Abstract
Zika virus (ZIKV) infection of pregnant women can result in fetal brain abnormalities. It has been established that ZIKV disrupts neural progenitor cells (NPCs) and leads to embryonic microcephaly. However, the fate of other cell types in the developing brain and their contributions to ZIKV-associated brain abnormalities remain largely unknown. Using intracerebral inoculation of embryonic mouse brains, we found that ZIKV infection leads to postnatal growth restriction including microcephaly. In addition to cell cycle arrest and apoptosis of NPCs, ZIKV infection causes massive neuronal death and axonal rarefaction, which phenocopy fetal brain abnormalities in humans. Importantly, ZIKV infection leads to abnormal vascular density and diameter in the developing brain, resulting in a leaky blood–brain barrier (BBB). Massive neuronal death and BBB leakage indicate brain damage, which is further supported by extensive microglial activation and astrogliosis in virally infected brains. Global gene analyses reveal dysregulation of genes associated with immune responses in virus-infected brains. Thus, our data suggest that ZIKV triggers a strong immune response and disrupts neurovascular development, resulting in postnatal microcephaly with extensive brain damage.
KEY WORDS: ZIKV, Microcephaly, Blood–brain barrier (BBB), Astrogliosis, Microglial activation
Highlighted article: A postnatal model for ZIKV infection reveals blood-brain barrier leakage, neuronal death, apoptosis and cell cycle arrest of NPCs, leading to microcephaly with brain damage in ZIKV-infected pups.
INTRODUCTION
The Zika virus (ZIKV) outbreak reported in 2015 in South and Central America has quickly reached pandemic status as it spreads through 66 countries (Ramos da Silva and Gao, 2016; Saiz et al., 2016). A major concern associated with ZIKV infection is the severe abnormalities in babies born to mothers infected with ZIKV, including microcephaly, CNS injury, fetal growth restriction, and stillbirth, among others (Brasil et al., 2016; Marrs et al., 2016). In 2015, the Brazilian Ministry of Health reported a 20-fold increase in reported neonatal microcephaly, which is now attributed to ZIKV infection. On 1 February 2016, the World Health Organization announced that ZIKV-associated microcephaly and other neurological disorders are a public health emergency of international concern (PHEIC) (Heymann et al., 2016; Marrs et al., 2016). Therefore, there is an urgent need for improved understanding of ZIKV pathogenesis in the developing brain.
Emerging evidence suggests a causative relationship between ZIKV infection and microcephaly. An increase of the number of cases of fetal microcephaly coincides with the ZIKV outbreak, and ZIKV has been detected in the amniotic fluid of infected pregnant woman as well as in microcephalic brain tissues (Brasil et al., 2016; Calvet et al., 2016; Driggers et al., 2016; Mlakar et al., 2016; Oliveira Melo et al., 2016). Precise control of neural progenitor cell (NPC) self-renewal and differentiation is essential for brain development, disruption of which is sufficient to cause microcephaly (Kriegstein and Alvarez-Buylla, 2009; Manzini and Walsh, 2011; Nigg and Raff, 2009). Indeed, it has been reported that ZIKV infects human NPCs and impairs their growth (Dang et al., 2016; Garcez et al., 2016; Qian et al., 2016; Tang et al., 2016), which could lead to smaller brain size. Recent studies show that ZIKV infection disrupts NPCs and leads to embryonic microcephaly and growth restriction in mice (Cugola et al., 2016; Li et al., 2016; Miner et al., 2016). However, current approaches to model ZIKV infection in rodents results in lethality during pregnancy, although human babies survive and display microcephaly. In addition, a newly born mouse brain is relatively immature, akin to the developmental stage of the human brain at mid-gestation (Semple et al., 2013), and mouse brain development includes a major postnatal component. Thus, it has been suggested that examination of neurodevelopmental defects of ZIKV in mice requires postnatal analysis (Miner et al., 2016). Hence, despite considerable rapid progress, fundamental questions remain regarding ZIKV-associated fetal brain abnormalities. It remains unknown whether ZIKV infection in mice can be used to study postnatal microcephaly. Although microcephaly has been the predominant focus, a wide spectrum of fetal brain abnormalities are described in humans, including neuronal loss, axonal rarefaction, astrogliosis, and microglial activation (Driggers et al., 2016; Mlakar et al., 2016). So far, there are no reported animal models that recapitulate these fetal brain abnormalities associated with ZIKV. Most importantly, NPC disruption is considered to be the major cause of microcephaly (Nigg and Raff, 2009; Thornton and Woods, 2009), and NPC abnormalities are suggested to be the cause of ZIKV-induced embryonic microcephaly (Li et al., 2016). However, it remains unknown whether other cell types, including differentiated neurons and vascular cells, are also damaged in the developing brain, contributing to the brain abnormalities associated with ZIKV infection.
To fill in these knowledge gaps, we established a mouse model of fetal brain abnormalities associated with human ZIKV infection. Our mouse model exhibits massive neuronal death, in addition to cell cycle arrest and apoptosis of NPCs, as well as global growth restriction. Interestingly, our model survives birth, allowing us to study postnatal microcephaly and leading to the discovery of neuronal loss and axonal rarefaction, which phenocopy fetal brain abnormalities reported in humans (Driggers et al., 2016; Mlakar et al., 2016). Most notably, ZIKV infection leads to abnormal vascular density and diameter in brains, resulting in a leaky blood–brain barrier (BBB). Extensive microglial activation and astrogliosis were detected in virally infected brains, which is further supported by dysregulation of genes associated with the immune response in the developing brain after ZIKV infection. Thus, our data suggest that ZIKV triggers a strong immune response, disrupts NPCs and neurovascular development, and leads to postnatal microcephaly with extensive brain damage.
RESULTS
ZIKV causes postnatal microcephaly and growth restriction
To investigate whether ZIKV can infect NPCs in developing mouse brains, we injected ∼1 μl of 1.7×106 TCID50/ml ZIKV virus (Mexican isolate MEX1-44) into the cerebral ventricles of embryonic day (E) 14.5 mouse brains. Zika MEX1-44 belongs to the Asian lineage, and was isolated in Chiapas, Mexico from an infected Aedes aegypti mosquito. The virus was passaged six times in Vero cells before inoculation of embryonic mouse brains. We sequenced the virus (passage 5), and found that the sequence of ZIKV-MEX1-44 is 99% identical at the nucleotide level to the sequence of the ZIKV strain isolated in Brazil (ZIKV PE243/2015).
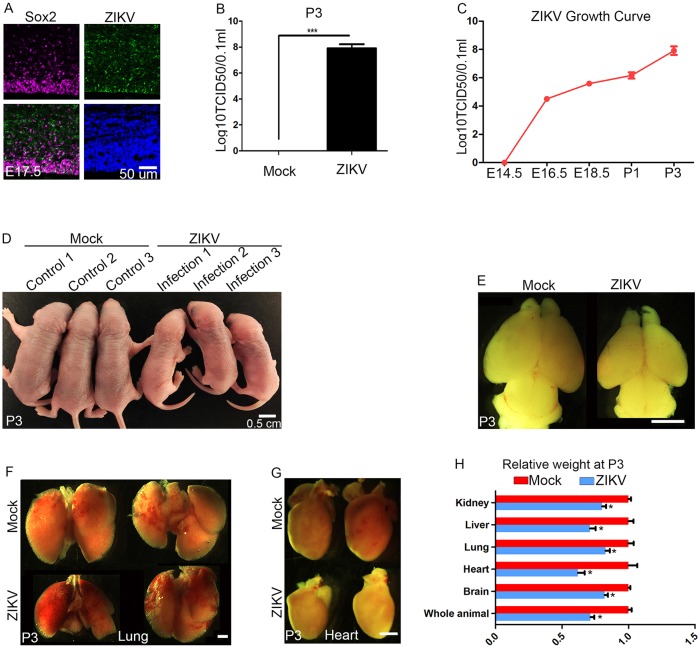
Staining E17.5 cerebral cortex with antibodies against a Flavivirus group antigen reveals ZIKV infection (ZIKV, green) of NPCs labeled by Sox2 (Fig. 1A). We also detected ZIKV in Pax6-positive apical NPCs and newly formed neurons labeled by TuJ1 (Tubb3) (data not shown). We measured viral titers at different stages after intracerebral inoculation of E14.5 embryonic brains. The ZIKV growth curve shows that ZIKV can effectively replicate in mouse brains from developmental to postnatal stages (Fig. 1B,C). Pups can survive until postnatal day (P) 3, and a lower titer ZIKV infection (3.4×105TCID50/ml) allowed recovery of P5 living pups. At P3, ZIKV-infected pups had a significantly smaller body (Fig. 1D,H) and brain (Fig. 1E,H) compared with controls. Previous studies on microcephaly genes suggest that NPCs are selectively vulnerable to genetic or environmental disturbances, resulting in smaller brains yet normal size of other organs (Gruber et al., 2011; Nigg and Raff, 2009; Thornton and Woods, 2009). To investigate whether the brain is selectively smaller after ZIKV infection, we dissected and weighed different organs, including brain, heart, lung, liver and kidney (Fig. 1F-H). Experimental results showed that individual organ masses are decreased in addition to total body weight (Fig. 1H). ZIKV-induced growth restriction can be observed as early as P1 for the brain and other organs compared with P1 controls (Fig. S1). Together, these results suggest that ZIKV infection is sufficient to cause postnatal microcephaly and growth restriction in mice.
Fig. 1.
ZIKV causes postnatal microcephaly and growth restriction. (A) Confocal imaging of infected E17.5 cerebral cortex stained with antibodies against Sox2 (labeling NPCs; magenta) and Flavivirus group antigen (ZIKV; green). Hoechst stains nuclei (blue). Scale bar: 50 μm. (B) Viral titers in P3 pup brains were determined using the TCID50 assay. A significantly higher titer of ZIKV (1×109.5 TCID50/ml) was detected in P3 pup brains compared with mock controls. Error bars indicate s.e.m. of three independent measurements with one mock and one ZIKV-infected brain in each measurement (***P<0.0001, Student's t-test). (C) Viral titers were determined in brains at different stages using the TCID50 assay. Error bars indicate the s.e.m. of three independent measurements with one mock and one ZIKV-infected brain in each measurement. Analysis of variance (ANOVA) detects a significant increase in viral titer as development proceeds. (D) Dorsal views of P3 pups. ZIKV (∼1 μl 1.7×106 TCID50/ml) was injected into cerebral ventricles of E14.5 brains followed by analyses at P3. Scale bar: 0.5 cm. (E) ZIKV-infected brains are smaller than controls at P3. Scale bar: 2 mm. (F,G) Dorsal views of P3 hearts and lungs after ZIKV intracerebral inoculation of E14.5 mouse brains. Scale bar: 1 mm. (H) Relative weights of different organs from control or ZIKV-infected pups at P3. Error bars indicate the s.e.m. of six independent experiments with one mock and one ZIKV-infected brain in each experiment (*P<0.01, Student's t-test).
ZIKV infection results in neuronal loss and cortical thinning
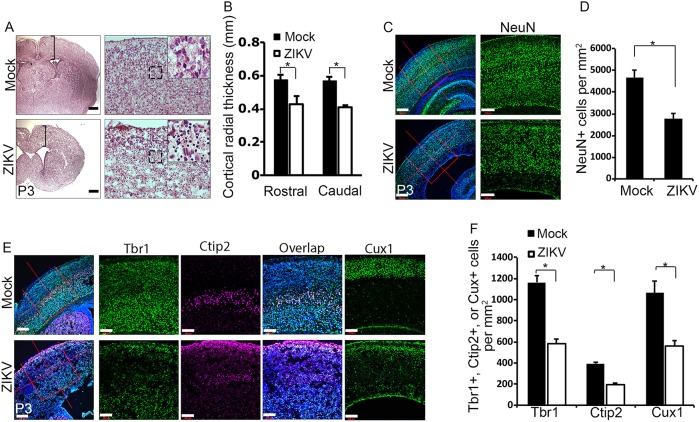
We focused our studies on brain development as microcephaly is a prominent fetal brain abnormality associated with ZIKV infection (Driggers et al., 2016; Mlakar et al., 2016; Oliveira Melo et al., 2016). As expected, cortical radial thickness is significantly reduced in ZIKV-infected brains compared with controls (Fig. 2A,B). Hematoxylin and Eosin (H&E) staining shows dead cell accumulation in ZIKV-infected brains, indicated by the presence of dark puncta (Fig. 2A, black box). Next, we measured total neuronal cell numbers using antibodies against NeuN (Rbfox3), a neuron-specific nuclear protein (Mullen et al., 1992). We found a drastic decrease in total number of neurons after ZIKV infection compared with controls (Fig. 2C,D). These results suggest that embryonic infection of ZIKV causes a significant neuronal loss in postnatal brains.
Fig. 2.
ZIKV infection results in neuronal loss and cortical thinning. (A) Coronal sections of P3 cerebral cortex stained with H&E. Scale bars: 0.2 mm (left panels); 50 μm (right panels). Black brackets indicate the measurement of cortical radial thickness. Black boxed areas are enlarged in insets and show a substantial number of dead cells indicated by dark staining in ZIKV-infected brains. (B) Quantification of cortical radial thickness from the experiment shown in A. Error bars indicate s.e.m. of nine sections from three independent experiments. *P<0.05 (Student's t-test). (C) Confocal imaging of P3 cerebral cortex stained with antibodies against NeuN (green). Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by red boxes in left panels. Scale bars: 200 μm (left panels); 100 μm (right panels). (D) Quantification of NeuN-positive cells per mm2 in a 3.385×105 μm2 boxed area of P3 cerebral cortex from the experiment shown in C. Error bars indicate s.e.m. of nine sections from three independent experiments. *P<0.05 (Student's t-test). (E) Confocal microscope images of coronal sections from P3 cortex stained with antibodies against Tbr1 (green), Ctip2 (magenta) and Cux1 (green). Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by red boxes in left panels. Scale bars: 200 μm (left panels); 100 μm (right panels). (F) Quantification of percentage of Tbr1-, Ctip2- and Cux1-positive cells per mm2 in a 5.274×105 μm2 boxed area of P3 cerebral cortex from the experiment shown in E. Error bars indicate s.e.m. of nine sections from three independent experiments (*P<0.05, Student's t-test).
The cerebral cortex is composed of a six-layer structure generated through the well-described ‘inside-out’ mechanism of corticogenesis. Neurons born earlier reside in deeper layers, whereas later-born neurons migrate over existing layers to form the more superficial layers (Angevine and Sidman, 1961; Marín and Rubenstein, 2003). To determine whether cortical lamination is disrupted and which layer(s) of neurons were affected in ZIKV-infected brains, we examined the well-established layer markers Tbr1 and Ctip2 (Bcl11b) to label layers V-VI, and Cux1 to label layers II-IV (Hevner et al., 2001; Nieto et al., 2004). The overall organization of cortical layers appears to be normal in ZIKV-infected brains compared with controls (Fig. 2E). However, viral infection results in a significant decrease in total Tbr1-, Ctip2- and Cux1-labeled neurons (Fig. 2E,F). Together, these data indicate that the cytoarchitecture of the cortex is largely preserved after ZIKV infection, suggesting that radial migration and lamination are not severely disrupted in the developing brain.
ZIKV infection leads to massive neuronal death and axonal rarefaction
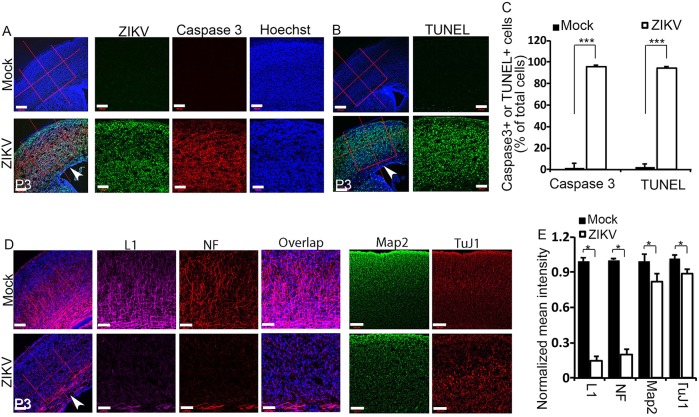
Extensive neuronal cell death is detected in human fetal brains infected with ZIKV (Driggers et al., 2016; Mlakar et al., 2016). Our H&E staining also suggests cell death in ZIKV-infected mouse brains (Fig. 2A). To identify the causes of cortical thinning and postnatal microcephaly associated with ZIKV, we first examined cell death. We stained P3 cerebral cortex using antibodies against activated caspase 3 and found that there is a drastic increase in caspase 3-positive cells in cerebral cortex after ZIKV infection (Fig. 3A, red; Fig. 3C). ZIKV was abundant throughout the cerebral cortex of P3 brains (Fig. 3A, green). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining further confirmed the dramatic increase of apoptotic cells in the virally infected cerebral cortex (Fig. 3B, green; Fig. 3C). Together, these results suggest that ZIKV infection results in extensive neuronal death in the P3 cerebral cortex, which could lead to the smaller brain phenotype.
Fig. 3.
ZIKV infection leads to massive neuronal death and axonal rarefaction. (A) Confocal imaging of P3 cerebral cortex stained with antibodies against cleaved caspase 3 (red) and Flavivirus group antigen (ZIKV, green). Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by red boxes in left panels. White arrowhead indicates the ventricular zone (VZ) with fewer viruses detected. Scale bars: 200 μm (left panels); 100 μm (right panels). (B) TUNEL staining (green) on coronal sections of P3 cortex reveals extensive apoptotic cell labeling in the ZIKV-infected cortex compared with controls. Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by red boxes in left panels. White arrowhead indicates the ventricular zone (VZ) with less viruses detected. Scale bars: 200 μm (left panels); 100 μm (right panels). (C) Quantification of percentage of caspase-3- and TUNEL-positive cells out of total cells in a 5.625×105 μm2 boxed area in the experiments shown in A and B. Error bars indicate s.e.m. of nine sections from three independent experiments. ***P<0.001 (Student's t-test). (D) Confocal microscope images of coronal sections from P3 cortex stained with antibodies against L1 (magenta; labels axons), NF (neurofilament; red; labels mature neurons), Map2 (green; labels dendrites) and TuJ1 (red; labels newly generated neurons). Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by red boxes in left panels. Note the drastically reduced L1 and NF staining indicated by white arrowhead in ZIKV-infected cerebral cortex. Scale bars: 200 μm (left panels); 100 μm (right panels). (E) Quantification of relative signal intensities from L1, NF, Map2 and TuJ1 staining using ImageJ. Error bars indicate s.e.m. of nine sections from three independent experiments (*P<0.05, Student's t-test).
To determine whether axons are impaired by ZIKV infection, we used L1 (L1cam) to label callosal axons and found that there is dramatic axon reduction in virally infected brains (Fig. 3D, magenta; Fig. 3E). Similarly, callosal axons labeled with antibodies for neurofilament (NF) were nearly absent from ZIKV-infected P3 cerebral cortex (Fig. 3D, red; Fig. 3E), which suggests a corpus callosum defect (Fig. 3D, white arrowhead). By contrast, staining with the dendritic marker Map2 revealed a relatively minor reduction of dendrites in the cortex compared with axons in ZIKV-infected brains (Fig. 3D, green; Fig. 3E). These results suggest that axons are more severely affected than dendrites in the developing brain. Next, we used antibodies against TuJ1 to label newly generated neurons. We found a relatively minor reduction in newly generated neurons in virally infected brains (Fig. 3D,E). Together, these results suggest that ZIKV infection leads to massive neuronal death and axonal rarefaction.
Cell cycle arrest and apoptosis in ZIKV-infected NPCs
ZIKV infects human NPCs and causes their cell cycle arrest (Dang et al., 2016; Garcez et al., 2016; Qian et al., 2016; Tang et al., 2016). Next, we examined NPCs in ZIKV-infected embryonic brains. We did not detect significant growth reduction or microcephaly in E16.5 embryos after ZIKV inoculation at E14.5 (Fig. S2A,B) and ZIKV was not detected in the cerebral cortex sections of E16.5 brains (Fig. S2C). Therefore, we focused on NPCs located at the ventricular zone and subventricular zone (VZ/SVZ) of the E17.5 cerebral cortex.
Phosphorylation of histone H3 (p-H3) is tightly correlated with chromosome condensation and serves as a marker for mitosis. We found that p-H3-positive cells are significantly reduced in ZIKV-infected brains compared with controls (Fig. S3A,B). Next, we used Ki67 (Mki67) to label all cycling cells and found that there is a slight but significant increase in Ki67-positive cells in virally infected brains compared with controls (Fig. S3A,C). Cell proliferation can be measured with the thymidine analog 5-chloro-2′-deoxyuridine (CldU) following its incorporation into newly synthesized DNA and its subsequent detection with antibodies against CldU. We performed CldU pulse experiments and found that there is a significant decrease in CldU-positive cells in the virally infected brains (Fig. S3D,E). Finally, we measured cell cycle length. Control NPCs exhibit a cell cycle length of around 20 h in E17.5 embryonic brains, which is consistent with published studies (Siegenthaler et al., 2008). However, virally infected NPCs display an extended cell cycle length of around 30 h (Fig. S3F). Together, these results suggest that there is a cell cycle arrest of NPCs after ZIKV infection in the developing brain.
To examine NPC survival, we used Pax6 to label apical neural progenitor cells, and Tbr2 (Eomes) to label intermediate neural progenitors in the cerebral cortex (Kriegstein and Alvarez-Buylla, 2009). TUNEL staining shows that ZIKV infection leads to a significant increase in apoptotic cells in both Pax6- and Tbr2-positive NPCs (Fig. S4A,B). However, we noticed that there is only a small fraction of TUNEL-positive NPCs (<2.5% for Pax6-positive NPCs; <1% for Tbr2-positive cells) in virally infected brains at E17.5. Therefore, we measured total Pax6- and Tbr2-positive cells and found that there is no significant difference between ZIKV-infected brains and controls (Fig. S4C). Together, these results suggest that ZIKV causes cell cycle arrest and apoptosis, but there is no substantial depletion of the NPC pool in E17.5 infected brains.
ZIKV infection leads to abnormal vasculature and a leaky blood–brain barrier (BBB)
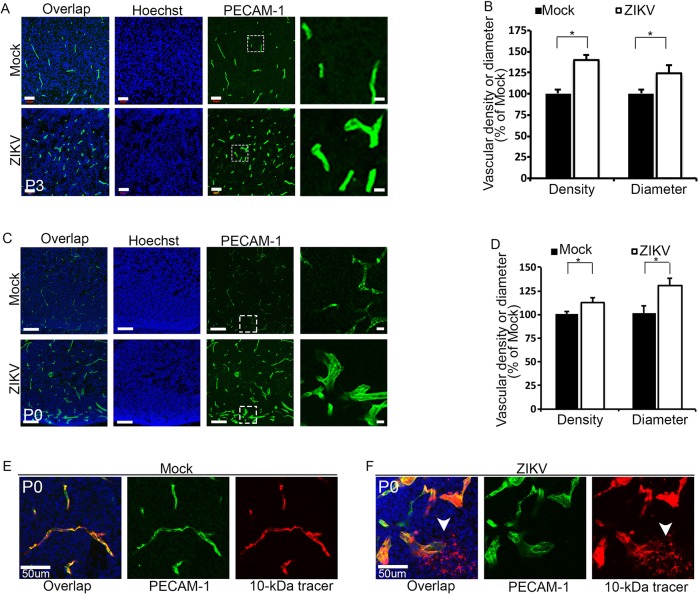
Brain calcification is a prominent fetal brain pathology associated with ZIKV infection in humans (Driggers et al., 2016; Mlakar et al., 2016; Oliveira Melo et al., 2016). BBB dysfunction has been reported to cause calcification in neurons (Keller et al., 2013; Miklossy et al., 2005). Therefore, we examined the integrity of the vasculature in ZIKV-infected brains. We labeled blood vessels in the developing brain using antibodies against PECAM-1. Immunostaining results show that there is a significant increase in vessel density and vessel diameter in P3 virally infected cerebral cortex compared with controls (Fig. 4A,B). We also examined vessels at P0 and found a similar increase in vessel density and diameter (Fig. 4C,D). Thus, these results suggest that ZIKV infection results in abnormal vasculature in the developing brain.
Fig. 4.
Abnormal vasculature and leaky BBB in ZIKV-infected brains. (A,C) Confocal micrographs of P3 (A) or P0 (C) coronal cortical sections stained with antibodies against PECAM-1 (green) after viral infection at E14.5. Hoechst stains nuclei (blue). Right panels are enlargements of the white boxed areas. Scale bars: 50 μm (left panels in A); 20 μm (right panels in A), 100 μm (left panels in C) and 10 μm (right panels in C). (B,D) Quantification of relative vessel density and diameter from the experiments shown in A and C. Measurements of vessel density and diameter in ZIKV-infected brain sections were normalized to that in controls. Error bars indicate s.e.m. of nine sections from three independent experiments. *P<0.05 (Student's t-test). (E,F) Confocal micrographs of coronal sections of P0 brains stained with antibodies against PECAM-1 (green) together with 10 kDa dextran tracer (red). Hoechst stains nuclei (blue). Scale bars: 50 μm. Dextran tracer (10 kDa) revealed a leaky BBB (white arrowheads) in P0 brains after ZIKV infection at E14.5.
The mouse BBB becomes functional at E15.5 (Ben-Zvi et al., 2014). Altered vasculature in ZIKV-infected brains prompted us to investigate whether the BBB is damaged after viral infection. To address this question, we performed a postnatal BBB permeability assay according to published methods (Ben-Zvi et al., 2014). Dextran tracer (10 kDa) was injected intracardially at P0 and antibodies against PECAM-1 subsequently used to visualize the blood vessels against the distribution of the dye. The tracer was confined to the capillaries in control brains (Fig. 4E) whereas ZIKV-infected brains showed obvious tracer leakage (Fig. 4F, white arrowheads) in the developing caudate. These results indicate that ZIKV alters vasculature integrity and results in a leaky BBB in the developing brain. Together, massive neuronal death and BBB leakage phenotypes suggest that ZIKV infection causes extensive damage in the developing brain.
ZIKV-infected brains exhibit extensive microglial activation and astrogliosis
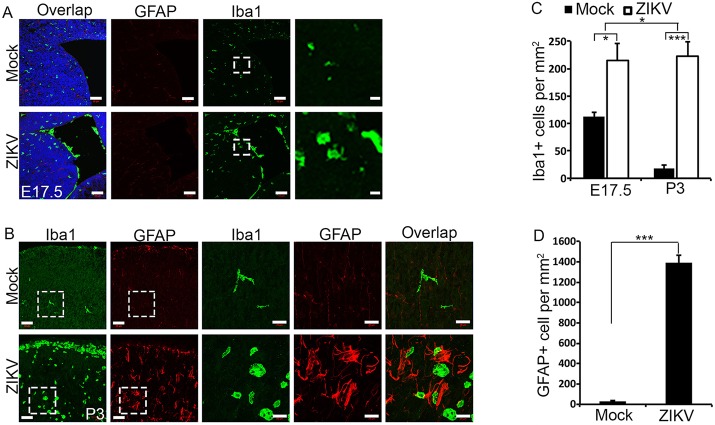
Microglia are brain-resident innate immune cells that respond swiftly to viral infection by transforming into an activated state and performing their functions (Nayak et al., 2014). In their activated state, microglia will change from a ramified morphology to a rounded one, as well as increase in number. Using Iba1 as a marker of microglia, we found both morphological alterations and a significant increase in number of Iba1-positive cells as early as E17.5 in virally infected mouse brains in subcortical structures and ventricles (Fig. 5A,C), suggesting microglial activation 3 days post viral infection. P3 infected brains exhibited sustained microglial activation and morphological changes, with a drastic increase in Iba1-positive cells compared with controls (Fig. 5B,C), spread across the brain parenchyma. Importantly, there is a significant increase in Iba1-positive cells at P3 compared with E17.5 in virally infected brains (Fig. 5A-C), suggesting a continued and progressive increase in microglial activation after ZIKV infection.
Fig. 5.
Extensive microglial activation and astrogliosis in ZIKV-infected brain. (A,B) Confocal micrographs of coronal sections of E17.5 (A) and P3 (B) brains stained with antibodies against Iba1 (green) or GFAP (red). Hoechst stains nuclei (blue). Right panels are enlargements of the regions outlined by white boxes in left panels. Scale bars: 50 μm (left panels in A and B); 10 μm (right panels in A) and 20 μm (right panels in B). (C) Quantification of Iba1-positive cells per mm2 in a 1.314×105 μm2 boxed area of E17.5 cerebral cortex from the experiment shown in A, and a 1.314×105 μm2 boxed area of P3 cerebral cortex from the experiment shown in B. Error bars indicate s.e.m. of nine sections from three independent experiments. *P<0.05, ***P<0.001 (Student's t-test for comparison of mock- versus ZIKV-infected brains). Two-way ANOVA analysis detected a significant difference in the increase of Iba1-positive cells between E17.5 and P3 (*P<0.05). (D) Quantification of GFAP-positive cells in a 1.314×105 μm2 boxed area of P3 cerebral cortex from the experiment shown in B. Error bars indicate s.e.m. of nine sections from three independent experiments. ***P<0.001 (Student's t-test).
Astrocytes are the most abundant cell type in the CNS and provide neuronal support by supplying nutrients, maintaining the chemical environment, and clearing neurotransmitters. Astrogliosis is the abnormal increase and change in morphology and behavior of astrocytes in the brain, typically in response to brain injury including viral infection (Sofroniew, 2009). To confirm that ZIKV infection induces brain damage, we examined the astrocyte population and morphology. Using GFAP staining to identify reactive astrocytes in the cortex at P3, we observed a significant increase in the number of astrocytes in ZIKV-infected brains (Fig. 5B,D), suggesting astrogliosis and brain injury in P3 brains. In healthy brains, GFAP is only expressed in the radial glia and fibrous astrocytes of the white matter, but strong GFAP expression in the cortex and changes in morphology indicate a change of protoplasmic astrocytes into reactive astrocytes (Fig. 5B). We did not detect significant changes in GFAP-positive cells at E17.5 (data not shown). Together, these results indicate that ZIKV infection results in progressive microglial activation and astrogliosis, further supporting the notion that ZIKV induces brain damage.
ZIKV induces dysregulation of genes involved in immune response
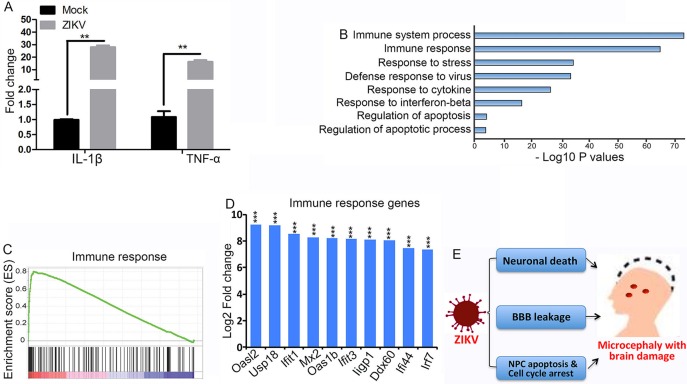
ZIKV triggers inflammation and an antiviral response in human skin fibroblasts, including the upregulation of interleukin-1 beta (IL-1β; IL1B) (Hamel et al., 2015). It has been reported that IL-1β and tumor necrosis factor-α (TNF-α) induce damages to the vasculature and neurons (Friedl et al., 2002; Lu et al., 2005; Puhlmann et al., 2005; Takeuchi et al., 2006). Therefore, we hypothesize that NPCs will generate an immune and inflammation response after ZIKV infection. To test this hypothesis, we infected NPCs, isolated from developing brains, with ZIKV for 48 h followed by RT-PCR analyses. We found that expression of IL-1β and TNF-α was dramatically increased after viral infection (Fig. 6A), suggesting that ZIKV induced an immune response in the NPCs.
Fig. 6.
ZIKV induces dysregulation of genes involved in the immune response. (A) RT-PCR analysis of expression of IL-1β and TNF-α in NPCs. NPCs isolated from E14.5 developing brains were infected with ZIKV (multiplicity of infection: 5) followed by RNA isolation at 48 h post-infection. Error bars indicate s.e.m. of three independent experiments. **P<0.01 (Student's t-test). (B) RNA-Seq analyses of E17.5 brains after ZIKV infection at E14.5. The dysregulated genes in ZIKV-infected brains compared with controls were subjected to GO analysis. (C) Gene set enrichment analysis (GSEA) reveals dysregulated genes in immune response in ZIKV-infected brains. x-axis represents the gene ranks in ordered immune response dataset. (D) RNA expression analyses of RNA-Seq data (Table S1) show that the top ten most significantly upregulated genes in infected brains are involved in immune response. ***P<0.001 (Student's t-test). (E) A diagram showing that ZIKV causes neuronal death, abnormal vasculature and BBB leakage, and cell cycle arrest and apoptosis of NPCs, resulting in postnatal microcephaly with brain damage.
To improve our understanding of ZIKV-induced immune responses in the developing brain at genome-wide levels, we performed global transcriptome analyses (RNA-Seq) using RNAs isolated from E17.5 brains after viral infection at E14.5. A large number of differentially expressed genes after viral infection were identified with RNA-Seq analysis (Table S1). We next performed Gene Ontology analyses with those genes for which expression was significantly altered after viral infection. Our results revealed a set of genes that are associated with the immune response and apoptosis pathways (Fig. 6B). Gene set enrichment analyses (GSEA) further show significant enrichments on both the immune system response and apoptosis pathways (Fig. 6C; data not shown). Most notably, the top ten most upregulated genes are associated with interferon response (Fig. 6D). Together, these results suggest that ZIKV infection triggers an aggressive immune response, which has the potential to cause exacerbation of brain damage by enhancing neuronal death and generating vascular abnormalities.
DISCUSSION
In this study, we have established a mouse model of fetal brain abnormalities associated with ZIKV, and provide a direct causative link between ZIKV and postnatal microcephaly in vivo. ZIKV triggers a strong immune response in the developing brain. In addition to disrupting NPCs, ZIKV infection leads to massive neuronal death and BBB leakage, resulting in postnatal microcephaly with extensive brain damage (Fig. 6E).
Studies from our mouse model provide novel insights into microcephaly associated with ZIKV infection during pregnancy. First, ZIKV infection is sufficient to cause postnatal microcephaly. Previous studies have all described embryonic lethality, whereas our ZIKV-infected pups survived after birth. Importantly, ZIKV-infected human fetuses can be born alive exhibiting microcephaly, which suggests that our postnatal microcephaly animal model is disease relevant. In addition to microcephaly, our postnatal mouse model recapitulates major aspects of fetal brain abnormalities associated with ZIKV in humans, including extensive neuronal apoptosis and loss, axonal rarefaction, and reactive astrocyte and microglial cell accumulation (Driggers et al., 2016; Mlakar et al., 2016; Oliveira Melo et al., 2016), which have not been described in previous animal models. Second, our data suggest that neuronal cell death contributes significantly to the microcephaly associated with ZIKV, consistent with the selective neuronal vulnerability to ZIKV observed in humans (Driggers et al., 2016). Interestingly, these observations are in contrast with classic microcephaly conditions, in which NPC depletion is the major cause of the smaller brain phenotype based on studies from other animal models for microcephaly (Chen et al., 2014; Gruber et al., 2011; Thornton and Woods, 2009). Our current studies cannot yet determine whether ZIKV directly infects terminally differentiated neurons, resulting in their death, or if ZIKV-infected NPCs differentiate into neurons followed by neuronal death. Future studies should address the relative contributions of neuronal death and reduced neuronal production due to defective NPCs in causing microcephaly. Another limitation of our studies is the lack of a control viral infection such as dengue virus, which would allow us to distinguish ZIKV-specific effects from general toxicity of viral infection. Although we observed consistent growth restriction and microcephaly from ZIKV-infected pups compared with controls (Fig. 1H, n=6), future studies should increase the numbers of mice analyzed in each experiment. Third, ZIKV-associated microcephaly is coupled with global growth restriction. It is intriguing that ZIKV intracerebral inoculation causes global growth restriction of mice. It is possible that local brain infection of ZIKV can result in a global effect on growth restriction due to the inflammatory and antiviral immune responses of the embryo.
Our studies have uncovered that ZIKV causes BBB leakage in the developing brain, which could contribute to brain damage. Previous studies suggest that BBB deficiency leads to calcification in neurons (Keller et al., 2013), probably due to the formation of a nidus from plasma proteins accumulating in the parenchyma from a leaky BBB (Miklossy et al., 2005). We found altered vasculature and a leaky BBB in ZIKV-infected mouse brains. However, our multiple attempts failed to detect calcification in the ZIKV-infected brains, which could be due to the relatively short pregnancy period in mice compared with humans. Future studies should reveal whether BBB deficiency does indeed cause or contribute to brain calcification associated with ZIKV infection in humans. It will be of interest to determine what causes vascular abnormalities in ZIKV-infected brains, although inflammation associated with ZIKV infection is likely to play important roles (Friedl et al., 2002; Puhlmann et al., 2005). Future studies should also reveal potential detrimental effects of BBB leakage on NPCs and differentiated neurons.
We found that ZIKV causes extensive brain damage. Classical microcephaly is characterized by smaller brain size without other obvious neuronal and brain damage (Nigg and Stearns, 2011; Thornton and Woods, 2009). Previous studies examining ZIKV-induced microcephaly have focused on NPC behaviors and do not describe other negative contributions to brain development. Therefore, it remained unclear from the current literature whether animal models of ZIKV pathogenesis can be used to recapitulate brain abnormalities other than microcephaly. Our discovery of massive neuronal death, leaky BBB, and astrogliosis in ZIKV-infected brains is the first study to suggest that ZIKV causes extensive brain damage.
ZIKV has been detected in amniotic fluid, placental tissues, and fetal and newborn brains (Calvet et al., 2016; Driggers et al., 2016; Mlakar et al., 2016; Noronha et al., 2016), suggesting that ZIKV can cross the placenta and infect fetal brains. Furthermore, it has been reported that ZIKV can infect human placental macrophages and trophoblasts (Quicke et al., 2016). Mouse model studies suggest that ZIKV can infect and damage the placenta, and cause microcephaly and growth restriction (Cugola et al., 2016; Miner et al., 2016). Our intracerebral inoculation method bypasses the placenta and infects fetal brains directly. We acknowledge the limitations of our animal model in studying ZIKV disease mechanisms in early pregnancy, in particular in investigating if and how ZIKV crosses the placenta. However, the unique survival of our ZIKV-infected pups after birth due to a different virus dosage, infection methods and timing provides advantages for studying the wide spectrum of brain abnormalities associated with ZIKV infection.
MATERIALS AND METHODS
Ethics statement
All animals were handled according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Georgia Athens (UGA). All of the experiments related to ZIKV were conducted by the following protocols approved by the UGA Institutional Biosafety Committee.
ZIKV virus (Mexican isolate MEX1-44)
Zika MEX1-44 was isolated in Chiapas, Mexico in January 2016 from an infected Aedes aegypti mosquito. The virus was passaged by the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) four times in Vero cells. We obtained this virus with permission through the University of Texas Medical Branch at Galveston (UTMB). We then amplified the stock an additional two passages in Vero cells, so the virus used in the experiments had been passaged six times in Vero cells from the time it was isolated from the mosquito. We have sequenced the virus (passage 5) and found that the sequence of ZIKV-MEX1-44 is 99% identical at the nucleotide level to the sequence of the ZIKV strain from Brazil (ZIKV PE243/2015).
Viral inoculation of embryonic brains
For timed pregnant mating, noon of the day after mating was considered E0.5. Pregnant C57BL/6J or 129S1/SvImJ mice with E14.5 embryos were treated with ketamine hydrochloride and xylazine to induce anesthesia. ZIKV virus (∼1 μl 1.7×106 TCID50/ml Mexican isolate MEX1-44) was injected into the lateral ventricles of E14.5 embryo brains. Control media was used as a sham injection. To improve retention of the pregnancy, we avoided viral injection of the two embryos next to the ovaries and the two embryos next to the upper vagina. Injected embryos were placed back to pregnant dams and allowed to develop after surgery for varied times according to individual experiments.
Viruses and titration
Vero cells (African green monkey kidney epithelial cells) were obtained from ATCC. Vero cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with penicillin/streptomycin and 10% fetal bovine serum at 37°C with 5% CO2. ZIKV stocks were propagated on Vero cells after inoculating at a multiplicity of infection of 0.01 and harvesting supernatants at 96 h and 120 h post-infection. The viral titer of ZIKV for brains and other organs were determined as 50% tissue culture infectious doses (TCID50) on Vero cells. Briefly, tissues were homogenized in ten volumes PBS and centrifuged at 3000 rpm (845 g) for 10 min. The supernatant was serially diluted tenfold in DMEM. A 100 μl aliquot of each diluted sample (10 dilutions in total) was added to 96-well plates, containing a monolayer of Vero cells. Cells were cultured for 96-120 h at 37°C in a tissue culture incubator. Cytopathic effect of endpoint dilutions was monitored.
Quantitative real-time PCR of ZIKV RNA
Total RNA was extracted with TRIzol (Invitrogen). Then, 1 µg RNA was reverse transcribed into cDNA utilizing SuperScript III first-strand synthesis for RT-PCR (Invitrogen) in a 20 µl reaction system. The cDNA was analyzed by qRT-PCR with Master mix (Taqman). The primers and probe specific for Zika virus have been previously described (Lanciotti et al., 2008). qRT-PCR was conducted under the following cycling conditions: 95°C for 10 min for pre-denaturation, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The last step was conducted at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 sec, which is necessary to acquire a melting curve for the PCR products to confirm the specificity of amplification. The relative mRNA abundances were analyzed utilizing the 2−ΔΔCt method with Gapdh as a reference and plotted as fold changes compared with the mock-treated samples. Specific ZIKV primers and probes were: ZIKV-1086 GCCCAACACAAG; ZIKV-1162c CCACTAACGTTCTTTTGCAGACAT; ZIKV-1107-FAM AGCCTACCTTGACAAGCAGTCAGACACTCAA.
Mouse brain phenotype analysis
Histological processing, TUNEL assay, and immunohistochemical labeling of cryosections were performed as described previously (Chen et al., 2014). Coronal sections of the cerebral cortex from different stages of embryos as indicated in the figure and text were used. The primary antibodies used are listed in Table S2. The secondary antibodies used were Alexa 488 and Alexa 555 conjugated to specific IgG types (Invitrogen Molecular Probes). All the experiments have been repeated at least three times, and representative images are shown in the individual figures.
CldU labeling study
After ZIKV inoculation of E14.5 brains, pregnant dams with E17.5 embryos were injected intraperitoneally with CldU at 10 mg/kg body weight. The animals were sacrificed 2 h after the injection. The brains were dissected out and fixed in 4% paraformaldehyde (PFA) overnight. Subsequently, the brains were stored in 30% sucrose for 16 h and embedded in Tissue-Tek O.C.T. Compound (Sakura). Cortical coronal sections were prepared for immunohistochemical staining using antibodies against CldU (Abcam, ab6326, 1:200).
Cell cycle length analysis
Cell cycle kinetics were determined by a dual-labeling approach as described previously (Martynoga et al., 2005; Siegenthaler et al., 2008). On E17.5, the pregnant mouse was injected with CldU (10 mg/ml, Sigma; 100 μl per 100 g body weight) at a time designated as T=0 h, such that all cells at S phase from the beginning of the experiment were labeled with CldU. At T=1.5 h, the pregnant mouse was injected with EdU (1 mg/ml, Invitrogen; 100 μl for per 100 g body weight) to label all cells in S phase. The animal was killed at T=2 h and its embryos were collected immediately. Embryo sections were immunostained using an anti-CldU antibody (Abcam, ab6326, 1:200) and Click-iT EdU Alexa Fluor 555 Imaging Kit (Life Technologies, C10338). Images were obtained with a Zeiss LSM 710 inverted confocal microscope. The length of S phase (Ts) and total length of cell cycle (Tc) were determined based on the relative number of cells that incorporated one or both of CldU and EdU (Martynoga et al., 2005). The ratio of the length of any one period of the cell cycle to that of another period is equal to the ratio of the number of cells in the first period to the number in the second period (Nowakowski et al., 1989). So Ts was calculated as the interval between both injections (Ti=1.5 h) divided by the quotient of the density of CldU+EdU− cells (Lcells, i.e. cells that have been in but left S phase before EdU injection) and CldU+EdU+ cells (Scells, i.e. cells remaining in S phase at the end of the experiment), i.e. Ti/Ts=Lcells/Scells. Using the same logic, the total cell cycle length can be calculated as below: Ts/Tc=Scells/Pcells where Pcells is estimated by counting the total numbers of cells in the assessed area.
Postnatal BBB permeability assay
This method was based on the well-established adult BBB dye-injection permeabilization assay, with modifications to perform on P0 pups (Ben-Zvi et al., 2014). In brief, P0 pups were deeply anesthetized and 10 μl of 10 mg/ml 10 kDa dextran tetramethylrhodamine (D3312, Molecular Probes) was injected into the left ventricle using a Hamilton syringe. After allowing for 5 min of circulation, brains were dissected without perfusion in order to avoid damage or washout of the dye, and fixed in 4% PFA overnight. Brains were then rinsed 3×5 min in PBS and cryopreserved in 25% sucrose for overnight. Brains were embedded coronally in Tissue-Tek O.C.T. Compound (Sakura) and sectioned at 25 μm. Sections were stained with PECAM antibody (550274, BD Biosciences, 1:200) to visualize the blood vessels against the distribution of the dye.
RNA isolation and high-throughput sequencing
Total RNAs were extracted from two mock and two ZIKV-infected E17.5 brains using TRIzol reagent (Life Technologies). Genomic DNA and ribosomal RNA was removed with Turbo DNA-free kit and RiboMinus Eukaryote Kit (Life Technologies). The resulting RNA fractions were subjected to strand-specific library preparation using NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs). Sequencing was performed on a Nextseq500 (Illumina).
Acknowledgements
We thank Chen lab colleagues for stimulating discussions. Zika isolates were provided by the World Reference Center for Emerging Viruses and Arboviruses at UTMB.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Q.S., S.H., S.-L.Y., F.L. conceived and performed all experiments. J.M.M. and M.A.B. prepared virus and helped with the manuscript writing. J.-F.C. designed and interpreted the experiments and wrote the manuscript.
Funding
This work was supported by funds from the Office of the Vice President for Research (OVPR) at University of Georgia; and by the National Institutes of Health [R00HD073269, R01NS096176 and R01NS097231 to J.C.; HD046860 and AI111242 to J.M.M.]. Deposited in PMC for release after 12 months.
Data availability
RNA-Seq data have been deposited in the NCBI Sequence Read Archive (SRA) under accession number GSE89069.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.143768.supplemental
References
- Angevine J. B. and Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766-768. 10.1038/192766b0 [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B. J., Mayshar Y., Yan H. and Gu C. (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507-511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P., Pereira J. P. Jr, Raja Gabaglia C., Damasceno L., Wakimoto M., Ribeiro Nogueira R. M., Carvalho de Sequeira P., Machado Siqueira A., Abreu de Carvalho L. M., Cotrim da Cunha D. et al. (2016). Zika virus infection in pregnant women in rio de janeiro-preliminary report. N. Engl. J. Med. doi.org/10.1056/NEJMoa1602412. 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet G., Aguiar R. S., Melo A. S. O., Sampaio S. A., de Filippis I., Fabri A., Araujo E. S. M., de Sequeira P. C., de Mendonça M. C. L., de Oliveira L. et al. (2016). Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect. Dis. 16, 653-660. 10.1016/S1473-3099(16)00095-5 [DOI] [PubMed] [Google Scholar]
- Chen J.-F., Zhang Y., Wilde J., Hansen K. C., Lai F. and Niswander L. (2014). Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 5, 3885 10.1038/ncomms4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola F. R., Fernandes I. R., Russo F. B., Freitas B. C., Dias J. L. M., Guimarães K. P., Benazzato C., Almeida N., Pignatari G. C., Romero S. et al. (2016). The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267-271. 10.1038/nature18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J., Tiwari S. K., Lichinchi G., Qin Y., Patil V. S., Eroshkin A. M. and Rana T. M. (2016). Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258-265. 10.1016/j.stem.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers R. W., Ho C.-Y., Korhonen E. M., Kuivanen S., Jääskeläinen A. J., Smura T., Rosenberg A., Hill D. A., DeBiasi R. L., Vezina G. et al. (2016). Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142-2151. 10.1056/nejmoa1601824 [DOI] [PubMed] [Google Scholar]
- Friedl J., Puhlmann M., Bartlett D. L., Libutti S. K., Turner E. N., Gnant M. F. X. and Alexander H. R. (2002). Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood 100, 1334-1339. [PubMed] [Google Scholar]
- Garcez P. P., Loiola E. C., Madeiro da Costa R., Higa L. M., Trindade P., Delvecchio R., Nascimento J. M., Brindeiro R., Tanuri A. and Rehen S. K. (2016). Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816-818. 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- Gruber R., Zhou Z., Sukchev M., Joerss T., Frappart P.-O. and Wang Z.-Q. (2011). MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat. Cell Biol. 13, 1325-1334. 10.1038/ncb2342 [DOI] [PubMed] [Google Scholar]
- Hamel R., Dejarnac O., Wichit S., Ekchariyawat P., Neyret A., Luplertlop N., Perera-Lecoin M., Surasombatpattana P., Talignani L., Thomas F. et al. (2015). Biology of Zika virus infection in human skin cells. J. Virol. 89, 8880-8896. 10.1128/JVI.00354-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner R. F., Shi L., Justice N., Hsueh Y.-P., Sheng M., Smiga S., Bulfone A., Goffinet A. M., Campagnoni A. T. and Rubenstein J. L. R. (2001). Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29, 353-366. 10.1016/S0896-6273(01)00211-2 [DOI] [PubMed] [Google Scholar]
- Heymann D. L., Hodgson A., Sall A. A., Freedman D. O., Staples J. E., Althabe F., Baruah K., Mahmud G., Kandun N., Vasconcelos P. F. C. et al. (2016). Zika virus and microcephaly: why is this situation a PHEIC? Lancet 387, 719-721. 10.1016/S0140-6736(16)00320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Westenberger A., Sobrido M. J., García-Murias M., Domingo A., Sears R. L., Lemos R. R., Ordoñez-Ugalde A., Nicolas G., da Cunha J. E. G. et al. (2013). Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 45, 1077-1082. 10.1038/ng.2723 [DOI] [PubMed] [Google Scholar]
- Kriegstein A. and Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149-184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kosoy O. L., Laven J. J., Velez J. O., Lambert A. J., Johnson A. J., Stanfield S. M. and Duffy M. R. (2008). Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging Infect. Dis. 14, 1232-1239. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Xu D., Ye Q., Hong S., Jiang Y., Liu X., Zhang N., Shi L., Qin C.-F. and Xu Z. (2016). Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120-126. 10.1016/j.stem.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Lu K.-T., Wang Y.-W., Yang J.-T., Yang Y.-L. and Chen H.-I. (2005). Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J. Neurotrauma 22, 885-895. 10.1089/neu.2005.22.885 [DOI] [PubMed] [Google Scholar]
- Manzini M. C. and Walsh C. A. (2011). What disorders of cortical development tell us about the cortex: one plus one does not always make two. Curr. Opin. Genet. Dev. 21, 333-339. 10.1016/j.gde.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O. and Rubenstein J. L. R. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441-483. 10.1146/annurev.neuro.26.041002.131058 [DOI] [PubMed] [Google Scholar]
- Marrs C., Olson G., Saade G., Hankins G., Wen T., Patel J. and Weaver S. (2016). Zika virus and pregnancy: a review of the literature and clinical considerations. Am. J. Perinatol. 33, 625-639. 10.1055/s-0036-1580089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B., Morrison H., Price D. J. and Mason J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 283, 113-127. 10.1016/j.ydbio.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Miklossy J., Mackenzie I. R., Dorovini-Zis K., Calne D. B., Wszolek Z. K., Klegeris A. and McGeer P. L. (2005). Severe vascular disturbance in a case of familial brain calcinosis. Acta Neuropathol. 109, 643-653. 10.1007/s00401-005-1007-7 [DOI] [PubMed] [Google Scholar]
- Miner J. J., Cao B., Govero J., Smith A. M., Fernandez E., Cabrera O. H., Garber C., Noll M., Klein R. S., Noguchi K. K. et al. (2016). Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081-1091. 10.1016/j.cell.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodušek V. et al. (2016). Zika virus Associated with Microcephaly. N. Engl. J. Med. 374, 951-958. 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Buck C. R. and Smith A. M. (1992). NeuN, a neuronal specific nuclear protein in vertebrates. Development 116, 201-211. [DOI] [PubMed] [Google Scholar]
- Nayak D., Roth T. L. and McGavern D. B. (2014). Microglia development and function. Annu. Rev. Immunol. 32, 367-402. 10.1146/annurev-immunol-032713-120240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Monuki E. S., Tang H., Imitola J., Haubst N., Khoury S. J., Cunningham J., Götz M. and Walsh C. A. (2004). Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J. Comp. Neurol. 479, 168-180. 10.1002/cne.20322 [DOI] [PubMed] [Google Scholar]
- Nigg E. A. and Raff J. W. (2009). Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663-678. 10.1016/j.cell.2009.10.036 [DOI] [PubMed] [Google Scholar]
- Nigg E. A. and Stearns T. (2011). The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 13, 1154-1160. 10.1038/ncb2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha L. d., Zanluca C., Azevedo M. L. V., Luz K. G. and Santos C. N. D. D. (2016). Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz 111, 287-293. 10.1590/0074-02760160085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski R. S., Lewin S. B. and Miller M. W. (1989). Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 18, 311-318. 10.1007/BF01190834 [DOI] [PubMed] [Google Scholar]
- Oliveira Melo A. S., Malinger G., Ximenes R., Szejnfeld P. O., Alves Sampaio S. and Bispo de Filippis A. M. (2016). Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol. 47, 6-7. 10.1002/uog.15831 [DOI] [PubMed] [Google Scholar]
- Puhlmann M., Weinreich D. M., Farma J. M., Carroll N. M., Turner E. M. and Alexander H. R. (2005). Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J. Transl. Med. 3, 37 10.1186/1479-5876-3-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., Yao B., Hamersky G. R., Jacob F., Zhong C. et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238-1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke K. M., Bowen J. R., Johnson E. L., McDonald C. E., Ma H., O'Neal J. T., Rajakumar A., Wrammert J., Rimawi B. H., Pulendran B. et al. (2016). Zika virus infects human placental macrophages. Cell Host Microbe 20, 83-90. 10.1016/j.chom.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos da Silva S. and Gao S.-J. (2016). Zika virus: an update on epidemiology, pathology, molecular biology and animal model. J. Med. Virol. 88, 1291-1296. 10.1002/jmv.24563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz J.-C., Vázquez-Calvo Á., Blázquez A. B., Merino-Ramos T., Escribano-Romero E. and Martin-Acebes M. A. (2016). Zika virus: the latest newcomer. Front. Microbiol. 7, 496 10.3389/fmicb.2016.00496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B. D., Blomgren K., Gimlin K., Ferriero D. M. and Noble-Haeusslein L. J. (2013). Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106-107, 1-16. 10.1016/j.pneurobio.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler J. A., Tremper-Wells B. A. and Miller M. W. (2008). Foxg1 haploinsufficiency reduces the population of cortical intermediate progenitor cells: effect of increased p21 expression. Cereb. Cortex 18, 1865-1875. 10.1093/cercor/bhm209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M. V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638-647. 10.1016/j.tins.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Sonobe Y., Mizuno T. and Suzumura A. (2006). Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 281, 21362-21368. 10.1074/jbc.M600504200 [DOI] [PubMed] [Google Scholar]
- Tang H., Hammack C., Ogden S. C., Wen Z., Qian X., Li Y., Yao B., Shin J., Zhang F., Lee E. M. et al. (2016). Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18, 587-590. 10.1016/j.stem.2016.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton G. K. and Woods C. G. (2009). Primary microcephaly: do all roads lead to Rome? Trends Genet. 25, 501-510. 10.1016/j.tig.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]