs cardiac Ca2+ homeostasis and reliable heartbeat.
INTRODUCTION
Cardiac excitation–contraction coupling, where an action potential elicits cardiomyocyte contraction, is mediated by ‘Ca2+-induced Ca2+ release’. Upon plasma membrane depolarisation, Ca2+ influx through voltage-gated Ca2+ channels triggers Ca2+ release through the sarcoplasmic reticulum (SR)-located RyR2 to increase cytoplasmic Ca2+ concentration and transduce sarcomere contraction (Bers, 2002). Termination of SR Ca2+ release is an incompletely understood process but appears to involve intra-SR luminal Ca2+ regulation of RyR2 channel activity (Radwański et al., 2013; Terentyev et al., 2002). Luminal Ca2+ exerts its effects directly on RyR2 (Chen et al., 2014) and through the cardiac SR Ca2+-binding protein CSQ2 (Beard et al., 2009; Gaburjakova et al., 2013; Gyorke and Terentyev, 2008). Abnormal RyR2 regulation by luminal Ca2+ causes diastolic SR Ca2+ leak, resulting in arrhythmias and heart failure (Gyorke and Terentyev, 2008; Priori and Chen, 2011). Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an outstanding example of how arrhythmia induces sudden cardiac death by perturbing Ca2+ handling (Cerrone et al., 2009). CPVT type 1 is linked to RyR2 mutations inherited in an autosomal-dominant manner, whereas CPVT type 2 is linked to both dominant and recessive mutations of calsequestrin 2 (CSQ2) (http://triad.fsm.it/cardmoc/).
RyR2 exists as a macromolecular complex composed of CSQ2 and the SR integral membrane proteins triadin and junctin (encoded by ASPH), which together form the luminal Ca2+ sensor (Zhang et al., 1997). CSQ2 interacts directly with both triadin and junctin in a Ca2+-dependent manner, with 1–5 mM Ca2+ inhibiting these interactions (Shin et al., 2000; Zhang et al., 1997). Their binding sites have been mapped to the Asp-rich region at the C-terminus of CSQ2 and to the KEKE motif (residues 200–224) of triadin (Kobayashi et al., 2000; Shin et al., 2000). Triadin and junctin interactions with RyR1 and RyR2 are also known to be direct, with the RyR2–junctin interaction being Ca2+-independent (Guo and Campbell, 1995; Zhang et al., 1997). The triadin KEKE motif interacts with negatively charged residues within the second luminal loop of RyR1 (Goonasekera et al., 2007; Lee et al., 2004), whereas junctin association with the RyR1 or RyR2 involves both luminal and cytoplasmic sites (Altschafl et al., 2011; Li et al., 2015). Evidence for direct RyR–CSQ association is very scarce. Interaction of calsequestrin 1 (CSQ1) with purified native skeletal muscle RyR1 has been reported (Herzog et al., 2000), which could have differential effects on channel activity depending on the presence of triadin and/or junctin (Beard et al., 2002; Szegedi et al., 1999). To our knowledge, there is no biochemical data for direct physical association between RyR2 and CSQ2 in the heart. Thus, although the functional association of RyR2 and CSQ2 is well established, this is generally believed to be indirectly mediated through triadin and junctin interactions (Beard et al., 2009; Gaburjakova et al., 2013; Gyorke and Terentyev, 2008).
RESULTS AND DISCUSSION
Direct association of calsequestrin 2 with RyR2
Initially, we investigated the native RyR2 interaction with recombinant purified CSQ2 using GST pull-down assays. GST alone was used as negative control and GST–FKBP12.6 (also known as FKBP1B) was used as a positive control. Purified (1 µM) GST-fusion protein was incubated with CHAPS-solubilised RyR2 from pig cardiac SR and isolated with glutathione Sepharose (Fig. 1A, bottom); the presence of co-precipitated RyR2 was analysed by immunoblotting using Ab1093 (Fig. 1A, top). RyR2 was efficiently co-precipitated by GST–CSQ2 and GST–FKBP12.6 but not by GST alone. The interaction of recombinant GST–CSQ2 with pig cardiac RyR2 might be direct or be mediated through the native-RyR2-associated proteins triadin, junctin and calsequestrin 2. To exclude an indirect interaction, we used recombinant human RyR2 expressed in HEK293 cells – a non-cardiac cell line lacking endogenous junctin, triadin and CSQ2, as revealed by immunoblot analysis (Fig. S1). Using GST pull-down assays, we found strong GST–CSQ2 binding to recombinant human RyR2 (Fig. 1B), indicating that triadin and junctin are non-essential for the RyR2–CSQ2 association. These experiments made use of purified CSQ2 expressed in bacteria that lack the machinery for glycosylation and phosphorylation, which are potential post-translational modifications for native calsequestrin 2. We therefore assessed the RyR2–CSQ2 interaction by performing co-immunoprecipitation assays following their co-expression in mammalian HEK293 cells. Hemagglutinin (HA)-tagged CSQ2 was immunoprecipitated with an antibody against HA from CHAPS-solubilised HEK293 cell lysates (Fig. 1C, bottom), and the presence of co-precipitated RyR2 was analysed by immunoblotting using Ab1093 (Fig. 1C, top). RyR2 was recovered from the anti-HA immunoprecipitate but not from the negative control using non-immune rabbit IgG. In the reverse experiment, immunoprecipitation of RyR2 with Ab1093 (Fig. S2, bottom) resulted in co-precipitation of HA–CSQ2 (Fig. S2, top), providing further evidence for direct complex formation between RyR2 and CSQ2. Our findings are consistent with previous work that indicates a physical association between the skeletal muscle isoforms, RyR1 and CSQ1, and that made use of purified native RyR1 with no detectable levels of endogenous triadin or junctin (Beard et al., 2002; Herzog et al., 2000; Szegedi et al., 1999).
Fig. 1.
CSQ2 displays a direct interaction with RyR2. (A) GST pull-down experiments (n=3) of native RyR2 incubated with purified GST-fusion proteins as indicated. Solubilised pig cardiac SR vesicles were incubated with purified GST-fusion proteins that had been captured on glutathione beads. Beads were precipitated by centrifugation, and the presence of associated RyR2 was analysed by SDS-PAGE (4% gel) and immunoblotting using the Ab1093 antibody (top). As negative control, pull-down assays were performed with GST alone. To detect the isolated GST-fusion proteins, one-tenth of pull-down samples was analysed by SDS-PAGE (12% gel) and immunoblotting using an antibody against GST (AbGST, bottom). An aliquot of solubilised pig cardiac SR corresponding to 1% (5 μg) of the amount processed in the pull-down assay was also included in the gels. As a negative control, pulldown assays were performed with GST alone; GST–FKBP12.6 was used as positive control for RyR2 interaction. (B) GST pull-down experiments (n=3) of recombinant human RyR2 incubated with purified GST-fusion proteins as indicated. Solubilised HEK293 microsomes expressing RyR2 (HEK micro) were incubated with purified GST-fusion proteins captured on glutathione beads, and analysed as described in A above. (C) Co-immunoprecipitation (co-IP) analyses (n=3) of recombinant human RyR2 co-expressed with CSQ2 in mammalian HEK293 cells. HA–CSQ2 was immunoprecipitated with an antibody against HA (AbHA) from solubilised HEK293 lysates, and the presence of associated RyR2 was analysed by SDS-PAGE (4% gel) and immunoblotting using Ab1093 (top). As negative control, co-immunoprecipitation assays were performed with non-immune rabbit IgG (Non-immune). To detect isolated HA–CSQ2, one-tenth of the immunoprecipitate was analysed by SDS-PAGE (12% gel) and western blotting (WB) using an antibody against HA (AbHA, bottom). An aliquot of HEK293 cell lysate corresponding to 1% (20 μg) of the amount processed in the co-immunoprecipitation assay was also included in the gels.
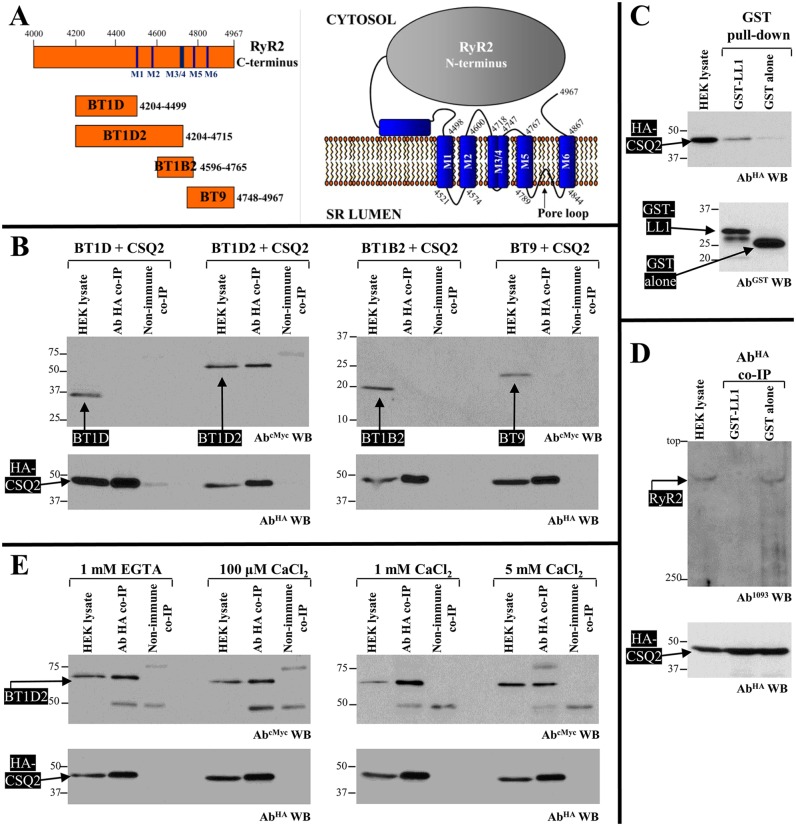
CSQ2 interacts with the first luminal loop of RyR2
The direct interaction of CSQ2 can only occur with RyR2 peptide sequences that face the SR lumen. To map the CSQ2-binding site, we used four overlapping fragments covering the RyR2 C-terminus containing all the transmembrane domains and intervening luminal loops (BT constructs shown in Fig. 2A). HA–CSQ2, co-expressed with Myc-tagged BT1D, BT1D2, BT1B2 and BT9 constructs (see Fig. 2A) in HEK293 cells, was immunoprecipitated with an antibody against HA from CHAPS-solubilised cell lysates (Fig. 2B, bottom), and the presence of co-precipitated BT constructs was analysed by immunoblotting using an antibody against Myc (Fig. 2B, top). BT1D2 was the only RyR2 fragment recovered in the anti-HA immunoprecipitate but not in the negative control using non-immune rabbit IgG. The shorter BT1D construct that lacks the first two transmembrane domains and intervening luminal loop was found to be negative for an interaction with CSQ2. To further test whether the CSQ2-binding site is contained within the RyR2 first luminal loop (LL1), we generated the RyR2 LL1 fragment (residues 4521–4573) as a GST-fusion protein (GST-LL1) purified from bacteria for use in pull-down assays; we were unable to use the LL1 construct in coimmunoprecipitation assays because it was expressed very poorly in HEK293 cells. Purified (1 µM) GST-fusion protein was incubated with CHAPS-solubilised HEK293 cell lysate expressing HA–CSQ2 and isolated with glutathione Sepharose (Fig. 2C, bottom); the presence of co-precipitated HA–CSQ2 was analysed by immunoblotting using an antibody against HA (Fig. 2C, top). HA–CSQ2 was efficiently pulled down by GST–LL1, whereas binding to GST alone, serving as negative control, was negligible. To further assess whether the CSQ2-binding site is contained within the RyR2 first luminal loop, we conducted competition binding experiments to test whether GST–LL1 disrupted the CSQ2 interaction with the full-length RyR2. RyR2 was co-expressed with HA–CSQ2 in HEK293 cells, and co-immunoprecipitation assays were performed in the presence of 1 µM GST–LL1 or GST alone. As seen in Fig. 2D, in the presence of GST alone, RyR2 was efficiently recovered in the anti-HA immunoprecipitate, whereas the inclusion of GST–LL1 abolished the RyR2 interaction with HA–CSQ2.
Fig. 2.
Mapping the CSQ2-interacting site on RyR2. (A) Human RyR2 SR membrane topology (right) and diagram of overlapping BT1D, BT1D2, BT1B2 and BT9 constructs covering the RyR2 C-terminus containing all the transmembrane domains and intervening luminal loops. M indicates membrane-spanning domains. (B) Co-immunoprecipitation (co-IP) experiments (n=4) of recombinant human RyR2 fragments co-expressed with CSQ2 in HEK293 cells. HA–CSQ2 was immunoprecipitated with an antibody against HA (AbHA) from solubilised HEK293 lysates that co-expressed Myc-tagged BT constructs. As negative control, co-immunoprecipitation assays were performed with non-immune rabbit IgG (non-immune). Immunoprecipitated proteins were resolved in separate SDS-PAGE gels (12% for BT1D and BT1D2, 15% for BT1B2 and BT9, and 12% for HA–CSQ2) loaded with nine-tenths or one-tenth of the immunoprecipitates and were analysed by immunoblotting using antibodies against Myc (AbcMyc, top) or HA (AbHA, bottom), respectively. An aliquot of HEK293 cell lysate corresponding to 1% (20 μg) of the amount processed in the co-immunoprecipitation assay was also included in the gels. (C) GST pull-down experiments (n=4) of recombinant human CSQ2 incubated with purified GST–LL1 (RyR2 residues 4521–4573) or GST alone serving as negative control. Solubilised HEK293 lysates expressing HA–CSQ2 were incubated with purified GST-fusion proteins captured on glutathione beads. Precipitated proteins were resolved in separate 12% SDS-PAGE gels loaded with nine-tenths or one-tenth of the pull-down samples and were analysed by immunoblotting for HA (AbHA, top) or GST (AbGST, bottom), respectively. (D) Competition binding experiments (n=3) to test the RyR2–CSQ2 interaction. HA–CSQ2 was immunoprecipitated with an antibody against HA (AbHA) from solubilised HEK293 lysates co-expressing RyR2 in the presence of GST–LL1 or GST alone (negative control). Immunoprecipitated proteins were resolved in separate 4% or 12% SDS-PAGE gels loaded with nine-tenths or one-tenth of the immunoprecipitates and were analysed by immunoblotting with Ab1093 (top) and for HA (AbHA, bottom), respectively. (E) Co-immunoprecipitation experiments (n=4) of BT1D2 co-expressed with CSQ2 in HEK293 cells, performed under different Ca2+ concentrations. HA–CSQ2 was immunoprecipitated with an antibody against HA (AbHA) from solubilised HEK293 lysates containing either 1 mM EGTA, 100 µM CaCl2, 1 mM CaCl2 or 5 mM CaCl2, as indicated. The presence of co-precipitated BT1D2 was analysed by SDS-PAGE (10% gels) and immunoblotting for Myc (AbcMyc, top) or HA (AbHA, bottom), respectively.
The experiments described above were conducted using assay buffers containing residual, micromolar Ca2+. Further investigation of the Ca2+ sensitivity of the RyR2–CSQ2 interaction was performed in the presence of near-physiological SR Ca2+ concentrations (1 mM CaCl2), or in the absence of Ca2+ (1 mM EGTA), as well as under conditions of high (5 mM CaCl2) or low (100 µM CaCl2) SR Ca2+ load. Using co-immunoprecipitation assays, we found comparable amounts of BT1D2 binding to HA–CSQ2 in the presence of 1 mM EGTA, and 100 µM and 1 mM CaCl2; however, recovery of BT1D2 in the anti-HA immunoprecipitate was diminished with 5 mM CaCl2 (Fig. 2E). Quantitative data (n=4) following densitometry analysis and normalisation for the amount of protein expressed in the cell lysate indicate equivalent amounts of HA–CSQ2 binding to BT1D2 for Ca2+ concentrations up to 1 mM (1 mM EGTA, 154.2±20.8; 100 µM CaCl2, 126.7±30.5; 1 mM CaCl2, 149.0±43.5; mean±s.e.m.); however, 5 mM CaCl2 substantially reduced binding by >50% (71.5±36.9, P<0.05 two-tailed paired Student's t-test compared to 1 mM CaCl2). These results suggest that at Ca2+ levels within the physiological SR range (≤1 mM), the RyR2–CSQ2 interaction remains intact but that it is diminished at supra-millimolar Ca2+ levels, in agreement with previous reports (Beard et al., 2009; Gaburjakova et al., 2013). Our data are also consistent with recent single-channel recordings demonstrating that CSQ2 effects on RyR2 activity are unaffected by luminal Ca2+ concentration changes up to 1 mM (Chen et al., 2013; Tencerová et al., 2012). CSQ2 undergoes structural changes upon Ca2+ binding and polymerises at >1 mM Ca2+ concentrations; it is believed that a front-to-front CSQ2 dimer is first formed, with these dimers forming a back-to-back stack to produce a polymeric structure (Gaburjakova et al., 2013). Our findings suggest that the RyR2-interacting site on CSQ2 assumes a Ca2+-independent conformation that might be obstructed if CSQ2 is in its polymeric form. They further indicate that monomeric CSQ2 is sufficient for direct RyR2 interaction.
Direct CSQ2 association with RyR2 regulates cellular Ca2+- release dynamics
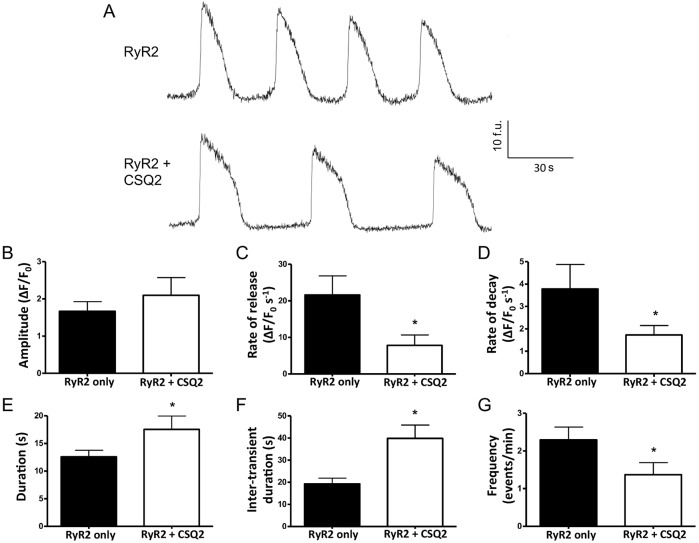
Expression of RyR2 in HEK293 cells is known to result in spontaneous global Ca2+-release events (Jiang et al., 2002; Seidel et al., 2015). To assess the functional impact of CSQ2 on RyR2 in the absence of triadin and junctin, we generated HEK293 cells that stably expressed CSQ2, verified by immunoblotting (Fig. S1). Co-expression of RyR2 in the CSQ2-expressing stable cell line resulted in an increase in the duration between Ca2+-release events (inter-transient duration), decreasing their overall frequency (Fig. 3A,F,G), compared to that in cells that expressed RyR2 alone. Rates of transient rise and decay were also slower (Fig. 3C,D), lengthening the overall duration of Ca2+ release (Fig. 3E), with no significant change in amplitude (Fig. 3B). These whole-cell functional results provide further evidence for a direct RyR2–CSQ2 interaction and corroborate our biochemical findings. Notably, our results are consistent with the previously reported inhibitory action of this luminal accessory protein in cardiomyocytes, where CSQ2 would associate with RyR2 both directly and indirectly through junctin and triadin. The decrease in Ca2+-release event frequency (Fig. 3G) that we observed agrees with that reported in most CSQ2-overexpression studies (Jones et al., 1998; Sato et al., 1998; Terentyev et al., 2003; Wang et al., 2000). The longer inter-transient duration (Fig. 3F) is also consistent with the increase in Ca2+-binding sites within the store provided by CSQ2, indicating that more time is required to reach a high-enough level of free luminal Ca2+ to trigger the next Ca2+-release event (Jiang et al., 2002). In addition, it has been reported that although RyR2 activity decreases when CSQ2 is dissociated (Wei et al., 2009), the relative open probability increases more steeply with luminal Ca2+ after CSQ2 dissociation (Dulhunty et al., 2012), in agreement with our findings that the rate of spontaneous Ca2+ release is increased in the absence of CSQ2 (Fig. 3C). Thus, although we cannot exclude the involvement of junctin and triadin, our data obtained in a cell line devoid of these accessory proteins suggest that the functional effects of CSQ2 are mediated primarily through its direct structural interaction with RyR2.
Fig. 3.
Direct interaction of CSQ2 with RyR2 alters intracellular Ca2+ release. Traces from Fluo-3-AM-loaded single HEK293 cells expressing RyR2 alone or RyR2 together with CSQ2, showing spontaneous Ca2+ release under conditions with 1.3 mM extracellular Ca2+ (A). Parameters of Ca2+-release events, including amplitude (B), rate of release (C), rate of decay (D), duration (E), inter-transient duration (F) and frequency (G) were analysed for n=18– 24 cells from three or four separate experiments. *Significant difference by unpaired Student's t-test, where P<0.005. Data are means±s.e.m. F denotes fluorescence; f.u. denotes fluorescence units.
In conclusion, our data indicate a direct functional RyR2 interaction with CSQ2, shedding more light on the quaternary structural assembly of the SR luminal Ca2+ sensor. Elucidation of the CSQ2-binding site within the first luminal loop of RyR2 should help in further understanding the pathologic mechanism(s) underlying arrhythmias and sudden cardiac death.
MATERIALS AND METHODS
Materials
Cell culture reagents and Fluo-3 AM were obtained from Life Technologies, electrophoresis equipment and reagents from Bio-Rad, protease inhibitor cocktail (Complete) from Roche, CHAPS from Merck, nProtein-A Sepharose and glutathione Sepharose 4B from GE Healthcare, secondary antibodies from Santa Cruz Biotechnology, enhanced chemiluminescence reagents from Pierce, DNA restriction endonucleases from New England Biolabs, Pfu DNA polymerase from Promega, oligonucleotides and all other reagents were from Sigma. Antibodies used for immunoprecipitation: (1 μg) rabbit anti-HA antibody (Y-11, cat. no. sc-805, 1:40 dilution for immunoprecipitation) and non-immune rabbit IgG were from Santa Cruz Biotechnology. Antibodies used in immunoblotting were mouse anti-Myc (9E10, cat. no. sc-40, 1:500 dilution for western blotting) from Santa Cruz Biotechnology, mouse anti-HA (16B12, cat. no. MMS-101, 1:1000 dilution for western blotting) from Covance; rabbit anti-GST (1:2000) (Zissimopoulos et al., 2012, 2006), and rabbit RyR2-specific (Ab1093, epitope at residues 4454-4474; 1:500) (Zissimopoulos et al., 2012, 2006) antibodies.
Plasmid construction
Human CSQ2 cDNA containing the entire open reading was generated by performing PCR amplification from a human heart cDNA library (Clontech) and was cloned into the bacterial expression vector pGEX6P1 (GE Healthcare). For transient mammalian expression, CSQ2 cDNA was subcloned into a modified pCR3 vector (Life Technologies) containing an N-terminal HA peptide epitope tag, whereas for stable expression, it was cloned into pcDNA3.1(+)hygro (Life Technologies). Human RyR2 constructs were generated by performing PCR amplification [using human (h)RyR2 cDNA as template] and cloning into a modified pCR3 vector containing an N-terminal Myc peptide epitope tag. For bacterial expression of the LL1 construct, the cDNA was subcloned into the pGEX6P1 vector. Oligonucleotide primers and restriction enzyme cloning sites used are available upon request. All plasmids were verified by direct DNA sequencing (BigDye, Perkin-Elmer).
Mammalian cell culture and transfection
HEK293 cells (ATCC®) were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine and 10% fetal bovine serum under a humidified atmosphere of 5% CO2 at 37°C. On reaching ∼70% confluence, cells growing on a 100-mm Petri dish were transiently transfected with 24 μg of plasmid DNA using the calcium phosphate precipitation method, as described elsewhere (Stanczyk et al., 2016). Cells were collected 24 h post-transfection and stored at −80°C. Transfected cells were also used to generate a clonally-derived CSQ2 stable cell line using limiting dilution with selection using hygromycin (400 µg ml−1). For Ca2+ imaging, stable cells from this clonal population were transiently transfected with eGFP–hRyR2 using Effectene® (Qiagen) according to the manufacturer's instructions.
Single cell Ca2+-imaging
Cells stably expressing CSQ2 (∼1×105) were seeded onto poly-lysine-coated glass-bottomed dishes (MatTek™) before transient transfection with eGFP–hRyR2. After 48 h, cells were loaded with Fluo-3 AM (10 µM) for 45 min at 30°C. Following de-esterification, cells were immersed in Krebs-Ringer-Hepes buffer for imaging (120 mM NaCl, 5.5 mM glucose, 25 mM Hepes, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2, pH 7.4). RyR2-mediated spontaneous Ca2+-release events were monitored using a laser scanning confocal microscope (Leica SP5) with an oil immersion 63× objective lens with excitation at 488 nm, and Ca2+-dependent fluorescence emission was detected over a 520±28 nm range. Using Leica Microsystems software (LAS-AF), Ca2+-release events were recorded for 2 min (five frames per second) at 512×512 pixel resolution, and acquired regions of interest representing global Ca2+ environments (typically ∼50 μm2) were selected. Amplitude and temporal characteristics of spontaneous Ca2+ release were quantified and are displayed as means±s.e.m. Statistical analysis was performed using unpaired Student's t-test.
Other methods
Bacterial expression and purification of GST-fusion proteins, GST pull-down and co-immunoprecipitation assays, SR preparation and immunoblotting were performed as described previously (Stanczyk et al., 2016; Zissimopoulos et al., 2012, 2006). Densitometry analysis was performed using a GS-700 scanner (Bio-Rad) and Quantity One software (Bio-Rad); data are expressed as means±s.e.m. and statistical analysis was performed using paired Student's t-test.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.H. initiated the study and together with S.Z. conducted the experiments and analysed the results shown in Figs 1 and 2. C.E.O. created the stable cell line and performed Ca2+ imaging with N.L.T., and analysed the results presented in Fig. 3. C.B. performed part of the experiments shown in Fig. 2. A.H., C.E.O., N.L.T. and S.Z. wrote the paper. F.A.L. and S.Z. designed the study and, together with A.J.W., edited the paper before submission.
Funding
This work was supported by British Heart Foundation fellowships to S.Z. [grant number FS/08/063 and FS/15/30/31494] and funding to N.L.T. and A.J.W. [grant number RG/15/6/31436]; and by an Egyptian Ministry of Higher Education Visiting Scholarship to A.H. Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.191643.supplemental
References
- Altschafl B. A., Arvanitis D. A., Fuentes O., Yuan Q., Kranias E. G. and Valdivia H. H. (2011). Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. J. Physiol. 589, 6063-6080. 10.1113/jphysiol.2011.215988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard N. A., Sakowska M. M., Dulhunty A. F. and Laver D. R. (2002). Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 82, 310-320. 10.1016/S0006-3495(02)75396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard N. A., Wei L. and Dulhunty A. F. (2009). Ca(2+) signaling in striated muscle: the elusive roles of triadin, junctin, and calsequestrin. Eur. Biophys. J. 39, 27-36. 10.1007/s00249-009-0449-6 [DOI] [PubMed] [Google Scholar]
- Bers D. (2002). Cardiac excitation–contraction coupling. Nature 415, 198-205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Cerrone M., Napolitano C. and Priori S. G. (2009). Catecholaminergic polymorphic ventricular tachycardia: a paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm 6, 1652-1659. 10.1016/j.hrthm.2009.06.033 [DOI] [PubMed] [Google Scholar]
- Chen H., Valle G., Furlan S., Nani A., Gyorke S., Fill M. and Volpe P. (2013). Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J. Gen. Physiol. 142, 127-136. 10.1085/jgp.201311022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Wang R., Chen B., Zhong X., Kong H., Bai Y., Zhou Q., Xie C., Zhang J., Guo A. et al. (2014). The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat. Med. 20, 184-192. 10.1038/nm.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Wium E., Li L., Hanna A. D., Mirza S., Talukder S., Ghazali N. A. A. and Beard N. A. (2012). Proteins within the intracellular calcium store determine cardiac RyR channel activity and cardiac output. Clin. Exp. Pharmacol. Physiol. 39, 477-484. 10.1111/j.1440-1681.2012.05704.x [DOI] [PubMed] [Google Scholar]
- Gaburjakova M., Bal N. C., Gaburjakova J. and Periasamy M. (2013). Functional interaction between calsequestrin and ryanodine receptor in the heart. Cell. Mol. Life Sci. 70, 2935-2945. 10.1007/s00018-012-1199-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera S. A., Beard N. A., Groom L., Kimura T., Lyfenko A. D., Rosenfeld A., Marty I., Dulhunty A. F. and Dirksen R. T. (2007). Triadin binding to the C-terminal luminal loop of the ryanodine receptor is important for skeletal muscle excitation–contraction coupling. J. Gen. Physiol. 130, 365-378. 10.1085/jgp.200709790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. and Campbell K. (1995). Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 270, 9027-9030. 10.1074/jbc.270.16.9027 [DOI] [PubMed] [Google Scholar]
- Gyorke S. and Terentyev D. (2008). Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77, 245-255. 10.1093/cvr/cvm038 [DOI] [PubMed] [Google Scholar]
- Herzog A., Szegedi C., Jona I., Herberg F. W. and Varsanyi M. (2000). Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca2+ release channel/ryanodine receptor with calsequestrin. FEBS Lett. 472, 73-77. 10.1016/S0014-5793(00)01431-9 [DOI] [PubMed] [Google Scholar]
- Jiang D., Xiao B., Zhang L. and Chen S. R. W. (2002). Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ. Res. 91, 218-225. 10.1161/01.RES.0000028455.36940.5E [DOI] [PubMed] [Google Scholar]
- Jones L. R., Suzuki Y. J., Wang W., Kobayashi Y. M., Ramesh V., Franzini-Armstrong C., Cleemann L. and Morad M. (1998). Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J. Clin. Invest. 101, 1385-1393. 10.1172/JCI1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Alseikhan B. and Jones L. (2000). Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 275, 17639-17646. [DOI] [PubMed] [Google Scholar]
- Lee J., Rho S.-H., Shin D., Cho C., Park W., Eom S., Ma J. and Kim D. (2004). Negatively charged amino acids within the intraluminal loop of ryanodine receptor are involved in the interaction with triadin. J. Biol. Chem. 279, 6994-7000. 10.1074/jbc.M312446200 [DOI] [PubMed] [Google Scholar]
- Li L., Mirza S., Richardson S. J., Gallant E. M., Thekkedam C., Pace S. M., Zorzato F., Liu D., Beard N. A. and Dulhunty A. F. (2015). A new cytoplasmic interaction between junctin and ryanodine receptor Ca2+ release channels. J. Cell Sci. 128, 951-963. 10.1242/jcs.160689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S. G. and Chen S. R. W. (2011). Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ. Res. 108, 871-883. 10.1161/CIRCRESAHA.110.226845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwański P. B., Belevych A. E., Brunello L., Carnes C. A. and Györke S. (2013). Store-dependent deactivation: cooling the chain-reaction of myocardial calcium signaling. J. Mol. Cell. Cardiol. 58, 77-83. 10.1016/j.yjmcc.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Ferguson D. G., Sako H., Dorn G. W., Kadambi V. J., Yatani A., Hoit B. D., Walsh R. A. and Kranias E. G. (1998). Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J. Biol. Chem. 273, 28470-28477. 10.1074/jbc.273.43.28470 [DOI] [PubMed] [Google Scholar]
- Seidel M., Thomas N. L., Williams A. J., Lai F. A. and Zissimopoulos S. (2015). Dantrolene rescues aberrant N-terminus intersubunit interactions in mutant pro-arrhythmic cardiac ryanodine receptors. Cardiovasc. Res. 105, 118-128. 10.1093/cvr/cvu240 [DOI] [PubMed] [Google Scholar]
- Shin D. W., Ma J. and Kim D. H. (2000). The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin. FEBS Lett. 486, 178-182. 10.1016/S0014-5793(00)02246-8 [DOI] [PubMed] [Google Scholar]
- Stanczyk P. J., Lai F. A. and Zissimopoulos S. (2016). Genetic and biochemical approaches for in vivo and in vitro assessment of protein oligomerization: the ryanodine receptor case study. J. Vis. Exp. 113, e54271 10.3791/54271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi C., Sárközi S., Herzog A., Jóna I. and Varsányi M. (1999). Calsequestrin: more than ‘only’ a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 337, 19-22. 10.1042/bj3370019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencerová B., Zahradníková A., Gaburjáková J. and Gaburjáková M. (2012). Luminal Ca2+ controls activation of the cardiac ryanodine receptor by ATP. J. Gen. Physiol. 140, 93-108. 10.1085/jgp.201110708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Valdivia H. H., Escobar A. L. and Györke S. (2002). Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ. Res. 91, 414-420. 10.1161/01.RES.0000032490.04207.BD [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Gyorke I., Volpe P., Williams S. C. and Gyorke S. (2003). Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA 100, 11759-11764. 10.1073/pnas.1932318100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Cleemann L., Jones L. R. and Morad M. (2000). Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J. Physiol. 524, 399-414. 10.1111/j.1469-7793.2000.00399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Hanna A. D., Beard N. A. and Dulhunty A. F. (2009). Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 45, 474-484. 10.1016/j.ceca.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Zhang L., Kelley J., Schmeisser G., Kobayashi Y. and Jones L. (1997). Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor: proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 272, 23389-23397. 10.1074/jbc.272.37.23389 [DOI] [PubMed] [Google Scholar]
- Zissimopoulos S., West D. J., Williams A. J. and Lai F. A. (2006). Ryanodine receptor interaction with the SNARE-associated protein snapin. J. Cell Sci. 119, 2386-2397. 10.1242/jcs.02936 [DOI] [PubMed] [Google Scholar]
- Zissimopoulos S., Seifan S., Maxwell C., Williams A. J. and Lai F. A. (2012). Disparities in the association of the ryanodine receptor and the FK506-binding proteins in mammalian heart. J. Cell Sci. 125, 1759-1769. 10.1242/jcs.098012 [DOI] [PubMed] [Google Scholar]