Abstract
Background
In the Federated States of Micronesia (FSM) and then the Republic of the Marshall Islands (RMI), levofloxacin pharmacokinetics (PK) were studied in children receiving directly observed once-daily regimens (10 mg/kg, age >5 years; 15–20 mg/kg, age ≤5 years) for either multidrug-resistant tuberculosis (MDR TB) disease or latent infection after MDR TB exposure, to inform future dosing strategies.
Methods
Blood samples were collected at 0 (RMI only), 1, 2, and 6 hours (50 children, aged 6 months to 15 years) after oral levofloxacin at >6 weeks of treatment. Clinical characteristics and levofloxacin Cmax, elimination half-life (t1/2), and area under the curve from 0 to 24 hours (AUC0–24 hours * µg/mL) were correlated to determine optimal dosage and to examine associations. Population PK and target attainment were modeled. With results from FSM, dosages were increased in RMI toward the target maximal drug concentration (Cmax) for Mycobacterium tuberculosis, 8–12 µg/ml.
Results
Cmax correlated linearly with per-weight dosage. Neither Cmax nor t1/2 was associated with gender, age, body mass index, concurrent medications, or pre-dose meals. At levofloxacin dosage of 15–20 mg/kg, Cmax ≥ 8 µg/ml was observed, and modeling corroborated a high target attainment across the ratio of the area under the free-concentration-versus-time curve to minimum inhibitory concentration (fAUCss,0–24/MIC) values.
Conclusions
Levofloxacin dosage should be 15–20 mg/kg for Cmax ≥ 8 µg/ml and a high target attainment across fAUCss,0–24/MIC values in children ≥2 years of age.
Keywords: tuberculosis, multidrug-resistant, pharmacokinetics, levofloxacin, children
Introduction
Levofloxacin is a broad-spectrum, fluoroquinolone antibacterial agent [1,2] with activity against Mycobacterium tuberculosis in vitro and in humans [3]. It is a well-tolerated, potentially effective drug for treating latent tuberculosis infection (LTBI) after exposure to multidrug-resistant tuberculosis (MDR TB), defined as TB that is resistant to both isoniazid and rifampin, the two most potent first-line TB drugs [4].
Definitive TB pharmacokinetic / pharmacodynamic (PK/PD) targets are lacking for levofloxacin. In a murine TB model, the ratio of the area under the free-concentration-versus-time curve to minimum inhibitory concentration (fAUC/MIC) predicted efficacy for moxifloxacin, ofloxacin and sparfloxacin [5]. Most adult TB patients who received 1000 mg levofloxacin had fAUC/MIC > 125, a typical target for Gram-negative bacilli [3].
Levofloxacin undergoes renal clearance [6]. The PK profile of levofloxacin has not been well described following chronic administration in children. One study reported that body weight (WT)-normalized clearance in children <5 years was nearly twice as fast as that in adults [7]; those authors recommended levofloxacin 10 mg/kg once daily for children ≥5 years, and 20 mg/kg divided into twice daily doses for children 6 months to <5 years. A recent study showed that serum concentrations in children, following oral doses of levofloxacin (15 mg/kg), were lower than expected, possibly because of faster elimination than in adults [8]. For adults, levofloxacin doses of 750 to 1000 mg once daily yield the target maximal drug concentration (Cmax), 8–12 µg/ml [3,5] and, on the basis of this, the same target has been suggested for children.
During outbreaks of MDR TB, first in Chuuk, Federated States of Micronesia (FSM) [9], and then in Majuro, the Republic of the Marshall Islands (RMI), 50 children received levofloxacin-based regimens for MDR TB disease or LTBI that was presumed to be MDR. In FSM, plasma concentrations were measured at the end of a 1-year regimen of levofloxacin as monotherapy or in combination with ethambutol (depending on drug susceptibility results for index patients) for LTBI or with multiple medications for MDR TB disease. These findings were used to adjust the levofloxacin dosage in RMI children who were receiving levofloxacin and ethambutol for LTBI after exposure to MDR TB. Levofloxacin PK data from both groups of children were analysed to inform future pediatric dosing strategies.
Materials and Methods
Ethics and human subjects protections
Ethics approval was obtained from the Institutional Review Board (IRB) at the Centers for Disease Control and Prevention (CDC) and from the IRB of the RMI Ministry of Health (MOH). The FSM MOH approved the protocol and relied on the CDC IRB. Pediatric assent with parental consent was obtained for children at least 7 years old, and only parental consent was obtained for younger children.
Study design
Patients, treatment, and specimen collection
In FSM, at the time of testing, 33 children aged 0·5 to 15 years (median 8 years; 16 girls, 17 boys) were receiving 5–20 (median 8·7) mg/kg levofloxacin once daily as an oral solution (25mg/ml) [7], either as treatment for MDR TB (n=8) or for presumed MDR LTBI (n=25) as household contacts to infectious MDR TB patients with tuberculin skin test > 5 mm; all participated in this study. Ten of 33 (30%) children were ≤5 years at treatment initiation. The daily dosages at the start of treatment were as recommended (10 mg/kg, age >5 years; 15–20 mg/kg, age ≤5 years), but WT gain during treatment, without dose recalculation, lessened the per-WT amounts when the study was performed. Dosages were not decreased to 10 mg/kg from 15–20 mg/kg daily for three children who had their sixth birthday during treatment. The eight with MDR TB disease received multiple medications in addition to levofloxacin; of those with LTBI, 8 (24%) also received ethambutol 11–37 (median 17) mg/kg with the levofloxacin. After directly observed therapy (DOT) for 1 year, blood specimens were collected into heparinized tubes at 1, 2, and 6 hours after the dose of levofloxacin, 1–3 hours after breakfast at home.
In a separate MDR TB outbreak in RMI, the FSM findings were used to adjust levofloxacin dosing for children ≥5 years to 12 mg/kg, and the doses for all children were recalculated quarterly for WT changes. Of 17 participating children, age 1–15 years (median 11 years; 8 girls, 9 boys) being treated for presumed MDR LTBI as household contacts to infectious MDR TB patients with tuberculin skin test > 5 mm, 3 (18%) were ≤5 years at treatment initiation. All 17 had been receiving 6 weeks–6 months of a 1-year regimen of levofloxacin 11–16 (median 12) mg/kg, once daily as an oral solution (25mg/ml) [7]. None had TB disease; all the children also received ethambutol 15 mg/kg daily, at the same time that they received levofloxacin. After DOT for >6 weeks, blood specimens were collected into heparinized tubes at 0, 1, 2, and 6 hours after the dose of levofloxacin, which was given at the study site 1–3 hours after a breakfast at home.
Blood specimens at both sites were centrifuged at a speed of 6000 rpm for 5 minutes, and the plasma was collected, immediately for storage at −70°C and shipment on dry ice to the University of Florida (Gainesville, FL, USA), where levofloxacin concentrations were measured by a high-pressure liquid chromatography assay [3]. All children at both sites received levofloxacin as an oral solution; the potency was verified for two vials randomly picked from stock at each site (verified by the University of Florida laboratory).
Data sources were clinic medical records covering the entire treatment regimens, including the periods after blood collection, data gathered specifically for this study during patient encounters, and logbooks maintained by clinic and study staff. Data verification was done on all observations and variables. All data were imported for statistical analysis into SAS, version 9·2 (SAS Institute Inc., Cary, NC, USA).
Pharmacokinetic and statistical analyses
i) Noncompartmental analysis
A noncompartmental PK analysis was performed using the software WinNonlin (Pharsight Corporation Version 5·3, St. Louis, MO, USA). Estimates were generated for (abbreviation, unit): first-order elimination rate constant (Lambda_Z, hours−1), volume of distribution (V/F, L/kg), clearance (CL/F, L/hour/kg), elimination half-life (t1/2, hours), maximal drug concentration (Cmax, µg/mL), time at maximal drug concentration (Tmax, hours), and area under the time concentration curve from 0 to 6 hours (AUC0–6, hours * µg/mL), calculated using the linear trapezoidal rule [10]. F denotes the levofloxacin’s bioavailability, which is unknown because only oral administration was studied.
Demographic and clinical factors including age, gender, body mass index (BMI), active disease or LTBI status, pre-dose food intake, complete drug regimen, and PK parameters were tested by univariate or linear regression analysis for correlation with levofloxacin Cmax, t1/2 and AUC0–6. HIV co-infection was not included in the model as no patients were HIV-infected.
ii) Population PK analysis
A population PK analysis including data from all children was performed using the software nonlinear mixed effects modeling (NONMEM, Version 7·2, Icon Development Solutions, Ellicott City, Maryland, USA). NONMEM execution, run management, bootstrapping and generation of prediction-corrected visual predictive checks (pcVPC) were performed using Perl speaks NONMEM (PsN) and Pirana [11, 12, 13]. Goodness of fit and pcVPC plots were generated using the R (Version 2·15·2) packages lattice and Xpose (Version 4·3·3) [14, 15]. The first-order conditional estimation method (FOCE) with interaction was used. Model development was guided using goodness of fit plots, visual predictive checks, plausibility of parameter estimates, as well as objective function and shrinkage values.
One- and two-compartment PK models were tested. For a one-compartment model, the population PK parameters estimated included the first-order absorption rate constant (KA, hours−1), clearance (CL/F, L/h), and volume of distribution (V/F, L). Initially no covariates were accounted for in the model, but were later tested for inclusion in an effort to explain (at least) part of the inter-individual variability. To account for size-based differences using WT, an allometric model with the exponent fixed to 0·75 and 1 was applied for CL/F and V/F, respectively, both using a 70 kg standardized WT. Thus, the population parameters CL/F and V/F in the case of a 70 kg patient can be related to CL/FGRP and V/FGRP, the group estimates where all subjects with the same WT have the same parameter value (Equation 1 and 2).
| (1) |
| (2) |
WTi represents the individual WT of each patient. Inter-individual variability was estimated for each PK parameter using an exponential relationship. A random variable, denoted as η, was normally distributed with mean zero and variance ω2. Thus, an individual PK parameter estimate can be estimated by relating the group population parameter value and accounting for inter-individual variability (Equation 3).
| (3) |
Pij denotes the estimate of parameter j in the ith individual, θGRP,j is the group value for parameter j, and ηij denotes the deviation from the group value for parameter j in the ith individual. To estimate residual error, a combined additive and proportional residual model was applied. In addition to accounting for size-based differences in PK parameters, a maturation function accounting for age-dependent changes in drug clearance also was tested for inclusion [16].
Additions of covariates to the PK model were tested using generalized additive modeling (GAM) implemented in the Xpose R package. Covariates available for all subjects included post-natal age, gender, WT, height, and breakfast status. For nested models, a p-value of 0·05 was used to assess statistical significance. Using the final model, one thousand bootstrap runs were performed, and the 2·5th, median, and 97·5th percentiles were calculated based on all samples generated.
Data from all 50 children (33 FSM, 17 RMI) were included in the analysis. With the exception of two 0-hour samples, all concentrations were greater than the assay quantification limit (0·2 µg/mL). For the two samples, the concentrations were approximately half the cutoff limit, and we retained them in the analysis.
iii) Dosing simulations and target attainment analysis
The final population PK model was used to perform simulations that can help inform dosing recommendations. Briefly, four dosing scenarios were considered: 5, 10, 15 and 20 mg/kg administered daily. For each dose, the dataset utilized in the model development phase was used to generate simulated PK measurements for an additional 200 replicate datasets (i.e., 50 subjects/dataset*200 datasets = 10,000 virtual subjects); whereby additional measurements were simulated for the 12, 18 and 24 hour time points. The area under the free concentration versus time curve from 0 to 24 hours at steady state (fAUCss,0–24) was calculated using the trapezoidal rule and an unbound fraction of 0·75 [17]. Box and whisker plots were generated in an effort to relate pediatric and adult levofloxacin exposure following multiple dosing. Estimates of median adult exposure were obtained from U.S. Food and Drug Administration clinical pharmacology review documents (http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM252889.pdf). Next, a target attainment analysis was performed whereby plausible MIC values for M. tuberculosis (0·25, 0·5, 1, and 2 µg/mL) were related to drug exposure at steady state (fAUCss,0–24/MIC). Since a validated target is not available for this population, target attainment was evaluated across a range of target values (40, 80, 100, and 125).
Results
Patient characteristics, treatment doses, and noncompartmental pharmacokinetics
The levofloxacin-based treatment regimens lasted 18 months for MDR TB and 12 months for MDR LTBI, and all 50 patients completed treatment without side effects necessitating interruptions or discontinuation. Twenty-five of 33 (76%) of FSM patients had WT recorded after treatment was started; WT increased a median of 4 kg (range −1 to 13) during the year as illustrated in Supplemental Digital Content 1 (Figure). Levofloxacin dosages had not been adjusted for WT change for any of these participants, and the day of blood collection coincided with the final days of treatment for all 33.
Descriptive data on study participants are presented in Tables 1 and Supplemental Digital Content 2 (table). In both FSM and RMI, there was representation of children ≤5 years to 17 years of age (Table 1). In both island groups, gender distribution (males:females) of participants was similar (Table 1). The majority (84%) of participants reported food intake on the morning of plasma specimen collection (Table 1). Forty-eight had WT and height measurements, and BMI was normal for 34 of 48 (71%), with 11 of 48 (23%) being overweight or obese and two of 48 (4%) being underweight (Supplemental Digital Content 2, table).
Table 1.
Characteristics of study participants at time of blood specimen collection, Federated States of Micronesia (FSM), and Republic of the Marshall Islands (RMI), 2009–2011.
| FSM (N=33) | RMI (N=17) | Total (N=50) | |
|---|---|---|---|
| Characteristic | |||
| Age (years), mean±SD | 8·5 ± 3·7 | 11·0 ± 4·8 | 9·3 ± 4·2 |
| ≤ 5 years, n (%) | 10 (30) | 3 (18) | 13 (26) |
| 5 – <11 years, n (%) | 12 (36) | 4 (24) | 16 (32) |
| 11 – 17 years, n (%) | 11 (34) | 10 (59) | 21 (42) |
| Weight (kg), mean±SD | 26·2 ± 9·2 | 34·9 ± 17·2 | 29·2 ± 13·1 |
| Height (m), mean±SD | 1·18 ± 0·20 | 1·31 ± 0·28 | 1·23 ± 0·24 |
| Gender, n (%) | |||
| Male | 17 (52) | 9 (53) | 26 (52) |
| Female | 16 (49) | 8 (47) | 24 (48) |
| Food intake 1–3 hours before, n (%) | |||
| Yes | 29 (91) | 12 (71) | 41 (84) |
| No | 3 (9) | 5 (29) | 8 (16) |
| Participant status, n (%) | |||
| Case | 8 (24) | 0 (0) | 8 (16) |
| Contact (all) | 25 (76) | 17 (100) | 42 (84) |
| Drug regimen, n (%) | |||
| Levofloxacin | 17 (52) | 0 (0) | 17 (35) |
| Levofloxacin/Ethambutol | 8 (24) | 17 (100) | 32 (65) |
| MDR regimen | 8 (24) | 0 (0) | 8 (24) |
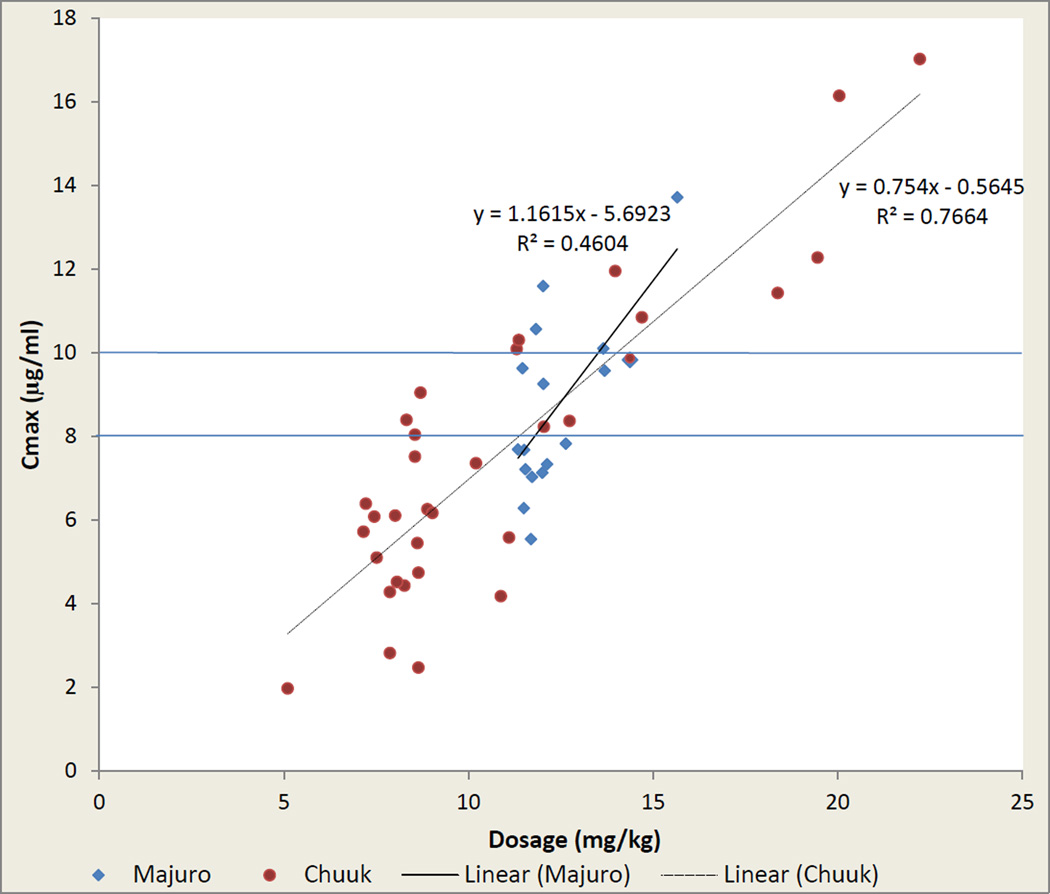
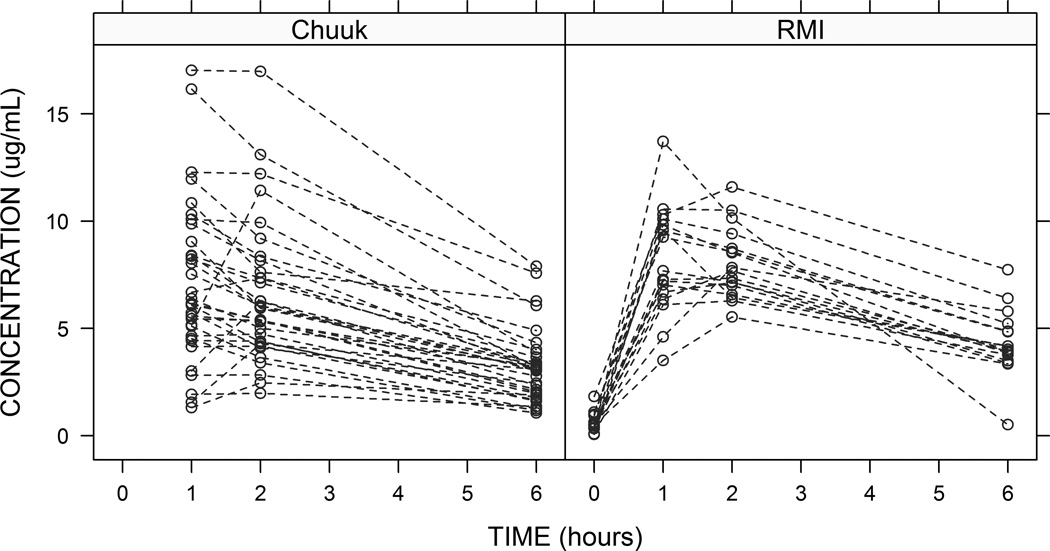
For the 23 FSM children >5 years at treatment initiation, median Cmax was 6.04 µg/ml (range 1·97–10·31) from a median dosage of 8·55 mg/kg (range 5·10–12·73). For 14 RMI children >5 years, clinicians increased the dosage to 12 mg/kg and recalculated for WT gain one week prior to blood sample collection; this resulted in median dosages of 11·76 mg/kg (range 11·33–13·65) and a median Cmax of 7·69 µg/ml (range 5·54–11·59). For 11 of 13 children ≤5 years at both sites who received ≥12 mg/kg, Cmax was ≥8·0 µg/ml (Figure 1). Cmax correlated linearly with per-WT dosage (R2=0·72), and the per-patient variance from the linear relationship was not explained by gender, age, BMI, concomitant medication administration, or prior food intake. T1/2 did not correlate with age (R2=0·03) or other demographic factors. Cmax was unaffected by ethambutol intake at both sites (Supplemental Digital Content 3, table). Levofloxacin concentration-versus-time plots stratified by study site are shown in Figure 2. A non-compartmental analysis was performed and the results were stratified by age (Table 2). Only three children were <2 years. Findings were largely consistent across the age groups, particularly for children ≥2 years.
FIGURE 1.
Maximal levofloxacin concentration (Cmax, µg/mL) as a function of drug dosage in children treated with levofloxacin for ≥ 6 weeks, Federated States of Micronesia (FSM; n=33) and Republic of the Marshall Islands (RMI; n=17). Regression lines from least-squares (see Supplemental Digital Content).
FIGURE 2.
Levofloxacin concentration over time, by study site, Federated States of Micronesia (FSM; n=33) and Republic of the Marshall Islands (RMI; n=17).
TABLE 2.
Levofloxacin noncompartmental pharmacokinetic parameters in 50 study participants1, Federated States of Micronesia (FSM), and Republic of the Marshall Islands (RMI), 2009–2011.
| AGE (years) |
n | LAMBDA_Z (hours−1) |
V/F (liters/kg) |
CL/F (liters/hour/kg) |
Half-life (hours) |
CMAX (µg/ml) |
TMAX (hours) |
AUC0–6 (hours*µg/ml) |
|---|---|---|---|---|---|---|---|---|
| 0·5 – <2 | 3 | 0·39 (0·31) | 0·74 (0·46) | 0·26 (0·15) | 2·46 (1·38) | 13·54 (3·58) | 1 (0) | 52·47 (23·18) |
| 2 – <5 | 7 | 0·19 (0·05) | 1·24 (0·43) | 0·24 (0·11) | 3·83 (1·12) | 10·58 (3·62) | 1·14 (0·38) | 44·79 (15·20) |
| 5 – <10 | 18 | 0·18 (0·07) | 1·49 (0·73) | 0·23 (0·07) | 4·89 (3·08) | 6·77 (2·69) | 1·44 (0·51) | 28·18 (10·22) |
| 10 – <12 | 5 | 0·18 (0·05) | 1·36 (0·30) | 0·25 (0·12) | 4·03 (0·89) | 6·95 (1·97) | 1·2 (0·45) | 29·37 (9·25) |
| 12 – <17 | 17 | 0·14 (0·05) | 1·38 (0·44) | 0·18 (0·06) | 5·45 (1·69) | 7·41 (2·17) | 1·35 (0·49) | 32·17 (10·90) |
Parameter estimates are shown as mean (standard deviation). The upper bound for each age group is inclusive.
Population pharmacokinetics and simulation studies
A one compartment PK model described the data well (Table 3). Of the available covariates, only WT was included in the final model. A maturation function accounting for age-dependent changes in clearance did not improve the data fit. Population estimates for KA, CL/F, and V/F (the latter two parameters for a 70-kg standardized WT) were 2·69 hours−1, 11·61 liters/hour, and 88.39 liters, respectively. Inter-individual variability (%CV) was estimated as 82·46%, 33·17%, and 24·49% for KA, CL/F, and V/F, respectively. Goodness of fit plots and a visual predictive check are shown in Supplemental Digital Content 4, 5, and 6 (figures)
TABLE 3.
Population parameter estimates from the final pharmacokinetic model and a bootstrap analysis (n=1,000).
| Parameter Estimate |
RSE (%) | Bootstrap (n=1,000) Median (95% CI)a |
||
|---|---|---|---|---|
| Structural Model | ||||
| KA (h−1) | 2·69 | 22 | 2·62 (1·89–5·31) | |
| CL/F (L/h)b | 11·61 | 5 | 11·61 (10·34–12·82) | |
| V/F (L)b | 88·39 | 4 | 87·77 (80·8–95·26) | |
| Inter-individual Variability (%CV) | ||||
| ω (KA) | 82·46 | 42 | 79·93 (46·9–119·5) | |
| ω (CL/F) | 33·17 | 28 | 32·25 (22·36–41·47) | |
| ω (V/F) | 24·49 | 50 | 23·23 (10–33·76) | |
| Residual Error | ||||
| Proportional (%) | 12 | 38 | 11·83 (4·69–16·73) | |
| Additive (µg/mL) | 0·29 | 56 | 0·29 (0·01–0·53) | |
RSE, Relative standard error; CI, Confidence interval
Bootstrap: median (2·5–97·5 percentile)
CL/F and V/F were scaled using a 70 kg standardized weight.
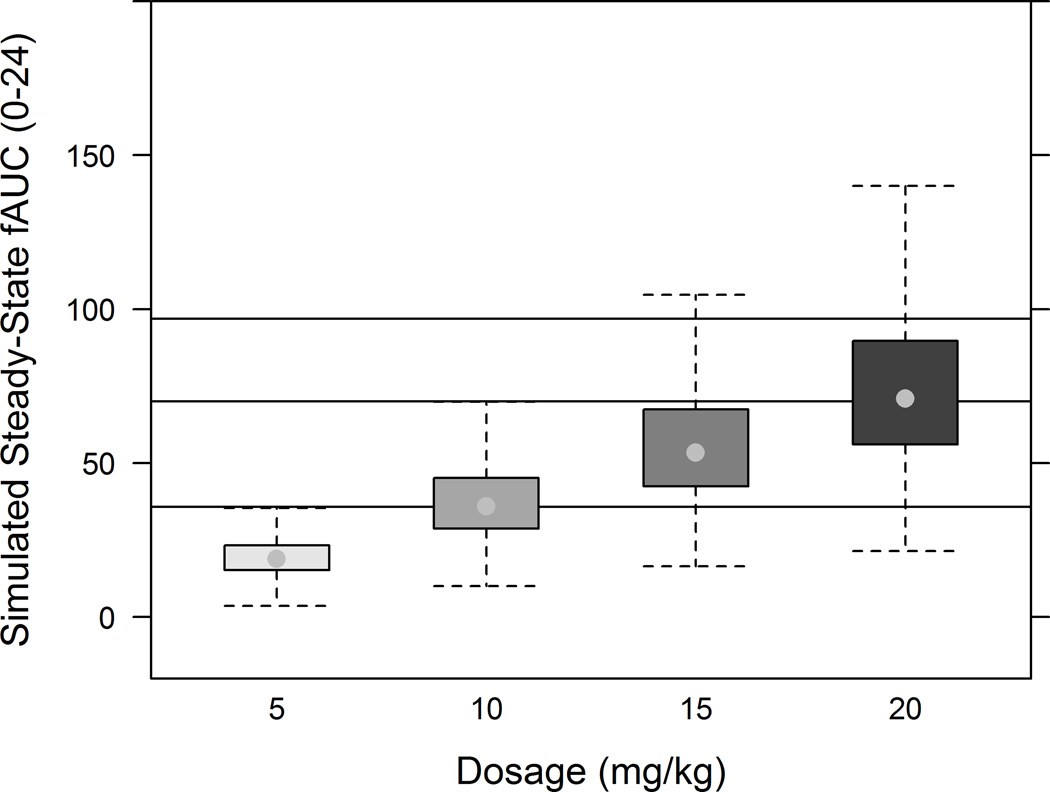
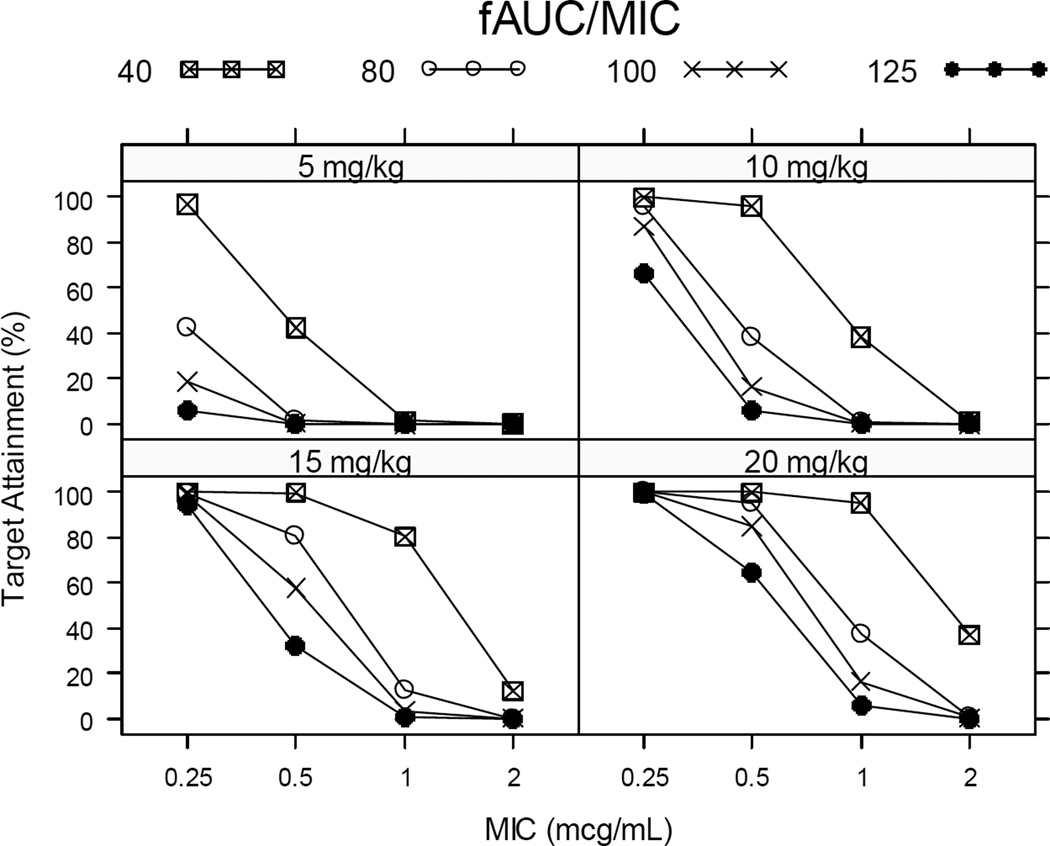
Utilizing the final population PK model (Figure 3), median free drug exposure, i.e., area under the free concentration versus time curve 0–24 hours at steady state (fAUCss,0–24), for simulated pediatric data following 10 and 20 mg/kg administered once daily closely approximated reported median steady-state adult exposure following 500 and 750 mg levofloxacin doses [18]. A dose of 5 mg/kg/day resulted in suboptimal exposure. Using simulated pediatric exposure and typical minimal inhibitory concentrations (MIC), fAUCss,0–24/MIC was calculated, and target attainment was related for various potential target values (Figure 4). In general, a 5 mg/kg dose resulted in poor target attainment regardless of the target. With the exception of a fAUCss,0–24/MIC target of 40, for MIC values ≥ 0·5 µg/mL, a poor, albeit dose-dependent, target attainment was observed across doses. For MIC < 0·5 µg/mL, a 15 mg/kg dose administered once daily achieves a high target attainment for a fAUCss,0–24/MIC target of 40. For a MIC 0·5 µg/mL, a 20 mg/kg dose administered once daily achieves a high target attainment across fAUCss,0–24/MIC target values.
FIGURE 3.
Area under the concentration versus time curve for the unbound drug obtained at steady state (fAUCss,0–24) following for various daily doses of levofloxacin (5, 10, 15, and 20 mg/kg). The lower, middle, and upper solid lines represent the median fAUCss,0–24 reported for adults following 500 mg once daily dosing, 750 mg once daily, and 1,000 mg once daily, respectively. An estimate for the 1000 mg daily dose was obtained from Peloquin et al. (1), while the estimates for 500 mg and 750 mg daily doses were obtained from clinical review documents [3 and 18]. A fraction unbound of 0.75 was assumed to calculate unbound exposure.
FIGURE 4.
Target attainment analysis performed by applying the developed PK model to simulate 10,000 virtual children (50 children/dataset * 200 simulations) and calculate likelihood of attaining various fAUC/MIC targets (40, 80, 100 or 125) following steady state dosing of levofloxacin (5, 10, 15 or 20 mg/kg).
Discussion
In the context of two MDR TB outbreaks, 50 children received levofloxacin-based regimens for MDR TB or MDR LTBI, and analyses of PK data derived from plasma specimens showed that (1) 15–20 mg/kg dosing daily may be required to achieve a Cmax of ≥ 8 µg/ml in children ≥2 years, (2) children aged 2 to 5 years might not need a different dosage or dosage schedule, because the half-life of elimination was similar to that of older children, (3) additional studies are needed in children <2 years to clarify the developmental PK(/PD) in this age group, and (4) in pediatric patients, WT should be checked every 3 months for dose recalculations. Our results differ from two other studies suggesting that children achieve lower plasma levofloxacin concentrations compared to adults using the same dosage structure [7,8]. The differences may reflect our study population and the drug formulation (i.e., oral suspension in our study).
Treatment of 32 adult MDR TB contacts in FSM with 400 mg daily of moxifloxacin necessitated obtaining plasma concentrations to confirm adequate dosing (data not shown); these efforts revealed similar plasma concentrations of moxifloxacin to those shown in other adult patient populations [6], suggesting that the metabolism of fluoroquinolones in Micronesians is similar to that in other patients. In this study, the lack of significant gender-based differences in levofloxacin PK was consistent with reports elsewhere [6]. A limitation of the study is that the data included only three children <2 years, and thus were too sparse to be definitive for that age group.
Due to their safety and bactericidal activity against M. tuberculosis, fluoroquinolones have become a preferred treatment for MDR LTBI in adults [19], despite the absence of clinical trials of their use for MDR-TB treatment. In a systematic review of levofloxacin used as treatment for LTBI [20], six studies with a total of eight study arms were included, and no severe adverse events were reported. The literature on long-term use of fluoroquinolones in children is sparse, hampered in part by concern of risk of injury to the musculoskeletal system and other serious adverse reactions [21–23]; many children have been treated with fluoroquinolones for short durations without arthropathy or bone abnormalities [24, 25]. Case reports of children treated with fluoroquinolones for >6 months have found no specific toxicity of these agents that is unique to children and no evidence of cartilage effects [24], and rates of reversible arthralgia have been similar to those in adults [24, 25]. In the data presented here, all of the 50 children received levofloxacin for at least a year without interruptions for severe adverse events or discontinuation in FSM [9] and RMI.
Estimates for clearance and volume of distribution obtained for the non-compartmental analysis were reasonably close to those previously reported following administration of a single 7 mg/kg dose to children ages 0·5 to 16 years, in which a two-fold difference in clearance was noted between children <5 years and adults [7]. Differences of this magnitude in clearance were not noted between younger and older children in our analyses, potentially due to the limited number of children 0·5 to 2 years of age. Typically, 90% of the adult glomerular filtration rate is attained by 1 year of age. With a larger sample size in this age range, the maturation in renal clearance, and therefore in levofloxacin clearance, might have been apparent [26].
Two publications have used PK data in children to make dosage recommendations that would match levofloxacin exposures observed in adults following administration of 500 mg once daily. In one, children < 5 years cleared levofloxacin nearly twice as fast as adults and, as a result, have a total systemic exposure approximately half that of adults; hence, to provide similar levofloxacin exposures, children ≥ 5 years would need a daily dose of 10 mg/kg, while younger children should receive 10 mg/kg every 12 hours [7]. In the other, when pharmacometric analyses were used to make recommendations for treatment of post-exposure inhalational anthrax in children, a once daily dose of 15 mg/kg resulted in levofloxacin exposures comparable to those seen in adults, but Cmax at steady state was higher than that reported in adults; as a result, 8 mg/kg taken twice daily was recommended [27].
Simulations were performed in an effort to relate pediatric and adult levofloxacin exposure at steady-state, with once daily regimens for convenience of directly observed therapy. Median simulated exposure following daily 10 and 20 mg/kg dosages matched the exposure observed in adults following 500 and 750 mg doses, respectively. A dosage of 15 mg/kg may be preferred to match a 500 mg adult dose, as fewer children would have less than the median exposure observed in adults. If higher drug exposures are needed, then 20 mg/kg should be considered. This sample included few children <2 years, and thus changes in clearance with age were unlikely to be captured.
Target attainment analyses were performed using simulated pediatric exposure, with multiple targets, as no validated drug exposure target exists for this population. Some have suggested that when target attainment rates fall below 90%, the probability of antimicrobial drug efficacy is reduced [28]. In our simulations, for the lower levofloxacin dose (5 mg/kg), regardless of the target selected, poor target attainment was predicted. For MIC values <0·5 µg/mL, high target attainment was observed when a fAUCss,0–24/MIC of 40 was sought with 15 mg/kg dosage. The wild-type in vitro MIC of levofloxacin is 0·125–0·5 µg/mL, suggesting that the cutoff for levofloxacin susceptibility could be MIC ≤0·5 µg/mL. These MIC data have correlated with BACTEC 960 MGIT and BACTEC 460 results [29]. Our data support once daily levofloxacin 15 mg/kg for children. However, for higher MIC values (≥0·5 µg/mL), high target attainment was observed for 20 mg/kg and a fAUCss,0–24/MIC greater than 40. For MIC 0·5 µg/mL, once daily 20 mg/kg would be preferred across target values. These analyses underscore the need for investigating appropriate targets for PK/PD indices that can be used to optimize levofloxacin dosing.
In these outbreaks, lack of TB disease among all 42 levofloxacin-treated pediatric patients with LTBI who were followed for at least 2 years after treatment completion, and the development of active TB disease in 3 of 15 untreated children with LTBI [9], suggest that these empirical levofloxacin regimens may prevent progression to MDR TB. Experience with more patients, with long-term follow-up, will be essential to prove effectiveness of MDR LTBI regimens, but based on the experience in FSM [9] and RMI, levofloxacin-based MDR LTBI treatment is adequately tolerated, potentially effective, and feasible even in a resource-poor setting.
To achieve a target Cmax ≥ 8 µg/ml in children, revision of the current dosage recommendations for levofloxacin should be considered; 15–20 mg/kg of levofloxacin once per day appears to be indicated, at least for children ≥2 years of age. Based on modeling, for MIC < 0·5 µg/mL, high target attainment was achieved with 15 mg/kg daily dosing. For MIC ≥ 0·5 µg/mL, 20 mg/kg daily should be considered. Further clinical research is needed to evaluate appropriate targets for PK/PD indices that can be used to optimize drug dosing and to refine dosage recommendations for children <2 years.
Supplementary Material
Acknowledgments
The authors acknowledge and thank the patients of FSM and RMI and their parents, the clinicians, DOT workers, and administrative staff of the Chuuk State TB Program and the RMI National TB Program, and the support of FSM and RMI national governments. Many experts have contributed to this study: we would like to particularly acknowledge Krista Powell, Risa Bukbuk, Andrew Heetderks, Roylinne Wada, Kasian Otoko, Matthew Bankowski, Mayleen Ekiek, Ann Buff, Maryam Haddad, Kinisalote Tikomaidraubuta, Kashef Ijaz and Amy Sandul.
Financial Support: Centers for Disease Control and Prevention. National Institute of General Medical Sciences; D.G. is funded by training grant T32GM086330 from the National Institute of General Medical Sciences.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose
Disclaimer
The opinions expressed by authors contributing to this journal do not necessarily represent the official position of the Centers for Disease Control and Prevention or the other respective institutions.
SDC 1. Observed WT changes during 1-year treatment among study participants for whom WTs were recorded FSM
SDC 2. BMI percentiles of study participants1 at time of plasma specimen collection, by gender, Federated States of Micronesia and Republic of the Marshall Islands, 2009–2011.
SDC 3. Levofloxacin pharmacokinetic parameters for study participants with MDR LTBI on levofloxacin and ethambutol or levofloxacin alone, Federated States of Micronesia (FSM), and Republic of the Marshall Islands (RMI), 2009–2011.
SDC 4. Observations versus individual and population predictions
SDC 5. Conditional weighted residuals (CWRES) versus time
SDC 6. Prediction-corrected visual predictive check
1 Two of 50 patients did not have height and weight measurements performed
Contributor Information
Sundari R. Mase, Email: fyy0@cdc.gov.
John A. Jereb, Email: jxj4@cdc.gov.
Daniel Gonzalez, Email: gonzald@email.unc.edu.
Fatma Martin, Email: haneefmartin@hotmail.com.
Charles L. Daley, Email: DaleyC@NJHealth.org.
Dorina Fred, Email: dfred@fsmhealth.fm.
Ann Loeffler, Email: ALoeffle@LHS.ORG.
Lakshmy Menon, Email: lakshmy.menon@gmail.com.
Sapna Bamrah Morris, Email: feu3@cdc.gov.
Richard Brostrom, Email: hld4@cdc.gov.
Terence Chorba, Email: tlc2@cdc.gov.
Charles A. Peloquin, Email: peloquin@cop.ufl.edu.
References
- 1.Liu H, Mulholland SG. Appropriate antibiotic treatment of genitourinary infections in hospitalized patients. Am J Med. 2005;118(Suppl 7A):14S20S. doi: 10.1016/j.amjmed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall C, Guglielmo BJ, Maselli J, Gonzales R. Antimicrobial drug prescribing for pneumonia in ambulatory care. Emerging Infect Dis. 2005;11:380–384. doi: 10.3201/eid1103.040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peloquin CA, Hadad DJ, Molino LP, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC) Management of contacts of MDR TB and XDR TB patients. Stockholm: ECDC; 2012. [Google Scholar]
- 5.Shandil RK, Jayaram R, Kaur P, et al. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob Agents Chemother. 2007 Feb;51(2):576–582. doi: 10.1128/AAC.00414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–119. doi: 10.2165/00003088-199732020-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chien S, Wells TG, Blumer JL, et al. Levofloxacin pharmacokinetics in children. J Clin Pharmacol. 2005;45:153–160. doi: 10.1177/0091270004271944. [DOI] [PubMed] [Google Scholar]
- 8.Thee S, Garcia-Prats AJ, McIlleron HM, et al. Pharmacokinetics of ofloxacin and levofloxacin for the prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother. 2014;58(5):294851. doi: 10.1128/AAC.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamrah S, Brostrom R, Fred D, et al. Treatment for Latent Tuberculosis Infection in Contacts to Multidrug-Resistant Tuberculosis- Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014;18(8):912918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. [DOI] [PubMed] [Google Scholar]
- 11.Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Comput. Methods Programs Biomed. 2004;75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 2011;101:72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar D. Lattice: Multivariate Data Visualization with R. 1st. New York: Springer; 2008. [Google Scholar]
- 15.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Nandy P, Chien S, Noel GJ, Tornoe CW. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob. Agents Chemother. 2010;54:375–379. doi: 10.1128/AAC.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peloquin CA, Hadad DJ, Molino LPD, Palaci M, Boom WH, Dietze R, Johnson JL. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 2008;52:852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levofloxacin Pharmacometrics Review: New Drug Application. [Accessed March 2013]; http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM252889.pdf.
- 19.Iseman M. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 20.Fraser A, Paul M, Attamna A, Leibovici L. Treatment of latent tuberculosis in persons at risk for multidrug-resistant tuberculosis: systematic review. Int J Tuberc Lung Dis. 2006;10(1):1923. [PubMed] [Google Scholar]
- 21.Dolui SK, Das M, Hazra A. Ofloxacin-induced reversible arthropathy in a child. J Postgrad Med. 2007;53:144–145. doi: 10.4103/0022-3859.32220. [DOI] [PubMed] [Google Scholar]
- 22.Chalumeau M, Tonnelier S, D'Athis P. Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France. Pediatrics. 2003;111(6 Pt 1):e714–e719. doi: 10.1542/peds.111.6.e714. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt JE, Hill MA, Turek JJ, Carlton WW. Ultrastructural changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1992;29:230238. doi: 10.1177/030098589202900307. [DOI] [PubMed] [Google Scholar]
- 24.Blumer JL. Fluoroquinolone use in children: resistance and safety implications. Contemp. Pediatr. 2003;20:97–113. [Google Scholar]
- 25.Smith KC, Seaworth BJ. Drug-resistant tuberculosis: controversies and challenges in pediatrics. Expert Review of Anti-infective Therapy. 2005;3:9951010. doi: 10.1586/14787210.3.6.995. [DOI] [PubMed] [Google Scholar]
- 26.Rubin M, Bruck E, Rapoport M. Maturation of renal function in childhood: clearance studies. J Clin Invest. 1949;28(5 Pt 2):114462. [PubMed] [Google Scholar]
- 27.Li F, Nandy P, Chien S, Noel GJ, Tornoe CW. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob. Agents Chemother. 2010;54:375–379. doi: 10.1128/AAC.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol Rev. 2007;20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angeby KA, Jureen P, Giske CG, et al. Wild-type MIC distributions of four fluoroquinolones active against Mycobacterium tuberculosis in relation to current critical concentrations and available pharmacokinetic and pharmacodynamic data. J. Antimicrob. Chemother. 2010;65:946–952. doi: 10.1093/jac/dkq091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.