Abstract
Heparin-based hydrogels are attractive for controlled growth factor delivery, due to the native ability of heparin to bind and stabilize growth factors. Basic fibroblast growth factor and vascular endothelial growth factor are heparin-binding growth factors that synergistically enhance angiogenesis. Mild, in situ encapsulation of both basic fibroblast growth factor and vascular endothelial growth factor and subsequent bioactive dual release has not been demonstrated from heparin-crosslinked hydrogels, and the combined long-term delivery of both growth factors from biomaterials is still a major challenge. Both basic fibroblast growth factor and vascular endothelial growth factor were encapsulated in poly(vinyl alcohol)-heparin hydrogels and demonstrated controlled release. A model cell line, BaF32, was used to show bioactivity of heparin and basic fibroblast growth factor released from the gels over multiple days. Released basic fibroblast growth factor promoted higher human umbilical vein endothelial cell outgrowth over 24 h and proliferation for 3 days than the poly(vinyl alcohol)-heparin hydrogels alone. The release of vascular endothelial growth factor from poly(vinyl alcohol)-heparin hydrogels promoted human umbilical vein endothelial cell outgrowth but not significant proliferation. Dual-growth factor release of basic fibroblast growth factor and vascular endothelial growth factor from poly(vinyl alcohol)-heparin hydrogels resulted in a synergistic effect with significantly higher human umbilical vein endothelial cell outgrowth compared to basic fibroblast growth factor or vascular endothelial growth factor alone. Poly(vinyl alcohol)-heparin hydrogels allowed bioactive growth factor encapsulation and provided controlled release of multiple growth factors which is beneficial toward tissue regeneration applications.
Keywords: Poly(vinyl alcohol), heparin, hydrogel, vascular endothelial growth factor, basic fibroblast growth factor
Introduction
Growth factors are promising molecules for the treatment of skin wounds and for tissue regeneration applications because they are proteins that can drive cell migration, proliferation, and/or differentiation. Growth factors have had little success in clinical applications because of challenges with intravenous (IV) administration of supraphysiological concentrations. When using IV delivery, extremely high initial concentrations are required to impart a physiological response. However, it has been shown that these levels of growth factors may lead to severe side effects. Conversely IV delivery may not allow sufficient levels of the factors to be sensed by the target tissue for the necessary time frame owing to their rapid denaturation, degradation, or cleaving.1–3 Interestingly, the clinical trials that have shown successful outcomes from growth factor treatment have predominantly involved use of a material carrier. These studies suggest that spatio-temporal control over the location and bioactivity of growth factors is crucial to achieve a therapeutic effect.1 A growth factor–releasing material platform that has robust mechanical properties, allows for encapsulation of bioactive growth factors, and provides for controlled release of multiple growth factors would aid in wound healing and tissue regeneration applications.
The extracellular matrix (ECM) is a source of inspiration for designing growth factor delivery systems because the ECM plays a fundamental role in coordinating growth factor signaling in vivo.4,5 Several growth factors possess specific interactions with heparin-containing molecules within the ECM and they are often described as heparin-binding growth factors.6,7 Heparin-based growth factor delivery systems have demonstrated the ability to provide sustained release of growth factors.8–10 Moreover, binding of growth factors to heparin is known to extend their half-lives, thus extending their bioactivity.11 Therefore, strategies using heparin-based matrices that can interact with growth factors are appealing.
Heparin can easily be modified to enable covalent crosslinking and the formation of hydrogels for growth factor binding and release. Hydrogels are an excellent candidate for wound treatment because they maintain moisture at the wound site and are capable of drug delivery.12 Heparin has been crosslinked to a variety of natural polymers including chitosan, alginate, arginine-based polycations, chondroitin sulfate, and hyaluronan to provide controlled release a variety of growth factors including bone morphogenetic protein 2 (BMP-2), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF).9,13–17 For example, hydrogel films crosslinked with heparin and other glycosaminoglycans have been used to deliver bFGF in a wound model in diabetic mice and showed acceleration of dermis formation and vascularization.18 Of particular interest for tissue regeneration are growth factors that promote angiogenesis, specifically bFGF and VEGF. VEGF is necessary to promote endothelial cell migration and induce the growth of blood vessels that sustain granulation tissue and bFGF is important for endothelial cell migration, wound reepithelization, and angiogenesis.1,19–22 Tissue repair involves the signaling of multiple growth factors and the delivery of a single type of growth factor may be insufficient.8 Dual release of these growth factors is known to have synergistic effects on endothelial cells, which are critical to wound angiogenesis.8,23,24 Although heparin has been used in the design of biomaterials for growth factor delivery,8,9,25 researchers have not demonstrated mild, in situ encapsulation of both bFGF and VEGF in crosslinked heparin hydrogels.
Hydrogels made from purely natural polymers, such as heparin, often have low mechanical properties and can be degraded uncontrollably by enzymes.26 Therefore, researchers have explored biosynthetic materials, whereby the desired biological polymer provides bioactivity, but a synthetic polymer is able to provide robust strength.27 For example, star-shaped poly(ethylene glycol) (PEG)-heparin hydrogels were designed such that the synthetic polymer PEG provided structural and mechanical hydrogel network characteristics, while the biological polymer heparin enabled reversible immobilization of growth factors.27 Recently, poly(vinyl alcohol) (PVA)-heparin hydrogels have been designed that crosslink to form hydrogels in situ using a mild, hydrazone bonding strategy (Figure 1). PVA is a hydrophilic synthetic polymer that is easily synthetically modified to enable crosslinking into hydrogels28 and is US Food and Drug Administration (FDA) approved for use in a variety of pharmaceutical and biotechnology applications. A challenge with growth factor encapsulation is that these sensitive proteins may be damaged during encapsulation reducing their bioactivity.29 Because of harsh encapsulation conditions, other studies have used pre-formed hydrogels into which growth factors have been subsequently absorbed. For example, starPEG-heparin hydrogels have been crosslinked via a harsh chemical reaction (i.e. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysulfosuccinimide, EDC/NHS), which required that the growth factors (bFGF and VEGF) had to be absorbed at high concentrations (µg), which results in growth factor loss from incomplete absorption, high cost, and involves additional processing steps.8 Hydrazone bonding is thought to be a mild crosslinking strategy because it occurs via the orthogonal reaction of polymers functionalized with aldehydes and hydrazides within solutions at physiological pH and temperature and the only reaction byproduct is water.30 Moreover, hydrazone bonding is advantageous because it provides for injectable, in situ hydrogel formation within a clinically relevant time frame of several minutes.28
Figure 1.
Schematic of PVA-heparin injectable hydrogels for growth factor release. Macromer precursor solutions form into hydrogel networks upon hydrazone bond formation between hydrazide (HY, red arrows) and aldehyde (AL, purple circles) groups.
In this study, PVA-heparin hydrogels were formed via hydrazone bonding for their ability to encapsulate and provide sustained dual release of the angiogenic, heparin-binding growth factors bFGF and VEGF. Release of bFGF and VEGF from the hydrogels was evaluated for independent as well as dual release. Subsequently, a model cell line (BaF32) was used to evaluate if the synthetically modified heparin and encapsulated, released growth factors have retained their signaling ability over multiple days. To demonstrate the angiogenic potential of these bFGF- and VEGF-releasing PVA-heparin hydrogels, endothelial cell proliferation and migration was monitored over several days. It was demonstrated that heparin-containing hydrogels can form via mild, in situ reactions and enable sustained release of bioactive growth factors, which has broad applications across wound healing and tissue regeneration.
Experimental section
Materials
PVA (13–23 kDa, 98% hydrolyzed), glycine ethyl ester hydrochloride, triethylamine, 1,1′-carbonyldiimidazole (CDI), hydrazine monohydrate, 3-amino-1,2-propanediol, sodium periodate (NaIO4), ammonium hydroxide solution (28%–30% NH3), heparin (heparin sodium salt from porcine intestinal mucosa, grade I-A, 17–19 kDa), N-hydroxybenzotriazole (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), sodium chloride (NaCl), hydrochloric acid (HCl), cellulose membrane dialysis tubing Molecular Weight Cut Off (MWCO 12 kDa), Dulbecco’s phosphate-buffered saline (PBS, pH 7.4), bovine serum albumin (BSA), Dulbecco’s Modified Eagle’s Medium (DMEM), Penicillin-Streptomycin, sodium bicarbonate, and trypsin-EDTA solution were purchased from Sigma-Aldrich. Dimethyl sulfoxide (DMSO) dried over 4 Å molecular sieves (100%), ethanol (100%), and diethyl ether (100%) were bought from Ajax Chemicals. Fetal bovine serum (FBS) was purchased from Moregate Biotech. bFGF, VEGF, the bFGF enzyme-linked immunosorbent assay (ELISA) kits, and the VEGF ELISA kits were purchased from ThermoFisher Scientific. MTS reagent (CellTiter 96® AQueous One Solution Cell Proliferation Assay) was purchased from Promega. Endothelial cell media was the phenol-red free Endothelial Cell Growth Medium 2 KIT from PromoCell. ThinCert™ transwells were purchased from Greiner Bio-One. Fences (migration assay kit, metal-silicone inserts) were purchased from Aix Scientifics® Clinical Research Organisation.
Macromer synthesis
Hydrazide-modified PVA (PVA-HY),30,31 aldehyde-modified PVA (PVA-AL),30,31 and aldehyde-modified heparin (heparin-AL)9 were synthesized as described previously.28 Briefly, to synthesize PVA-HY, PVA was dissolved in DMSO with CDI. Glycine ethyl ester hydrochloride and triethylamine were added and the reaction was stirred overnight. Hydrazine monohydrate was added and the mixture was stirred at room temperature for 24 h. PVA-HY was precipitated in 80/20 diethyl ether/ethanol and then dialyzed against water followed by lyophilization.
PVA-AL was synthesized in a two-step reaction. First, PVA was dissolved in DMSO with CDI. A solution of 3-amino-1,2-propanediol and triethylamine were added to the reaction and the reaction was stirred at 60°C for 18 h. Concentrated aqueous NH3 was then added and stirred at room temperature for 1 h. Modified PVA was precipitated in 80/20 mixture of diethyl ether/ethanol, redissolved in deionized water, dialyzed against deionized water, and lyophilized. Second, to create the aldehyde-modified PVA by oxidation, the amino-glycerol-modified PVA was dissolved in an ice bath and NaIO4 and the mixture was stirred for 1 h. The solution was then dialyzed against deionized water and lyophilized.
Heparin-AL was also synthesized in a two-step reaction. Heparin, HOBt, and 3-amino-1,2-propanediol were dissolved in deionized water. The pH of the reaction mixture was then adjusted to 6.0 and EDC was added for overnight reaction. The solution was dialyzed and lyophilized. To form heparin-AL, amino-glycerol-modified heparin was dissolved in deionized water. Predissolved NaIO4 was added to the reaction mixture and stirred for 30 min. The reaction was quenched with excess ethylene glycol. The solution was dialyzed against deionized water and lyophilized to obtain heparin-AL.
The PVA-HY, PVA-AL, and heparin-AL were characterized using 1H NMR and found to contain 5 HY or 5 AL per PVA or heparin chain.
Hydrogel formation
Macromers of PVA-HY, PVA-AL, and heparin-AL were dissolved in PBS. HY and AL macromers were dissolved separately and then combined at a ratio of 1 mol HY:1 mol AL to achieve a final polymer concentration of 10% (w/w) with 0% or 1% (w/w) heparin-AL and a volume of 50 µL (2.5 mm height × 5 mm diameter). Specifically, gel formulations were 10% (w/w) PVA (PVA gel) or 1% (w/w) heparin:9% (w/w) PVA (PVAHep gel). Hydrogels were allowed to polymerize for 15 min at room temperature and then immediately submersed in solution.
Growth factor release kinetics
To assess growth factor release kinetics, bFGF (500 ng/mL, 25 ng/gel) and/or VEGF (500 ng/mL, 25 ng/gel) were mixed into sterile macromer solutions. Sterile macromers were prepared through filtration of a dilute macromer solution through a 0.22-µm filter and subsequent sterile lyophilization. Sterile HY and AL macromers were dissolved separately in PBS and then combined 1 mol HY:1 mol AL to a final polymer concentration of 10% (w/w) with 0% (w/w) or 1% (w/w) heparin-AL and a volume of 50 µL (2.5 mm height × 5 mm diameter). Hydrogels were allowed to polymerize for 15 min at room temperature and then submersed in PBS containing 0.5% (w/w) BSA at 37°C. At predetermined time points, the release solution was collected, stored at −20°C for later analysis, and replenished with fresh PBS containing 0.5% (w/w) BSA. Release profiles of bFGF and VEGF were analyzed using ELISA kits following the manufacturer’s instructions.
Hydrogel swelling
After polymerization, hydrogel samples were placed in PBS containing 0.5% (w/w) BSA at 37°C, with the exception of time 0 samples which were taken immediately. At 12, 24, 48, and 72 h, hydrogels were removed and gently blotted for the removal of excess PBS and weighed. This swollen mass (ms) was recorded and then samples were lyophilized to obtain the dry polymer mass (mp). The swelling ratio (equation (1)) was determined as follows
| (1) |
BaF32 cell proliferation assay for heparin and bFGF releasate bioactivity
The BaF32 cell line used in this study are an interleukin 3 (IL-3)-dependent and heparin sulfate proteoglycan–deficient myeloid B cell line that has been transfected with fibroblast growth factor receptor 1c (FGFR1c).32,33 BaF32 cells are a model system used to identify bioactive heparin structures that interact with fibroblast growth factors and their receptors. The result of this assay is cell proliferation that indicates the formation of ternary complexes between the cells, bioactive heparin, and bioactive bFGF. BaF32 cells were maintained in RPMI 1640 medium containing 10% (v/v) FBS, 10% (v/v) WEHI-3BD conditioned medium, and 1% (v/v) penicillin/streptomycin. WEHI-3BD cells were maintained in RPMI 1640 medium supplemented with 2 g/L sodium bicarbonate, 10% (v/v) FBS, and 1% (v/v) penicillin/streptomycin and the conditioned medium was collected three times per week and stored at −20°C until required.
Hydrogels (PVAHep gel) containing 500 ng/mL bFGF were formed as described above and placed in RPMI 1640. At 0, 12, 24, 48, or 72 h, the cumulative releasate was collected and stored frozen at −20°C until the BaF32 assay.
Prior to the mitogenic assays, the BaF32 cells were transferred into IL-3-depleted medium for 24 h. BaF32 cells were then seeded into 96-well plates at a density of 2 × 104 cells/well in the presence of bFGF (0–0.3 nM), heparin (0–30 nM), and/or hydrogel releasate. Cells were incubated for 48 h in 5% CO2 at 37°C and the number of cells present was assessed using the MTS reagent (10% (v/v)) by adding to the cell cultures for 6 h prior to measuring the absorbance at 490 nm.
Endothelial cell proliferation in response to hydrogel growth factor release
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell media (phenol-red free endothelial basal media, 2% (v/v) FBS, 5 ng/mL epidermal growth factor, 10 ng/mL bFGF, 20 ng/mL insulin-like growth factor, 0.5 ng/mL VEGF, 1 µg/mL ascorbic acid, 22.5 µg/mL heparin, 0.2 µg/mL hydrocortisone). Prior to the proliferation assay, HUVECs were trypsinized and plated (5 × 103 cells/well) in endothelial cell media in 24-well plates for 24 h to allow attachment. Wells were rinsed with PBS and basal media (phenol-red free endothelial basal media with 0.5% (v/v) FBS) was added to the wells. PVAHep hydrogels containing 500 ng/mL bFGF and/or 500 ng/mL VEGF were placed in transwells (8 µm pores) and suspended in the media above the cells. Cells were incubated for 0–72 h in 5% CO2 at 37°C and proliferation was assessed using the MTS reagent (10% (v/v)) by adding to the cell cultures for 6 h prior to measuring the absorbance at 490 nm.
Endothelial cell outgrowth in response to hydrogel growth factor release
HUVECs were trypsinized and plated (3 × 104 cells/well, a confluent monolayer) in endothelial cell media inside fences (7 mm diameter) in 24-well plates for 4 h to allow attachment. Wells were rinsed with PBS and basal media (phenol-red free endothelial basal media with 0.5% (v/v) FBS) was added to the wells. PVAHep hydrogels containing 500 ng/mL bFGF and/or 500 ng/mL VEGF were placed in transwells (8 µm pores) and suspended in the media above the cells. Cells were incubated for 24 h in 5% CO2 at 37°C. To view cellular outgrowth, HUVECs were fixed with 80% ethanol for 1 h at room temperature, followed by staining for 1 h with 0.3% crystal violet in 10% methanol:90% diH2O. Images of the gels were taken using a Leica M80 stereo microscope at 1.25× magnification. Cellular outgrowth was quantified using ImageJ software by comparing the area of cellular coverage at 0 h to cellular coverage at 24 h post-treatment with hydrogels.
Statistics
Significance was tested using GraphPad Prism 6 (GraphPad Software). Quantitative data are expressed as mean with error bars representing standard error of the mean (mean ± SEM). Data were compared using analysis of variance (ANOVA) with Tukey’s post hoc analysis. Significance was established for p < 0.05. All samples were prepared in triplicate and all studies were repeated three times.
Results and discussion
Gel formation and growth factor release
PVA and heparin were functionalized with reactive crosslinking groups to enable covalent crosslinking of the polymers. PVA was functionalized with a hydrazide or an aldehyde (i.e. oxidized amino-glycerol) as previously described.28,30,31 Heparin was functionalized with an aldehyde of the same oxidized amino-glycerol moiety, using a regio-selective modification method that does not oxidize (i.e. cleave) the native polymer.9,28 Retaining the native heparin polymer structure may be important to provide sufficient heparin-binding sites for encapsulated growth factors.34,35 The hydrazide and aldehyde containing macromers were dissolved separately from each other and with any molecules for encapsulation (e.g. drugs such as growth factors), and upon mixing the hydrazide and aldehyde macromers hydrazone bonding and gelation occurred (Figure 1). It has previously been established that these PVA and heparin macromer solutions combine to form handleable, mechanically robust (shear modulus (G*) = 1–10 kPa) hydrogels within the clinically relevant time frame of under 15 min.28
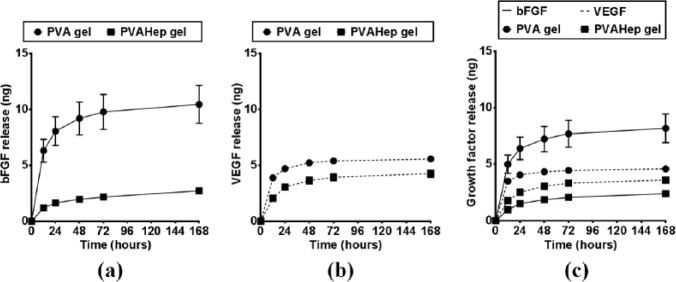
Growth factor release from biosynthetic, PVA-heparin hydrogels was compared to that of purely synthetic PVA hydrogels in order to observe how heparin, as a bioactive polymer, impacts growth factor release. To enable comparison between the hydrogels, both the purely synthetic and biosynthetic hydrogels were formed at the same total polymer concentration (10% (w/w)), using the same initial hydrazone crosslinking density, and with the same growth factor concentrations. In the absence of heparin, PVA-only gels have a burst release, with over 60% of the total bFGF released over 7 days being released within the first 12 h (Figure 2(a)). PVA-heparin hydrogels had a significantly lower fraction of burst release of bFGF in the first 12 h (p < 0.05), as well as significantly lower release overall with 3 ng bFGF releasing from PVA-heparin gels versus 10 ng bFGF release from PVA-only gels over 7 days (p < 0.05). This lower release at 7 days is beneficial, as it suggests that more growth factor remains in the hydrogel long-term for sustained release. Similarly when an alternative growth factor, VEGF, was released from the PVA-only gels, there was a significant burst release with 70% of the total VEGF released over 7 days being released within the first 12 h (p < 0.05, Figure 2(b)). VEGF released from PVA-heparin hydrogels had a significantly lower fraction (20% lower) of burst release in the first 12 h (p < 0.05). Additionally, PVA-heparin gels had significantly lower total release of VEGF over 7 days (4.3 ng), whereas the PVA-only gels released 5.6 ng (p < 0.05). Although many hydrogels exhibit excellent biocompatibility and can be used as drug releasing systems, the reduced burst and more sustained release of growth factors from biosynthetic, PVA-heparin hydrogels as compared to purely synthetic PVA hydrogels is advantageous. Moderating the burst release of drugs has the potential to make therapies more efficient and reduce cost, while limiting the inherent side effects related to systemic administration of drugs, such as drug dosing at supraphysiological levels or outside of the therapeutic window.36
Figure 2.
Growth factor release profiles from PVA (circles) and PVA-heparin (squares) gels over 7 days: (a) bFGF release (ng; solid lines), (b) VEGF release (ng, dashed lines), and (c) dual release of bFGF and VEGF (ng).
PVA-only hydrogels swell less after submersion in aqueous buffer than PVA-heparin gels (Table 1) likely because the anionic carboxyls and sulfates in heparin make it highly hydrophilic.28 Higher swelling in PVA-heparin hydrogels would suggest that growth factor should diffuse out more rapidly because of a larger mesh size. The swelling ratio of a hydrogel is known to correspond to its mesh size, where a higher mass swelling ratio indicates a larger mesh size, and therefore a higher rate of diffusion of encapsulated molecules would be expected.37 However, in this study, the higher growth factor release from the PVA hydrogels as compared to the PVA-heparin hydrogels is not likely due to differences in diffusion due to hydrogel swelling. The lower growth factor release in PVA-heparin gels combined with the fact that PVA-heparin gels are known to have higher swelling suggests that heparin is interacting with the growth factors, resulting in higher growth factor retention. This retention likely occurs because bFGF and VEGF are known as heparin-binding growth factors.8,27,35,38 Heparin-growth factor interactions are predominately based on ionic or H-bonding forces between specific basic amino acid residues in the heparin-binding protein and the sulpho and carboxyl groups in the saccharide backbone.6 For example, hyaluronan-heparin hydrogels released approximately 30% less bFGF than alternative hyaluronan-based hydrogels, showing that heparin binding is critical to growth factor interactions.9
Table 1.
Mass swelling ratio (q) of PVA and PVA-heparin hydrogels.
| Time (h) | Mass swelling ratio (q) | |
|---|---|---|
| PVA hydrogels | PVA-heparin hydrogels | |
| 0 | 10.0 ± 0.2 | 10.0 ± 0.3 |
| 12 | 13.2 ± 0.3 | 14.1 ± 0.5 |
| 24 | 13.9 ± 0.8 | 15.5 ± 0.7 |
| 48 | 14.5 ± 0.7 | 17.0 ± 0.5 |
| 72 | 13.2 ± 0.9 | 16.0 ± 0.5 |
PVA: poly(vinyl alcohol).
Although individual release of growth factors from hydrogels is able to produce a biological response, it has been shown that delivery of multiple growth factors may have a synergistic impact on cell behavior.8 When bFGF and VEGF were simultaneously released (Figure 2(c)), the release profiles were similar to when they are released alone (Figure 2(a) and (b)), suggesting dual, but independent release of these pro-angiogenic growth factors. It can be seen that both growth factors have significantly higher release from PVA-only gels when compared to PVA-heparin gels (p < 0.05), despite the same amount of growth factor incorporation into the both gel types. When looking at release from PVA hydrogels after 7 days, the larger growth factor (VEGF) which has a molecular weight of 38.8 kDa and radius of 30.2 Å39 releases 45% less than the smaller growth factor (bFGF) which has a molecular weight of 17.2 kDa and radius of 14.5 Å.40 This suggests that these growth factors may diffuse out of the PVA-only hydrogel based on size. Interestingly, the release of bFGF and VEGF from PVA-heparin hydrogels was opposite of the PVA-only hydrogels, with a significantly higher release of VEGF than bFGF over 7 days (p < 0.05). This may be due to the relative strength of binding between heparin and the different growth factors. The dissociation constant between bFGF and heparin is Kd ~3.9 × 10−8 M,41 whereas for VEGF and heparin it is Kd ~8.0 × 10−8 M.35 The dissociation constant corresponds to the concentration of protein bound to heparin and indicates the strength of the binding. These results suggest that bFGF binds more tightly to heparin than VEGF binds to heparin, despite the same initial amount of growth factor being in each hydrogel (500 ng/mL). Additionally, these results suggest that the heparin has bioactive binding sites available, even after being modified with aldehyde moieties for covalent crosslinking. This interaction between heparin and the growth factor is ideal, since binding between heparin and growth factors is not only critical for bioactivity, but in many cases also for stabilization.42 Stabilized growth factors have longer half-lives than non-stabilized growth factors,43 thus potentially increasing the duration of bioactive growth factor delivery within the therapeutic window for these costly protein drugs. Additionally, the ability of these gels to bind multiple growth factors and provide dual release over several days is beneficial if multiple effects are desired. For example, bFGF is known to more strongly encourage endothelial cell proliferation, whereas VEGF is known to promote migration.22
Bioactivity of bFGF and heparin after release from PVA-heparin hydrogels
It is known that the PVA-heparin hydrogels can degrade over time via hydrazone-bond hydrolysis.44 Therefore, it is expected that there will be release of PVA and heparin macromers simultaneously with growth factor release.28 To further demonstrate that the heparin remains bioactive following aldehyde modification, hydrazone bonding, and subsequent release from the gel, a cellular assay was performed. BaF32 cells are a model cell line which proliferate only in response to the formation of a ternary complex between bFGF, heparin, and the cells.33 If the bFGF or heparin is degraded, denatured, or otherwise unable to bind the cells, proliferation will not occur. It can be seen that the cells alone, with bFGF, or with heparin produce a threshold BaF32 cell number (Figure 3). However, when the cells are placed with fresh bFGF and heparin (controls), there is a twofold or higher increase in cell number due to proliferation, indicative of the ternary complex formation. The cumulative releasate from PVA-heparin bFGF gels up to 72 h of release was collected, and it can be seen that bFGF and modified-heparin retain their ability to bind and form ternary complexes with the BaF32 cells as evidence by the increased proliferation (Figure 3). Although this cellular assay does not give a quantitative measure of how much bFGF and heparin remains bioactive, it can be seen that there is a sufficient amount of both bioactive heparin and bioactive bFGF to promote BaF32 proliferation. Similarly, others have demonstrated PVA-heparin photocrosslinked hydrogels can promote BaF32 proliferation in the presence of bFGF;45 however, these studies did not demonstrate prolonged bioactive heparin and growth factor releasate as seen here. This long-term retention of bioactivity is impressive, given that the heparin and bFGF had been released cumulatively for up to 3 days prior to being placed in to the BaF32 media for a further 2 days to promote cell proliferation. Additionally, the bFGF bioactivity suggests that hydrazone crosslinking is a mild growth factor encapsulation strategy. This also shows that the aldehyde modification does not reduce heparin’s bioactivity.
Figure 3.
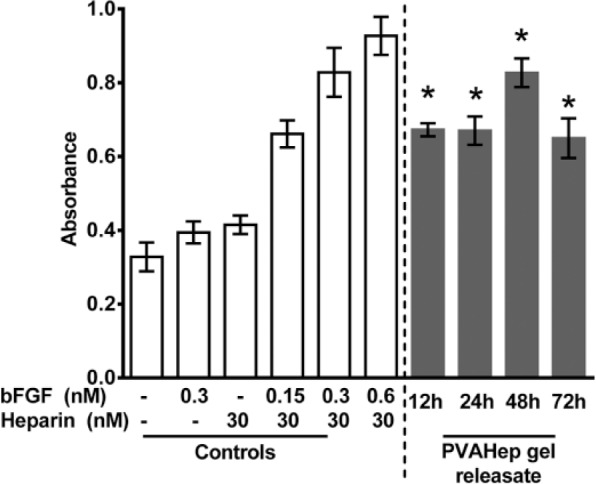
Growth factor (bFGF) was released from PVA-heparin hydrogels for 12–72 h. The releasate was subsequently placed with BaF32 cells and proliferation was monitored after 48 h with the MTS assay (gray bars). Negative controls contained cells in medium with no heparin/bFGF, bFGF only (0.3 nM), or heparin only (30 nM) which give a threshold proliferation. Positive controls contained cells in medium with fresh heparin (30 nM) and bFGF (0.15–0.6 nM) and show increased absorbance because of the formation of ternary complexes (cell, heparin, bFGF) that promote cell proliferation. Controls are represented by white bars. BaF32 cell proliferation in response to PVAHep gel releasate was found to be significantly higher when compared to threshold controls without bFGF, heparin, or both (*p < 0.05).
Endothelial cell proliferation in response to growth factor release
Cellular assays provide a sensitive measure to understanding if biomaterial-released growth factors can guide a variety of critical cellular activities including proliferation, migration, and differentiation. Because applications such as wound healing and tissue regeneration rely heavily on the influx of blood vessels for new tissue growth, these growth factor delivering PVA-heparin hydrogels were evaluated for their ability to promote proliferation of endothelial cells using bFGF and VEGF. The presence of PVA-heparin hydrogels resulted in a 0.7-fold higher proliferation than the basal media control after 3 days (p < 0.05). The significant increase in proliferation in the presence of PVA-heparin gels may occur for several reasons. Heparin is known to bind and stabilize a large variety of growth factors6,42 and thus could increase the stability of growth factors present in the FBS. Alternatively, the PVA and heparin released into solution likely increases the osmotic pressure surrounding the cells, which is known to impact endothelial cell proliferation.46 VEGF released from PVA-heparin hydrogels did not result in increased cell proliferation over the hydrogels alone, with only 1.1-fold increase in cell proliferation over basal media at 3 days. However, the release of bFGF from PVA-heparin hydrogels resulted in significant proliferation over PVA-heparin hydrogels, with a greater than 3.6-fold increase in cell proliferation compared to basal media (p < 0.05). Similarly, the dual release of bFGF and VEGF from PVA-heparin hydrogels resulted in 4.2-fold increase in cell proliferation over basal media conditions over 3 days. This proliferation response is not surprising given that HUVEC proliferation is known to be significantly higher in response to bFGF, as compared to VEGF. For example, at concentrations ranging from 5 to 1000 ng/mL, bFGF results in approximately 2.5-fold higher HUVEC proliferation as compared to 5–1000 ng/mL VEGF.22 StarPEG-heparin hydrogels that released bFGF and VEGF demonstrated that bFGF encapsulated at 1 µg/mL resulted in higher cell proliferation than VEGF at 1 µg/mL; however, they further demonstrated that the combination of the growth factors provided a synergistic effect on proliferation.8 The absence of a significant synergistic effect of the dual release of bFGF and VEGF from PVA-heparin hydrogels in this study may be due to the significantly lower growth factor concentrations encapsulated.22 Overall, these results further demonstrate that PVA-heparin hydrogels release bioactive bFGF and that the released growth factor results in proliferation of endothelial cells.
Endothelial cell migration in response to growth factor release
In addition to cellular proliferation, cellular migration is critical to wound healing and tissue regeneration applications. Angiogenesis involves the migration of endothelial cells to the site where new vessel formation is needed. Therefore, the ability of the angiogenic growth factors, bFGF and VEGF, released from PVA-heparin hydrogels to induce HUVEC motility was probed. When placed in solution with HUVECs, PVA-heparin hydrogels encourage a slight, 3%, outgrowth of cells and the cells appear to have a lower density than those in the presence of released growth factors (Figure 5). However, when the growth factors bFGF and VEGF were released separately from PVA-heparin hydrogels, there was significantly increased outgrowth of HUVECs with 9% and 8% outgrowth, respectively (p < 0.05). The independent release of these growth factors indicates several biological effects. First, it suggests that the heparin is bioactive and present at the correct saccharide length to induce cell migration in combination with bFGF and VEGF. This is important because studies have suggested that, depending on the size of heparin (e.g. oligosaccharides), heparin can suppress endothelial cell migration induced by bFGF and VEGF.47 Second, the independent release of bFGF and VEGF give an indication of the migration inducing capabilities of each growth factor. Different studies present inconsistent results as to whether bFGF or VEGF is the most potent initiator of endothelial cell motility.8,22,48 In this study, both bFGF and VEGF result in cellular migration; however, since proliferation was not blocked, the endothelial cell outgrowth due to bFGF may be in part due to proliferation, as seen in Figure 4. In this study, it was seen that VEGF was not a potent inducer of HUVEC proliferation at 24 h when compared to PVA-heparin gels alone (Figure 4), but VEGF did promote migration (Figure 5), a finding similar to others.22 The dual release of bFGF and VEGF had a synergistic impact on HUVEC outgrowth with significantly higher outgrowth than either growth factor alone (p < 0.05). Increased cell migration due to the synergistic effects of bFGF and VEGF has been also observed by others.8,49 These results demonstrate that PVA-heparin hydrogels can provide release of multiple growth factors and that both bFGF and VEGF are bioactive, resulting in cellular migration. Delivery of multiple heparin-binding growth factors from PVA-heparin hydrogels enables multiple effects on cellular behavior (e.g. proliferation and migration), as well as the ability to act synergistically (i.e. bFGF with VEGF enhances migration), increasing the potential of these PVA-heparin hydrogels for growth factor delivery for wound treatment and tissue regeneration.
Figure 5.
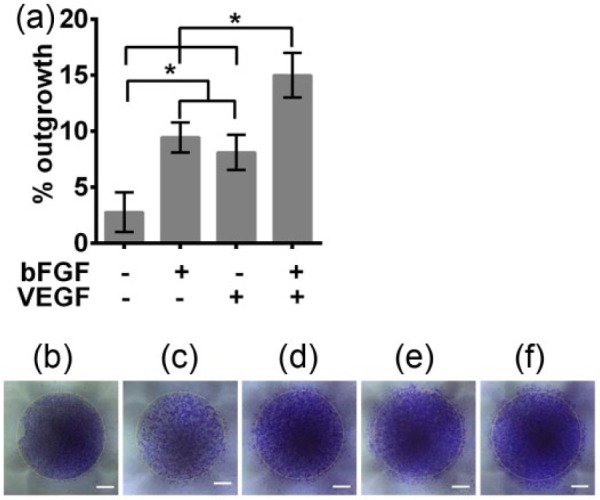
HUVEC migration in response to growth factor releasing hydrogels was monitored via an outgrowth assay. HUVECs were plated confluently within a fence and after fence removal transwells with PVA-heparin hydrogels containing no growth factor, 500 ng/mL bFGF, 500 ng/mL VEGF or 500 ng/mL bFGF, and 500 ng/mL VEGF were placed above the cells and the HUVECs were cultured for 24 h. Cells were stained with crystal violet and imaged and compared to the initial time point (0 h). (a) HUVEC outgrowth was quantified as a percent increase in area over 24 h. Significant differences are noted by an asterisk (*p < 0.05). (b) Initial confluent cell monolayer (0 h). Cell coverage after 24 h in the presence of PVA-heparin gels containing (c) no growth factor, (d) 500 ng/mL bFGF, (e) 500 ng/mL VEGF, or (f) 500 ng/mL bFGF and 500 ng/mL VEGF. Scale bars are 2 mm. Yellow dashed line indicate the initial cell coverage area.
Figure 4.
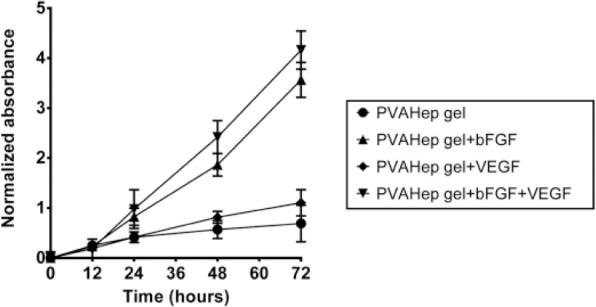
PVA-heparin hydrogels containing no growth factor, 500 ng/mL bFGF, 500 ng/mL VEGF or 500 ng/mL bFGF, and 500 ng/mL VEGF were placed in transwells above plated HUVECs and the HUVECs were cultured over 3 days. Absorbance was normalized to the negative control, basal media only, at each time point. HUVEC proliferation was quantified with an MTS assay.
Conclusion
A unique, ECM-inspired growth factor-releasing material platform based on hydrazone bonded PVA-heparin hydrogels was evaluated. These PVA-heparin hydrogels allowed for growth factor encapsulation under mild environmental conditions and provided controlled release of multiple growth factors which could aid in wound healing and tissue regeneration applications. The addition of heparin to PVA-based hydrogels altered the release profile of the growth factors, demonstrating that the crosslinked heparin was bioactive and could interact with the growth factors. PVA-heparin hydrogels had delayed growth factor release compared to PVA-only gels, as would be expected since heparin is a native ECM molecule known to bind growth factors. The hydrazone bonding approach for fabricating PVA-heparin gels enabled encapsulation of growth factors during the fabrication step, and the growth factors retain their bioactivity, as evidenced by cell responsiveness to released growth factors. Although native bFGF is known to have a plasma half-life on the order of minutes, bFGF released from PVA-heparin hydrogels remained bioactive for at least 3 days after encapsulation as demonstrated by BaF32 cell proliferation. PVA-heparin hydrogels also promoted endothelial cell proliferation and outgrowth in response to released bFGF and/or VEGF. Overall, this study demonstrates that hydrazone-bonded PVA-heparin hydrogels can provide for mild, in situ encapsulation of multiple growth factors and that those growth factors retain bioactivity for multiple days of release to encourage cellular proliferation and outgrowth. These heparin-containing hydrogels have widespread potential toward controlled, sustained drug delivery for applications such as wound healing and tissue regeneration.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors would like to acknowledge a Whitaker International Scholarship Grant and funding from a UNSW Early Career Researcher Grant awarded to J.J.R.
References
- 1. Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011; 8(55): 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishnamurthy R, Manning MC. The stability factor: importance in formulation development. Curr Pharm Biotechnol 2002; 3(4): 361–371. [DOI] [PubMed] [Google Scholar]
- 3. Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res 1989; 6(11): 903–918. [DOI] [PubMed] [Google Scholar]
- 4. Rice JJ, Martino MM, De Laporte L, et al. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater 2013; 2(1): 57–71. [DOI] [PubMed] [Google Scholar]
- 5. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005; 23(1): 47–55. [DOI] [PubMed] [Google Scholar]
- 6. Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl 2002; 41(3): 391–412. [DOI] [PubMed] [Google Scholar]
- 7. Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev 2007; 59(13): 1366–1381. [DOI] [PubMed] [Google Scholar]
- 8. Zieris A, Chwalek K, Prokoph S, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J Control Release 2011; 156(1): 28–36. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Oommen OP, Yan H, et al. Mild and efficient strategy for site-selective aldehyde modification of glycosaminoglycans: tailoring hydrogels with tunable release of growth factor. Biomacromolecules 2013; 14(7): 2427–2432. [DOI] [PubMed] [Google Scholar]
- 10. Mario Cheong GL, Lim KS, Jakubowicz A, et al. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater 2014; 10(3): 1216–1226. [DOI] [PubMed] [Google Scholar]
- 11. Zakrzewska M, Wiedlocha A, Szlachcic A, et al. Increased protein stability of FGF1 can compensate for its reduced affinity for heparin. J Biol Chem 2009; 284(37): 25388–25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slaughter BV, Khurshid SS, Fisher OZ, et al. Hydrogels in regenerative medicine. Adv Mater 2009; 21(32–33): 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zern BJ, Chu H, Wang Y. Control growth factor release using a self-assembled [polycation:heparin] complex. PLoS ONE 2010; 5(6): e11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KW, Yoon JJ, Lee JH, et al. Sustained release of vascular endothelial growth factor from calcium-induced alginate hydrogels reinforced by heparin and chitosan. Transplant Proc 2004; 36(8): 2464–2465. [DOI] [PubMed] [Google Scholar]
- 15. Bhakta G, Rai B, Lim ZX, et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 2012; 33(26): 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho YC, Mi FL, Sung HW, et al. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. Int J Pharm 2009; 376(1–2): 69–75. [DOI] [PubMed] [Google Scholar]
- 17. Jeon O, Powell C, Solorio LD, et al. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release 2011; 154(3): 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Cai S, Shu XZ, et al. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen 2007; 15(2): 245–251. [DOI] [PubMed] [Google Scholar]
- 19. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83(3): 835–870. [DOI] [PubMed] [Google Scholar]
- 20. Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014; 22(5): 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16(5): 585–601. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida A, Anand-Apte B, Zetter BR. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 1996; 13(1–2): 57–64. [DOI] [PubMed] [Google Scholar]
- 23. Pepper MS, Ferrara N, Orci L, et al. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 1992; 189(2): 824–831. [DOI] [PubMed] [Google Scholar]
- 24. Goto F, Goto K, Weindel K, et al. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest 1993; 69(5): 508–517. [PubMed] [Google Scholar]
- 25. Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater 2014; 10(4): 1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011; 8(5): 607–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zieris A, Prokoph S, Levental KR, et al. Sustainable growth factor delivery through affinity-based adsorption to starPEG-heparin hydrogels (Proteins at Interfaces III State of the Art. ACS Symposium Series. 1120). Washington, DC: American Chemical Society, 2012, pp. 525–541. [Google Scholar]
- 28. Roberts JJ, Naudiyal P, Juge L, et al. Tailoring stimuli responsiveness using dynamic covalent cross-linking of poly(vinyl alcohol)-heparin hydrogels for controlled cell and growth factor delivery. ACS Biomater Sci Eng 2015; 1(12): 1267–1277. [DOI] [PubMed] [Google Scholar]
- 29. McCall JD, Anseth KS. Thiol-ene photopolymerizations provide a facile method to encapsulate proteins and maintain their bioactivity. Biomacromolecules 2012; 13(8): 2410–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alves MH, Young CJ, Bozzetto K, et al. Degradable, click poly(vinyl alcohol) hydrogels: characterization of degradation and cellular compatibility. Biomed Mater 2012; 7(2): 024106. [DOI] [PubMed] [Google Scholar]
- 31. Ossipov DA, Brannvall K, Forsberg-Nilsson K, et al. Formation of the first injectable poly(vinyl alcohol) hydrogel by mixing of functional PVA precursors. J Appl Polym Sci 2007; 106(1): 60–70. [Google Scholar]
- 32. Ornitz DM, Xu JS, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem 1996; 271(25): 15292–15297. [DOI] [PubMed] [Google Scholar]
- 33. Ornitz DM, Yayon A, Flanagan JG, et al. Heparin is required for cell-free binding of basic fibroblast growth-factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol 1992; 12(1): 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jayson GC, Gallagher JT. Heparin oligosaccharides: inhibitors of the biological activity of bFGF on Caco-2 cells. Br J Cancer 1997; 75(1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao W, McCallum SA, Xiao Z, et al. Binding affinities of vascular endothelial growth factor (VEGF) for heparin-derived oligosaccharides. Biosci Rep 2012; 32(1): 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos A, Aw MS, Bariana M, et al. Drug-releasing implants: current progress, challenges and perspectives. J Mater Chem B 2014; 2(37): 6157–6182. [DOI] [PubMed] [Google Scholar]
- 37. Bryant SJ, Anseth KS. Photopolymerization of hydrogel scaffolds. Scaffolding in tissue engineering. Boca Raton, FL: CRC Press, 2005, pp. 71–90. [Google Scholar]
- 38. Baird A, Schubert D, Ling N, et al. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A 1988; 85(7): 2324–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stefanini MO, Wu FT, Mac Gabhann F, et al. A compartment model of VEGF distribution in blood, healthy and diseased tissues. BMC Syst Biol 2008; 2(1): 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dowd CJ, Cooney CL, Nugent MA. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. J Biol Chem 1999; 274(8): 5236–5244. [DOI] [PubMed] [Google Scholar]
- 41. Harmer NJ. Chapter 14. Role of heparan sulfate in fibroblast growth factor signaling. In: Hales HG, Linhardt RJ, Hales CA. (eds) Chemistry and biology of heparin and heparan sulfate. Amsterdam: Elsevier Science, 2005, pp. 399–434. [Google Scholar]
- 42. Nguyen TH, Kim SH, Decker CG, et al. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat Chem 2013; 5(3): 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Damon DH, Lobb RR, D’Amore PA, et al. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J Cell Physiol 1989; 138(2): 221–226. [DOI] [PubMed] [Google Scholar]
- 44. Oommen OP, Wang SJ, Kisiel M, et al. Smart design of stable extracellular matrix mimetic hydrogel: synthesis, characterization, and in vitro and in vivo evaluation for tissue engineering. Adv Funct Mater 2013; 23(10): 1273–1280. [Google Scholar]
- 45. Nilasaroya A, Poole-Warren LA, Whitelock JM, et al. Structural and functional characterisation of poly(vinyl alcohol) and heparin hydrogels. Biomaterials 2008; 29(35): 4658–4664. [DOI] [PubMed] [Google Scholar]
- 46. He SH, Zhang J, Qi X, et al. Neuregulin protects human umbilical vein endothelial cell via activating CD98hc through MAPK pathway. Int J Clin Exp Med 2015; 8(5): 6702–6712. [PMC free article] [PubMed] [Google Scholar]
- 47. Cole CL, Hansen SU, Barath M, et al. Synthetic heparan sulfate oligosaccharides inhibit endothelial cell functions essential for angiogenesis. PLoS ONE 2010; 5(7): e11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donohue PJ, Richards CM, Brown SAN, et al. TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol 2003; 23(4): 594–600. [DOI] [PubMed] [Google Scholar]
- 49. Yan Q, Li Y, Hendrickson A, et al. Regulation of retinal capillary cells by basic fibroblast growth factor, vascular endothelial growth factor, and hypoxia. In Vitro Cell Dev Biol Anim 2001; 37(1): 45–49. [DOI] [PubMed] [Google Scholar]