Abstract
Background
Previous studies have demonstrated that glial cells play an important role in the generation and maintenance of neuropathic pain. Activated glial cells produce numerous mediators such as proinflammatory cytokines that facilitate neuronal activity and synaptic plasticity. Similarly, bladder pain syndrome/interstitial cystitis shares many characteristics of neuropathic pain. However, related report on the involvement of spinal glia in bladder pain syndrome/interstitial cystitis-associated pathological pain and the underlying mechanisms are still lacking. The present study investigated spinal glial activation and underlying molecular mechanisms in a rat model of bladder pain syndrome/interstitial cystitis.
Results
A rat model of bladder pain syndrome/interstitial cystitis was established via systemic injection with cyclophosphamide. Mechanical allodynia was tested with von Frey monofilaments and up-down method. Moreover, Western blots and double immunofluorescence were used to detect the expression and location of glial fibrillary acidic protein, OX42/Iba1, P-P38, NeuN, interleukin (IL)-1β, phosphorylation of N-methyl-D-aspartate receptor 1 (P-NR1), and IL-1 receptor I (IL-1RI) in the L6-S1 spinal cord. We found that glial fibrillary acidic protein rather than OX42/Iba1 or P-P38 was significantly increased in the spinal cord of cyclophosphamide-induced cystitis. L-alpha-aminoadipate but not minocycline markedly attenuated the allodynia. Furthermore, we found that spinal IL-1β was dramatically increased in cyclophosphamide-induced cystitis, and activated astrocytes were the only source of IL-1β release, which contributed to allodynia in cystitis rats. Besides, spinal P-NR1 was statistically increased in cyclophosphamide-induced cystitis and only localized in IL-1RI positive neurons in spinal dorsal horn. Additionally, NR antagonist significantly attenuated the cystitis-induced pain. Interestingly, the time course of the P-NR1 expression paralleled to that of IL-1β or glial fibrillary acidic protein.
Conclusions
Our results demonstrated that astrocytic activation but not microglial activation contributed to the allodynia in cyclophosphamide-induced cystitis and IL-1β released from astrocytes might bind to its endogenous receptor on the neurons inducing the phosphorylation of NR1 subunit, leading to sensory neuronal hyperexcitability and pathological pain.
Keywords: Bladder pain syndrome, cystitis, glia, cytokines, N-methyl-D-aspartate receptor, pain, spinal cord
Introduction
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a debilitating chronic syndrome, manifested by bladder filling-related pain and increased urinary frequency without infection or other identifiable pathology.1 Notably, visceral pain is the most troubling symptom for BPS/IC patients,2 and 94% of registrants in a BPS/IC database complain pain in the pelvic area.3 Although several pathophysiological mechanisms have been proposed for BPS/IC, including mast cell activation, urothelial glycosaminoglycan layer defects, autoimmune disease, and neuroendocrine immune interactions,4 the cause of BPS/IC-associated pain remains unclear.
In recent years, neural mechanisms have been considered as vital factors in the pathogenesis of BPS/IC-related pain. Our previous study reveals that increased transient receptor potential vanilloid 1 positive nerve fibers and enhanced expression of nerve growth factor in the bladder tissues are associated with pain scores of BPS/IC patients.5 In addition, an intriguing study suggests a critical role of enhanced afferent nerve activity from the lower urinary tract in the etiology of BPS/IC.6 Visceral inflammation is involved in elevated levels of neurotransmitters and increased neuronal activity in the primary afferent pathways.7,8 Release of excitatory neurotransmitters at the spinal dorsal horn level induces neuronal plasticity leading to spinal central sensitization.9
Several lines of evidence have shown that glial cells play an important role in the generation and maintenance of chronic pain.10–13 Activated glial cells produce numerous mediators such as proinflammatory cytokines that facilitate neuronal activity and synaptic plasticity.14 Several studies have strongly identified spinal cord glia and proinflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor-α, as key factors in the induction and maintenance of pathological pain.14,15 IL-1β signaling may facilitate neuronal excitability via enhancing the activity of N-methyl-D-aspartate receptor (NR).10,16 Previous studies show that activation of spinal NR, a glutamate receptor localized on neurons, plays a pivotal role in neuropathic pain.17,18 Similarly, BPS/IC-associated pain shares many characteristics of neuropathic pain. In cats diagnosed with feline IC, Birder et al.19 report that glial fibrillary acidic protein (GFAP), a astrocytic activation marker, is significantly elevated at the level of the spinal cord.19 Coincidentally, a study performed at same time with us shows that acute cystitis induced by a single cyclophosphamide (CYP) injection in mice led to astrocyte activation in lumbar spinal cord, but microglial activation was sparsely observed.20 But they do not explore the association between astrocytes with pain and the underlying mechanisms. To the best of our knowledge, related report on the involvement of spinal glia in BPS/IC-associated chronic pain and the underlying mechanisms are still lacking.
Systemic injection with CYP induced a reproducible dose-dependent chronic cystitis in rats, which has been used as BPS/IC models.16,21–23 In the present study, rats received three doses of 100 mg/kg CYP via intraperitoneal (i.p.) injection on the first, third, and fifth days, in order to install a chronic cystitis.23 Thereafter, L-α-aminoadipate (LAA) and minocycline were used to inactivate astrocyte and microglia, respectively, to identify their roles in the development of mechanical allodynia in CYP-induced cystitis. In addition, the underlying molecular mechanisms were detected through investigating the role of inflammatory cytokines such as IL-1β, which may released by astrocytes, in neuronal NR activation.
Material and methods
Animal and experimental design
Female Sprague Dawley rats (220–260 g in weight) were obtained from the Institute of Experimental Animals of Sun Yat-Sen University. Animals were housed in separated cages at a room temperature kept at 24 ± 1℃ and 50%–60% humidity, under a 12:12 h light/dark cycle. They had access to food and water ad libitum. Experimental procedures were approved by the Animal Care Committee of Sun Yat-Sen University (No.: IACUC-2013-0805; Gunangzhou, China) and adhered to the guidelines of the Committee for Research and Ethical Issues of IASP. All efforts were made to minimize animal numbers and potential suffering.
The experimental design was as follows: First, rats were divided into naïve group, sham group, cystitis group. Pain behavior, immunostaining of GFAP or OX42/Iba1, and expression of GFAP, OX42, P-P38, IL-1β, or P-NR1 were detected at different time points (n = 5/group). Second, saline, LAA, minocycline, pentoxifylline, IL-1 receptor antagonist (IL-1ra), AP5, or MK-801 was injected intrathecally in sham group and cystitis group on sixth day from first CYP injection (d6). After injection, pain behavior was immediately measured (n = 5/group). Third, LAA was injected intrathecally in sham group and cystitis group on d6, and 1 h later, spinal cords were harvested for Western blot analysis of IL-1β (n = 5/group). Double-labeling immunofluorescence of IL-1β with GFAP, OX42, or NeuN was performed in the L6-S1 spinal cord sections. Fourth, LAA, pentoxifylline, or IL-1ra was injected intrathecally in cystitis group on d6, and 1 h later, the spinal cords were harvested for Western blot analysis of P-NR1 (n = 5/group). Double-labeling immunofluorescence of IL-1RI and P-NR1 was performed in the L6-S1 spinal cord sections.
Induction of cystitis
Systemic injection with CYP induced a reproducible dose-dependent cystitis in rats, which has been used as BPS/IC models.21,23 Rats received three doses of 100 mg/kg CYP via intraperitoneal (i.p.) injection on the first, third, and fifth days, in order to install a chronic urinary cystitis.23
Drug administration
Intrathecal injection (i.t.) was performed as described previously.24 Sprague Dawley rats were anesthetized with 3% isoflurane for induction and maintenance. After clipping the fur on the rat lower back, the skin was sterilized with 75% alcohol. A 30-gauge needle was inserted into the L5-L6 intervertebral space. Sudden tail twist or leg kick was considered as an indicator of successful penetration of the needle tip into the vertebral canal. The drug was delivered slowly, and the needle was left in situ for more 15 s before withdrawal. The dosage of each drug used in the present study was according to the previous reports. Treatment group received intrathecal injection of LAA (100 nmol),25 minocycline (100 µg),26 pentoxifylline (150 nmol),27 IL-1ra (100 µg),28 AP5 (40 pmol),29 or MK-801 (100 pmol),29 while the same volume of saline was injected in control group. Each drug was dissolved in 10 µL of saline and injected intrathecally by the way of a single acute administration.
Drugs information as follows: LAA (astrocytic specific inhibitor; Sigma), minocycline (microglial specific inhibitor; Sigma), pentoxifylline (cytokine inhibitor; Polfilin, Polfarma, Poland), IL-1ra (Amgen, Thousand Oaks, CA, USA), 5-aminophosphonovaleric acid (NMDA receptor antagonist; Sigma), and (5R,10 S)-(++)- 5-methyl-10,11-dihydro-5H- dibenzo [a,d]cyclo-hepten-5,10- imine hydrogen maleate (MK-801, non-competitive NMDA receptor antagonist; Sigma).
Mechanical hypersensitivity
The mechanical thresholds (MTs) were tested with the Von Frey monofilaments using the up-down method. Rats were placed individually in a clear plastic box with six small chambers on a metal grid floor. Rats were allowed to acclimatize to the environment for 30 min before testing. Somatic sensitivity was analyzed in the lower abdomen, known to be areas of referred pain associated with visceral pathologies.30 The lower abdominal region was touched with one of a series of von Frey monofilaments (rated at 2, 4, 6, 8, 15, 26, and 60 g). Care was taken to stimulate different areas within the region to avoid desensitization. Starting at 2 g, filaments were applied perpendicularly to the abdominal region until there was bending of the filament. Sharp retraction of the abdomen and immediate licking or scratching of the stimulation area was considered a positive response. If there was no response, the next stronger filament in the sequence was tested. If a positive response was elicited, the next weaker filament was tested. The threshold requires six successive tries with different filaments for per animal.
Immunofluorescence
The rats were anesthetized using sodium pentobarbital (50 mg/kg, i.p.) and perfused with 4% paraformaldehyde through the ascending aorta.
The L6-S1spinal cord were removed and post-fixed in the same fixative overnight and transferred to 30% sucrose overnight. Cryostat sections (14 µm) were cut and processed for immunohistochemistry using primary antibodies against GFAP (1:500, Chemicon), OX42 (1:200, Chemicon), Iba1(1:500; Abcam), NeuN (1:1000, Chemicon), IL-1β (1:300, Endogen), P-ser896 NR1 (1:500, Millipore), and IL-1RI (1:600, R&D Systems). Following incubation overnight at 4℃, the sections were incubated in Cy3-conjugated (Chemicon) and FITC-conjugated secondary antibodies (Chemicon) for 1 h at room temperature. Five rats were included in each group for immunohistochemical quantification, and five spinal cord tissue sections per animal were randomly selected for analysis. The stained sections were examined with an Olympus IX71 (Olympus Optical) fluorescence microscope, and images were captured with a CCD spot camera.
Western blot analysis
The dorsal quadrants of L6-S1 spinal cord were harvested and mechanically homogenized and centrifuged. The supernatant was collected and stored at −80℃. The protein concentrations were estimated using the bicinchoninic acid method. Proteins of interest were separated by SDS-PAGE electrophoresis (20 mg of total protein per well) and transferred onto nitrocellulose membranes. The membranes were placed in a blocking solution (Tris-buffered saline with 0.02% Tween and 5% non-fat dry milk powder) for 1 h and incubated overnight with mouse anti-GFAP IgG (1:500, Chemicon), mouse anti-OX42 IgG (1:200, Chemicon), mouse anti-phospho-p38 (1:500, Cell Signaling), rabbit anti- IL-1β IgG (1:300, Endogen) or rabbit anti-P-ser896 NR1 IgG (1:500, Millipore), and mouse anti-β-actin (1:1000, loading control; Sigma). After washing, the membranes were incubated in peroxidase-conjugated secondary antibody (1:10000; Santa Cruz) for 1 h. An enhanced chemiluminescence solution (Pierce, USA) was used to detect the immunocomplexes. Each band was quantified using a computer-assisted imaging analysis system (KONTRON IBAS 2.0).
Statistical analysis
All data were collected by experimenters blinded to the treatment protocol, and statistical analyses were done using SPSS software (version 13, USA). All data are expressed as means ± SEM. For analysis of the Western blot data, the differences between groups were compared using one-way analysis of variance followed by Fisher’s protected least significant difference post hoc analysis. For behavioral analysis, the data were compared using two-way analysis of variance followed by post hoc analysis. Statistical significance was set at p ≤ 0.05 for all tests.
Results
Mechanical allodynia occurred in CYP-treated rats and was induced by spinal astrocytic activation
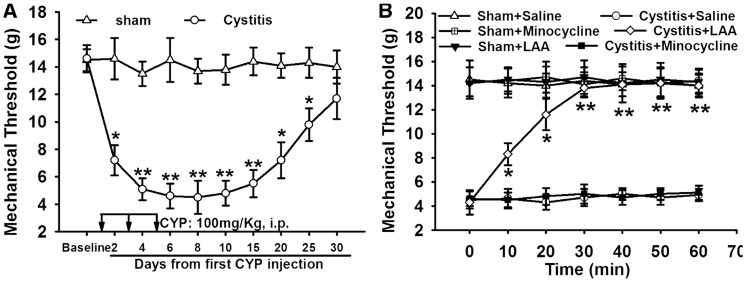
Compared to Sham group (14.6 ± 1.5 g, d2), the nociceptive threshold of cystitis group significantly decreased (7.2 ± 1.1 g, p < 0.05, d2), reached the lowest value on sixth day (d6) (4.5 ± 0.9 g), and thereafter maintained at low level till 20 days (7.1 ± 1.3 g, p < 0.05). After 20 days, the MT in cystitis group gradually increased to the basal level (11.7 ± 1.5 g, 30 days) (Figure 1(A)). The astrocytic specific toxin LAA or the microglial specific inhibitor minocycline was injected intrathecally and observed their effects on mechanical allodynia in cystitis rats (d6). LAA significantly attenuated the allodynia (p < 0.001). However, minocycline failed to reverse the mechanical allodynia (p > 0.05). The MT of rats in the sham group was not influenced by LAA or minocycline (Figure 1(B)).
Figure 1.
The role of glial blockers in cyclophosphamide (CYP)-induced mechanical allodynia. (A) Compared with Sham group, the nocicepive threshold of CYP group was significantly decreased, reached the lowest value on sixth day, and thereafter maintained at low level till 20 days. After 20 days, the paw withdrawal threshold of cystitis group gradually increased to the basal level. (B) Astrocytic specific inhibitor LAA but not microglial specific inhibitor minocycline attenuated mechanical allodynia. The mechanical allodynia of the rats in the sham group was not influenced by LAA or minocycline. All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group.
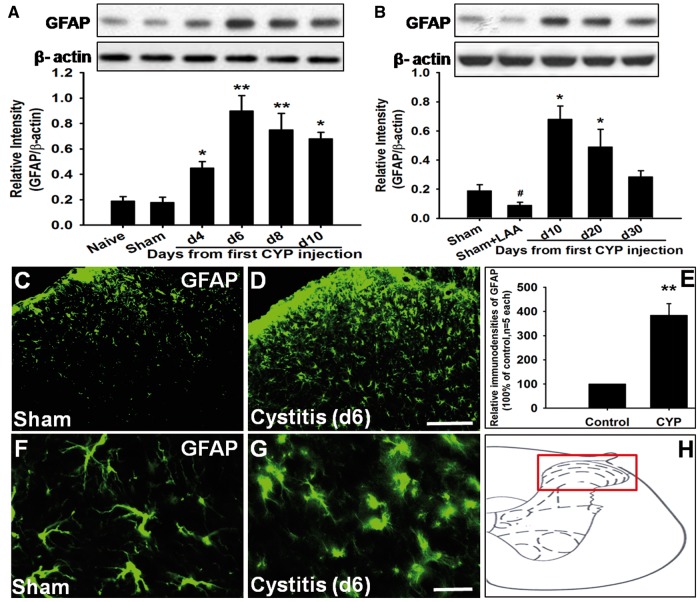
Using Western blot, we detected that compared to naive group (0.19 ± 0.04) and Sham group (0.18 ± 0.04), GFAP expression was significantly increased in cystitis group on fourth day (0.45 ± 0.05, p < 0.05). GFAP upregulation peaked on sixth day (0.90 ± 0.12) and thereafter persisted at high level till 20 days (0.50 ± 0.12, p < 0.05). After 20 days, GFAP expression gradually decreased to the basal level (0.28 ± 0.04, 30 days) (Figure 2(A) and (B)). Thus, the time course of GFAP expression was similar to that of mechanical allodynia. Our immunostaining results indicated that compared to sham group, GFAP staining was significantly increased in the spinal cord of cystitis group (p < 0.001) (Figure 2(C) to (E)). Staining of GFAP appeared to be enhanced throughout the spinal dorsal horn. Activated astrocytes had hypertrophied cell bodies and thickened processes with enhanced GFAP-immunoreactivity (Figure 2(F) and (G)). Scheme presenting an overview over the detected region (Figure 2(H)).
Figure 2.
Spinal astrocyte was activated in cystitis group. (A) and (B) Western blot analysis showed that GFAP expression was significantly increased in cystitis group on fourth day and upregulation peaked on d6 and thereafter persisted at high level till 20 days. After 20 days, GFAP expression gradually decreased to the basal level. LAA downregulated the expression of GFAP in the sham group. (C) to (E) Compared to Sham group, GFAP staining was significantly increased in the spinal cord of cystitis group. (F) and (G)Activated astrocytes had hypertrophied cell bodies and thickened processes with enhanced GFAP immunoreactivity. (H) Scheme presenting an overview over the detected region. Bar = 100µm in (C) and (D) and 20 µm in (F) and (G). All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group.
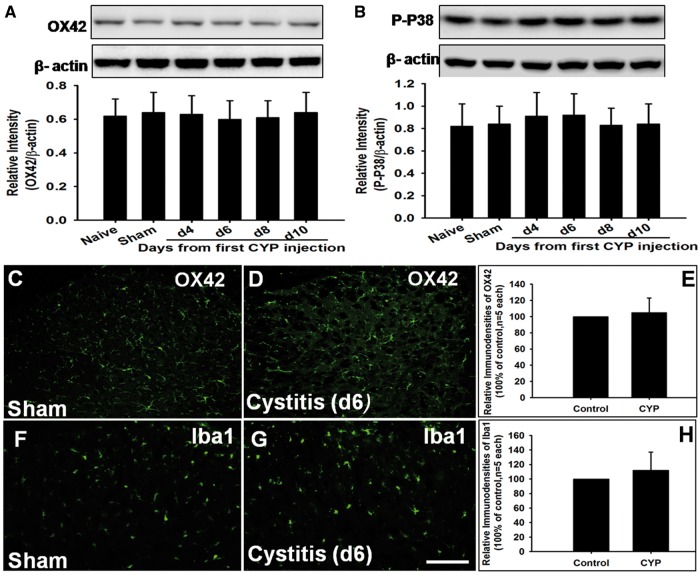
With regard to microglia are critical for pathological pain, our Western blot showed that the expression of microglial marker OX42 and their activated indicator P-P38 had no difference among naive group, sham group, and cystitis group at any time points from first CYP injection (Figure 3(A) and (B)). And the immunostaining of OX42 or Ibal1 showed the similar results (Figure 3(C) to (H)).
Figure 3.
Spinal microglia was not activated in cystitis group. (A) and (B) Western blot analysis showed that no significant difference in the expression of OX42 and P-P38 in spinal cord was observed among Naive group, Sham group, and CYP group through the period tested. (C) to (H) OX42-like immunoreactivity and Iba1-like immunoreactivity in spinal dorsal horn had no difference between Sham group and cystitis group (d6). Bar = 100 mm. All data were calculated as mean ± SEM (n = 5/group).
Spinal astrocytes dramatically increased the expression of IL-1β which is related to mechanical allodynia
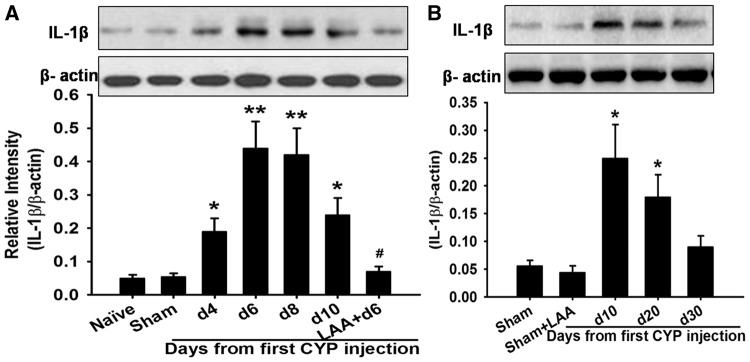
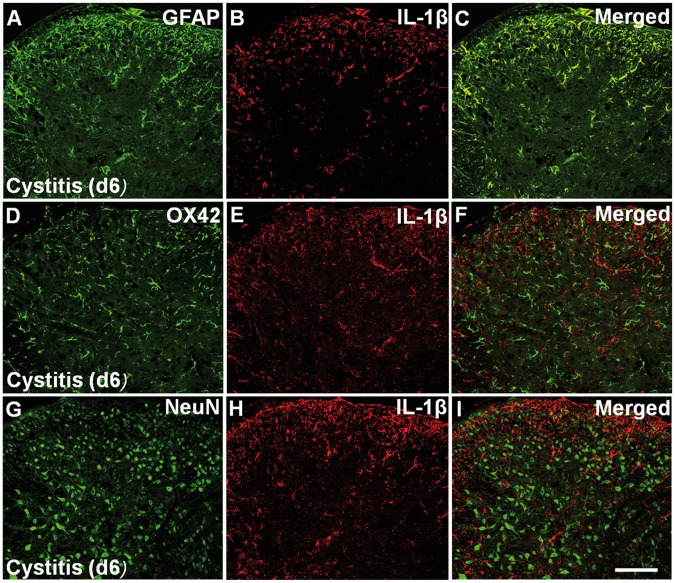
Since activated glia can release cytokines and there cytokines, such as IL-1β, as key factors in the induction and maintenance of neuropathic pain,14,15 so we wonder whether IL-1β contribute to the development of cystitis-induced pain. Here, our Western blot results showed that compared to Naive group (0.05 ± 0.01) and Sham group (0.055 ± 0.01), IL-1β expression was significantly increased in cystitis group on fourth day (0.19 ± 0.04, p < 0.05). IL-1β upregulation reached a peak on d6 (0.44 ± 0.08), and then remained at high level till 20 days (0.19 ± 0.04, p < 0.05). After 20 days, IL-1β expression gradually decreased to the basal level (0.09 ± 0.02, 30 days) (Figure 4(A) and (B)). Thus, the time course of IL-1β expression paralleled to that of GFAP expression. In the cystitis group (d6), intrathecally administered the astrocytic specific toxin LAA significantly reversed IL-1β overexpression (p < 0.001; Figure 4(A)). Additionally, our double immunofluorescent staining showed that IL-1β-immunoreactivity was only localized in GFAP-immunopositive cells but not in NeuN- immunopositive cells or OX42-immunopositive cells (Figure 5(A) to (I))
Figure 4.
The expression of IL-1β in different group. Western blot results showed that compared to Naive group and Sham group, IL-1β expression was significantly increased in cystitis group on fourth day and reached a peak on d6 and then remained at high level till 20 days. After 20 days, IL-1β expression gradually decreased to the basal level. In the cystitis group (d6), intrathecally administered the astrocytic specific toxin LAA significantly reversed IL-1β overexpression. LAA did not affect the expression of IL-1β in the sham group. All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group. #p < 0.01 vs. Cystitis group (d6).
Figure 5.
The cellular location of IL-1β in spinal cord. Double immunofluorescent staining showed that IL-1β- immunoreactivity was only localized in GFAP immunopositive cells (A) to (C) but not in OX42-immunopositive cells (D) to (F) or NeuN-immunopositive cells (G) to (I) in the cystitis group (d6). Bar = 100 mm.
In the cystitis group (d6), we injected intrathecally pentoxifylline (cytokine inhibitor) or IL-1ra and observed their effects on mechanical allodynia. Both pentoxifylline and IL-1ra significantly attenuated the allodynia (all p < 0.001; Figure 6).
Figure 6.
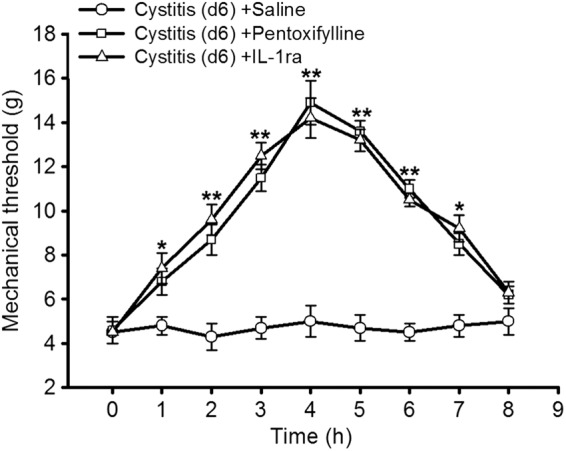
The role of IL-1β in cystitis associated pain. In the cystitis group (d6), we injected intrathecally pentoxifylline (cytokine inhibitor) or IL-1ra (interleukin-1 receptor antagonist) and observed their effects on mechanical allodynia. Both pentoxifylline and IL-1ra significantly attenuated the allodynia. All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group.
IL-1β released from astrocyte induced NMDA receptor phosphorylation in spinal dorsal horn neurons
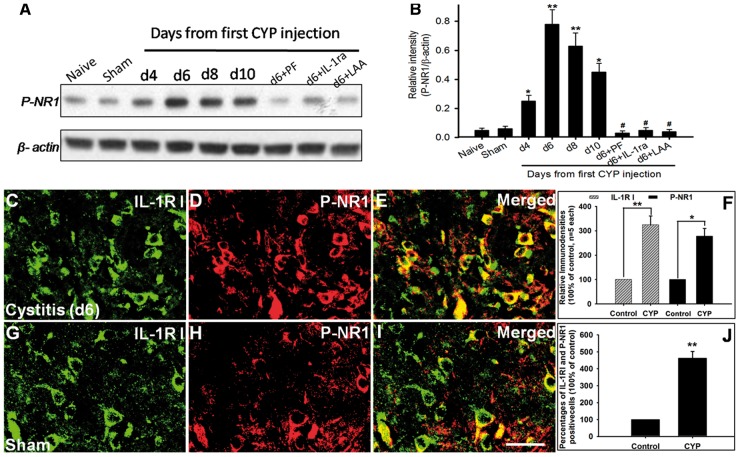
Since several studies showed that spinal NR activation is prominently involved in the neuropathic pain,17,18 and IL-1β signaling may facilitate NR phosphorylation to enhance neuronal activity and induce pain,10,31 so we wonder whether IL-1β contribute to the cystitis-induced pain through facilitating NR phosphorylation. Here, our Western blot analysis showed that compared to naive group (0.05 ± 0.015) and sham group (0.06 ± 0.017), P-NR1 expression was significantly increased in cystitis group on fourth day (0.25 ± 0.04, p < 0.05). P-NR1 upregulation reached a peak on d6 (0.78 ± 0.10). And thereafter, P-NR1 expression maintained at high level. Thus, the changing course of the level of P-NR1 was similar to that of IL-1β or GFAP. In the cystitis group (d6), intrathecally administered astrocytic specific toxin LAA, pentoxifylline (cytokine inhibitor), or IL-1ra significantly reversed the overexpression of P-NR1 (all p < 0.001; Figure 7(A) and (B)). Our double immunofluorescent staining of spinal cord showed that P-NR1- immunoreactivity and IL-1RI-immunoreactivity were totally co-localized, and the percentages of IL-1RI and P-NR1 positive cells in spinal cord dorsal horn of cystitis group were significantly higher than that in the sham group (Figure 7(C) to (J)).
Figure 7.
The expression and cellular location of P-NR1 in spinal cord. (A) and (B) Western blot analysis showed that compared to naive group and sham group, P-NR1 expression was significantly increased in cystitis group on fourth day and reached a peak on d6. And thereafter, P-NR1 expression maintained at high level. In the cystitis group (d6), intrathecally administered astrocytic specific toxin LAA, pentoxifylline (cytokine inhibitor), or IL-1ra (interleukin-1 receptor antagonist) significantly reversed the overexpression of P-NR1. (C) to (J) Double immunofluorescent staining of spinal cord showed that P-NR1-immunoreactivity and IL-1RIimmunoreactivity were totally co-localized, and the percentages of IL-1RI and P-NR1 positive cells in spinal cord dorsal horn of cystitis group were significant higher than that in sham group. Bar = 20µm in (C) to (E) and (G) to (I). All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group, #p < 0.01 vs. Cystitis group (d6).
In the cystitis group (d6), we injected intrathecally AP5 (NR antagonist) or MK-801 (non-competitive NR antagonist) and observed their effects on mechanical allodynia. We found that both AP5 and MK-801 significantly attenuated the allodynia (all p < 0.001; Figure 8).
Figure 8.
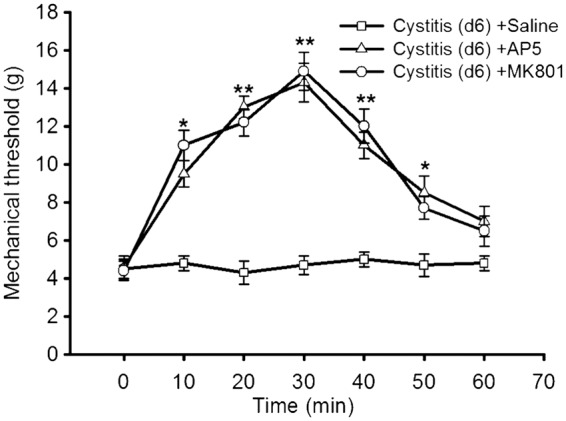
The role of NR in cystitis associated pain. In the cystitis group (d6), we injected intrathecally AP5 (NR antagonist) or MK-801 (non-competitive NR antagonist) and observed their effects on mechanical allodynia. We found that both AP5 and MK-801 significantly attenuated the allodynia. All data were calculated as mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 vs. Control group.
Discussion
In the present study, we found that GFAP (astrocytic activation marker) rather than OX42/Iba1(microglial activation marker) or their activated indicator P-P38 was significantly increased in the spinal cord of CYP-induced cystitis rats. LAA (astrocytic specific inhibitor) but not minocycline (microglial specific inhibitor) significantly attenuated the cystitis-associated allodynia. Furthermore, we found that spinal IL-1β expression was dramatically increased in CYP-induced cystitis compared to control rats, and LAA could reverse its overexpression. Activated astrocytes were the only source of IL-1β release that contributed to mechanical allodynia. Besides, we found that spinal P-NR1 was significantly increased in cystitis group, and LAA, pentoxifylline, or IL-1ra significantly reversed its overexpression. P-NR1-immunoreactivity neurons and IL-1RI- immunoreactivity neurons were almost co-localized, and the percentages of IL-1RI and P-NR1 positive cells in spinal cord dorsal horn of cystitis group were significant higher than that in the sham group. In addition, NR antagonist such as AP5 or MK-801 significantly attenuated the cystitis-induced pain. The time course of the P-NR1 expression paralleled to that of IL-1β or GFAP. Taken together, our results indicated that astrocytic activation but not microglial activation contributed to allodynia in CYP-induced cystitis, and IL-1β released from astrocytes might bind to its endogenous receptor on the neurons inducing to the phosphorylation of NR1 subunit, leading to enhanced sensory neuronal excitability and pathological pain.
CYP-induced urinary bladder inflammation is a well-established experimental model for BPS.16,21–23 And this model has the potential to be a useful tool to examine the complex and poorly understood mechanisms of pain in BPS/IC patients.32 For animal model, direct measurement of bladder pain is still difficult. Bon et al.33 report that visceral inflammation was accompanied by increased sensitivity of somatic structures to noxious stimuli, and the somatic hyperalgesia was used as a surrogate measurement for visceral pain in experimental cystitis. In this study, we evaluated bladder pain in CYP-induced cystitis through measuring MT of low abdomen.
Several previous studies have reported that central sensitization is involved in development of cystitis-associated pain. Visceral inflammation is involved in elevated levels of neurotransmitters and increased neuronal activity in the primary afferent pathways.7,8 Release of excitatory neurotransmitters at the spinal dorsal horn level induces neuronal plasticity leading to spinal central sensitization.9 Additionally, Fos protein, a marker of neural activation, was increased at the spinal cord after noxious irritation of acetic acid or CYP.34,35 Nociceptive information from the urinary bladder is transmitted to second-order neurons in the spinal cord dorsal horn by sensory neurons in the lumbosacral DRG, leading to central hyperexcitability.36 Therefore, it was hypothesized that the central sensitization may be one of mechanisms for cystitis-induced pain.
Glial cells in central nervous system are now considered as important regulators of synaptic activity.37 Recent studies have strongly supported that spinal cord glia (including astrocytes and microglia) and proinflammatory cytokines, such as IL-1β, are involved in the induction and maintenance of neuropathic pain.11–13,15 LAA is a specific astrocytic inhibitor,38 and minocycline is a specific microglial inhibitor.39 Several reports indicate that intraperitoneal or intrathecal administration of minocycline could relieve neuropathic pain induced by nerve injury40,41 or peripheral inflammation.42 Similarly, several studies reported that LAA produces dose-dependent inhibition of nerve injury-induced mechanical allodynia43,44 and chronic pancreatitis-induced pain.25 To identify which subtype of glial cell (astrocyte or microglia) was involved in cystitis-associated pain, we tested the expression of their markers (GFAP and OX42/Iba1, respectively) in the spinal cord and the influence of their inhibitors (LAA and minocycline, respectively) on mechanical allodynia in CYP-treated rats. Here, we found that GFAP rather than OX42/Iba1 or P-P38 was significantly increased in the spinal cord of CYP-treated rats, LAA but not minocycline significantly attenuated the allodynia, which indicated that astrocytic activation but not microglial activation contributed to the allodynia in CYP-treated rats. To the best of our knowledge, this study is the first to use the astrocytic specific inhibitor LAA to attenuate the cystitis-associated pain. Similar to our findings, several studies report that it is spinal astrocytic activation but no microglial activation involved in pain pathogenesis in animal models.20,25,45–48 Previous studies have documented that compared with microglia contributing to the initiation of chronic pain, while astrocytes may response more to the maintenance of chronic pain.49,50
Under physiological condition, astrocytes do not or only release very low level of neuroexcitatory substances. However, under pathological condition, activated astrocytes release numerous proinflammatory cytokines.51 Several researches have strongly identified spinal cord glia and cytokines, such as IL-1β and tumor necrosis factor-α, as key factors in the induction and maintenance of neuropathic pain.14,15 Among these cytokines, IL-1β has become the research focus.52,53 We found that spinal IL-1β expression was significantly increased in CYP-treated rats compared to control group. Activated astrocytes were the only source of IL-1β release, which contributed to cystitis-associated mechanical allodynia. In support of our findings, several studies also reported selective localization of increased IL-1β in spinal astrocytes in bone cancer pain model,54 diabetic neuropathic pain,47 chemotherapy-induced neuropathic pain model,48 and complete Freunds adjuvant-induced inflammatory pain model.55
Several studies showed that IL-1β signaling may facilitate NR phosphorylation to enhance neuronal activity.10,31 It is generally believed that NR, a glutamate receptor localized on neurons, plays a crucial role in neuropathic pain.17,18 Functional NRs are heteromeric complexes including the NR1 subunit and one or more of the NR2A-D subunits,56 and all functional NMDARs include at least one NR1 subunit and NR1 is required for receptor activity.57 Additionally, the phosphorylation of NR1 subunit in the spinal dorsal horn neurons is strongly correlated with induction and maintenance of chronic pain.58,59 Herein, we found that spinal P-NR1 was significantly increased in CYP-treated rats compared to control group and intrathecally administered LAA, pentoxifylline, or IL-1ra reversed its overexpression. Furthermore, intrathecal injection of NR antagonist such as AP5 or MK-801 significantly attenuated cystitis-induced pain. Besides, the time course of the upregulation of P-NR1 paralleled to that of IL-1β or GFAP. Therefore, the above results indicated that spinal-activated astrocytes significantly increased the expression of IL-1β which induced NR phosphorylation in spinal neurons, leading to neuronal hyperexcitability and pathological pain.
In order to explore how IL-1β contributes to the NR phosphrylation, we examined the expression of IL-1 receptor (IL-1R) in the spinal cord via immunofluorescent staining. IL-1R is the endogenous receptor for IL-1β. IL-1R contains two subtypes: the type I IL-1R (IL-1RI) and the type II IL-1R (IL-1RII). IL-1RI is a transmembrane molecule that is responsible for IL-1b signaling, whereas IL-1RII lacks an intracellular domain and is incapable of signal transduction.60 Here, we found that IL-1RI-immunoreactivity was only localized in spinal neurons in CYP-treated rats. Interestingly, our double immunostaining showed that the IL-1RI was co-localized with P-NR1 in this study, which strongly supported a close interaction of IL-1β signaling with neuronal NR. Thus, it was hypothesized that IL-1β released from astrocytes might bind to its endogenous receptor on neurons inducing phosphorylation of NR1 subunit, leading to nociceptive neuronal excitability and pathological pain. In support of our findings, a previous study showed that phosphorylation of the NR1 was attenuated by LAA in spinal nerve ligation-induced neuropathic pain model.61 Besides, several previous studies reported that IL-1ra blocked the phosphorylation of the NR1 in inflammatory pain models,10,28 diabetic neuropathic pain model,47 and chemotherapy-induced neuropathic pain model.48 Taken together, the above results indicated that spinal-activated astrocytes dramatically increased the expression of IL-1β which binds to IL-1RI to induce NMDA receptor phosphorylation in spinal neurons, leading to enhanced neural excitability and pathological pain.
Conclusions
Our results were the first to provide evidence that LAA (astrocytic specific inhibitor) but not minocycline (microglial specific inhibitor) exerted analgesic effects in CYP-indued cystitis. Our data suggested that “Astrocyte- IL-1β- NMDAR-Neuron” pathway might be the underlying molecular mechanisms for the role of astrocytic activation in cystitis-induced pain. These findings suggest that spinal astrocytic inhibition may be a novel strategy for treating cystitis-induced pain.
Author Contributions
Bolong Liu and Minzhi Su contributed equally to this paper. Bolong Liu and Minzhi Su were responsible for executing the entire research project and writing the manuscript. ShaoJun Tang critically reviewed the work. Xiangfu Zhou conceived the study and participated in its design and coordination. Hailun Zhan and Tengcheng Li helped provide the material and facilities. Wenbiao Li and Juncong Xie were responsible for executing the statistical analyses. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the General Program of National Natural Science Foundation (81670688) and the Guangdong Province Natural Science Foundation Projects of China (S2013010016625 and 2016A030313192). Bolong Liu was supported by International Program for PhD Candidates, Sun Yat-Sen University (02300-52094201). Shaojun Tang was supported by NIH grants 5R01DA036165 and 5R01NS079166.
References
- 1.Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015; 193: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 2.van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 2008; 53: 60–67. [DOI] [PubMed] [Google Scholar]
- 3.Kirkemo A, Peabody M, Diokno AC, et al. Associations among urodynamic findings and symptoms in women enrolled in the Interstitial Cystitis Data Base (ICDB) Study. Urology 1997; 49(5A Suppl): 76–80. [DOI] [PubMed] [Google Scholar]
- 4.Bouchelouche K, Nordling J. Recent developments in the management of interstitial cystitis. Curr Opin Urol 2003; 13: 309–313. [DOI] [PubMed] [Google Scholar]
- 5.Liu BL, Yang F, Zhan HL, et al. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int 2014; 92: 202–208. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura N, Seki S, Chancellor MB, et al. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 2002; 59(5 Suppl 1): 61–67. [DOI] [PubMed] [Google Scholar]
- 7.Benemei S, Nicoletti P, Capone JG, et al. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 2009; 9: 9–14. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci 2010; 30: 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol 2009; 194: 451–491. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 2007; 27: 6006–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Zhu MD, Zhang X, et al. NFkappaB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation 2014; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen W, Hu XM, Liu YN, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Gelman BB, Lisinicchia JG, et al. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci 2012; 32: 10833–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, Zhu C, Li ZH, et al. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J Neuroinflammation 2015; 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger JV, Deumens R, Goursaud S, et al. Enhanced neuroinflammation and pain hypersensitivity after peripheral nerve injury in rats expressing mutated superoxide dismutase 1. J Neuroinflammation 2011; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn 2011; 30: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christoph T, Schiene K, Englberger W, et al. The antiallodynic effect of NMDA antagonists in neuropathic pain outlasts the duration of the in vivo NMDA antagonism. Neuropharmacology 2006; 51: 12–17. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 19.Birder LA, Wolf-Johnston AS, Chib MK, et al. Beyond neurons: involvement of urothelial and glial cells in bladder function. Neurourol Urodyn 2010; 29: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitas RD, Costa KM, Nicoletti NF, et al. Omega-3 fatty acids are able to modulate the painful symptoms associated to cyclophosphamide-induced-hemorrhagic cystitis in mice. J Nutr Biochem 2016; 27: 219–232. [DOI] [PubMed] [Google Scholar]
- 21.Auge C, Chene G, Dubourdeau M, et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol 2013; 707: 32–40. [DOI] [PubMed] [Google Scholar]
- 22.Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 2010; 298: F589–F600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meotti FC, Forner S, Lima-Garcia JF, et al. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact 2013; 203: 440–447. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz ES, Kim HY, Wang J, et al. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci 2009; 29: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng QX, Wang W, Feng XY, et al. Astrocytic activation in thoracic spinal cord contributes to persistent pain in rat model of chronic pancreatitis. Neuroscience 2010; 167: 501–509. [DOI] [PubMed] [Google Scholar]
- 26.Hua XY, Svensson CI, Matsui T, et al. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci 2005; 22: 2431–2440. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Feng X, Yu M, et al. Pentoxifylline attenuates the development of hyperalgesia in a rat model of neuropathic pain. Neurosci Lett 2007; 412: 268–272. [DOI] [PubMed] [Google Scholar]
- 28.Zhang RX, Li A, Liu B, et al. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 2008; 135: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanchet EM, Longo I, Cury Y. Involvement of spinal neurokinins, excitatory amino acids, proinflammatory cytokines, nitric oxide and prostanoids in pain facilitation induced by Phoneutria nigriventer spider venom. Brain Res 2004; 1021: 101–111. [DOI] [PubMed] [Google Scholar]
- 30.Studeny S, Cheppudira BP, Meyers S, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci 2008; 36: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardoni F, Boraso M, Zianni E, et al. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1beta and NMDA stimulation. J Neuroinflammation 2011; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita M, Kasai E, Omachi S, et al. A novel method for assessing bladder-related pain reveals the involvement of nerve growth factor in pain associated with cyclophosphamide-induced chronic cystitis in mice. Eur J Pain 2016; 20: 79–91. [DOI] [PubMed] [Google Scholar]
- 33.Bon K, Lichtensteiger CA, Wilson SG, et al. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol 2003; 170: 1008–1012. [DOI] [PubMed] [Google Scholar]
- 34.Dinis P, Charrua A, Avelino A, et al. Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci 2004; 24: 11253–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 2000; 278: R1027–R1039. [DOI] [PubMed] [Google Scholar]
- 36.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994; 107: 271–293. [DOI] [PubMed] [Google Scholar]
- 37.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science 2002; 298: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. Glia 1996; 16: 351–358. [DOI] [PubMed] [Google Scholar]
- 39.Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 1998; 95: 15769–15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei XP, Xu H, Xie C, et al. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neurosci Res 2011; 70: 305–312. [DOI] [PubMed] [Google Scholar]
- 41.Guasti L, Richardson D, Jhaveri M, et al. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Mol Pain 2009; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005; 115: 71–83. [DOI] [PubMed] [Google Scholar]
- 43.Zhuang ZY, Wen YR, Zhang DR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 2006; 26: 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Wang W, Mei X, et al. Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS One 2009; 4: e6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011; 176: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Yoon SY, Zhang H, et al. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain 2012; 13: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao YH, Zhang GH, Jia D, et al. Spinal astrocytic activation contributes to mechanical allodynia in a mouse model of type 2 diabetes. Brain Res 2011; 1368: 324–335. [DOI] [PubMed] [Google Scholar]
- 48.Ji XT, Qian NS, Zhang T, et al. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS One 2013; 8: e60733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci 2001; 24: 450–455. [DOI] [PubMed] [Google Scholar]
- 50.Zhuang ZY, Gerner P, Woolf CJ, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. [DOI] [PubMed] [Google Scholar]
- 51.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 2005; 6: 626–640. [DOI] [PubMed] [Google Scholar]
- 52.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 2003; 521: 1–21. [PubMed] [Google Scholar]
- 53.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001; 90: 1–6. [DOI] [PubMed] [Google Scholar]
- 54.Zhang RX, Liu B, Wang L, et al. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 2005; 118: 125–136. [DOI] [PubMed] [Google Scholar]
- 55.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 2004; 20: 467–473. [DOI] [PubMed] [Google Scholar]
- 56.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 2007; 7: 39–47. [DOI] [PubMed] [Google Scholar]
- 57.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology 1995; 34: 1219–1237. [DOI] [PubMed] [Google Scholar]
- 58.Brenner GJ, Ji RR, Shaffer S, et al. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci 2004; 20: 375–384. [DOI] [PubMed] [Google Scholar]
- 59.Ultenius C, Linderoth B, Meyerson BA, et al. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett 2006; 399: 85–90. [DOI] [PubMed] [Google Scholar]
- 60.Dayer JM. The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2003; 42(Suppl 2): ii3–ii10. [DOI] [PubMed] [Google Scholar]
- 61.Mei XP, Wang W, Wang W, et al. Combining ketamine with astrocytic inhibitor as a potential analgesic strategy for neuropathic pain ketamine, astrocytic inhibitor and pain. Mol Pain 2010; 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]