Abstract
Immunotherapy for castration resistant prostate cancer has continued to be an area of active research over the last several years. The enthusiasm of this approach has been based on the assumption of better tolerability and that using the body’s own immune system may be more effective than either hormonal or chemotherapy. Sipuleucel-T, a dendritic cell based vaccine is the only approved agent in this class for the management of castrate resistant prostate cancer. Although Sipuleucel-T increases overall survival without any significant changes in progression free survival, other forms of immunotherapy such as PSA-TRICOM, Ipilimumab and CAR-T cell therapy are in advanced stages of clinical development. Immune biomarkers are being developed to assess response to these treatments and also to understand how the immune system responds to these respective therapies. Combinations of immunotherapy with androgen deprivation, radiation therapy, and chemotherapy have also been explored with varying results. This review discusses the mechanisms, key preclinical and clinical data, and perspectives for immunotherapeutic agents in the treatment scheme for castrate resistant prostate cancer.
Introduction
Patients with castrate resistant prostate cancer are living longer with a defined natural history in most cases. As such, efforts to develop new therapeutic modalities that are as equally efficacious as Androgen Receptor (AR)-directed therapy and chemotherapy and have fewer adverse side effects are ongoing. Over the last decade, Cabazitaxel (Jevtana®) [1], Sipuleucel-T (Provenge®) [2], Abiraterone (Zytiga®) [3], Enzalutamide (Xtandi®) [4], and Radium-223 (Xofigo®) [5] have been approved for the treatment of men with castration resistant prostate cancer based on evidence of improved overall survival. Of these, only Sipuleucel-T is an immunotherapeutic agent. Cancer immunotherapy can be broadly classified as therapeutic cancer vaccines [6], checkpoint inhibitors [7] or adoptive cellular therapy [8]. Unlike cytotoxic chemotherapy and androgen deprivation therapy which act directly against the tumor, immunotherapy attempts to recruit the host's immune system to recognize the tumor as foreign and reject the tumor through direct cytolytic effect. Vaccine-based immunotherapy relies on the innate ability of antigen presenting cells (APC) to capture and process antigens for display to T cells. These APC’s can be harnessed to capture and present one or more prostate cancer tumor associated antigens (TAA) leading to the generation of a humoral and cytotoxic T cell response to the cancer (Fig. 1). Checkpoint inhibition relies on T cells that already recognize the TAA but have either been inhibited by signals intended to prevent autoimmunity or by a suppressive tumor microenvironment. These drugs act by uncoupling inhibitory signals or “brakes” to facilitate full T-cell activation (Fig. 2). Adoptive transfer of engineered T cells obviates the need for APCs or endogenous TAA-recognizing T cells and instead relies on engineered synthetic single chain variable fragments (scFv) that recognize the TAA in an HLA-independent fashion to facilitate T cell mediated cytotoxicity (Fig. 3). Research into the mechanisms of action of these immunotherapeutic agents and how best to integrate them into current treatment paradigms of CRPC management is continuing. This review discusses promising immune therapy agents, their mechanisms of action and current approaches to combination therapy with chemotherapy, radiation and AR-directed therapy.
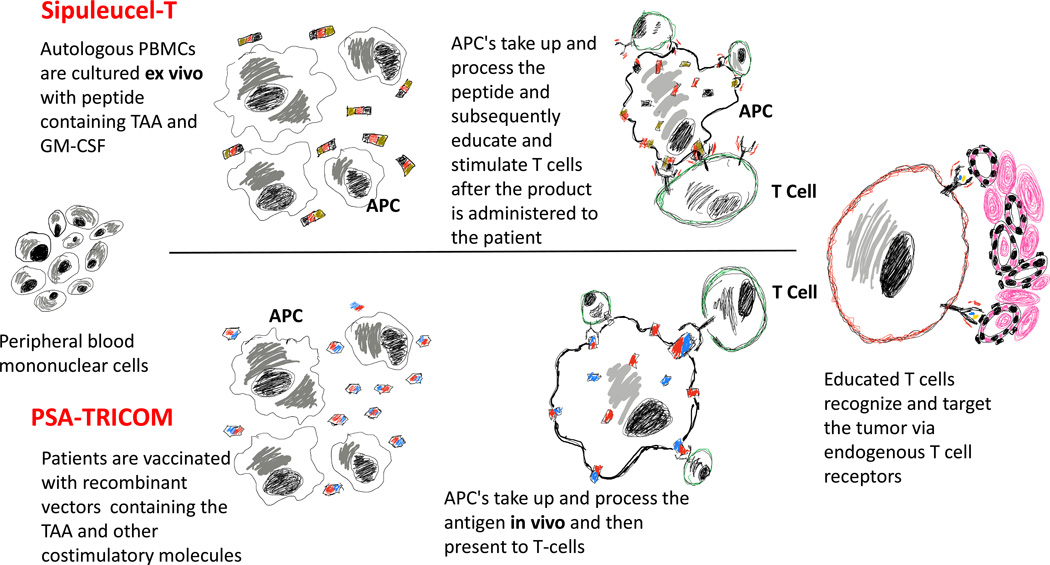
Figure 1.
Overview of vaccine-based immune therapy. Top panel (Sipuleucel-T): Autologous peripheral mononuclear cells are co-incubated with a synthetic peptide and costimulatory molecules. APC’s capture and process the antigen and costimulatory molecules ex vivo. Upon administration back to the patient, activated APC’s engage and educate T cells. Educated T cells seek out and eradicate tumor. Bottom panel (PSA-TRICOM): The vaccine containing the tumor associated antigen and costimulatory molecules are administered to the patient. The vaccine is processed by APC’s in vivo and the antigens/costimulatory molecules are presented to T cells. Educated T cells seek out and eradicate tumor.
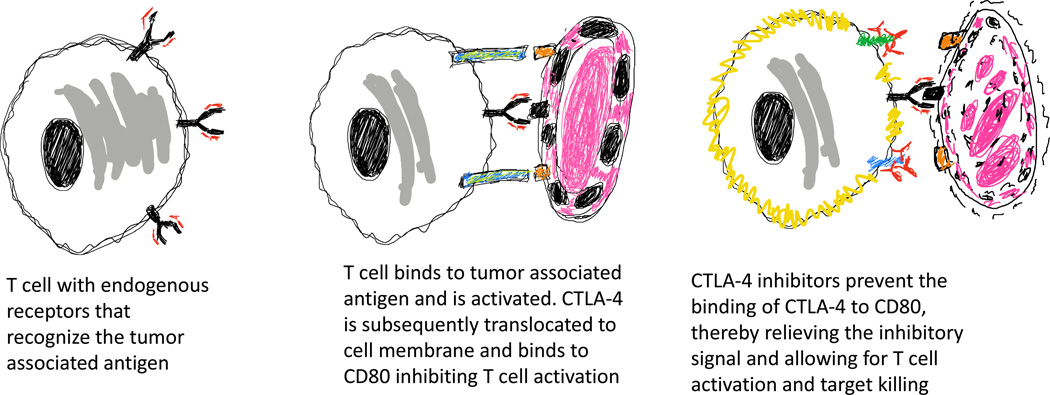
Figure 2.
Overview of checkpoint blockade. Endogenous T cells with T cell receptors that recognize tumor antigens initially engage the tumor and become activated. After activation, CTLA-4 is translocated to the cell membrane and binds to CD80 on the tumor. This CTLA-4/CD80 engagement leads to T cell inactivation. CTLA-4 inhibitors prevent interaction with CD80 leading to persistent T cell activation and tumor eradication.
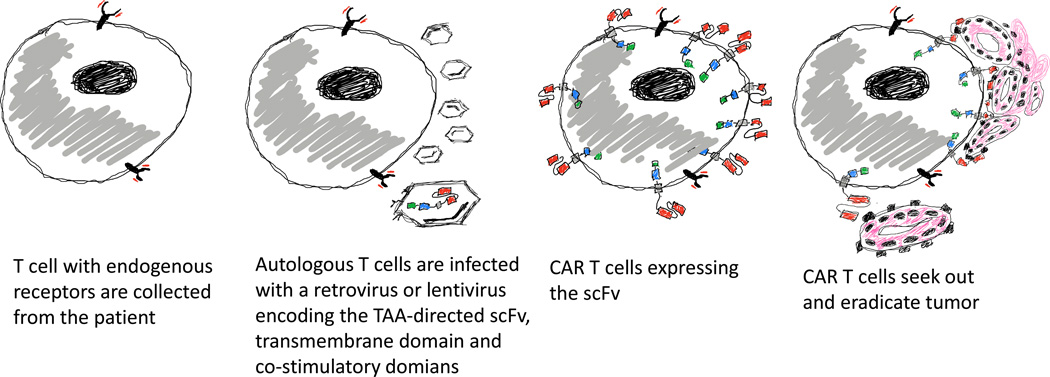
Figure 3.
Overview of CAR T immune therapy. Autologous T cells collected from the patient are transduced with a retrovirus or lentivirus that contains the chimeric antigen receptor construct. Transduced T cells are infused back to the patient where they seek out and eradicate tumor cells.
Sipuleucel-T
Sipuleucel-T (Provenge®) is currently the only approved cellular product immune therapy for the treatment of patients with asymptomatic or minimally symptomatic castrate resistant prostate cancer [6]. Sipuleucel-T is an autologous product derived from ex vivo co-incubation of a patient's peripheral blood mononuclear cells and antigen presenting cells with a recombinant fusion protein consisting of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony stimulating factor (GM-CSF), collectively referred to as PA2024 [9] (Fig. 1 top panel). This ex vivo modified product is administered intravenously every 2 weeks apart for a total of 3 doses. FDA approval of Sipuleucel-T was based on a multicenter, double-blind, placebo-controlled phase III trial published by Kantoff et al [2] showing improved overall survival in treated patients (table 1). Patients treated with Sipuleucel-T had an increased mean overall survival by 4.1 months compared to the placebo-treated group (25.8 months vs 21.7 months). Despite improvement in overall survival, time to clinical (HR: 0.92; 95% CI, 0.75 – 1.12; P=0.40) and objective disease progression (HR: 0.05; 95% CI, 0.77 – 1.17; P=0.63) was similar between the treatment and placebo groups. Consistent with a vaccination response, antibody titers to the immunogen and prostatic acid phosphatase were increased in the Sipuleucel-T group as well as T-cell proliferation index after 6 weeks, suggestive of a persistent pool of T cells that recognized the antigen. Another phase III study by Small et al [10] also did not show any differences in time to progression but did show an overall survival benefit of 4.5 months.
Table 1.
Table highlighting selected clinical trials of immunotherapy for castration-resistant prostate cancer.
| Study | Intervention | Outcome |
|---|---|---|
| Kantoff at al (IMPACT).2 |
Multicenter, double-blind, placebo-controlled phase 3 trial in men with castrate resistant prostate cancer |
22% relative reduction in the risk of death (HR 0.78, 95% CI 0.61–0.98, P=0.02) |
| Improved OS by 4.1 months in treatment group (25.8 months vs 21.7 months) |
||
| Small et al.10 | Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer |
Median OS 25.9 months vs 21.4 months (HR 1.70, 95% CI 1.13–2.56, P=0.01)1 |
| Time to disease progression 11.7 weeks vs 10 weeks (HR 1.45, 95% CI 0.99–2.11, P=0.052) |
||
| Kantoff et al.27 | Phase II randomized controlled trial of PROSTVAC-VF in metastatic castration-resistant prostate cancer |
Median OS 25.1 months vs 16.6 months (HR 0.56, 95% CI 0.37–0.85, P=0.0061)* |
| Median progression-free survival (PFS) 3.8 vs 3.7 months (HR 0.88, 95% CI 0.57–1.38, P=0.60)** |
||
| Slovin et al.43 | Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study |
Median OS 17.4 months (95% CI: 11.5–24.7) |
| PSA decline > 50% in 8 (16%0 patients at 10 mg/kg dose ± radiation. 1 patient had CR and 6 patients had SD | ||
| Kwon et al.44 | Multicenter, randomized phase III trial of Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel. The results of the phase III trial in the pre-chemotherapy space are currently pending publication. |
Median OS 11.2 months for Ipilimumab group vs 10 months for placebo (HR 0.85, 95% CI 0.72–1.00, P=0.05). |
| Increased PFS 4 months for ipilimumab group vs 3.1 months for placebo (HR 0.70, 95% CI 0.61–0.82, P<0.0001) | ||
OS was not the primary presepcified endpoint
Treatment vs Placebo
Mechanism of action
In an effort to understand the mechanism of Sipuleucel-T efficacy, Sheikh et al performed a detailed characterization of the post-activated immune cells subsets, post treatment humoral compartment, T-cell activation and cytokine analysis of patients treated on 3 phase III trials [11]. After ex vivo coculture with the fusion protein PA2024, the autologous derived product was rich in CD14+ APC's, CD3+ T-cells, CD56+ NK cells and a minority of CD19+ B cells. ELISPOT assays also demonstrated increased PA202- specific T cell proliferation and IFN-γ production. Supernatants from the PA2024 treated product had increased cytokines such as IL-2, IL-12, TNF-α, and IFN-γ [11]. In vivo, patients treated with Sipuleucel-T showed cellular and humoral responses against PA2024 and or PAP. While the data on the improved OS with Sipuleucel-T has been replicated, the absence of a clear mechanism of action has led to critiques on the experimental design [12], specifically the absence of a control arm that includes a GMCSF- expressing construct alone without the fused antigen product. Activated dendritic cells secrete GMCSF and GMCSF has been suggested to have antitumor activity based on a phase II clinical trial showing PSA responses in patients with castrate-resistant prostate cancer treated with exogenous GMCSF [13]. Another hypothesis for the mechanism of action of Sipuleucel-T involves modification or reshaping of the immune landscape. Analysis of patient samples from the IMPACT trial revealed evidence of significantly elevated serum IgG levels at weeks 2, 10 and 22 [14]. Further, these IgG molecules were directed against prostate cancer tumor associated antigens. Some of these antigens were confirmed by Luminex xMAP and included PSA, KLK2, E-ras and K-ras. None of these antigens were included in the PA202 fusion protein. Taken together with data from McNeel et al [15] which showed increased polyclonal gamma globulins, these findings are suggestive of antigen spreading via broader responses to non-PAP/PSAP antigens. The vast majority of studies attempting to elucidate the mechanisms of Sipuleucel-T activity have been correlative and hypothesis generating at best [15, 16, 17]. Fong et al [18] undertook a prospective approach by conducting an open label phase II trial of neoadjuvant Sipuleucel-T prior to radical prostatectomy. Histologic examination of post-treatment prostatectomy samples revealed increased infiltration of CD3+, CD8+, CD4+FoxP3− and CD4+FoxP3+ T cells at the tumor interface. Despite elevated PD-1 expression in the infiltrating CD3+ T cells, equally elevated Ki67 suggested that elevated PD-1 was more a marker of T-cell activation than exhaustion. It should be noted however that despite being on a continuum, hormone-sensitive prostate cancer and castrate-resistant metastatic prostate cancer are biologically distinct entities [19], and as such, the results of this study may not be applicable to castrate resistant disease.
Enhancing efficacy
Sipuleucel-T is generally well tolerated [9]. The most common side effects were grade 1 or 2 chills, fever, fatigue, nausea and headache, with most of these reactions occurring within 1–2 days following infusion [2]. Despite a survival benefit, it remains unclear how long a patient should be observed to determine whether or not there is an antitumor effect. Radiographic and biochemical responses to the treatment have been suboptimal; therefore, it is reasonable to combine Sipuleucel-T with other FDA approved agents. Kantoff et al reported improved overall survival attributable to Sipuleucel-T in the patients who went on to receive docetaxel chemotherapy [2]. Intriguing as these findings are, there are no currently active clinical trials exploring the combination of Sipuleucel-T and chemotherapy. This is perhaps due in part to the rationale that newer agents such like Sipuleucel-T are envisioned to delay or avoid the use of chemotherapy in men with castrate-resistant prostate cancer. Abiraterone is an orally bioavailable potent CYP17 inhibitor that significantly lowers circulating testosterone levels and is approved for the management of castrate resistant prostate cancer based on phase III clinical trials showing improved PFS and OS [3]. Small and colleagues [20] recently published the results of a phase II clinical trial examining the safety and immunological profile of patients treated with Sipuleucel-T and either sequentially or concurrently with Abiraterone. In the concurrent treatment arm, abiraterone was started 1 day after the first Sipuleucel-T infusion and in the sequential arm, abiraterone was started 6 weeks after the 3rd infusion of Sipuleucel-T. Treatment was well tolerated in both arms with muscle spasms, fatigue, back pain and nausea being the most adverse effects reported. The authors reported ex vivo APC activation and comparable in vivo cellular and humoral responses between both arms. There were no differences in IgG levels against PSA and LGALS3 between both arms. Patients from both arms had a ≥ 50% PSA decline and the percentage of patients responding in the concurrent and sequential treatment arms were not statistically significant (65.7% vs 58.8%; P= 0.6624). This report suggests that Abiraterone can be combined with Sipuleucel-T without compromising efficacy. A case report of a single patient with CRPC with metastasis to the bone responding to the addition of Sipuleucel-T to Enzalutamide has also been reported [21].
Perspectives
There are several currently active or recruiting clinical trials looking at the combination of Sipuleucel-T and radiation therapy (NCT01807065, NCT01833208), radium-223 (NCT02463799), anti-PD1 antibody (NCT01420965), CTLA-4 blockade (NCT01804465), recombinant IL-7 (NCT01881867) and IDO inhibitors (NCT01560923). Combining Sipuleucel-T with radiation therapy or Radium-223 might generate neoantigens that can be captured by APC’s that have been primed by Sipuleucel-T therapy. Alternatively, the efficacy of “educated” T cells might be further improved with the addition of CTLA-4 inhibitors, or cytokine therapy to Sipuleucel-T therapy. As these trials result, clinical correlates and translational research will provide more insight into the mechanism of Sipuleucel-T activity and help refine its role in the algorithm of management of men with castrate resistant prostate cancer.
PSA-TRICOM
PSA-TRICOM (Prostvac®) is a peptide antitumor vaccine utilizing a prime boost regimen based on a recombinant vaccinia vector containing PSA, B7.1, ICAM-1 and LFA3 (TRICOM) as the prime, followed by a fowlpox PSA-TRICOM vector as the boost [22,23]. Similar to the mechanism of action of many vaccines, the PSA-TRICOM viral construct is expected to infect dendritic cells and other immune cells whereupon the PSA antigen and TRICOM costimulatory molecules are presented by the APC to effector T cells [24] (Fig. 1 Bottom panel). A phase I study by DiPaola et al [23] reported 10 patients treated with 1 dose of vaccinia PSA-TRICOM on day 1 followed by another dose of fowlpox PSA-TRICOM on day 29. Examination for toxicity performed on day 57 showed that the treatment was well tolerated with injection site reactions and fatigue being the most common adverse effects. In another phase I trial by Arlen and colleagues [25], 3 doses of fowlpox PSA-TRICOM boosts with or without vector-based or recombinant GM-CSF was evaluated. Similar to the prior phase I study, PSA-TRICOM was well tolerated with injection site reactions, sweating and bone pain being the most common reactions, the latter of which is likely due to GM-CSF administration. A phase II randomized study in patients with chemotherapy naive metastatic CRPC randomized to receive PSA-TRICOM with or without vector-based or recombinant GM-CSF [26] has been reported. The authors reported “absolute” PSA declines in 12 of 32 (37.5%) patients, durable PSA response > 50% in 1 patient for over 30 months and 2 objective responses as assessed by regression of a soft tissue mass and lymphadenopathy. Kantoff et al [27] conducted a double blind placebo-controlled trial with PSA-TRICOM and recombinant GM-CSF. Patients with visceral metatstasis or symptomatic disease were excluded from study participation. PSA responses were infrequent on this study with only 1 patient achieving a > 80% PSA decline. There were no lymph node regressions and at 180 days, 23% of the patients in the control arm and 25% of the patients in the treatment arm had progressed. Median OS was increased in the treatment arm compared to the placebo-treated cohort (25.1 vs 16.6 months, HR=0.56, p=0.0061). A phase III randomized clinical trial in men with CRPC is completed and pending analysis (NCT01322490).
Mechanism of action
Examination of immune responses pre and post vaccination did not reveal any differences in PSA-specific T-cell responses among patients treated with PSA-TRICOM and either vector-based or recombinant GM-CSF [26]. In a separate report, a detailed quantitative and qualitative regulatory T cell (Treg) analysis was performed on patient samples [28]. The authors did not find any significant differences in CD4+CD25+FoxP3+ Treg numbers in patients with improved OS. However, most patients with improved OS had decreased Treg suppressive activity. Despite elaboration of antibody responses to vaccinia, no humoral response to PSA was observed by Kantoff et al [27]. There is speculation that similar to Sipuleucel-T, PSA-TRICOM improves overall survival by altering tumor growth dynamics [29]. It has also been proposed that perhaps PSA response, and progression free survival are not adequately suited to measuring response in vaccine based immunotherapy [29]. In contrast to these studies, Jochems et al reported differences in T cell cell populations in patients treated with combination PSA-TRICOM and ipilimumab (Yervoy®) [30]. However, the additive or synergistic effect of ipilimumab makes it challenging to attribute which treatment led to what finding. As previously mentioned, PSA-TRICOM is well tolerated which lends well to combinatorial therapy due to a reduced risk for additive toxicities.
Enhancing efficacy
In the absence of robust effector T cell activity in patients treated with PSA-TRICOM, combination with checkpoint blockade is a rational choice. Madan et al conducted a phase I dose escalation study of PSA-TRICOM in patients with metastatic CRPC, the majority of whom had not had previous chemotherapy (24/30) [31]. Treatment was generally well tolerated with only a few cases of grade 3/4 colitis, rash and endocrinopathies likely related to ipilimumab therapy. Of the patients who had not received prior chemotherapy, 25% (6/24) had a PSA decline > 50% from baseline and 58% had PSA decrease from baseline. In patients who had previous chemotherapy exposure, only 1 (of 6) had a PSA decline from baseline. Since this was a safety and tolerability trial, phase II trials are needed to explore efficacy.
Perpectives
The role of PSA-TRICOM in the algorithm of metastatic CRPC management is yet to be defined. Combining chemotherapy with PSA-TRICOM might lead to both a rapid anti-tumor response and could potentially augment the vaccination effect via elaboration of neoantigens upon tumor lysis. Furthermore, androgen ablation has been reported to reverse T-cell tolerance [32] and positively augment vaccine responses [33]. Clinical trials exploring combination therapy with docetaxel (NCT02649855) and enzalutamide (NCT01867333) are ongoing.
Ipilimumab
Checkpoint blockade has been collectively used to describe CTLA-4 inhibitors, PD-1 and PD-L1 inhibitors. Checkpoint inhibitors were catapulted into therapeutic relevance based on data showing improved progression free and overall survival in patients with metastatic melanoma treated with ipilimumab (Yervoy®) [34]. Further evidence to support efficacy for this class of drugs came with the approval of nivolumab (Opdivo®) in metastatic melanoma [35] and non-small cell lung cancer [36, 37] and more recently renal cell cancer [38] and with pembrolizumab (Keytruda®) for non small cell lung cancer [39]. The first agent in this class and perhaps the most studied is ipilimumab, an anti- CTLA-4 inhibitor. CTLA-4 is expressed on activated T cells [7]. CD80 (B7.1) is expressed on APC's and binds to its cognate receptor CD28 on T cells. This interaction provides an important costimulatory signal for T cell activation. CTLA-4 also binds to CD80 but instead provides an inhibitory signal. Ipilimumab binds to CTLA-4 and inhibits the interaction between CTLA-4 and CD80 thereby abolishing this negative regulation and promoting a persistent T cell activation [7] (Fig. 2). Achievement of a durable response, especially in patients with metastatic melanoma treated with ipilimumab [40] has prompted intensive investigation into its efficacy in prostate cancer. Small et al [41] treated 14 patients with castrate resistant prostate cancer with a single 3 mg/kg dose of ipilimumab. Only 2 of these patients had a PSA response, defined as a PSA decline of ≥ 50%. Both of these patients were re-treated upon relapse, but there were no further responses. A phase II study of patients with chemotherapy-naive castration resistant prostate cancer treated with 4 doses of ipilimumab given every 4 weeks and another arm that included a single dose of docetaxel was presented [42]. Although there were no radiographic responses, 3 patients in each treatment arm had a ≥ 50% PSA response. In another phase I/II report by Slovin et al [43], 71 patients were treated with either ipilimumab alone, or ipilimumab and external beam radiation therapy. Of 11 patients who received ipilimumab alone, 6 had a psa decline of ≥ 50%. Although there were some serious adverse immune related toxicities reported in the trial, the authors were able to reach the prespecified maximum dose of 10 mg/kg. The median overall survival was 17.4 months (95% CI: 11.5–24.7). The only published phase III trial for ipilimumab was conducted by Kwon et al [44]. In this 799 patient multicenter clinical trial, patients with castrate resistant prostate cancer with at least one bone metastasis were randomized to receive either ipilimumab (10 mg/kg every 3 weeks) or placebo after radiation. The prespecified primary endpoint was overall survival. The 6-month progression free survival was superior in the ipilimumab treatment group compared to placebo (30.7%, 95% CI 26.0–35.3 vs 18.1%, 14·2–22·0) and the frequency of psa responses were greater in the ipilimumab arm as well 13.1% vs 5.2% (95% CI 9.5–17.5 vs 3·0–8·4). However, the primary endpoint of OS was not met (ipilimumab: 11.2 months, 95% CI 9.5–12.7 vs placebo: 10.0 months, 95% CI 8.3–11.0; HR 0.85, CI 0.72–1.00; p=0·053). Subgroup analysis suggested that patients with no visceral metastasis, low bone alkaline phosphatase (< 1.5× ULN), and higher hemoglobin (> 11.0 g/dl) were more likely to benefit from ipilimumab therapy. These findings are thought provoking but ultimately can only be interpreted as hypothesis generating. A randomized phase III trial in patients with asymptomatic or minimally symptomatic metastatic castration resistant prostate cancer who have not had previous chemotherapy has completed and results are eagerly anticipated (NCT01057810).
Mechanism of action
The reason ipilimumab works in some solid tumors but not others is currently an area of intense investigation. Differences in doses and concurrent treatments (i.e docetaxel, EBRT) have made cross-trial comparisons challenging in prostate cancer ipilimumab trials. T-cell infiltration has been reported in prostatectomy samples [45] and CD4+CD25+FoxP3+ regulatory T cells have also been found in high numbers in peripheral blood and prostatectomy samples [46]. It has been hypothesized that these infiltrating effector T cells are suppressed by neighboring regulatory T cells. Preclinical in vivo models in mice show quite elegantly that prostate cancer cell lines modified to express the CTLA-4 cognate receptor B7 are rejected by the host's immune system [47]. In this model, treatment of wild type prostate cancer cell lines with anti-CTLA-4 antibodies led to significant growth inhibition or outright tumor eradication. Extended to a mouse model of residual disease after surgical resection, anti-CTLA-4 antibodies significantly decreased relapse after resection of the primary tumor by eradicating residual nodal metastasis after surgery [48]. The weight of this preclinical evidence makes the failure of ipilimumab in prostate cancer clinical trials all the more perplexing. A detailed characterization of the immunological landscape after ipilimumab treatment was reported by Jochems et al [30]. This report examined immune correlates of patients treated with a single dose of PSA-TRICOM and escalating doses of ipilimumab. A PSA response was reported in 25% (6/24) of treated patients. Comparison of cellular immunophenotypes at baseline and up to 13 and 70 days post treatment showed a positive correlation between increased activated CD8 T cells (PD-1NegTIM-3+CD8+), lower effector memory CD4 T cells (PD-1+TIM-3NEGCD4EM), increased mature NK cells (CD16+CD56DIM), and lower regulatory T cells (CD4+CD25HiFoxP3+CD127NEG) with an increase in overall survival [30]. One caveat in the interpretation of this study is that vaccination therapy was also used; this might confound delineation of the effects of ipilimumab alone on modulation of the immunophenotypic landscape. Although correlative in nature, identification of an immunophenotypic profile that correlates with survival might one day be used as a tool to identify patients who might benefit in the long term from ipilimumab. Lessons learned from this and other correlative studies can then be retroactively applied to preclinical animal models to better understand why ipilimumab works for some patients and more importantly, how it can be improved.
Enhancing efficacy
Due to the unique mechanism of action of ipilimumab, clinical trials evaluating synergy between ipilimumab and radiation [43, 44], PSA-TRICOM [31], GVAX [49], and docetaxel [42] have been explored. However, one of the primary concerns regarding ipilimumab has been its adverse effect profile [50]. These so called immune-related adverse events include grade 3 rash, dizziness and asthenia at the 3 mg/kg dose [41]. In one trial involving a single dose of radiation therapy and 10 mg/kg of ipilimumab [43], there was significant grade 3/4 diarrhea, colitis and hepatitis, including one treatment-related mortality at the 5 mg/kg dose secondary to sepsis. Notably in this trial, there was one patient who achieved a complete and durable remission. The phase III trial by Kwon et al also involving radiation reported 4 treatment related mortalities and 5 gastrointestinal perforations in addition to colitis, rash and pruritus [44]. Although it is tempting to draw an association with the use of radiotherapy and increased severity of the adverse events reported in these trials, similar immune related toxicities have been observed in melanoma clinical trials and these patients did not receive radiation therapy [50]. Similarly, colitis, rash, adrenal insufficiency and panhypophysitis have been reported in the vaccine combination arms [31, 49]. Prompt recognition and intervention has mitigated many of the on-target off-tumor toxicities reported in the early trials of ipilimumab therapy.
Perspectives
Despite some of the setbacks with checkpoint blockade therapy for prostate cancer in general and for ipilimumab in particular, efforts are ongoing to determine which combination of available or novel agents will synergize with ipilimumab. There are currently trials exploring the role of combining immediate or delayed CTLA-4 inhibition with Sipuleucel-T (NCT01804465), combination ipilimumab and nivolumab (NCT02601014), and MGA271, a novel monoclonal antibody directed against B7-H3, in combination with ipilimumab in patients with castrate resistant prostate cancer (NCT02381314). Clinical trials exploring PD-1 inhibition in prostate cancer are also currently in progress (NCT01420965, NCT02499835, NCT02312557, NCT02475213).
CAR T therapy
Chimeric antigen receptor (CAR) T cells are autologous T cells that have been engineered ex vivo to express a chimeric antigen receptor directed against a tumor associated antigen [51] (Fig. 3). CAR T cells consist of a synthetic T cell receptor or single chain variable fragment (scFv) consisting of the variable regions of an antibody heavy and light chain directed against a tumor associated antigen. This sequence is followed by one or more signaling domains designed to facilitate T cell activation. Inclusion of the cluster of differentiation 3ς (CD3ς) signaling domain provides activation signal 1 that is necessary, but not sufficient for complete T cell activation. CAR T cells with this design are designated "first generation" CAR T cells and while they showed promising preclinical efficacy in hematologic malignancy tumor models [52], they were largely ineffective in subsequent clinical trials [53]. Attempts to improve on this design involve engineering CD28 in series with CD3ς to provide the important signal 2 necessary to avoid activation-induced cell death in CAR T cells. These "second generation" CAR T cells have shown great promise in the management of B-cell hematologic cancers [54]. Other molecules such as 4-1BB have been used to provide signal 2 [55] and combinations of 3 costimulatory molecules have been engineered to generate third generation CAR T cells [56]. Additionally, CAR T cells can be further modified to secrete cytokines or express ligands that further enhance their antitumor efficacy [57]. Overall, CAR T cell therapy has not had the same success in the management of solid tumor malignancies [58] and this therapy is not yet approved for the management of any solid or hematologic malignancy. Junghans et al [59] reported a PSA response in 2 out of 5 patients treated with first generation Prostate Specific Membrane Antigen-directed (PSMA) CAR T cells. This approach required non-myeloablative preconditioning and concurrent IL-2 administration. Second generation and armored CAR T cells have been designed to reduce or eliminate the requirement for preconditioning and continuous cytokine administration. A phase I dose escalation trial of PSMA- directed second generation CAR T cells has recently concluded (NCT01140373).
Mechanism of action
The two most commonly utilized tumor associated antigens for prostate cancer CAR T cell therapy are PSMA [56] and PSCA (prostate stem cell antigen) [60] due to their relatively restricted expression to the tumor and tumor associated vasculature. Hillerdal et al [60] reported robust PSCA-directed third generation (CD3ς/CD28/OX40) CAR T cell proliferation and surface expression of surrogate degranulation markers (CD107a) when exposed to PSCA-expressing tumor cells. Furthermore, treatment of tumor-bearing mice with PSCA-directed CAR T cells led to impaired tumor growth and improved overall survival in a xenograft model. In another report using PSMA-directed third generation CAR T cells (CD3ς/CD28/4-1BB), Zhong et al [56] showed significantly improved overall survival compared to second generation PSMA-directed CAR T cells. CAR T cell therapy is generally well tolerated based on inferred data from hematologic malignancy clinical trials. However, cytokine release syndrome (CRS), characterized by fever, myalgias, flu-like symptoms, encephalopathy and even seizures have been reported [61]. Furthermore, there has been a correlation between disease burden at the time of treatment and the severity of CRS [61]. Due to the paucity of ongoing clinical trials for CAR T cell therapy in solid tumors, the likelihood and potential severity of CRS is unknown. Additionally, it is unclear if the correlation between disease burden and CRS severity will be similar between solid and hematologic malignancies.
Enhancing efficacy
Clinical trials examining combination therapy with CAR T cells are forthcoming, likely pending results of maximum tolerated dose, requirement for chemotherapy preconditioning and safety from the soon-to-be-resulted phase I trial. There is suggestion, however, that combination with anti-PD1 checkpoint blockade can enhance CAR T cell therapy [62]. This synergy was found to be partly due to increased proliferation and function of CAR T cells in addition to decreased PD-1 expressing MDSCs at the tumor. Whether this rationally designed combination can be extended to prostate cancer is a question yet to be answered.
Perspectives
CAR T cell therapy for prostate cancer is in its clinical infancy compared to tumor vaccines or checkpoint inhibitors. Besides the aforementioned phase I trial in patients with castrate-resistant prostate cancer (NCT01140373), there is only 1 other trial in patients with advanced castrate resistant prostate cancer after non-myeloablative conditioning (NCT00664196) [63]. There is rationale for combining CAR T cell therapy with checkpoint blockade, AR-direceted therapy or cytotoxic chemotherapy in an effort to augment CAR T cell efficacy, decrease tumor burden or facilitate antigen spreading. These avenues can be explored once optimal CAR T cell dose and single agent efficacy is established.
Conclusions
Immunotherapy options for the management of castrate resistant prostate cancer remain a partially achieved goal. Except for Sipuleucel-T, there are no other FDA-approved immunotherapy agents for the management of castrate-resistant prostate cancer. The absence of evidence of an antitumor response, as evaluated by PSA decline and radiological regression of disease, gives some clinicians pause over prescribing Sipuleucel-T despite data showing improved overall survival. Some of these concerns might be assuaged when more is understood about how Sipuleucel-T modulates the immune system and perhaps modifies the tumor microenvironment or the tumor biology. Also lacking is data regarding which patients might benefit from immunotherapy based on baseline and on-therapy immune parameters. PSA-TRICOM is in advanced stages of clinical development and clinical trials are ongoing with different agents to ascertain the best single and combinatorial approach for this modality. The clinical success of checkpoint blockade and adoptive T cell therapy in melanoma and B-cell hematologic malignancies have yet to be replicated in prostate cancer despite promising preclinical results and painstakingly designed clinical trials. Two commonly discussed hypotheses for the lack of efficacy in PSA-TRICOM and ipilimumab immunotherapy is the presence of an immunosuppressive tumor microenvironment [8] and a lack of validated biomarkers for immune-based therapies [64]. Preclinical and correlative markers from clinical trials are currently evaluating both of these claims.
All current forms of immunotherapy rely on harnessing the patient's immune system, whether it's via autologous ex vivo modified APC's or T cells, antibody administration or conventional vaccination, and much has been published regarding the role for synergistic chemotherapy administration [65]. What hasn't been fully explored is the fitness of the patient's immune system to effectively participate in immunotherapy, especially in patients with castrate resistant prostate cancer who may have received many previous lines of androgen deprivation and chemotherapy. Ultimately, rationally designed combination therapy with chemotherapy, radiation, androgen deprivation or other modalities of immunotherapy with non-overlapping toxicity might be the best approach [66].
Abbreviations
- APC
Antigen Presenting Cells
- TAA
Tumor Associated Antigens
- scFv
Single chain variable fragment
- HLA
Human Leucocyte Antigen
- PSA
Prostate Specific Antigen
- PAP
Prostatic Acid Phosphatase
- PSMA
Prostate Specific Membrane Antigen
- PSCA
Prostate Stem Cell Antigen
- ULN
Upper Limit of Normal
- GM-CSF
Granulocyte Macrophage- Colony Stimulating Factor
- OS
Overall Survival
- PFS
Progression Free Survival
- SD
Stable Disease
- NK
Natural Killer Cells
- KLK2
Kallikrein related peptidase 2
- E-ras
Embryonic stem cell expressed Rat sarcoma virus protein
- K-ras
Kirsten rat sarcoma virus protein
- PD-1
Programmed cell death protein 1
- IFN-γ
Interferon gamma
- LGALS3
Lectin Galactoside-Binding Soluble 3
- IDO
Indoleamine 2,3-dioxygenase
- CRPC
Castrate resistant prostate cancer
- CLA-4
Cytotoxic T-lymphocyte associated protein 4
- Treg
Regulatory T cell
- ICAM-1
Intracellular Adhesion Molecule 1
- CAR
Chimeric antigen receptor
- Her-2
Human Epidermal Growth factor receptor 2
References
- 1.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. The New England journal of medicine. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 6.Quinn DI, Shore ND, Egawa S, et al. Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. Urologic oncology. 2015;33:245–260. doi: 10.1016/j.urolonc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Hillerdal V, Essand M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2015;29:75–89. doi: 10.1007/s40259-015-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert review of anticancer therapy. 2006;6:1163–1167. doi: 10.1586/14737140.6.9.1163. [DOI] [PubMed] [Google Scholar]
- 10.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunology, Immunotherapy. 2012;62:137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longo DL. New Therapies for Castration-Resistant Prostate Cancer. New England Journal of Medicine. 2010;363:479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Reese DM, Um B, et al. Therapy of Advanced Prostate Cancer with Granulocyte Macrophage Colony-stimulating Factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 14.GuhaThakurta D, Sheikh NA, Fan L-Q, et al. Humoral Immune Response against Nontargeted Tumor Antigens after Treatment with Sipuleucel-T and Its Association with Improved Clinical Outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3619–3630. doi: 10.1158/1078-0432.CCR-14-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeel DG, Gardner TA, Higano CS, et al. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer immunology research. 2014;2:988–999. doi: 10.1158/2326-6066.CIR-14-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrylak D. Lower baseline PSA predicts greater benefit from sipuleucel-T. Clinical advances in hematology & oncology : H&O. 2013;11:377–381. [PubMed] [Google Scholar]
- 17.Small EJ, Higano CS, Kantoff PW, et al. Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate cancer and prostatic diseases. 2014;17:259–264. doi: 10.1038/pcan.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Small EJ, Lance RS, Gardner TA, et al. A Randomized Phase II Trial of Sipuleucel-T with Concurrent versus Sequential Abiraterone Acetate plus Prednisone in Metastatic Castration-Resistant Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3862–3869. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 21.Graff JN, Drake CG, Beer TM. Complete biochemical (prostate-specific antigen) response to sipuleucel-T with enzalutamide in castration-resistant prostate cancer: a case report with implications for future research. Urology. 2013;81:381–383. doi: 10.1016/j.urology.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer research. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 23.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. Journal of translational medicine. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert opinion on investigational drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. The Journal of urology. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 26.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer immunology, immunotherapy : CII. 2010;59:663–6674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergati M, Cereda V, Madan RA, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer immunology, immunotherapy : CII. 2011;60:197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madan RA, Schwaab T, Gulley JL. Strategies for optimizing the clinical impact of immunotherapeutic agents such as sipuleucel-T in prostate cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:1505–1512. doi: 10.6004/jnccn.2012.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jochems C, Tucker JA, Tsang K-Y, et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer immunology, immunotherapy : CII. 2014;63:407–418. doi: 10.1007/s00262-014-1524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. The Lancet Oncology. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake CG, Doody ADH, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh YT, Gray A, Higgins SA, et al. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. The Prostate. 2009;69:571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. New England Journal of Medicine. 2014;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 36.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2015;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 40.Delyon J, Maio M, Lebb eC. The ipilimumab lesson in melanoma: achieving long-term survival. Seminars in oncology. 2015;42:387–401. doi: 10.1053/j.seminoncol.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 42.Small E, Higano C, Tchekmedyian N, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. ASCO Meeting Abstracts. 2006;24:4609. [Google Scholar]
- 43.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. The Lancet Oncology. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van den Eertwegh AJM, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. The Lancet Oncology. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 50.Camacho LH. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer medicine. 2015;4:661–672. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer discovery. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brentjens RJ, Latouche J-B, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Medicine. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 53.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5:177. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong X-S, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochemical Society transactions. 2016;44:412–418. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamers CHJ, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 59.Junghans RP, Ma Q, Rathore R, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate. 2016;76:1257–1270. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- 60.Hillerdal V, Ramachandran M, Leja J, et al. Systemic treatment with CAR-engineered T cells against PSCA delays subcutaneous tumor growth and prolongs survival of mice. BMC cancer. 2014;14:30. doi: 10.1186/1471-2407-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh N, Frey NV, Grupp SA, et al. CAR T Cell Therapy in Acute Lymphoblastic Leukemia and Potential for Chronic Lymphocytic Leukemia. Current treatment options in oncology. 2016;17:28. doi: 10.1007/s11864-016-0406-4. [DOI] [PubMed] [Google Scholar]
- 62.John LB, Devaud C, Duong CPM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 63.Slovin SF, Wang X, Hullings M, et al. Chimeric antigen receptor (CAR+) modified T cells targeting prostate specific membrane antigen (PSMA) in patients (pts) with castrate metastatic prostate cancer (CMPC) J Clin Oncol. 2013;31 (suppl abstr TPS3115) [Google Scholar]
- 64.Slovin S. Biomarkers for immunotherapy in genitourinary malignancies. Urologic Oncology. 2015;34:205–213. doi: 10.1016/j.urolonc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galluzzi L, Buqu eA, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Burotto M, Singh N, Heery CR, et al. Exploiting Synergy: Immune-Based Combinations in the Treatment of Prostate Cancer. Frontiers in Oncology. 2014;4:1–10. doi: 10.3389/fonc.2014.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]