Abstract
Neuroplastic changes in the amygdala account for emotional-affective aspects of pain and involve neuropeptides such as calcitonin gene-related peptide and corticotropin-releasing factor. Another neuropeptide system, central arginine vasopressin, has been implicated in neuropsychiatric disorders, but its role in pain-related emotional expression and neuroplasticity remains to be determined. Here, we tested the hypothesis that arginine vasopressin in the amygdala contributes to pain-related emotional-affective responses, using stereotaxic applications of arginine vasopressin and antagonists for G-protein coupled vasopressin V1A and oxytocin receptors in adult male Sprague-Dawley rats. In normal animals, arginine vasopressin increased audible and ultrasonic vocalizations and anxiety-like behavior (decreased open-arm preference in the elevated plus maze). The facilitatory effects were blocked by a selective V1A antagonist (SR 49059, Relcovaptan) but not by an oxytocin receptor antagonist (L-371,257). L-371,257 had some facilitatory effects on vocalizations. Arginine vasopressin had no effect in arthritic rats (kaolin/carrageenan knee joint pain model). SR 49059 inhibited vocalizations and anxiety-like behavior (elevated plus maze) in arthritic, but not normal, rats and conveyed anxiolytic properties to arginine vasopressin. Arginine vasopressin, SR 49059, and L-371,257 had no significant effects on spinal reflexes. We interpret the data to suggest that arginine vasopressin through V1A in the amygdala contributes to emotional-affective aspects of pain (arthritis model), whereas oxytocin receptors may mediate some inhibitory effects of the vasopressin system.
Keywords: Vasopressin, oxytocin, pain, anxiety, amygdala
Background
Pain is a complex multidimensional experience with cognitive, sensory, and emotional-affective components that engage different regions of the central nervous system. Previous work from our lab demonstrated that emotional-affective pain behaviors arise from enhanced amygdala output as the consequence of neuroplasticity triggered by nociceptive inputs and multiple neuropeptide systems. Neuropeptides act as excitatory (calcitonin gene-related peptide and corticotropin releasing factor) and inhibitory (neuropeptide S) mediators of pain-related amygdala plasticity.1
The amygdala is also a major extra-hypothalamic site of expression of the neuropeptides arginine vasopressin (AVP) and oxytocin (OT) and their G-protein coupled receptors (V1A, V1B, and OTR),2 which are found at particularly high levels in the central nucleus (CeA).3 The CeA mediates key amygdala output functions, including pain production and modulation.1 AVP and OT have emerged as important regulators of stress responses and targets for neuropsychiatric disorders.2,4 For example, they are released from dendrites in the CeA in response to forced swim stress.5–7 Central AVP and OT can exert opposing effects. AVP has anxiogenic and depressive actions, whereas OT is anxiolytic and antidepressive.2 Related to this, differential effects have been observed on CeA neurons. For example, OTR activation inhibited neurons that were excited by V1A activation.3 Vasopressin receptor V1A and V1B blockade, knockdown, knockout, or gene polymorphism can alter anxiety- and depression-like behavior.2,8 Importantly, a possible link between single nucleotide polymorphisms in the V1A gene and pain sensitivity has been described in humans.9 It should be noted that in the brain, V1A is the primary and abundant receptor subtype8,10 and electrophysiological effects of AVP in the CeA were mediated by V1A but not V1B and V2.3
Given these observations, we tested the hypothesis that AVP in the amygdala (CeA) contributes to pain-related emotional responses (vocalizations) and anxiety-like behaviors (elevated plus maze (EPM) test) through V1A. We found that V1A, but not OTR, mediated facilitatory effects of exogenously administered AVP in normal rats. In an arthritis pain model (kaolin/carrageenan-induced knee joint arthritis), AVP effects were largely absent, but a V1A antagonist (SR49059, Relcovaptan) had inhibitory effects, suggesting that the endogenous AVP system is activated in the pain model, thus occluding the effects of exogenous AVP. Facilitatory effects of OTR blockade under normal conditions are consistent with opposing roles of the OT and AVP systems.
Materials and methods
Animals
Male Sprague Dawley rats (200–350 g) were housed in a temperature-controlled room and maintained on a 12-h-day/night light cycle with access to food and water ad libitum. On the day of experimental studies, the animals were transferred from the animal facility and allowed to acclimate to the laboratory for at least 1 h. All experimental procedures conformed to the guidelines of International Association for the Study of Pain and the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at the Texas Tech University Health Sciences Center.
Experimental protocol
On day 1, the animals underwent surgical implantation of a guide cannula into the brain for stereotaxic drug application by microdialysis. On day 2, animals randomly assigned to the arthritis group underwent arthritis induction in one knee (see Arthritis pain model section). The control group was left untreated but handled in a similar fashion. On day 3, behavioral tests were performed. After completion of the tests, the animals were euthanized, and tissues were obtained for histological verification of drug application sites.
Arthritis pain model
Animals were randomly assigned to the arthritis group or control group. For arthritis induction on day 2, rats were briefly anesthetized with isoflurane (2%–3%), and kaolin (4%, 100 μl) and carrageenan (2%, 100 μl) were injected into the left knee joint cavity through the patellar ligament followed by repetitive flexion and extension of the leg after each injection for 15 min and 5 min, respectively, as described previously.11 These injections reliably lead to a localized mono-arthritis in the knee with behavioral and electrophysiological changes that reach a peak after 5–6 h and persist at that level for two to three days, gradually declining for one to two weeks.12 For the control group, we used normal rats because our previous works showed that brain activity and behavior of normal rats were not different from rats that received intraarticular saline injection13 or needle insertion.14 Behavioral testing was done the next day (day 3).
Stereotaxic drug application by microdialysis
On day 1, rats were anesthetized with isoflurane (3.5%–4.5%, precision vaporizer) and placed into a stereotaxic apparatus (David Kopf Instr.) under continued anesthesia with isoflurane (2.0%–3.0%) as described previously.11 Using sterile procedures, a small craniotomy was made at the sutura-parietalis level, and a guide cannula was inserted into the CeA of the amygdala using following stereotaxic coordinates: 2.1 mm posterior to bregma, 3.7–4.0 mm lateral, and 6.5–7.0 mm ventral. The cannula was anchored to the bone with dental acrylic and a pair of small screws. Antibiotic ointment (bacitracin) was applied topically to prevent infection. After removal from the stereotaxic apparatus, the animal was allowed to recover for 1 h before being returned to its home cage. On day 3, a microdialysis probe (CMA/11) was inserted through the guide cannula so that the tip of the microdialysis fiber protruded by 1 mm. The probe was connected to an infusion pump (Harvard Apparatus) with PE50 tubing and perfused with oxygenated artificial cerebrospinal fluid (ACSF, pH 7.4) for at least 1 h to establish equilibrium in the tissue. ACSF contained the following (in mM): 125.0 NaCl, 2.6 KCl, 2.5 NaH2PO4, 1.3 CaCl2, 0.9 MgCl2, 21.0 NaHCO3, and 3.5 glucose.
The following compounds were administered into the amygdala (CeA) by microdialysis for a period of 15 min at a rate of 5 µl/min to determine their behavioral effects. ACSF (control), AVP, SR 49059 (Relcovaptan, selective non-peptide V1A receptor antagonist),15 and L-371,257 (potent, high-affinity non-peptide OT receptor antagonist with >800-fold selectivity over V1a and V2).16,17 AVP, SR 49059, and L-371,257 were purchased from Tocris Bioscience. AVP was dissolved in water to 1 mM and then in ACSF to a 100 µM solution (concentration in the microdialysis fiber). SR 49059 and L-371,257 were dissolved in DMSO for a 1 mM stock solution and diluted in ACSF to a final concentration of 100 µM in the microdialysis fiber. Concentrations were selected based on data in the literature,3,16–18 and our own work comparing drug effects in brain slices with drug application by microdialysis, which indicated that that microdialysis required a 100-fold higher concentration because of the concentration gradient across the dialysis membrane and diffusion in the tissue.19–22 For combined administration of AVP with one of the receptor antagonists, the antagonist was administered by itself for 5 min before the mixture of AVP and antagonist was administered for 15 min
Behavioral assays
The behavioral tests (EPM, vocalizations, and spinal reflexes) were conducted in shielded temperature- and light-controlled rooms as described in detail previously.11,12,14,22–24
Elevated plus maze
Exploration of the open and closed arms of the EPM (Columbus Instr.) was used to determine anxiety-like behaviors of normal and arthritis rats and drug effects in the amygdala. Animal movements in the open and closed arms were detected with photocells, and entries into the respective arm were measured by Multi-Varimex software (Columbus Instr.) as the disruption of a photobeam. After acclimation to the lab for 1 h and immediately after drug administration, the animal was transferred to the EPM and placed on the central area, facing an open arm. Anxiety-like behavior was measured as the ratio of open-arm entries to the total number of entries (expressed as %) during 5 min.
Vocalizations
Rats were briefly anesthetized with isoflurane (2%, precision vaporizer) and placed in a custom-designed recording chamber, and the microdialysis probe was inserted through the guide cannula. The chamber was designed to permit access to the hindlimbs for mechanical tissue compression. After habituation to the chamber, brief (15 s) mechanical stimuli of innocuous (300–500 g/30 mm2) and noxious (1200–1500 g/30 mm2) intensities were applied to the left knee joint using a calibrated forceps with a force transducer to monitor the applied force (in g). Audible (20–16 Hz) and ultrasonic (25 ± 4 kHz) vocalizations were measured with a condenser microphone and a bat detector, respectively, over a period of 1 min starting with the onset of mechanical stimulus. Placed in front of the animal at a fixed distance, the sound detectors were connected to a filter and amplifier (UltraVox four-channel system; Noldus Information Technology). Total duration and number of events were analyzed using UltraVox 2.0 software (Noldus Information Technology).
Spinal reflexes
Thresholds of hindlimb withdrawal reflexes were measured by applying mechanical stimuli of continuously increasing intensity to the knee, using a calibrated forceps equipped with a force transducer. Withdrawal threshold was defined as the minimum stimulus intensity that evoked a withdrawal reflex. Measurements were repeated two times at 5 min interval, and the average was taken as the final value.
Histology
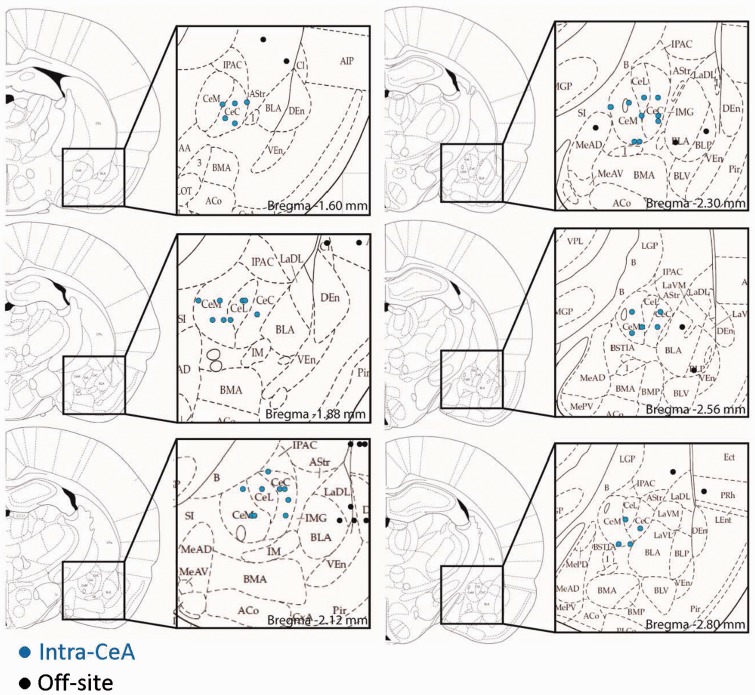
At the end of a behavioral experiment, the animal was sacrificed, and the brain was removed and submerged in 10% formalin. Tissues were stored in 20% sucrose before they were frozen sectioned at 10–50 µm. Sections were stained with hematoxylin and eosin, mounted on gel-coated slides, and coverslipped. Positions of the microdialysis fibers were identified under the bright-field microscopy and plotted on standard diagrams (Figure 1).
Figure 1.
Histological verification of drug application sites. Diagrams show coronal sections through the left hemisphere posterior to bregma, with insets showing the amygdaloid nuclei and placement of cannulas in the central nucleus (CeA) or off-site. Numbers indicate distance from bregma.
Statistics
All averaged values are given as the mean ± SE. Statistical significance was accepted at the level P < 0.05. GraphPad Prism 3.0 software was used for all statistical analyses. For multiple comparisons, one-way analysis of variance was used with Dunnett’s posthoc tests to compare all sets of data to a control value.
Results
The behavioral effects of AVP and antagonists for V1A (SR 49059, Relcovaptan) and OTR (L-371,257) in the amygdala were determined in normal and arthritic rats (24 h postinduction of arthritis). All drugs and ACSF (control) were administered stereotaxically into the CeA. Effects of off-site injections were also analyzed. Figure 1 shows the drug application sites.
Normal conditions
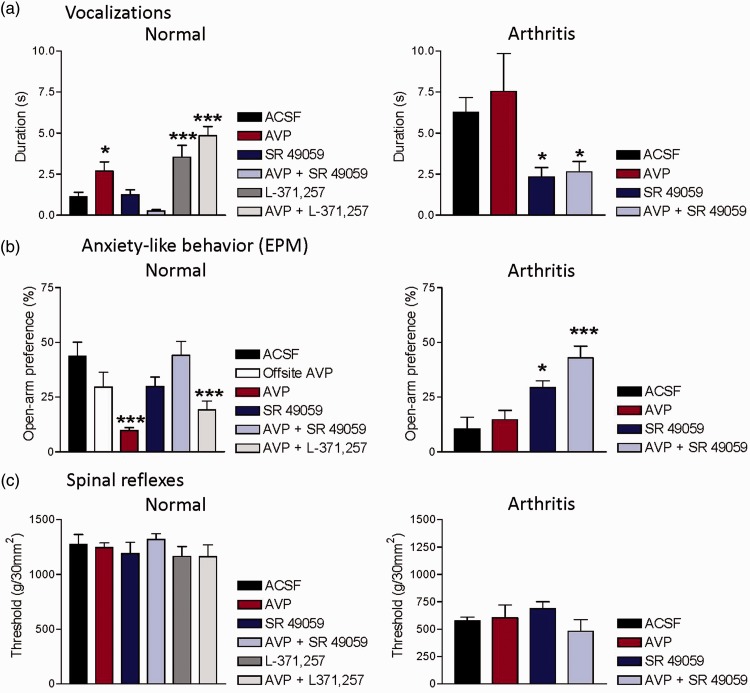
Vocalizations (Figure 2(a))
Figure 2.
Behavioral effects of vasopressin in normal animals (left column) and in the arthritis pain model (right column). (a) Vocalizations in the ultrasonic range were evoked by brief (15 s) noxious stimuli (compression of the knee joint with a calibrated forceps; 1500 g/30mm2). Total duration of vocalizations (in s) is shown for a recording period of 1 min starting with the onset of the mechanical stimulus. (b) Open-arm preference in the elevated plus maze (EPM) was measured as the number of entries into the open arm over the total number of entries for a period of 5 min. Decreased open-arm preference indicates anxiety-like behavior. (c) Hindlimb withdrawal thresholds for spinal reflexes were measured by mechanical compression of the knee with a calibrated forceps. Drugs were administered into the CeA by microdialysis for 15 min. For drug combinations, administration of the antagonists started 5 min before the mixture of agonist (AVP) combined with antagonist was administered for 15 min. *,***P < 0.05, 0.001 compared to ACSF (vehicle), one way analysis of variance with Dunnett’s multiple comparison tests. Bar histograms show means ± SEM.
Compared to ACSF control (n = 6 rats), stereotaxic administration of AVP (100 µM, concentration in microdialysis probe, see Materials and Methods section; n = 5 rats) into the CeA significantly increased emotional-affective pain responses (audible and ultrasonic vocalizations) evoked by brief (15 s) noxious mechanical stimuli (knee joint compression, see Materials and Methods section). Audible (not shown) and ultrasonic vocalizations were recorded for a period of 1 min following the onset of the mechanical stimulus. A selective V1A antagonist (SR 49059, 100 µM, concentration in microdialysis probe; n = 5 rats) had no effect on its own but blocked the facilitatory effect of AVP when both compounds were co-administered into the CeA (n = 5 rats). In fact, the drug combination decreased vocalizations below baseline, and so we tested the hypothesis that the V1A antagonist unmasked an inhibitory effect of AVP mediated through another receptor. Since there is evidence for opposing effects of V1A and OT receptors in the CeA,3 we measured the effect of an OTR antagonist (L-371,257). L-371,257 (100 µM, concentration in microdialysis probe; n = 6 rats) had significant facilitatory effects, which were even more pronounced in combination with AVP (n = 6 rats). The data suggest that V1A mediates the facilitatory effect of exogenous AVP, whereas OTR is endogenously activated to exert some inhibitory tone under normal conditions.
Anxiety-like behavior (Figure 2(b))
Open-arm preference in the EPM was measured for 5 min in normal rats. Compared to ACSF control (n = 5 rats), stereotaxic administration of AVP (100 µM, concentration in microdialysis probe) into the CeA (n = 6 rats), but not off-site (n = 5 rats), decreased open-arm preference significantly, indicating anxiety-like behavior. A selective V1A antagonist (SR 49059, 100 µM, concentration in microdialysis probe) administered alone (n = 6 rats) or in combination with AVP (n = 7 rats) had no significant effect. The anxiogenic-like effect of AVP was not blocked by an OTR antagonist (L-371,257, 100 µM, concentration in microdialysis probe; n = 5 rats). The data suggest that V1A, but not OTR, mediate the anxiogenic-like effects of AVP in normal rats.
Spinal reflexes (Figure 2(c))
Hindlimb withdrawal reflexes were evoked by mechanical compression of the knee joint with a calibrated forceps (see Materials and Methods section). Compared to ACSF control (n = 6 rats), stereotaxic administration of AVP (100 µM, concentration in microdialysis probe, n = 5 rats), SR 49059 (100 µM, concentration in microdialysis probe) alone (n = 5 rats) or together with AVP (n = 5 rats), and L-371,257 (100 µM, concentration in microdialysis probe) alone (n = 6 rats) or combined with AVP (n = 6 rats) had no significant effect. The data argue against a significant role of V1A and OTR in the amygdala in the descending modulation of spinal nociceptive behavior.
Arthritis pain model
Behavioral measurements were made one day after arthritis induction.
Vocalizations (Figure 2(a))
As shown in our previous work (for a recent review, see literature1), audible and ultrasonic vocalizations increased in the arthritis pain model. Different than in normal rats, stereotaxic administration of AVP (100 µM, concentration in microdialysis probe; n = 5 rats) into the CeA had no significant effect in arthritic rats compared to ACSF control (n = 5 rats). In contrast, a V1A antagonist (SR 49059, 100 µM, concentration in microdialysis probe) alone (n = 6 rats) or combined with AVP (n = 5 rats) inhibited vocalizations significantly. The data suggest that endogenous activation of V1A in the CeA contributes to increased emotional-affective responses in the arthritis pain model.
Anxiety-like behavior (Figure 2(b))
Arthritic rats showed decreased open-arm preference in the EPM, indicating anxiety-like behavior consistent with our previous studies (reviewed in literature1). Stereotaxic administration of AVP (100 µM, concentration in microdialysis probe; n = 5 rats) into the CeA had no significant effect compared to ACSF control (n = 5 rats). A V1A antagonist (SR 49059, 100 µM, concentration in microdialysis probe) alone (n = 5 rats) or combined with AVP (n = 6 rats) increased open-arm preference significantly. The data suggest that endogenous activation of V1A in the CeA contributes to increased anxiety-like behavior in the arthritis pain model.
Spinal reflexes (Figure 2(c))
Thresholds for hindlimb withdrawal reflexes were decreased in arthritic rats indicating hypersensitivity as described before (reviewed in literature1). Compared to ACSF control (n = 5 rats), stereotaxic administration of AVP (100 µM, concentration in microdialysis probe, n = 5 rats) or SR 49059 (100 µM, concentration in microdialysis probe) alone (n = 6 rats) or the combination of SR 49059 and AVP (n = 5 rats) had no significant effect. The data suggest that V1A in the CeA does not contribute to spinal nociceptive behavior in the arthritis pain model.
Discussion
The key novelties of this study are the identification of V1A in the amygdala (CeA) as a mediator of the facilitatory effects of AVP on emotional affective rather than sensory aspects of pain behaviors. Functional V1A is available in the CeA under normal conditions since its stimulation with exogenous AVP generates pain responses, and this effect is blocked by a selective V1A antagonist (SR 49059, Relcovaptan) but not by an OT receptor antagonist (L-371,257). In an arthritis pain model, emotional-affective behaviors are driven by the endogenous activation of V1A as revealed by the beneficial effects of V1A blockade. The fact that L-371,257 had some facilitatory effects on its own under normal conditions may suggest that vasopressin exerts some inhibitory tone through OTR, and that in the pain state, there is a shift from OTR-mediated inhibition to V1A-mediated facilitation. Synaptic and cellular mechanisms remain to be determined, but the significance of this study is to lay the groundwork for more mechanistic analyses.
A point of consideration here is the selection of pharmacological compounds and targets to probe the AVP system. The actions of AVP are mediated through two types of G-protein coupled receptors. V1 receptors (V1A and V1B) are coupled to phospholipase through Gq, whereas V2 receptors are coupled to Gs and adenylyl cyclase.4,10 We focused on V1A because it is the primary and abundant vasopressin subtype in the brain,8 and differential V1A and V1B knockout experiments suggest that V1A but not V1B was responsible for the mediation of anxiety-like behavior.10 Further, electrophysiological effects of AVP in the CeA were shown to be mediated by V1A but not V1B and V2.3 Our data suggest that V1A in the amygdala mediates pro-nociceptive and anxiogenic effects of AVP. We also tested the contribution of OTR because in the presence of a V1A antagonist, AVP actually had some anxiolytic effects (Figure 2(b)) and inhibited vocalizations (Figure 2(a)). AVP can act on OTR.8,10,18 We found that an OTR antagonist (L-371,257) alone increased vocalizations and increased the facilitatory effects of AVP (Figure 2(a)). The data suggest that OTR mediates an inhibitory tone in the amygdala, which is consistent with antinociceptive effects of central OTR.25 In contrast, the role of AVP in pain modulation has remained unclear, and both pro- and anti-nociceptive effects have been observed, possibly depending on the site of action and receptor involved.26 It should be noted that L-371,257 can also act on V1A in the rat.27 However, in our study, L-371,257 did not mimic the effects of the V1A antagonist, and so we interpret our data to suggest that effects of L-371,257 were due to blockade of OTR, and that V1A and OTR mediate differential effects of AVP in the amygdala (CeA).
Opposing roles of AVP and OT have been described with regard to anxiety and depression2 and could explain the failure in clinical trials of compounds that block V1 as well as OTR.4 V1A and OTR both couple to PLC through G-proteins, and so differential effects observed here are unlikely due to different signaling pathways but rather involve different types of neurons and circuits. For example, in the CeA, OTR seems to mediate inhibition of output neurons, whereas V1A activates these neurons; and expression of OTR was restricted to the lateral division of the CeA, whereas V1A was found in the medial CeA.3 Synaptic mechanisms of the behavioral effects of AVP in our study remain to be determined.
Author Contributions
BC and VN conceived the study, designed the experiments, and wrote the manuscript. BC carried out the experiments. All authors participated in the data analysis and interpretation of results and read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH Grants NS081121 and NS038261.
References
- 1.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015; 227: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 2012; 35: 649–659. [DOI] [PubMed] [Google Scholar]
- 3.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005; 308: 245–248. [DOI] [PubMed] [Google Scholar]
- 4.Beurel E, Nemeroff CB. Interaction of stress, corticotropin-releasing factor, arginine vasopressin and behaviour. Curr Top Behav Neurosci 2014; 18: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebner K, Wotjak CT, Landgraf R, et al. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci 2002; 15: 384–388. [DOI] [PubMed] [Google Scholar]
- 6.Ebner K, Bosch OJ, Kromer SA, et al. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology 2005; 30: 223–230. [DOI] [PubMed] [Google Scholar]
- 7.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 2004; 25: 150–176. [DOI] [PubMed] [Google Scholar]
- 8.Frank E, Landgraf R. The vasopressin system—from antidiuresis to psychopathology. Eur J Pharmacol 2008; 583: 226–242. [DOI] [PubMed] [Google Scholar]
- 9.Mogil JS, Sorge RE, LaCroix-Fralish ML, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci 2011; 14: 1569–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koshimizu TA, Nakamura K, Egashira N, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev 2012; 92: 1813–1864. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JM, Ji G, Neugebauer V. Small-conductance calcium-activated potassium (SK) channels in the amygdala mediate pain-inhibiting effects of clinically available riluzole in a rat model of arthritis pain. Mol Pain 2015; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neugebauer V, Han JS, Adwanikar H, et al. Techniques for assessing knee joint pain in arthritis. Mol Pain 2007; 3: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neugebauer V, Li W, Bird GC, et al. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 2003; 23: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregoire S, Neugebauer V. 5-HT2CR blockade in the amygdala conveys analgesic efficacy to SSRIs in a rat model of arthritis pain. Mol Pain 2013; 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahtaoui C, Balestre MN, Klotz P, et al. Identification of the binding sites of the SR49059 nonpeptide antagonist into the V1a vasopressin receptor using sulfydryl-reactive ligands and cysteine mutants as chemical sensors. J Biol Chem 2003; 278: 40010–40019. [DOI] [PubMed] [Google Scholar]
- 16.Ring RH, Malberg JE, Potestio L, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006; 185: 218–225. [DOI] [PubMed] [Google Scholar]
- 17.Williams PD, Clineschmidt BV, Erb JM, et al. 1-(1-[4-[(N-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]piperidin-4- yl)-4H-3,1-benzoxazin-2(1H)-one (L-371,257): a new, orally bioavailable, non-peptide oxytocin antagonist. J Med Chem 1995; 38: 4634–4636. [DOI] [PubMed] [Google Scholar]
- 18.Manning M, Misicka A, Olma A, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol 2012; 24: 609–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 2008; 28: 3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiritoshi T, Ji G, Neugebauer V. Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci 2016; 36: 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 2011; 31: 1114–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren W, Kiritoshi T, Gregoire S, et al. Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors. J Neurophysiol 2013; 110: 1765–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han JS, Bird GC, Li W, et al. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods 2005; 141: 261–269. [DOI] [PubMed] [Google Scholar]
- 24.Medina G, Ji G, Gregoire S, et al. Nasal application of neuropeptide S inhibits arthritis pain-related behaviors through an action in the amygdala. Mol Pain 2014; 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo R, D’Agostino G, Mattace RG, et al. Central administration of oxytocin reduces hyperalgesia in mice: implication for cannabinoid and opioid systems. Peptides 2012; 38: 81–88. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Hernandez A, Rojas-Piloni G, Condes-Lara M. Oxytocin and analgesia: future trends. Trends Pharmacol Sci 2014; 35: 549–551. [DOI] [PubMed] [Google Scholar]
- 27.Williams PD, Bock MG, Evans BE, et al. Nonpeptide oxytocin antagonists: analogs of L-371,257 with improved potency. Bioorg Med Chem Lett 1999; 9: 1311–1316. [DOI] [PubMed] [Google Scholar]