Abstract
Background
Most substance use disorders (SUD) treatment clinical trials are too short and small to reliably estimate the incidence of rare events like death.
Objective
The aim of this study is to estimate the overall mortality rates among a SUD treatment-seeking population by pooling participants from multiple clinical trials conducted through the National Institute on Drug Abuse (NIDA)-sponsored National Drug Abuse Treatment Clinical Trials Network (CTN).
Participants
Drug and or alcohol users (N=9,866) who sought treatment and participated in one of the twenty-two CTN trials.
Measurements
Data were collected through randomized clinical trials in national community treatment programs (CTPs) for SUD. Pooled analysis was performed to assess age- and gender-standardized mortality rate(s) (SM rate(s)), and mortality ratio(s) (SM ratio(s)) of CTN trial participants compared to the U.S. general population. We also assessed if there were differences in mortality rates across different types of substance of abuse.
Results
The age- and gender-SM rate among CTN trials participants was 1403 (95% CI: 862-2074) per 100,000 person years (PY) compared to 542 (95% CI: 541-543) per 100,000 PY among the U.S. general population in 2005. By gender, age-adjusted SM ratio for female CTN trial participants was over five times (SM ratio=5.35, 95% CI: 3.31-8.19)), and for male CTN trial participants was over three times (SM ratio=3.39, 95% CI: 2.25-4.90) higher than their gender comparable peers in the U.S. general population.
Conclusions
Age and gender-standardized mortality rates and ratios among NIDA CTN SUD treatment-seeking clinical trial participants are higher than the age and gender comparable U.S. general population. The overall mortality rates of CTN trial participants are similar to in-treatment mortality reported in large U.S. and non-U.S. cohorts of opioid users. Future analysis with additional CTN trial participants and risk times will improve the stability of estimates, especially within subgroups based on primary substance of abuse. These SUD mortality rates can be used to facilitate safety monitoring within SUD clinical trials.
Keywords: Substance use disorder, clinical trial, standardized mortality rate, standardized mortality ratio, pooled analysis
1. Introduction
Substance use is a serious public health problem in the U.S. associated with high rates of pre-mature deaths and high costs in health care and societal economics(Fenoglio, Parel, & Kopp, 2003; ONDCP, 2012; United Nations Office on Drugs and Crime, 2012).Mortality in SUD populations has been mainly investigated in prospective cohort studies (Arendt, Munk-Jorgensen, Sher, & Jensen, 2011; Degenhardt, Bucello, et al., 2011; Degenhardt, Singleton, et al., 2011). A meta-analysis of 58 cohort studies across many countries among opioid dependent or regular users reported a pooled all-cause mortality rate of 2,090 (95% CI: 1,930-2,260) deaths per 100,000 person-years (PY) and a pooled age- and gender-standardized mortality ratio (SM ratio) of 14.66 (95% CI: 12.82-16.50) compared to the general population (Degenhardt, Bucello, et al., 2011). Another systematic review that included seven cohort studies with problematic or dependent cocaine users suggests that crude mortality rates are highly variable across individual studies and countries, ranging from 530 (95% CI: 100-1580) to 6610 (95% CI: 5210-7110) per 100,000 PY(Degenhardt, Bucello, et al., 2011; Tyndall et al., 2001). Factors such as country of the study, SUD subpopulations (drug injectors versus non-injectors), cohort sizes, follow-up stages, and treatment phases (in-treatment versus post-treatment) likely contribute to the variability in mortality rates reported in the literature. In addition, mortality rates reported in the above meta-analysis were derived from longitudinal follow-up studies and may have limited generalizability to SUD patients who seek treatment primarily in community treatment programs through SUD treatment clinical trials. To our knowledge, there have been no reports estimating mortality in SUD treatment-seeking clinical trial participants.
The National Institute on Drug Abuse (NIDA)-sponsored National Drug Abuse Treatment Clinical Trials Network (CTN) was established in 1999. Through February 2012, twenty-three clinical trials involving pharmacological and/or psychosocial/behavioral interventions have been completed among SUD populations (Tai et al., 2010; Wells, Saxon, Calsyn, Jackson, & Donovan, 2010a). Participants enrolled in CTN research studies are self-identified substance users with a confirmed SUD diagnosis and seeking treatment at a community treatment program (CTP), with research trials typically having short follow-up (average six months) and sample sizes of a few hundred participants. When these trials are analyzed individually, it leads to imprecise estimates of rare events like death. The public availability of participant-level information from these individual CTN trials allow for pooling of data and provides more precise estimates of the overall mortality among SUD clinical trial participants. In addition to highlighting the issue of mortality in substance use populations, as studied in the CTN, the primary goal of estimating mortality in SUD clinical trials is to provide a reference to facilitate safety monitoring of SUD clinical trial interventions in the future.
The main objective of the current analysis is to estimate the overall mortality rates by pooling CTN clinical trial data and then compare these rates to the U.S. general population, and to assess the differences in mortality across different substances of abuse within the CTN trials.
2. Methods and Materials
Of the completed twenty-three trials, twenty-two trials were included in this analysis. The excluded trial (NIDA-CTN-0029) only enrolled cigarette smokers with attention deficit hyperactivity disorder (ADHD) and specifically excluded individuals with current drug abuse or dependence. The target population of the remaining twenty-two trials was drug and or alcohol abuse or dependence individuals. De-identified data of 9,866 randomized participants from these twenty-two multi-site clinical trials were retrieved from the NIDA Data Share (https://datashare.nida.nih.gov/). Data from various assessments collected on the case report forms of these trials were used to construct a database for analysis, which included adverse event (AE)/serious AE (SAE) forms, demographics, participant disposition, and questionnaires. In general, CTN trials recorded death events on AE, SAE, or special disposition case report forms (CRFs). The occurrence of a death could be discovered by study staff via reporting by family members, friends, hospital records, or newspaper obituaries. The causes of deaths recorded in CRFs were then uniformly MedDRA coded. Baseline primary substance of use and alcohol were identified through the Addiction Severity Index-Lite (ASI-Lite) (McLellan, Luborsky, Woody, & O'Brien, 1980) for twenty out of the twenty-two trials. For the remaining two trials without the ASI-Lite assessment tool, a self-reported substance use instrument or a global substance use measure was used to identify primary substance use at the baseline. For the purposes of analysis, we collapsed the smaller primary substance of use subgroups of cannabis, alcohol, other drugs, and no problem into a single subgroup named “All Others”. These primary substances of abuse had relatively low total risk times (from 83 to 570 PY), limiting our ability to examine differences in mortality, and further, none of included CTN trials specifically targeted these substances despite them being noted as a primary substance of use based on the ASI-Lite assessment.
Mortality rates were calculated as the number of observed deaths divided by cumulative time (days) at risk from all participants, and then standardized to 100,000 person-years (PY)(Rosner, 2000; Zhang & Yu, 2008). Risk time for each participant was calculated as the days from randomization to death (if died) or the last available contact day (if completed the trial or were lost to follow-up). Death and demographic information for the U.S. general population was retrieved from the “Human Mortality Database” (http://www.mortality.org/). To compare the mortality of CTN trial participants to the U.S. general population, both direct standardization (age- and gender-standardize mortality rates, SM rates) and indirect standardization methods (standardized mortality ratio, SM ratio) were used. Average age (range: 13-78) and gender distributions of the U.S. general population of the year 2001-2010 were used as the reference population structure. Specifically, an age- and gender-SM ratio was computed as the ratio of the observed number of deaths over the expected number of deaths in the CTN sample, where the age and gender-specific mortality rate of the reference population (i.e., U.S. population) was applied to the target population (i.e., CTN sample) to yield the expected number (Last, 1983). Ninety-five percent confidence intervals (95% CIs) around SM ratio were estimated using Byar’s approximation to Poisson-distributed deaths (Breslow & Day, 1987; Liddell, 1984; Sahai & Khurshid, 1993). An age- and gender-SM rate was calculated for both the CTN sample and the U.S. general population of the year 2005 by applying age- and gender-specific mortality rates of the target sample (i.e., CTN sample or U.S. population of the year 2005) to the age and gender profile-matched reference sample (i.e., average age and gender distributions of U.S. populations of the year 2001 to 2010). 95% CIs of SM rates were estimated assuming a Gamma distribution of the rate by an exact method exploiting the relationship between the Chi-squared and cumulative Poisson distributions (Fay & Feuer, 1997; Rosner, 2000; Zhang & Yu, 2008).
An advantage to pooling multiple trials together is to reduce the sampling error and improve the stability of estimates. In the pooled analysis, between-trial heterogeneity (i.e., random trial effect) was investigated by an extended proportional hazard (PH) Cox regression model (i.e., frailty model), assuming that the between-trial variance follows a normal distribution with zero mean and variance σ2 (Simmonds et al., 2005). In the Cox PH model, a participant either had a death event, or was censored at loss to follow-up (LTFU) or study completion date. For a LTFU participant, the censoring day was the last contact day before the participant was lost.
Both unadjusted (crude) and adjusted survival curves of the pooled CTN sample were calculated using the extended Cox PH model. The corrected group prognosis method (Chang, Gelman, & Pagano, 1982; Ghali et al., 2001), a method analogous to direct standardization, was applied to generating the adjusted survival curves. The reference population for the adjusted survival curves was the U.S. population of Year 2005, approximately the mid-time point when CTN trial participants were recruited (2001- 2010).
SAS 9.3 (SAS Institute, Inc., Cary, NC, USA) was used to perform all analyses.
3. Results
3.1 Demographics and Mortalities of Pooled CTN Trials
Table 1 characterizes the analysis population pooled across the 22 CTN trials. Mean age of the 9,866 participants randomized in these 22 trials was 37.1 years old. The majority of the study population was males (58.2%), white (52.2%), and non-Hispanic origin (82.6%). There were 23.5% African Americans/black. Primary drug substance of use at baseline was illicit or licit opioids among 26.3% participants, multi-drug/or combined drug/substance and alcohol among 24.0% participants, stimulant among 17.6% participants, alcohol among 14.1% participants, cannabis in 11.3%, other drugs among 2.1%, and no-primary drug/alcohol problem in 3.6% participants. A total of 49 deaths (0.5%) were reported over 4,730 PY of risk time in the 9,866 CTN participants for an overall pooled crude mortality rate of 1,036 (95% CI: 766-1,370) per 100,000 PY. Mortality rates did not apparently differ by gender, race, or ethnicity, and did not change linearly across different age groups, with the three highest mortality rates occurred in ≥ 55(3,122, 95% CI: 1,145-6,795, per 100,000 PY) , 45-54 (1,324, 95% CI: 705-2,263, per 100,000 PY) and < 18 age groups (1,181, 95% CI: 433-2,572 per 100,000 PY). Mortality rates were also characterized by primary substance of abuse at the baseline based on ASI-Lite categorization (Table 1), with mortality rates highest among multi-drug/alcohol users (1371, 95% CI: 813-2166, per 100,000 PY) and then primary opioid users (1067, 95% CI: 551-1863, per 100,000 PY).
Table 1.
Characteristics of CTN Trial Participants and Mortalities
| Characteristic | Category | Number of participants (%) |
Number of deaths |
Risk Time (PY) |
Crude Mortality Rates(95% CI, per 100,000 PY) |
|---|---|---|---|---|---|
| All | Overall | 9866 | 49 (0.5%) |
4730 | 1036 (766- 1370) |
| Sex * | Male | 5737 (58.2) | 28 | 2602 | 1076 (715- 1555) |
| Female | 4121 (41.8) | 21 | 2124 | 988 (612- 1511) |
|
|
Age
*
(37.1±10.2) |
<18 | 677 (6.9) | 6 | 508 | 1181 (433- 2572) |
| 18-24 | 1449 (14.7) | 4 | 654 | 611 (167- 1565) |
|
| 25-34 | 2469 (25.0) | 11 | 1078 | 1020 (509- 1826) |
|
| 35-44 | 2811 (28.5) | 9 | 1313 | 686 (314- 1301) |
|
| 45-54 | 2046 (20.7) | 13 | 982 | 1324 (705- 2263) |
|
| ≥55 | 406 (4.1) | 6 | 192 | 3122 (1145- 6795) |
|
| Race * | White | 5151 (52.2) | 32 | 2427 | 1318 (902- 1861) |
| Others | 2374 (24.1) | 7 | 1161 | 603 (242- 1243) |
|
| Black (African American) | 2318 (23.5) | 10 | 1133 | 883 (424- 1623) |
|
| Ethnicity * | Non-Hispanic | 8151 (82.6) | 44 | 3893 | 1130 (821- 1517) |
| Hispanic | 1711 (17.3) | 5 | 835 | 599 (194- 1398) |
|
|
Baseline
primary drug/alcohol |
Opioid | 2592 (26.3) | 12 | 1125 | 1067 (551- 1863) |
| Multi- drug/substance/alcohol |
2364 (24.0) | 18 | 1313 | 1371 (813- 2166) |
|
| Stimulant | 1736 (17.6) | 6 | 794 | 756 (277- 1645) |
|
| All Others* | 3069 (31.1) | 13 | 1471 | 883 (470- 1511) |
|
|
Cause of
death |
Cause | Cases | |||
|
SUD-related
or likely SUD related (40.8%) |
Overdose | 9 | |||
| Alcoholism | 1 | ||||
| Substance abuse | 2 | ||||
| Suicide | 2 | ||||
| Gunshot wound | 1 | ||||
| Head injury | 1 | ||||
| Road traffic accident | 1 | ||||
| Toxicity to various agents | 3 | ||||
|
Unlikely SUD-
related (30.6%) |
Cardiac failure, Congestive cardiac failure, Deep vein thrombosis, Dehydration, Endocarditis, Jaundice, Lung neoplasm malignant, Neoplasm malignant, Renal failure |
9 (1 case for each cause) |
|||
| Pre-existing disease | 3 | ||||
| Myocardial infarction | 3 | ||||
|
Unknown
cause, but unlikely SUD- related (28.6%) |
Death with unknown cause but unrelated to study intervention or drug abuse |
14 |
8 participants with unknown gender and age, 23 with unknown race, 4 with unknown ethnicity, 105 with unknown primary substance(s). “All Others” includes cannabis, alcohol, sedative/hypnotics/tranquilizers and no problem at baseline.
Table 1 also displays the known causes of deaths. A total of 20 deaths (20/49=40.8%) were SUD-related or likely related. Overdose was the most common cause (9/49=18.4%), with an additional 11 deaths (11/49=22.4%) likely SUD-related deaths (substance abuse, alcoholism, suicide, gunshot wound, head injury, traffic accident, and toxicity to various agents).
3.2 Characteristics of CTN Trials
Among the twenty-two CTN trials, fifteen involved only psychosocial/behavioral interventions, while the other seven trials either had pharmacological intervention only or pharmacological combined with psychosocial/behavioral intervention. Mean observed risk time of the 22 trials was 0.48 years per person. Table 2 shows that mean risk time per person varied by trial, ranging from 0.19 years per person (CTN-0003) to 1.14 years per person (CTN-0015). Fifteen trials reported at least one death. Of the seven trials reporting zero deaths, five had risk time less than 0.4 years per person. Two trials with primarily opioid users, CTN-0001 and CTN-0003, had observed higher mortality rates (4,150 and 2,983, per 100,000 PY respectively) than most of the other trials.
Table 2.
Characteristics of CTN Trials
| CTN Trial No. |
Sample Size (N) |
Intervention Type | Number of Deaths |
Total Observed Risk Time (Person Years) |
Average Years Per Person |
Crude Mortality Rate (per 100,000 PY) |
Primary drug of use (not reported if less than 1%) |
|---|---|---|---|---|---|---|---|
|
NIDA- CTN- 0001 |
113 | Pharmacological | 2 | 48.19 | 0.43 | 4150 | Opioids: 96% Multi- drug/substance/alcohol: 3% |
|
NIDA- CTN- 0002 |
230 | Pharmacological | 0 | 86.81 | 0.38 | 0 | Opioids: 99% |
|
NIDA- CTN- 0003 |
516 | Pharmacological | 3 | 100.58 | 0.19 | 2983 | Opioids: 84% Unknown: 15% |
|
NIDA- CTN- 0004 |
461 | Psychosocial/Behavior | 2 | 133.65 | 0.29 | 1497 | Alcohol: 28% Stimulants: 27% Other drug: 20% Cannabis: 15% Opioid: 9% |
|
NIDA- CTN- 0005 |
423 | Psychosocial/Behavior | 0 | 92.27 | 0.22 | 0 | Alcohol: 48% Stimulants: 26% Cannabis: 20% Opioid: 5% Other drug: 1% |
|
NIDA- CTN- 0006 |
454 | Psychosocial/Behavior | 0 | 183.53 | 0.40 | 0 | Stimulants: 57% Multi- drug/substance/alcohol: 30% Alcohol: 7% Cannabis: 2% Opioid: 2% No problem: 1% |
|
NIDA- CTN- 0007 |
403 | Psychosocial/Behavior | 1 | 187.00 | 0.46 | 535 | Opioid: 40% Stimulant: 34% Multi- drug/substance/alcohol: 22% Unknown: 2% |
|
NIDA- CTN- 0009 |
225 | Pharmacological and Psychosocial/Behavior |
1 | 108.30 | 0.48 | 923 | Opioid: 52% Stimulants: 17% Multi- drug/substance/alcohol: 11% Cannabis: 8% Alcohol: 7% Other drug:4% No problem: 1% |
|
NIDA- CTN- 0010 |
154 | Pharmacological | 1 | 113.45 | 0.74 | 881 | Opioids: 89% Multi- drug/substance/alcohol: 10% |
|
NIDA- CTN- 0011 |
339 | Psychosocial/Behavior | 2 | 86.82 | 0.26 | 2304 | Multi- drug/substance/alcohol: 45% Alcohol: 23% Stimulants: 21% Opioid: 7% Cannabis: 3% |
|
NIDA- CTN- 0013 |
200 | Psychosocial/Behavior | 0 | 64.75 | 0.32 | 0 | Stimulant: 31% Cannabis: 31% Opioids: 14% Multi- drug/substance/alcohol: 12% Alcohol: 11% Other drug: 2% |
|
NIDA- CTN- 0014 |
482 | Psychosocial/Behavior | 6 | 466.01 | 0.97 | 1288 | Cannabis: 45% No problem: 30% Multi- drug/substance/alcohol: 18% Stimulant: 4% Alcohol: 2% |
|
NIDA- CTN- 0015 |
353 | Psychosocial/Behavior | 4 | 401.89 | 1.14 | 995 | Multi- drug/substance/alcohol: 64% Stimulant: 19% Alcohol: 9% Opioid: 4% Cannabis: 3% |
|
NIDA- CTN- 0017 |
632 | Psychosocial/Behavior | 5 | 294.76 | 0.47 | 1696 | Multi- drug/substance/alcohol: 80% Opioid: 14% Stimulant: 6% |
|
NIDA- CTN- 0018 |
594 | Psychosocial/Behavior | 7 | 327.59 | 0.55 | 2137 | Multi- drug/substance/alcohol: 49% Stimulant: 16% Opioid: 14% Alcohol: 11% No problem: 5% Cannabis: 4% Other drug: 2% |
|
NIDA- CTN- 0019 |
515 | Psychosocial/Behavior | 5 | 296.68 | 0.58 | 1685 | Multi- drug/substance/alcohol: 42% Opioid: 23% Stimulant: 22% Alcohol: 5% No problem: 4% Cannabis: 2% Other drug: 2% |
|
NIDA- CTN- 0020 |
628 | Psychosocial/Behavior | 2 | 304.69 | 0.49 | 656 | Multi- drug/substance/alcohol: 51% Opioid: 23% Stimulant: 10% Alcohol: 10% No problem: 4% Cannabis: 2% |
|
NIDA- CTN- 0021 |
436 | Psychosocial/Behavior | 0 | 127.46 | 0.29 | 0 | Alcohol: 61% Stimulants: 25% Cannabis: 8% Opioid: 6% |
|
NIDA- CTN- 0028 |
303 | Pharmacological and Psychosocial/Behavior |
0 | 103.55 | 0.34 | 0 | Cannabis: 88% Alcohol: 8% Stimulant: 2% Unknown: 2% |
|
NIDA- CTN- 0030 |
653 | Pharmacological and Psychosocial/Behavior |
2 | 312.73 | 0.48 | 640 | Opioid: 91% Multi- drug/substance/alcohol:9% |
|
NIDA- CTN- 0031 |
471 | Psychosocial/Behavior | 0 | 234.81 | 0.50 | 0 | Stimulant: 55% Multi- drug/substance/alcohol: 37% Alcohol: 3% Cannabis: 2% Opioids: 2% |
|
NIDA- CTN- 0032 |
1281 | Psychosocial/Behavior | 6 | 654.46 | 0.51 | 917 | Alcohol: 32% Cannabis: 21% Opioid: 17% Stimulant: 13% No problem: 10% Other drug: 4% |
All CTN trials have group counseling as the standard of care (referred to as Treatment as Usual, TAU) of the CTPs, which is separate from “psychosocial” intervention indicated in this table.
3.3 Age- and Gender-SM Rates, Survival Probabilities and SM Ratios of CTN Trial Participants
No statistically significant between-trial heterogeneity was found (variance=0.03, standard error=0.14, p=0.3155), suggesting that pooled CTN trials can be treated as a single sample without accounting for between-trial variance with respect to estimating the pooled mortality rate. Table 3 provides the SM rates and SM ratios of the pooled CTN sample. The ageand gender-adjusted SM rate for the pooled CTN sample was 1403 (95% CI: 862-2074) per 100,000 PY, compared to 542 (95% CI: 541-543) per 100,000PY for the U.S. general population of 2005. Age-SM rates of both female (1141, 95% CI: 465-2080 per 100,000 PY) and male (1672, 95% CI: 869-2738 per 100,000 PY) participants of CTN trials were higher than their same gender counterparts from the U.S. general population of the year 2005 (444, 95% CI: 443-446 for females and 642, 95 % CI: 640-643 for males per 100,000 PY).
Table 3.
Age- and Gender-Standardized Mortality Rate per 100,000 PY and Standardized Mortality Ratio for CTN Trial Participants Compared with Comparable U.S. General Population
| Standardized Mortality Rate (95 % CI) | Standardized Mortality Ratio (95 % CI) | |||||
|---|---|---|---|---|---|---|
| Group | Overall | Females | Males | Overall | Females | Males |
| CTN trials | 1403 (862-2074) |
1141 (465-2080) |
1672 (869-2738) |
4.02 (2.97-5.32) |
5.35 (3.31-8.19) |
3.39 (2.25-4.90) |
| U.S.A. (2005) | 542 (541-543) |
444 (443-446) |
642 (640-643) |
|||
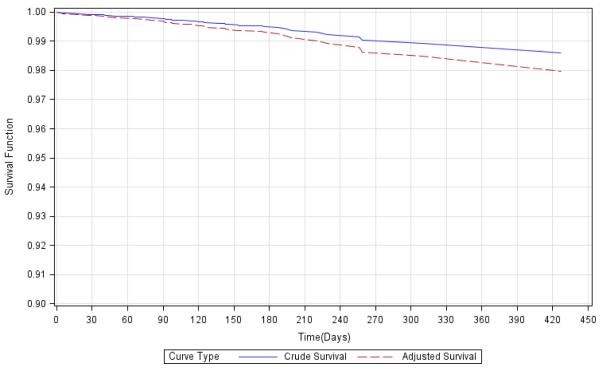
Figure 1 illustrates that overall survival function of the CTN trial participants adjusted by the age and gender structure of the U.S. population. The adjusted survival probability on the last death event day (Day 420) was approximately 98.0%.
Figure 1*. Crude and Age- and Gender-Adjusted Survival Functions of Pooled NIDA CTN Trial Participants, Using Corrected Prognosis Method.
*Reference population for the adjusted curve is the U.S. general population of the year 2005. 9858 participants with known age and gender were used for plotting.
CTN trial participants experienced four times (SM ratio=4.02, 95% CI: 2.97-5.32) as many deaths as expected for the U.S. general population. Specifically, females from the CTN sample experienced over five times, and males experienced over three times as many deaths as their counterparts from the U.S. population (SM ratio= 5.35, 95% CI: 3.31-8.19 for females, and 3.39, 95% CI: 2.25-4.90 for males).
4. Discussion
To our knowledge, this is the first investigation of within-trial mortality among SUD trial participants across multiple studies. As individual SUD clinical trials are too short and small to yield stable estimates, pooling participants across trials creates more stable estimates. Similarities in the CTN participant characteristics population provide a rationale for pooling across trial, such as socioeconomic status, racial and ethnic groups, employment status, and educational level (Calsyn et al., 2009; Campbell et al., 2010; Carroll et al., 2006; Donovan et al., 2013; Hien et al., 2010; Hien et al., 2009; Horigian, Robbins, Dominguez, Ucha, & Rosa, 2010; Korthuis et al., 2012; Kropp et al., 2013; Masson et al., 2013; Meade et al., 2010; Svikis et al., 2012; Weiss et al., 2011; Winhusen, Winstanley, Somoza, & Brigham, 2012; Woody et al., 2008). Participants were typically recruited from community-based substance abuse treatment programs (CTPs) within the CTN network (McCarty et al., 2008) and trials were designed by NIDA CTN investigators with common goals of bridging practice with substance abuse treatment research results (Tai et al., 2010; Wells, Saxon, Calsyn, Jackson, & Donovan, 2010b). In addition, there was no statistical evidence that mortality rates differed across these CTN trials. We also examined high HIV-risk behaviors or HIV infections in CTN trials with high mortality rates, or specifically targeting drug-injectors, or focusing on HIV-risk behaviors or HIV testing. We found no indication that death was related to high HIV-risk behaviors or HIV burdens in the deceased participants of these trials. Another rationale for pooling CTN trials was that no death event in these CTN trials was reported to be related to a study intervention, suggesting that interventions of these CTN trials did not directly impact the risk of death. For these reasons, the authors feel it is reasonable to pool CTN trial participants to improve the stability of the estimated mortality rates and mortality ratios among SUD treatment trial participants.
The overall mortality rate among the pooled CTN trial participants falls in the range of 1,000-2,000 deaths per 100,000 PY frequently reported in the literature for participants treated in drug abuse treatment programs (Gossop, Stewart, Treacy, & Marsden, 2002; Hall, Degenhardt, & Lynskey, 1999; Joe, Lehman, & Simpson, 1982; Rehm et al., 2005). The CTN mortality rate is lower than the mortality rate (2,090, 95% CI: 1,930-2,260 deaths per 100,000 PY) among opioid-dependent or regular users estimated through a meta-analysis of 58 cohort studies (Degenhardt, Bucello, et al., 2011), but similar to in-treatment mortality rate of a Swiss cohort of heroin users (1,060 per 100,000 PY, N=6281) (Rehm et al., 2005) and the Drug Abuse Reporting Program (DARP) (1,300 deaths overall, 1,400 deaths for males and 900 for females, per 100,000 PY, N=20,808) (Watterson, Simpson, & Sells, 1975). The Swiss cohort and the CTN trial population in this analyses had similar sample sizes (n=6281 and 9866 respectively) and total observed risk time (4623 versus 4730 PY respectively). In contrast, the DARP study was a larger cohort (N=50,489) with longer risk time (20,808 PY) (1970-1973) and more deaths (n=275) than the CTN and Swiss cohorts. Despite these differences with DARP, similar mortality rates were observed.
As suggested by both SM rates in Table 3 and the adjusted survival curves in Figure 1, CTN trial participants would have had higher mortality rates and lower survival probabilities had their age and gender profile matched the U.S. population, as the CTN trials had proportionally fewer older people (≥ 55 years, 4.1% for the pooled CTN sample versus 23% for the U.S. general population), but higher mortality rate among this age group (3,122 deaths for the CTN versus 1665 deaths for the U.S., per 100,000 PY).
As presented in Table 2, the observed mortality rates with the trials varied, especially among trials with shorter follow-up durations, indicating potential random sampling errors. Of note, the survival probabilities and mortality rates estimated in this analysis were conditional on the participants being within-trial, not post-trial regardless the length of the follow-up of a participant.
Characterizing mortality rates by substance of abuse is important to SUD researchers and clinicians in some subgroups. Since the majority of CTN trials targeted an opioid or stimulant use population, we had very limited information on cannabis, alcohol, or sedative/hypnotics/tranquilizer users in the present CTN participants and for these groups individually, mortality rates were not calculated, but rather pooled. The opioid and multi-drug/alcohol group had numerically, albeit not statistically significant, higher mortality rates than stimulant users and other drug users, and consistent with the literature that overdose deaths are more common in opioid users (Paulozzi, Weisler, & Patkar, 2011). However, small sample sizes and risk time limited our ability to make conclusive statements.
A 3 fold increase in death for males (SM ratio=3.39) and 5 fold for females (SM ratio=5.35) were found in CTN trial participants when compared to their peers from U.S. general population. Although unlikely statistically different due to the overlapping 95% CIs, relatively higher risk of death due to abusing drugs/alcohol among females than males is also reported in the literature suggesting that drug abuse elevates mortality, but more so among female drug users (Degenhardt, Bucello, et al., 2011; Rehm et al., 2005).
Consistent with the literature, overdose is the most frequently reported cause of death among CTN trial participants (Darke, Mills, Ross, & Teesson, 2011; Degenhardt, Bucello, et al., 2011; Gossop et al., 2002; Watterson et al., 1975). With 40.8% (20/49) of causes of deaths related or likely-related to SUD, it is likely a major contributory factor to the within-trial death events with overdose as an important and perhaps preventable cause of death. This estimate could underestimate the number of deaths related to SUD due to the large percentage of deaths for which the cause was unknown (14/49=28.6%). The information supports the need for clinicians and clinical trial researchers to focus attention on potential overdose, even during treatment and clinical trial period. Examination of case report form information among the 14 deaths with unknown cause did not identify cases with high HIV risk behaviors, and these deaths with unknown cause spanned all categories of baseline substances of abuse.
5. Limitations
To facilitate safety monitoring and improve implications to clinicians as to when a safety concern may arise and require attention, future analysis based on larger sample sizes, more events, and longer risk times among different substance use subgroups is necessary to establish more stable estimates. CTN trial participants were generally a group of SUD population highly motivated for treatment, differing from an unselected sample of SUD population who may not perceive the harmfulness of drugs or alcohol, and are not motivated to seek care. Therefore, our findings may not be generalizable to other SUD populations or even to SUD population post the participation of SUD treatment clinical trials.
Despite pooling data across 22 studies with close to 10,000 participants, a lack of precision of estimates and limited statistical power is still an issue due to the rarity of death events and relatively short follow-up durations for SUD clinical trials. This limitation is magnified when subgroup analyses based on primary substance of use were attempted. This limitation will lessen as future CTN trials are added to the pool and more death events and longer total risk times are observed in subgroups. Since the mean of the between-trial variance was not significantly different from zero, we did not account for this variance when estimating the pooled mortality rates. However, between-trial heterogeneity could still exist.
The CTN pooled sample had on average 17% of participants that ended trial participation before the end of trial-planned completion time. The mortality rates of the current pooled analysis could be underestimated if some of these participants were actually lost due to death and unknown to the research sites.
Lastly, although this pooled analysis of CTN trials provides a reference sample in the future to facilitate trial safety monitoring, mortality rates measured within any single trial will remain imprecise due to death being a rare event even if more common than the general population.
6. Conclusion
This pooled analysis suggests that the mortality rate of the CTN trial participants is significantly higher than their age and gender comparable U.S. general population. The number of deaths observed in the CTN trials is three to five times more than would have been expected had the mortality rates of CTN trial participants matched the age and gender comparable U.S. general population. These findings add to the existing literature quantifying mortalities among the SUD treatment-seeking population. For future meta-analysis of mortalities among the SUD population, age and gender-standardized mortality rates and ratios provided in this study are valuable to compare results. The pooled mortality rates and ratios may also help safety monitoring of SUD clinical trials by providing a potential reference in the future against which to assess rare death events in individual clinical trials. We plan to continue to update the mortality estimates, especially for different substances of abuse, to improve its implications to SUD treatment trials and clinicians as more NIDA CTN trials are entered into the public database and more death events are accumulated in subgroups.
Highlights.
Standardized mortality (SM) rate among substance abuse trials was assessed.
Standardized mortality (SM) ratio among substance abuse trials was assessed.
SM rate of substance abuse trial population is higher than general population.
SM ratio of substance abuse trial population is higher than general population.
Acknowledgements
This research was supported by the National Institute on Drug Abuse, National Drug Abuse Treatment Clinical Trials Network, National Institutes of Health, through Contract No. HHSN271200900034C.
Footnotes
Declarations of Interest: None of the authors have a connection to any of the researchers with the tobacco, alcohol, pharmaceutical or gaming industries or anybody substantially funded by one of these organizations. Additionally, they have no financial conflict of interest arising from involvement with organizations that seek to provide help with or promote recovery from addiction.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt M, Munk-Jorgensen P, Sher L, Jensen SO. Mortality among individuals with cannabis, cocaine, amphetamine, MDMA, and opioid use disorders: a nationwide follow-up study of Danish substance users in treatment. Drug and Alcohol Dependence. 2011;114(2-3):134–139. doi: 10.1016/j.drugalcdep.2010.09.013. doi: S0376-8716(10)00327-3 [pii] [DOI] [PubMed] [Google Scholar]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Scientific Publications. 1987;(82):1–406. [PubMed] [Google Scholar]
- Calsyn DA, Hatch-Maillette M, Tross S, Doyle SR, Crits-Christoph P, Song YS, Berns SB. Motivational and skills training HIV/sexually transmitted infection sexual risk reduction groups for men. J Subst Abuse Treat. 2009;37(2):138–150. doi: 10.1016/j.jsat.2008.11.008. S0740-5472(08)00221-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BK, Tillotson CJ, Choi D, Bryant K, DiCenzo J, Provost SE, McCarty D. Predicting outpatient treatment entry following detoxification for injection drug use: the impact of patient and program factors. J Subst Abuse Treat. 2010;38(Suppl 1):S87–96. doi: 10.1016/j.jsat.2009.12.012. S0740-5472(10)00024-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Woody GE. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81(3):301–312. doi: 10.1016/j.drugalcdep.2005.08.002. S0376-8716(05)00248-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35(8):669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- Darke S, Mills KL, Ross J, Teesson M. Rates and correlates of mortality amongst heroin users: findings from the Australian Treatment Outcome Study (ATOS), 2001-2009. Drug and Alcohol Dependence. 2011;115(3):190–195. doi: 10.1016/j.drugalcdep.2010.10.021. S0376-8716(10)00381-9 [pii] [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Singleton J, Calabria B, McLaren J, Kerr T, Mehta S, Hall WD. Mortality among cocaine users: a systematic review of cohort studies. Drug and Alcohol Dependence. 2011;113(2-3):88–95. doi: 10.1016/j.drugalcdep.2010.07.026. S0376-8716(10)00289-9 [pii] [DOI] [PubMed] [Google Scholar]
- Donovan DM, Daley DC, Brigham GS, Hodgkins CC, Perl HI, Garrett SB, Zammarelli L. Stimulant abuser groups to engage in 12-step: a multisite trial in the National Institute on Drug Abuse Clinical Trials Network. J Subst Abuse Treat. 2013;44(1):103–114. doi: 10.1016/j.jsat.2012.04.004. S0740-5472(12)00081-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Fenoglio P, Parel V, Kopp P. The social cost of alcohol, tobacco and illicit drugs in France, 1997. Eur Addict Res. 2003;9(1):18–28. doi: 10.1159/000067730. doi: 6773067730 [pii] [DOI] [PubMed] [Google Scholar]
- Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Knudtson ML. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286(12):1494–1497. doi: 10.1001/jama.286.12.1494. doi: jsc10095 [pii] [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Treacy S, Marsden J. A prospective study of mortality among drug misusers during a 4-year period after seeking treatment. Addiction. 2002;97(1):39–47. doi: 10.1046/j.1360-0443.2002.00079.x. doi: 79 [pii] [DOI] [PubMed] [Google Scholar]
- Hall WD, Degenhardt LJ, Lynskey MT. Opioid overdose mortality in Australia, 1964-1997: birth-cohort trends. Med J Aust. 1999;171(1):34–37. doi: 10.5694/j.1326-5377.1999.tb123495.x. [DOI] [PubMed] [Google Scholar]
- Hien DA, Jiang H, Campbell AN, Hu MC, Miele GM, Cohen LR, Nunes EV. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA's Clinical Trials Network. The American journal of psychiatry. 2010;167(1):95–101. doi: 10.1176/appi.ajp.2009.09091261. appi.ajp.2009.09091261 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Wells EA, Jiang H, Suarez-Morales L, Campbell AN, Cohen LR, Nunes EV. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. J Consult Clin Psychol. 2009;77(4):607–619. doi: 10.1037/a00162272009-11168-002. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigian VE, Robbins MS, Dominguez R, Ucha J, Rosa CL. Principles for defining adverse events in behavioral intervention research: lessons from a family-focused adolescent drug abuse trial. Clin Trials. 2010;7(1):58–68. doi: 10.1177/17407745093565757/1/58. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe GW, Lehman W, Simpson DD. Addict death rates during a four-year posttreatment follow-up. Am J Public Health. 1982;72(7):703–709. doi: 10.2105/ajph.72.7.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Feaster DJ, Gomez ZL, Das M, Tross S, Wiest K, Metsch LR. Injection behaviors among injection drug users in treatment: the role of hepatitis C awareness. Addictive Behaviors. 2012;37(4):552–555. doi: 10.1016/j.addbeh.2011.12.001. S0306-4603(11)00402-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp F, Somoza E, Lilleskov M, Moccasin MG, Moore M, Lewis D, Winhusen T. Characteristics of Northern Plains American Indians seeking substance abuse treatment in an urban, non-tribal clinic: a descriptive study. Community Ment Health J. 2013;49(6):714–721. doi: 10.1007/s10597-012-9537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JM, editor. A Dictionary of Epidemiology. Oxford University Press; New York: 1983. [Google Scholar]
- Liddell FD. Simple exact analysis of the standardised mortality ratio. Journal of Epidemiology and Community Health. 1984;38(1):85–88. doi: 10.1136/jech.38.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson CL, Shopshire MS, Sen S, Hoffman KA, Hengl NS, Bartolome J, Iguchi MY. Possible barriers to enrollment in substance abuse treatment among a diverse sample of Asian Americans and Pacific Islanders: opinions of treatment clients. J Subst Abuse Treat. 2013;44(3):309–315. doi: 10.1016/j.jsat.2012.08.005. S0740-5472(12)00140-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D, Fuller B, Kaskutas LA, Wendt WW, Nunes EV, Miller M, Edmundson E. Treatment programs in the National Drug Abuse Treatment Clinical Trials Network. Drug Alcohol Depend. 2008;92(1-3):200–207. doi: 10.1016/j.drugalcdep.2007.08.004. S0376-8716(07)00295-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meade CS, Weiss RD, Fitzmaurice GM, Poole SA, Subramaniam GA, Patkar AA, Woody GE. HIV risk behavior in treatment-seeking opioid-dependent youth: results from a NIDA clinical trials network multisite study. Journal of Acquired Immune Deficiency Syndromes. 2010;55(1):65–72. doi: 10.1097/QAI.0b013e3181d916db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONDCP [Retrieved January 18, 2012];National Drug Control Strategy 2012. Policy & Research. 2012 from http://www.whitehouse.gov/sites/default/files/ondcp/2012_ndcs.pdf.
- Paulozzi LJ, Weisler RH, Patkar AA. A national epidemic of unintentional prescription opioid overdose deaths: how physicians can help control it. Journal of Clinical Psychiatry. 2011;72(5):589–592. doi: 10.4088/JCP.10com06560. [DOI] [PubMed] [Google Scholar]
- Rehm J, Frick U, Hartwig C, Gutzwiller F, Gschwend P, Uchtenhagen A. Mortality in heroin-assisted treatment in Switzerland 1994-2000. Drug and Alcohol Dependence. 2005;79(2):137–143. doi: 10.1016/j.drugalcdep.2005.01.005. S0376-8716(05)00031-1 [pii] [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. 5th ed Duxbury Press; Pacific Grove, CA: 2000. [Google Scholar]
- Sahai H, Khurshid A. Confidence intervals for the mean of a Poisson distribution: a review. Biometrical Journal. 1993;35(7):857–867. [Google Scholar]
- Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2(3):209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Keyser-Marcus L, Stitzer M, Rieckmann T, Safford L, Loeb P, Zweben J. Randomized multi-site trial of the Job Seekers' Workshop in patients with substance use disorders. Drug Alcohol Depend. 2012;120(1-3):55–64. doi: 10.1016/j.drugalcdep.2011.06.024. S0376-8716(11)00302-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai B, Straus MM, Liu D, Sparenborg S, Jackson R, McCarty D. The first decade of the National Drug Abuse Treatment Clinical Trials Network: bridging the gap between research and practice to improve drug abuse treatment. Journal of Substance Abuse Treatment. 2010;38(Suppl 1):S4–13. doi: 10.1016/j.jsat.2010.01.011. S0740-5472(10)00025-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall MW, Craib KJ, Currie S, Li K, O'Shaughnessy MV, Schechter MT. Impact of HIV infection on mortality in a cohort of injection drug users. Journal of Acquired Immune Deficiency Syndromes. 2001;28(4):351–357. doi: 10.1097/00126334-200112010-00008. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report 2012. United Nations Publications; New York, NY, USA: 2012. pp. 7–57. [Google Scholar]
- Watterson O, Simpson DD, Sells SB. Death rates and causes of death among opioid addicts in community drug treatment programs during 1970-1973. American Journal of Drug and Alcohol Abuse. 1975;2(1):99–111. doi: 10.3109/00952997509002726. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. archgenpsychiatry.2011.121 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells EA, Saxon AJ, Calsyn DA, Jackson TR, Donovan DM. Study results from the Clinical Trials Network's first 10 years: where do they lead? Journal of Substance Abuse Treatment. 2010a;38(Suppl 1):S14–30. doi: 10.1016/j.jsat.2009.12.009. S0740-5472(10)00019-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells EA, Saxon AJ, Calsyn DA, Jackson TR, Donovan DM. Study results from the Clinical Trials Network's first 10 years: where do they lead? J Subst Abuse Treat. 2010b;38(Suppl 1):S14–30. doi: 10.1016/j.jsat.2009.12.009. S0740-5472(10)00019-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Winstanley EL, Somoza E, Brigham G. The potential impact of recruitment method on sample characteristics and treatment outcomes in a psychosocial trial for women with co-occurring substance use disorder and PTSD. Drug Alcohol Depend. 2012;120(1-3):225–228. doi: 10.1016/j.drugalcdep.2011.06.014. S0376-8716(11)00277-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, Fudala P. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574300/17/2003. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JB, Yu XF. [Retrieved August, 2012];A SAS® Program for Estimating Incidence Density for Cohort Studies with Multiple Follow-ups. 2008 PH008. from http://www.nesug.org/proceedings/nesug03/ph/ph008.pdf.