Abstract
Detecting infections of Strongyloides stercoralis is arduous and has low sensitivity. Clinically this is a major problem because chronic infections may disseminate in the host and lead to a life threatening condition. Epidemiologically, S. stercoralis is often missed in surveys as it is difficult to identify by standard stool examination procedures. We present, for the first time, evidence that the infection can be detected in filtered urine samples collected and processed in the field and subsequently assayed for the presence of parasite DNA. Urine specimens (~40 ml) were collected from 125 test and control individuals living in rural and peri-urban regions of Northern Argentina. From the same individuals, fresh stool specimens were processed using three different copropological methods. Urine specimens were filtered in the field through a 12.5cm Whatman No. 3 filter. The filters were dried and packed individually in sealable plastic bags with desiccant and shipped to a laboratory where DNA was recovered from the filter and PCR-amplified with primers specific to a dispersed repetitive sequence. Prevalence of S. stercoralis infection by stool culture and direct examination was 35/125 (28%), In contrast, PCR-based detection of parasite-specific trans-renal DNA in urine indicated that 56/125 (44.8%) carried the parasite. Of the patients that tested positive for urine-based parasite DNA, approximately half also tested positive in their stool specimens. There were 6.4% of cases where parasite larvae were seen in the stool but no DNA was amplified from the urine. As proof of principle, DNA amplification from urine residue reveals significantly more cases of S. stercoralis infection than the current standard stool examination techniques. Additional work is required to establish the relative utility, sensitivity and specificity of urine-based analysis compared to parasitological and nucleic acid detection from stool for clinical and epidemiological detection for S. stercoralis infection.
Keywords: Strongyloides stercoralis, diagnostic test, DNA detection, urine specimen, gastro-intestinal parasite
Graphical Abstract
The environment for Strongyloides stercoralis transmission. (photo: Dr A. Krolewiecki)
1 Introduction
Among the most neglected of the neglected tropical diseases is the infection caused by nematodes in the genus Strongyloides. Of the two species of Strongyloides that infect humans, S. stercoralis, which is mainly found in tropical and subtropical regions, is the most prevalent (Schad 1989). Human infections with S. fuelleborni and S. fuelleborni kellyi are restricted to Africa and New Guinea, respectively (Pampiglione and Ricciardi 1972, Ashford et al. 1992). The complicated life cycle of Strongyloides contributes to its persistence as a pathogen. Infection is initiated when the free-living infective third larval stage (L3) penetrate the skin. The larvae migrate, presumably via the blood and lungs, to the oropharyngeal area where they are swallowed and the parasite develops into reproductively active adult females in the small intestine where they release eggs. Within the intestinal tract, the first-stage larvae (L1) emerge from the eggs. The L1s have two main fates. They can be released into the environment and undergo L2-L4 development into rhabditiform male and female worms and establish a single sexually reproducing, free-living cycle that generates large numbers of long-lived L3 parasites capable of spreading the infection. Alternatively, the L1s can develop directly into an infective L3 within the intestine and initiate an autoinfection that, if uncontrolled as observed in immunocompromised individuals, can result in fulminant expansion of the parasite, multi-organ involvement and a fatal outcome. It is estimated that between 100 and 200 million individuals are infected with Strongyloides worldwide, however this is likely to be a serious underestimate because these infections are difficult to detect (Schar et al. 2013). The Kato-Katz and McMaster's procedures, the WHO recommended diagnostic techniques most commonly used to detect worm infections, focus on the identification of eggs in processed stool and hence miss larvae of Strongyloides spp. The low number of eggs or larvae released by females on a sporadic and unpredictable schedule compromises the sensitivity of these traditional diagnostic methods. Detection of specific cell-free DNA in urine appears to improve sensitivity over stool for some neglected tropical diseases (Ibironke et al. 2012, Lodh et al. 2014) as will be demonstrated here with Strongyloides.
Efforts to address this insensitivity gap have concentrated on serology to detect anti-Strongyloides antibodies and detection of parasite DNA in stool specimens (Pilotte et al. 2016). While antibody-based detection systems employing species-specific recombinant antigens can be a sensitive measure of exposure, the decline of antibody response over some months limits the utility of this approach for post-treatment assessment of incidence and prevalence. While DNA-based detection methods in stool specimens have been shown to be adequately sensitive (Basuni et al. 2011), the operational assumption that parasites are present in each stool sample may not be valid. An alternative approach that has shown validity for other pathogens is the detection of cell-free DNA in the urine (Ibironke et al. 2012). The utility of detecting parasite-specific trans renal DNA has been demonstrated for schistosomes (Ibironke et al. 2012) and Plasmodium (Mharakurwa et al. 2006).
The objective of this work is to demonstrate the feasibility of using a urine-based diagnostic test for S. stercoralis. To this end, a short repeat fragment of the S. stercoralis genome was tested for its practicality and diagnostic suitability among endemic communities in Northern Argentina.
2. Materials and Methods
2.1 Study Population
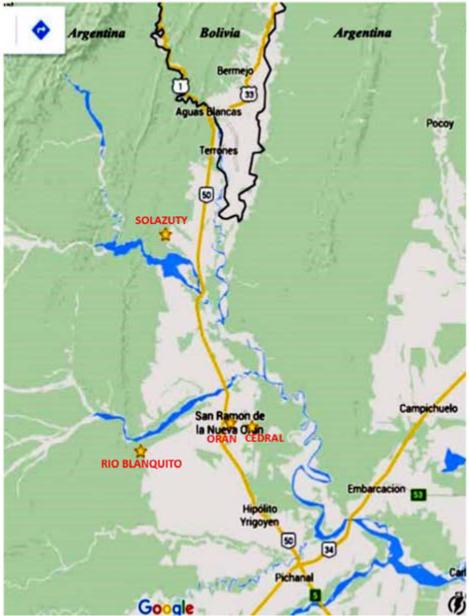
The study population was selected among rural and urban communities from Salta province, Argentina. Two rural villages, Solazuty and Rio Blanquito, (Fig.1) are agricultural communities with open or limited sanitary facilities and a lack of potable water in the houses. The peri-urban community surrounds the city of San Ramon de la Nueva (population of ~ 74,000 (Fig.1) where the population has access to potable water from taps but in the periphery latrines are open and often there is alfresco defaecation. Participating individuals were among those attending at the outpatient laboratory of the Instituto de Investigaciones en Enfermedades Tropicales at the Universidad Nacional de Salta as well as individuals under coverage by the primary public health care system in communities participating in a programme for the control of soil transmitted helminths (STH) infections. Demographic data of the participants in this study are presented in Table 1.
Figure 1.
Map of Northern Argentina, indicating locations of the three communities that participated in the study. These are represented by stars. (Image Google Earth)
Table 1.
Demographics of population that provided specimens
| Age | Female | Male | Total |
|---|---|---|---|
| 3-5 | 14 | 10 | 24 |
| 6-15 | 46 | 29 | 75 |
| 16-30 | 8 | 5 | 13 |
| ≥31 | 6 | 5 | 11 |
| Total | 74 | 49 | 123* |
2 specimens were not included as age and sex was unrecorded, however 125 samples were used in the study which did not require this information.
2.2 Ethical Consent
Approval for this work was obtained from Commité de Ética, Colegio Médico de Salta, Salta, Argentina, and Johns Hopkins University (IRB number 6199).
2.3 Sample Collection
: Samples consisting of a single stool and a single urine sample were collected from members of the rural and periurban communities. Fresh stool specimens were taken to the University of Salta laboratory, and processed using sedimentation-concentration, Harada Mori and Baermann techniques for hookworm and Strongyloides identification as outlined previously (Garcia 2001). Approximately 40 ml of urine was filtered through a 12.5 cm Whatman No. 3 filter disc. Filtration was done within 24 hours after passing the urine specimen. The filter disc was dried under a fly proof cover and stored in a sealed plastic bag with desiccant at 4°C and subsequently sent to Johns Hopkins University in Baltimore for processing and analysis for parasite DNA.
2.4 DNA Detection
Filter papers were processed and analysed in a blinded fashion. Fifteen 1.0 mm discs were punched from the central portion of the filter paper and were transferred to a 1.5 ml Eppendorf tube and 800 μL of water was added to each tube. After incubation at 95° C for 10 min the samples were subject to gentle agitation overnight at room temperature. Following the overnight stand, tubes were centrifuged at 4000 rpm to pack the paper and the supernatant was removed and processed from DNA extraction using the QIAmpDNA Blood Mini Kit (Qiagen, MD) according to manufacturer's protocol. The amount of recovered DNA was measured by NanoDrop ND-1000 spectrophotometer (Thermo Scientific Milwaukee, WI) and stored at −20°C.
2.5 DNA Standardization and amplification
Primers were designed specifically to amplify a 125 bp fragment from a S. stercoralis dispersed repetitive sequence (Fig 2). The forward primer (SSC-F) 5' CTC AGC TCC AGT AAA GCA ACA G 3' and reverse primer (SSC-R) 5' AGC TGA ATC TGG AGA GTG AAG A 3' were designed by using PrimerQuest Tool (IDT, Coralville, Iowa). PCR amplification was in a 15 μL volume with Taq 2X Mastermix (New England Biolabs, Ipswich, Mass.), 0.75 μL of 10 μM of each primer, 1 to 2 μL (20-100 ng/μL) of DNA and PCR-grade water (Sigma-Aldrich, St. Louis, Missouri). The amplification profile was initial denaturation at 95° C for 10 min and 35 cycles at 95°C for 1 min, 63°C for 1 min 30s, 72°C for 1 min and final extension at 72°C for 10 min. To confirm amplification and amplicon size the PCR products were resolved on a 2% agarose gel stained with Ethidium Bromide (Sigma-Aldrich, St. Louis. Missouri). These primers were tested against a series of helminths DNA specimens from three Ancylostoma spp, Schistosoma mansoni and S. haematobium and found only to amplify a product from S. stercoralis (data not shown).
Figure 2.
Repeat Sequence from GenBank: AY028262 the target and primers are indicated.
Plasmid Construction
The S. stercoralis DNA repeat sequence was synthesized (Genscript Biotech Corporation, Piscataway Township, NJ) and ligated into the EcoR1 site in pUC. The plasmid construct was transfected into E.coli and the amplified/purified plasmid was used at 236 ng/μL.
2.6 Titration experiments
Genomic DNA from S. stercoralis was diluted to 40 ng/μL for use. In order to assess the degree of sensitivity, a series of serial dilutions were made using genomic or plasmid DNA starting from the known concentrations given above using nuclease free water as diluent. A starting concentration of 5 ng/μL was diluted sequentially from 5 ng/μL through to 1 fg/μL to determine the extinction level. Amplification procedures were performed as outlined above.
DNA amplification was done in duplicate for each sample. Positivity was recorded when two amplifications were positive. Positivity was also confirmed by sequencing. PCR products were cleaned with ExoSAP-IT (Affymetrix Inc. Cleveland, OH) and sequenced and compared to the S. stercoralis repeat sequence in GenBank (AY028262).
3. Results
3.1 S. stercoralis DNA in urine
Preliminary studies revealed that small fragments of a parasite-specific dispersed DNA repeat sequence (GenBank accession AY028262; Fig 2) were able to traverse the kidneys and be detected in the urine. Experiments designed to determine the size range of the DNA fragments derived from the 765 bp S. stercoralis repeat that could be reliably detected as urine-based DNA, indicated an upper limit of ~150-170 bp (data not shown). Consequently, primers were designed to specifically amplify a 125 bp fragment from the S. stercoralis dispersed repetitive sequence (Fig. 2). For all samples with a PCR product of the correct size, sequence analysis showed that the product matched completely the sequence of the GenBank entry suggesting that this repeat is highly conserved and could serve as a consistent target across different geographic strains of S. stercoralis (data not shown).
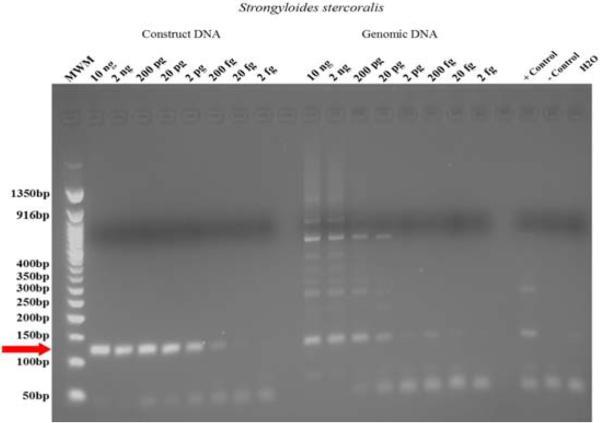
To gain some idea of the dynamic range of the base PCR assay, serial dilutions of S.stercoralis genomic DNA were made between 10 ng and 2 fg (Fig. 3). PCR products were robustly detectable out to 20 pg of parasite DNA. When a plasmid containing a single copy of the 765 bp S. stercoralis repeat was used as template, the sensitivity was increased to between 20 and 2 fg of plasmid DNA (Fig. 3).
Figure 3.
Demonstration of the cut off dilution for sensitivity to detect dilutions of the target fragment of DNA detected either in a construct fragment of the target or a dilution of entire genomic DNA prepared from standard. Red arrow indicates the 125 bp diagnostic DNA fragment
3.2 Detection of S. stercoralis DNA from urine of infected individuals
The current standard for diagnostics of S. stercoralis infection is the detection of larvae in a stool sample. This study was designed to compare the diagnostic efficacy of parasitological copro-diagnostic methods (concentration-sedimentation, Harada-Mori and Baermann) with DNA-based detection of the parasite-derived repeat DNA in urine. Of the samples derived from 125 individuals 61 were negative in both stool and urine based analyses (Table 2). For the urine samples, 56 had detectable levels of S. stercoralis repeat DNA. Interestingly only 27 of the 56 individuals who were positive for the S. stercoralis-repeat fragment were positive for matched stool samples. A further eight individuals had stool samples that were positive for larvae but were negative for parasite repeat DNA in a urine sample taken at the same time.
Table 2.
Results of diagnostic examination comparing: (a) detection of Strongyloides stercoralis larvae in stools with (b) parasite specific DNA fragment detected in urine residue on filter paper. These data were used to generate parasite prevalence in the sample analysed.
| N: 125 samples | Stool analysis | ||
|---|---|---|---|
| + 35 (28%) | − 90 (72%) | ||
| Urine PCR | + 56 (44.8%) | 27 (21.6%) | 29(23.2%) |
| − 69 (55.2%) | 8 (6.4%) | 61(48.8%) | |
Thus, DNA-based detection in urine indicates prevalence infection of 44.8% with a positive predictive value of 77.42% (95% CL 58.90-90.41%). By contrast the standard parasitological method calculated by detection of larvae suggests a prevalence of only 28% with a positive predictive value of 36.92% (95% CL 25.26-49.80%). Only 21.6 % of positive cases were congruent while 48.8% negative specimens were in agreement. The disparity is most likely to be increased sensitivity by detection of cell-free S. stercoralis DNA. Comparing urine positivity versus stool positivity the difference is significant P = 0.0058.
4. Discussion
This work was performed to determine the overall use and validity of detecting of cell-free DNA from the parasite S. stercoralis in urine after filtration done in the field. There appears to be a clear benefit to this approach in terms of sensitivity. The estimate of prevalence of infection was almost doubled when detecting the S. stercoralis specific repeat compared with coprological diagnosis of parasites. Although the estimates from the analysis of urine samples indicates a higher prevalence of S. stercoralis infection compared to parasitological analysis of stool, additional work is required to determine the true sensitivity and specificity of the two diagnostic approaches for clinical samples.
There is always an unknown factor in interpreting diagnostic tests that do not clearly meet the rigours of Koch's postulates, viz. isolation or demonstration of the infective organism. However with the large range of parasites where cell-free DNA has been detected and shown to be a useful diagnostic, one can argue that detection of pathogen specific DNA fragments in the host urine provides evidence that the pathogen is present. However, in the case of S. stercoralis, we have shown that in just over 6% of cases, there was no DNA detected from the urine but larvae were seen in the stool specimens. This may be due to technical error, but at this stage there is insufficient evidence to determine a biological basis to this observation. Studies done in the same region of Argentina as those reported here, have found that serological surveys using the recombinant antigen NIE in an ELISA platform in an untreated population, were able to detect twice as many infections as found in a comprehensive stool evaluation (Krolewiecki et al. 2010). These are of similar sensitivity as reported here, however antibody levels remain high even after treatment, this does not happen with cell-free DNA as shown by examining schistosome infected people who were found not to be passing parasite specific DNA in urine two weeks post-treatment with praziquantel (Ibironke et al. 2011).
Cell-free DNA has been detected in urine of patients with a number of blood-borne and tissue dwelling protozoan and helminth parasites (Lodh et al. 2013, Ibironke et al. 2011, Mharakurwa et al. 2006). Although S. stercoralis resides in the gut as an adult form, the parasite can maintain an autoinfection cycle in immunocompetent hosts that results in tissue dissemination (Schad et al. 1989, Schad et al. 1989). This happens when rhabditiform larvae emerge within the gut, moult twice and become filariform, then traverse the gut wall and enter the circulation system. They then pass through the lungs and lodge in various organs or tissues in the body. When this phenomenon occurs with high magnitude of migrating larvae it is called the Hyperinfection Syndrome (Marcos et al. 2008); however, at low levels this subclinical event is the key step for chronic infections for several decades (Krolewiecki et al. 2013). Detection of specific cell-free DNA is feasible, realistic, and applicable in most endemic countries with amplification either by conventional PCR or Loop mediated Isothermal Amplification (LAMP). This is crucial not only to determine the overall public health impact of the pathogen, but also to understand the extent of the infection in communities so that attempts to control or eliminate these pathogens are efficient and cost-effective.
Highlights.
We have shown that Strongyloides stercoralis DNA can be detected in urine.
There is a strong probability that this is a significant advance over fecal examination.
Urine specimens are filtered in the field, dried and packed with desiccant specimens are stable.
Diagnosis based on urine obviates the time expended and health risk of examining fresh stool.
Acknowledgements
Grateful thanks are due to Dr Sean Prigge and Hugo Jhun for cloning the repeat fragment. We would like to acknowledge the collaboration of the participating communities and their members. Adrian Vargas, Maria Canabire, Pamela Cajal and Marisa Juarez for their assistance in stool analysis. Funding for this work was provided through Grant 1R21 AI113475-01A1 from NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ashford RW, Barnish G, Viney ME. Strongyloides fuelleborni kellyi. Trends in Parasitology. 1992;8:318. doi: 10.1016/0169-4758(92)90106-c. [DOI] [PubMed] [Google Scholar]
- Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Rahumatullah A, Aziz FA, Zainudin NS, Noordin R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. Diagnostic Medical Parasitology. 4 ed. ASM Press; Washington. DC.: 2001. [Google Scholar]
- Ibironke OA, Koukounari A, Asaolu S, Moustaki I, Shiff C. Validation of a new test for Schistosoma haematobium based on detection of the Dra1 DNA repeat fragment in urine: evaluation through latent class analysis. PLoS Neglected Tropical Diseases. 2012;6:e1464. doi: 10.1371/journal.pntd.0001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibironke OA, Phillips AE, Garba A, Lamine SM, Shiff C. Diagnosis of Schistosoma haematobium by detection of specific DNA fragments from filtered urine samples. Am J Trop Med Hyg. 2011;84:998–1001. doi: 10.4269/ajtmh.2011.10-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki AJ, Lammie P, Jacobson J, Gabrielli AF, Levecke B, Socias E, Arias LM, Sosa N, Abraham D, Cimino R, Echazu A, Crudo F, Vercruysse J, Albonico M. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis. 2013;7:e2165. doi: 10.1371/journal.pntd.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, Juarez M, Di PA, Tapia L, Acosta N, Lee R, Lammie P, Abraham D, Nutman TB. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin. Vaccine Immunol. 2010;17:1624–1630. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh N, Mwansa JC, Mutengo MM, Shiff CJ. Diagnosis of Schistosoma mansoni without the stool: comparison of three diagnostic tests to detect Schistosoma mansoni infection from filtered urine in Zambia. Am J Trop Med Hyg. 2013;89:46–50. doi: 10.4269/ajtmh.13-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh N, Naples JM, Bosompem KM, Quartey J, Shiff CJ. Detection of Parasite-Specific DNA in Urine Sediment Obtained by Filtration Differentiates between Single and Mixed Infections of Schistosoma mansoni and S. haematobium from Endemic Areas in Ghana. PLoS One. 2014;9:e91144. doi: 10.1371/journal.pone.0091144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield LS, Niamatali S, Bhopale V, Volk SSG, Lok JB, Genta RM, Schad GA. Strongyloides stercoralis: maintenance of exceedingly chronic infections. Am. J. Trop. Med. Hyg. 1996;55:617–624. doi: 10.4269/ajtmh.1996.55.617. [DOI] [PubMed] [Google Scholar]
- Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans. R. Soc. Trop Med Hyg. 2008;102:314–318. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Mharakurwa S, Simoloka C, Thuma PE, Shiff CJ, Sullivan DJ. PCR detection of Plasmodium falciparum in human urine and saliva samples. Malar J. 2006;5:103. doi: 10.1186/1475-2875-5-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglione S, Ricciardi ML. Geographical distribution of Strpngyloides fuelleborni in humans in tropical Africa. Parasitologia. 1972;14:329–338. [Google Scholar]
- Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, Williams SA. Improved PCR-Based Detection of Soil Transmitted Helminth Infections Using Next-Generation Sequencing Approcah to Assay Design. PLoS NTD. 2016;10:e0004578. doi: 10.1371/journal.pntd.0004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad G. Strongyloidiasis, a major infection of man. Taylor and Francis; London: 1989. Morphology and life history of Strongyloides stercoralis. [Google Scholar]
- Schad G, Aikens L, Smith G. Strongyloides stercoralis: Is there a canonical migratory route through the host? J. Parasitol. 1989;75:740–749. [PubMed] [Google Scholar]
- Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7:e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff C. Accurate diagnostics for schistosomiasis: a new role for PCR? Reports in Parasitology. 2015;4:23–29. [Google Scholar]