Summary
Cyclotides are fascinating micro-proteins (≈30 residues long) present in several families of plants that share a unique head-to-tail circular knotted topology of three disulfide bridges, with one disulfide penetrating through a macrocycle formed by the two other disulfides and interconnecting peptide backbones, forming what is called a cystine knot topology. Naturally-occurring cyclotides have shown to posses various pharmacologically-relevant activities, and have been reported to cross cell membranes. Altogether, these features make the cyclotide scaffold an excellent molecular framework for the design of novel peptide-based therapeutics, making them ideal substrates for molecular grafting of biological peptide epitopes. In this chapter we describe how to express a native folded cyclotide using intein-mediated protein trans-splicing in live Escherichia coli cells.
Keywords: Cyclotides, CCK motif, split-intein, protein trans-splicing, Npu intein
1. Introduction
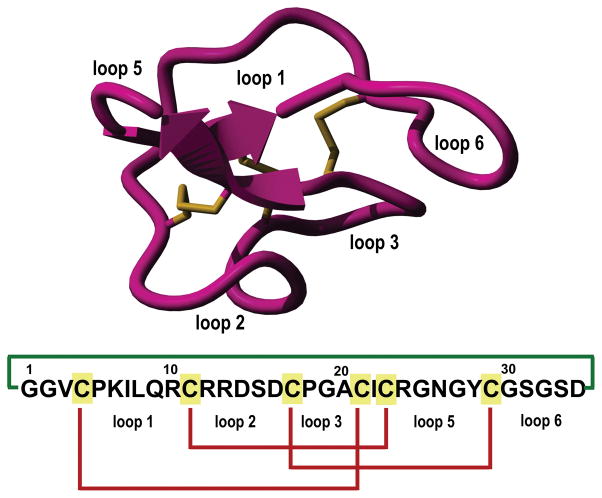
Cyclotides are small globular microproteins (ranging from 28 to 37 residues) containing a unique head-to-tail cyclized backbone topology that is stabilized by three disulfide bonds to form a cystine-knot (CCK) motif [1, 2] (Fig. 1). This CCK molecular framework provides a very rigid molecular platform [3–5] conferring an exceptional stability towards physical, chemical and biological degradation [1, 2]. In fact, the use of cyclotide-containing plants in indigenous medicine first highlighted the fact that the peptides are resistant to boiling and are orally bioavailable [6].
Figure 1.
Tertiary structure of the cyclotide MCoTI-II (PDB code: 1IB9) and primary structures of cyclotides used in this study. The backbone cyclized peptide (connecting bond shown in green) is stabilized by the three disulfide bonds (shown in red).
Cyclotides can be considered as natural combinatorial peptide libraries structurally constrained by the cystine-knot scaffold and head-to-tail cyclization but in which hypermutation of essentially all residues is permitted with the exception of the strictly conserved cysteines that comprise the knot [7–9]. The main features of cyclotides are a remarkable stability due to the cystine knot, a small size making them readily accessible to chemical synthesis, and an excellent tolerance to sequence variations. Naturally-occurring cyclotides have shown to posses various pharmacologically-relevant activities [1, 10]. Cyclotides have been also engineered to target extracellular [11–13] and intracellular [14] molecular targets in animal models. Some of these novel cyclotides are orally bioavailable [12] and are able to cross cellular membranes efficiently [15, 16]. Cyclotides thus appear as highly promising leads or frameworks for peptide drug design [10, 17].
Naturally-occurring cyclotides are ribosomally produced in plants from precursors that comprise between one and three cyclotide domains [18–21]. However, the mechanism of excision of the cyclotide domains and ligation of the free N- and C-termini to produce the circular peptides has not yet been completely elucidated although it has been speculated that asparaginyl endopeptidases are involved in the cyclization process [22–24]. Cyclotides can be also produced recombinantly using standard microbial expression systems by making use of modified protein splicing units [25–28] allowing for the first time the production of biologically-generated libraries of these microproteins [26].
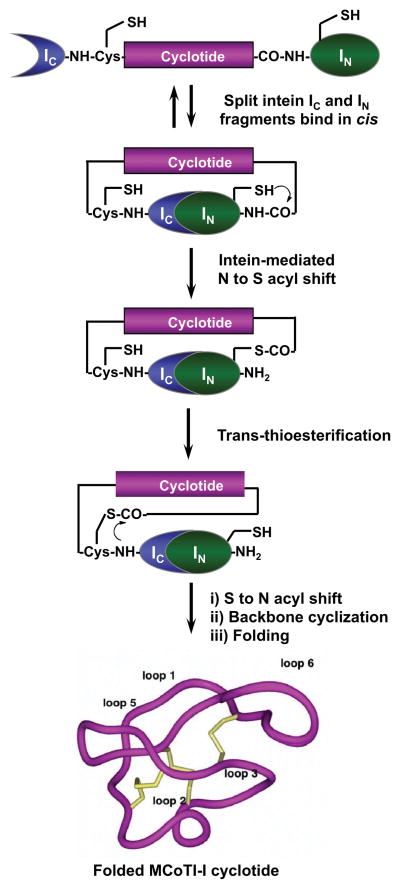
We will describe in this chapter how to produce cyclotide MCoTI-I (Fig. 1) in E. coli cells making use of protein trans-splicing. Cyclotide MCoTI-I is a very potent trypsin inhibitor (Ki ≈ 20 pM) [27] that has been recently isolated from dormant seeds of Momordica cochinchinensis, a plant member of the Cucurbitaceae family [29]. Trypsin inhibitor cyclotides are interesting candidates for drug design because they can cross mammalian cell membranes [15, 16] and their specificity for inhibition can be altered and their structures can be used as natural scaffolds to generate novel binding activities [11, 14]. Protein trans-splicing is a post-translational modification similar to protein splicing with the difference that the intein self-processing domain is split into N- (IN) and C-intein (IC) fragments. The split-intein fragments are not active individually, however, they can bind to each other with high specificity under appropriate conditions to form an active protein splicing or intein domain in trans [30]. PTS-mediated backbone cyclization can be accomplished by rearranging the order of the intein fragments. By fusing the IN and IC fragments to the C- and N-termini of the polypeptide for cyclization, the trans-splicing reaction yields a backbone-cyclized polypeptide (Fig. 2).
Figure 2.
In-cell expression of native folded cyclotide MCoTI-I using intein-mediated protein trans-splicing.
In cell cyclization and folding of cyclotide MCoTI-I will be accomplished using the naturally-occurring Nostoc puntiforme PCC73102 (Npu) DnaE split-intein. This DnaE intein has the highest reported rate of protein trans-splicing (τ1/2 ≈60 s) [31], high splicing yield [31, 32] and has shown high tolerance to the amino acid composition of the intein-extein junctions for efficient protein splicing [25, 33]. To accomplish this, we designed the split-intein construct 1 (Fig. 3). In this construct, the MCoTI-I linear precursor was fused in-frame at the C- and N-termini directly to the Npu DnaE IN and IC polypeptides. None of the additional native C- or N-extein residues were added in this construct. We used the native Cys residue located at the beginning of loop 6 of MCoTI-I (Fig. 1) to facilitate backbone cyclization. A His-tag was also added at the N-terminus of the construct to facilitate purification. In-cell expression of cyclotide MCoTI-I using PTS-mediated backbone cyclization was achieved by transforming the plasmid encoding the split-precursor 1 into Origami 2(DE3) cells to facilitate folding.
Figure 3.
Architecture of the intein precursor used for the expression of cyclotide MCoTI-I described in this protocol.
2. Materials
All solutions were prepared using ultrapure water with a resistivity of 18 MΩ x cm at 25° C and analytical grade reagents. All reagents and solutions were stored at room temperature unless indicated otherwise.
2.1 Instruments
Sonicator for cell lysis (e.g. Sonifier 250 Branson CA, USA).
5 ml Polypropylene Columns (QIAGEN).
Lyophilizer (e.g. Flexi Dry™ μp, Frigeco Inc. USA).
HPLC system equipped with gradient capability and UV-vis detection (e.g. Agilent 1100, Agilent Technologies, USA).
C18 reverse phase HPLC columns (e.g. Vydac C18 column 5 μm, 4.6 x 150 mm and 2.1 × 100 mm columns, Grace Discovery Sciences, USA).
Electrospray mass spectrometer (ES-MS) (e.g. API-3000, Applied Biosystems, USA).
2.2 Cloning of MCoTI-intein contruct 1
DNA ultramers encoding MCoTI-I, Npu DnaE IC and IN were ordered as synthetic oligonucleotides (20 nmol scale and purified by PAGE) (Table 1).
TE buffer: 10 mM Tris-HCl, 1 mM EDTA, pH 8.0.
Annealing buffer: phosphate buffer, 20 mM Na2HPO4, 300 mM NaCl buffer, pH 7.4.
QIAquick PCR Purification Kit (QIAGEN).
QIAprep Spin Miniprep Kit (QIAGEN).
QIAquick Gel Extraction Kit (QIAGEN).
Synthetic DNA primers used to amplify DNA encoding MCoTI-intein construct 1 (20 nmol scale, HPLC purified) (Table 2).
Vent DNA polymerase, TaqDNA polymerase, dNTPs solution, 10X thermopol PCR buffer, 10X TaqDNA polymerase buffer.
Restriction enzymes: Nco I and Hind III .
NEB buffer 2.1, 50mM NaCl, 10 mM Tris-HCl, 10 mM MgCl2, 100 μg/ml bovine serum albumin (BSA), pH 7.9 .
Chemical competent DH5α cells.
Expression plasmid pET28a (Novagen-EMD Millipore).
T4 DNA ligase buffer .
LB medium: 25 g of LB broth was dissolved in 1 L of pure H2O and sterilized by autoclaving at 120° C for 30 min.
LB medium-agar: 3.3 g of LB agar was suspended in 100 mL of pure H2O and sterilized by autoclaving at 120° C for 30 min. To prepare plates, allow LB medium-agar to cool to 50° C, then add 0.1 mL of kanamycin stock solution (25 mg kanamycin/mL in H2O, sterilized by filtration over a 45 μm filter), gently mix and pipet 20 mL into a sterile petri dish (100 mm diameter).
SOC Medium: 20 g of tryptone, 5 g yeast extract, 0.5 g NaCl and 0.186 g KCl was suspended into 980 ml of pure water and sterilized by autoclaving at 120° C for 30 min. Dissolve 4.8 g MgSO4, 3.603 g dextrose in 20 mL of pure H2O and filter sterilize over a 45 μm filter and add to the autoclaved medium.
Table 1.
ssDNA sequences used to generated the dsDNA fragments encoding Npu DnaE IC and IN, and MCoTI-I. DNA sequences were generated using optimal codons for expression in E. coli. Bases in red are as overhangs to facilitate ligation.
| Name of Sequence | Nucleotide sequence |
|---|---|
| P5-IC |
|
| P3-IC |
|
| P5-MCoTI |
|
| P3-MCoTI |
|
| P5-IN |
|
| P3-IN |
|
Table 2.
DNA oligonucleotides used to generate the dsDNA encoding the MCoTI-intein precursor construct 1.
| Name of Sequence | Nucleotide sequence |
|---|---|
| Forward IC primer | 5′-AAA ACC ATG GGC AGC AGC CAT CAT CAT -3′ |
| Reverse IN primer | 5′-TTT TAA GCT TAA TTC GGC AAA TTA TCA ACC C -3′ |
2.3 Cyclotide expression, purification and characterization
Chemical competent Origami2 (DE3) cells (EMD Millipore).
Isopropyl-thio-β-D-galactopyranoside (IPTG), analytical grade. Prepare a stock solution of 1 M in H2O and sterilize by filtration over 45 μm filter. Store at -20° C.
Sterile conical bottom flasks .
Ni-lysis buffer: 10 mM imidazole, 50 mM Na2HPO4, 150 mM NaCl, pH 8.
Ni-wash buffer: 20 mM imidazole, 50 mM Na2HPO4, 150 mM NaCl, pH 8.
Ni-elution buffer: 50 mM Na2HPO4, 150 mM NaCl, 250 mM imidazole, pH 8.
Ni-NTA agarose beads (EMD MilliPore).
100 mM phenylmethylsulphonyl fluoride (PMSF) in EtOH (better to prepare fresh before use).
4×SDS-PAGE sample buffer: 1.5 mL of 1 M Tris-HCl buffer, pH 6.8, 3 mL of 1 M DTT (dithiothreitol) in pure H2O, 0.6 g of sodium dodecyl sulfate (SDS), 30 mg of bromophenol blue, 2.4 mL of glycerol, bring final volume to 7.5 mL.
SDS-PAGE sample buffer: dilute 4 times 4×SDS-PAGE sample buffer in pure H2O and add 20% 2-mercaptoethanol (by volume). Prepare fresh.
SDS-4-20% PAGE gels, 1X SDS running buffer .
Gel stain: Gelcode® Blue (Thermo scientific).
N-hydroxy-succinimide ester (NHS)-activated sepharose beads (GE Healthcare life sciences).
Porcine pancreatic trypsin type IX-S (14,000 units/mg) (Sigma Aldrich).
Coupling buffer: 200 mM sodium phosphate, 250 mM NaCl, pH 6.0.
Washing buffer: 200 mM sodium acetate, 250 mM NaCl, pH 4.5.
Column buffer: 0.1 mM ethylenediaminetetraacetic acid (EDTA), 50 mM Na2HPO4, 150 mM NaCl, pH 7.4.
100 mM ethanolamine (Eastman Kodak).
8 M guanidium chloride in pure water for molecular biology .
Solid-phase extraction silica-C18 cartridge (820 mg of silica-C18, 55-105 μm particle size) (Sep-Pak C18 Plus Long Cartridge, Waters).
HPLC buffers. Buffer A: pure and filtered (over 45 μm filter) H2O with 0.1% trifluoroacetic acid (TFA) (HPLC grade). Buffer B: 90% acetonitrile (HPLC grade) in pure and filtered (over 45 μm filter) H2O with 0.1% TFA.
3. Methods
3.1 Construction of intein-MCoTI-I construct 1
3.1.1 Annealing of the DNA fragments encoding split intein Npu DnaE and cyclotide MCoTI-I
-
1
Dissolve each ultramer shown in Table 1 in TE buffer to a concentration of 1 μg/μL with TE buffer.
-
2
The annealing reaction for every DNA fragment (Npu DnaE IC and IN, and MCoTI-I) is carried out as follows: 5 μL of solution containing the upper DNA strand (P5) and 5 μL of solution containing the lower strand (P3) are added into a 0.5 mL centrifuge tube containing 2.5 μL of 10X annealing buffer and 12 μL of pure H2O. This should provide three annealing reactions for the DNA encoding regions corresponding to the DnaE IN and IC, and MCoTI-I polypeptides.
-
2
Incubate the above samples on a preheated water bath to 95° C for 15 min. Turn off the power of the water bath , and allow the samples to slowly cool down to room temperature. The cooling process should not take less than 60 min.
-
3
Purify the double strand DNA fragments by using the QIAGEN PCR clean up kit following the manufacturer instructions.
-
4
Double strand DNA fragments were obtained in TE buffer and quantified by UV-vis spectroscopy (for a 1-cm pathlength, an optical density at 260 nm (OD260) of 1.0 equals to a concentration of 50 μg/mL solution of dsDNA).
3.1.2 Ligation and amplification of the DNA fragment encoding construct 1
Mix equimolar amounts (≈20 nmol) of each dsDNA fragment encoding for the DnaE IC/IN and MCoTI-I polypeptides in a thin walled 0.5 mL centrifuge tube. Add enough pure sterile water to have a final volume reaction of 50 μL, add 5 μL of 10X T4 DNA ligase buffer, 1 μL of 10 mM ATP, 1 μL of 10 mM dNTP, and then add 1 μL (400 units) of T4 DNA ligase enzyme. Incubate the ligation reaction at 16° C overnight.
Purify the ligated DNA encoding construct 1 using Qiagen PCR Clean up kit according to the manufacturer instructions, and quantify it by UV-vis spectroscopy.
Amplify the construct by PCR using primers shown in Table 2, which introduce Nco I and Hind III restriction sites in the 5′ and 3′ positions of the coding DNA sequence. Carry out the PCR reaction as follows: 40 μL sterile pure H2O, 1 μL of ligated dsDNA (≈10 ng/μL), 5 μL of 10× thermopol reaction buffer, 1.0 μl of dNTP solution (10 mM each), 1 μL of forward IC primer solution (0.2 μM), 1 μl of IN reverse primer solution (0.2 μM), and 1 μl Vent DNA polymerase (2 units).
PCR cycle conditions used: initial denaturation at 94° C for 5 min followed by 30 cycles (94° C denaturation for 30 s, annealing at 56° C for 45 s, and extension at 72° C for 60 s) and final extension at 72° C for 10 min.
Purify the PCR amplified fragment encoding construct 1 using the QIAquick PCR purification kit following the manufacturer instructions and quantify it by UV-visible spectroscopy.
3.1.3 Preparation of expression plasmid pET28-MCoTI-TS
-
1
Digest the plasmid pET28a (Novagen-EMD Millipore) and the PCR-amplified gene encoding MCoTI-intein construct with restriction enzymes Nco I and Hind III. Use a 0.5 ml centrifuge tube and add 5 μl of NEB buffer 2.1 (New England Biolabs), add enough pure sterile water to have a final volume reaction of 50 μL, add ≈10 μg of the corresponding dsDNA to be digested add finally add 1 μL (20 units) of restriction enzyme Nco I (New England Biolabs). Incubate at 37° C for 3 h. Then, add 1 μL (20 units) of restriction enzyme Hind III (New England Biolabs) to the same tube and incubate at 37° C for 1 h.
-
2
Purify the double digested PCR-product and pET28a plasmid by agarose (0.8% and 2% agarose gels for pET28s and PCR product should be used, respectively) gel electrophoresis. The bands corresponding to the double digested DNA are cut and purified using the QIAquick Gel Extraction Kit (QIAGEN), eluted with TE buffer and quantified using UV-visible spectroscopy.
-
3
Ligate double digested pET28a and PCR-product encoding MCoTI-intein construct 1. Use a 0.5 mL centrifuge tube, add ≈100 ng of Nco I, Hind III-digested pET28a, ≈50 ng of Nco I, Hind III-digested PCR-amplified DNA encoding MCoTI-intein construct 1, enough pure sterile H2O to make a final reaction volume of 20 μL, 2 μL of 10X T4 DNA ligase buffer, 1 μL of 10 mM ATP and 1 μL (400 units) T4 DNA ligase. Incubate at 16° C overnight.
-
4
Transform the ligation mixture into DH5α competent cells. ≈100 μL of chemical competent cells are thawed on ice and mixed with the ligation mixture (20 μL) for 30 min. The cells are heat-shocked at 42° C for 45 s and then kept on ice for an extra 10 min. Add 900 μL of SOC medium and incubate at 37° C for 1 h in an orbital shaker. Plate 100 μL on LB agar plate containing kanamycin (25 μg/mL) and incubate the plate at 37° C overnight.
-
5
Pick up several colonies (most of the times 5 colonies should be enough) and inoculate into 5 ml of LB medium, 25μg/mL kanamycin. Incubate tubes at 37° C overnight in an orbital shaker.
-
6
Pellet down cells and extract DNA using the QIAprep Spin Miniprep Kit (QIAGEN) following the manufacturer protocol and quantify plasmid using UV-visible spectroscopy.
-
7
Verify the presence of DNA encoding MCoTI-intein construct in each colony using PCR. Carry out the PCR reaction as follows: 40 μL sterile pure H2O, 1 μL of plasmid DNA (≈50 ng/μL), 5 μL of 10× TaqDNA polymerase buffer, 1.0 μl of dNTP solution (10 mM each), 1 μL of forward IC primer solution (0.2 μM), 1 μl of IN reverse primer solution (0.2 μM), and 1 μl Taq DNA polymerase (5 units).
-
3
PCR cycle conditions used: initial denaturation at 94° C for 5 min followed by 30 cycles (94° C denaturation for 30 s, annealing at 56° C for 45 s, and extension at 72° C for 60 s) and final extension at 72° C for 10 min.
3.2 Expression and purification of cyclotides
3.2.1 Expression of precursor protein encoding the MCoTI-intein construct 1
Transform chemical competent Origami2(DE3) cells with plasmid containing the DNA encoding MCoTI-intein construct 1 (plasmid pET28-MCoTI-TS)(Note 1). Plate transformed cells on LB plate, 2 μg/mL kanamycin, and incubate at 37° C overnight as described in section 3.1.3 (Note 2).
Resuspend the colonies from 2 plates in 2 mL of LB medium and use resuspension to inoculate 1 L of LB medium, 25 μg/mL kanamycin, in a 2.5 L flask.
Grow cells in an orbital shaker incubator at 37° C for 2–3 h to reach mid-log phase (OD at 600 nm ≈0.5). Add IPTG to reach a final concentration of 0.3 mM. Adjust the temperature of the incubator to 25°C and incubate cells in shaker for 16 h.
Pellet cells by centrifugation at 6,000 x g for 15 min at 4° C. Discard the supernatant and process the pellet immediately. (Note 3).
3.2.2 Protein extraction
Resuspend cell pellet with 30 mL of Ni-lysis buffer containing 1 mM PMSF. Lyse cells by sonication on ice using 25 s bursts spaced 30 s each (Note 4). Repeat the cycle six times. (Note 5). Take two 100 μL aliquots. In one of the samples add 33 μL 4X SDS-PAGE sample buffer, 20% 2-mercaptoethanol, and heat it at 94° C for 5 min (label it total cell lysate, T). For the other aliquot, separate the insoluble and soluble fractions by centrifugation at 14,000 rpm in a microcentrifuge at 4° C for 30 min. Take the supernatant fraction and resuspend the pellet in 100 μl of Ni-lysis buffer. Add 33 μL of 4X SDS-PAGE sample buffer containing 20% 2-mercaptoethanol to both fractions and heat them at 94° C for 5 min (label them soluble and insoluble cell lysate samples, P and S, respectively). Save the samples for later SDS-PAGE analysis.
Separate the soluble cell lysate fraction by centrifugation at 15,000 x g for 20 min at 4° C. Stored the pellets at −80° C in case they need to be re-processed.
Transfer the soluble cell lysate fraction (≥ 30 mL) into a 50 mL centrifuge tube and add 1 mL of pre-equilibrated Ni-NTA-agarose beads. Incubate with gentle rocking for 30 min at 4° C.
Separate the beads from supernatant by centrifugation at 3,000 x g for 10 min at 4° C. Take the supernatant and save it at 4°C for later analysis (Section 3.2.4). Separate the beads and wash them in a 5 mL polypropylene column with no less than 15 column volumes of Ni-wash buffer. Take ≈ 100 μL of Ni-NTA-agarose beads into a 0.5 mL centrifuge tube, add 33 μL of 4X SDS-PAGE sample buffer, 20% 2-mercaptoethanol, and heat it at 94° C for 5 min (label it soluble cell lysate bound to Ni-NTA agarose beads, B). Save the sample for later SDS-PAGE analysis.
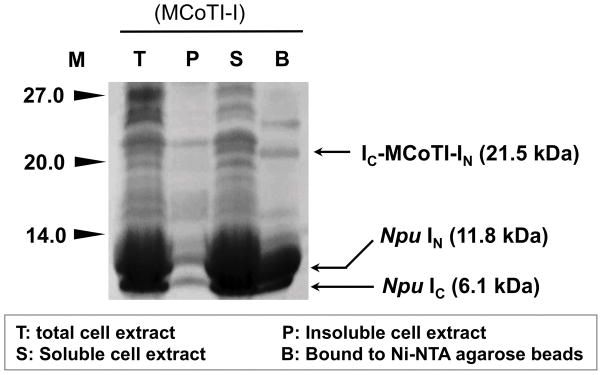
Analyze the expression level of the precursor protein 1 using SDS-PAGE (Fig. 4). Load 25 μL of samples labeled T, P, S and B (see above) onto an SDS-4-20% PAGE gel. Run the samples at 125 V for about 1 h and 30 min in 1X SDS running buffer. Remove SDS with pure water and stain the gel with 20 mL GelCode® Blue reagent (Note 6) using the manufacturer protocol (Fig. 4).
Figure 4.
SDS-PAGE analysis of the recombinant expression of cyclotide precursors 1 in Origami2(DE3) cells for in-cell production of cyclotide MCoTI-I. The bands corresponding to precursor 1, and IN and IC polypeptides are marked with arrows. Despite that only the IC polypeptide has a His-tag, the IN binds the IC polypeptide with low nM affinity and is co-purified during the Ni-NTA pre-purification step. M, stands for protein markers.
3.2.3 Preparation of trypsin-immobilized agarose beads for affinity chromatography
Wash ≈ 1 mL of NHS-activated sepharose with 15 column volumes of ice-cold 1 mM HCl using a 5 ml polypropylene column .
Equilibrate column with 15 volumes of coupling buffer.
Dissolve 4 mg of porcine pancreatic trypsin in 500 μL of coupling buffer using gentle rocking.
Add the trypsin solution to the equilibrated NHS-activated sepharose beads and incubate for 3 h with gentle rocking at room temperature.
Wash the sepharose beads with 10 volumes of coupling buffer containing 100 mM ethanolamine.
Incubate beads with 3 column volumes of coupling buffer containing 100 mM ethanolamine for 3 h with gentle rocking at room temperature.
Wash the sepharose beads with 50 column volumes of washing buffer and store a 4° C until use (Note 6).
3.2.4 Affinity-purification of cyclotide MCoTI-I from bacterial soluble cell lysate
-
1
Wash ≈500 μL of trypsin-sepharose beads with 10 column volumes of column buffer.
-
2
Incubate the washed beads with the soluble cell lysate flow-through from the Ni-NTA agarose beads purification step (Section 3.2.2, step 4) for 1 h at room temperature with gentle rocking.
-
3
Separate the supernatant by centrifugation at 3,000 x g for 10 min at 4° C.
-
4
Transfer the trypsin-beads to a 5 mL polypropylene column and wash the beads with 50 volumes of column buffer containing 0.1% Tween 20.
-
5
Wash trypsin-beads with 50 volumes of column buffer with no detergent added.
-
6
Elute bound cyclotide MCoTI-I with 3 volumes (3 x 500 μL) of 8 M guanidium hydrochloride at room temperature for 15 min by gravity.
-
7
Desalt the sample using a solid-phase extraction cartridge (SepPak, Waters) by following the manufacturer protocol. Briefly, pre-swell the SepPak cartridge with 20 mL of 50% acetonitrile in water containing 0.1% TFA. Equilibrate cartridge with 50 mL of HPLC buffer A. Load sample into cartridge using a plastic 20 mL syringe slowly with a flow rate of ≈1 mL/min. Collect flow-through and repeat this step for efficient binding. Wash the cartridge with 20 mL of 5% acetonitrile in H2O containing 0.1% TFA. Elute cyclotide MCoTI-I from the solid-phase extraction cartridge with 5 mL of HPLC buffer-B. Lyophilize to remove solvents.
-
10
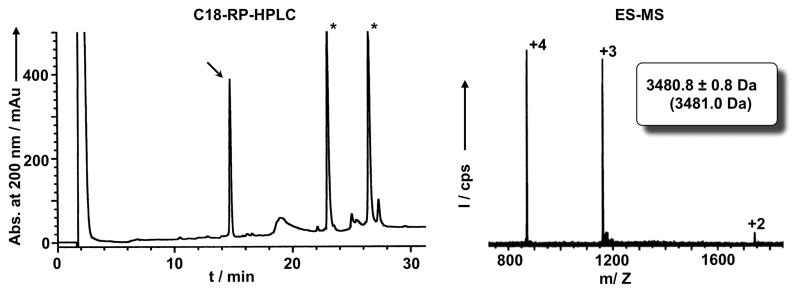
Dissolve in 5 mL of HPLC buffer A. Analyze sample by HPLC using an isocratic of 0% buffer B for 2 min and then a linear gradient of 0% to 70% buffer B in 30 min. Use detection at 220 and 280 nm. Using these conditions the retention time of the cyclotide should be around 15 min (Fig. 5). Collect the peak and analyze by mass spectrometry to confirm identity of cyclotide MCoTI-I (Fig. 5; expected molecular weight: 3481.0 Da).
-
11
Quantify the cyclotide using UV-visible spectroscopy and a molar absorptivity at 280 nm of 2240 M−1 x cm−1. Around 150 μg of folded cyclotide should be obtained per liter of LB culture.
Figure 5.
Analytical HPLC trace (left panel) of the soluble cell extract of bacterial cells expressing precursor 1 after purification by affinity chromatography on a trypsin-sepharose column. Folded MCoTI-I is marked with an arrow. Endogenous bacterial proteins that bind trypsin are marked with an asterisk. Mass spectrum (right panel) of affinity purified MCoTI-I. The expected average molecular weight is shown in parentheses.
Footnotes
We recommend to screen at least 3–4 different colonies for protein expression to make sure that the MCoTI-I intein construct 1 is expressed efficiently.
When plating the transformed cells with pET28-MCoTI-TS, it is better to aim for plates containing 200–300 colonies.
Cell pellets can be stored at -80° C for no more than 2–3 weeks before being processed.
During sonication, be sure the temperature of the sample does not overheat.
A french press can be also used to lyse cells, depending on the availability.
Coomassie brilliant blue can be also used for staining PAGE gels.
The trypsin-sepharose column should not be stored for more than 2 weeks. The loading of the trypsin-sepharose can be determined by incubating a small aliquot of the beads with a known amount of pure MCoTI-I and determining the amount of cyclotide captured on the beads using HPLC, see 3.2.4).
References
- 1.Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Adv Drug Deliv Rev. 2009;61:918–930. doi: 10.1016/j.addr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Gould A, Ji Y, Aboye TL, Camarero JA. Cyclotides, a novel ultrastable polypeptide scaffold for drug discovery. Curr Pharm Des. 2011;17:4294–4307. doi: 10.2174/138161211798999438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Backbone Dynamics of Cyclotide MCoTI-I Free and Complexed with Trypsin. Angew Chem Int Ed Engl. 2010;49:7030–7034. doi: 10.1002/anie.201002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Erratum in: Backbone Dynamics of Cyclotide MCoTI-I Free and Complexed with Trypsin. Angew Chem Int Ed Engl. 2011;50:6948–6949. doi: 10.1002/anie.201002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly NL, Thorstholm L, Greenwood KP, King GJ, Rosengren KJ, Heras B, Martin JL, Craik DJ. Structural insights into the role of the cyclic backbone in a squash trypsin inhibitor. J Biol Chem. 2013;288:36141–36148. doi: 10.1074/jbc.M113.528240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saether O, Craik DJ, Campbell ID, Sletten K, Juul J, Norman DG. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry. 1995;34:4147–4158. doi: 10.1021/bi00013a002. [DOI] [PubMed] [Google Scholar]
- 7.Austin J, Kimura RH, Woo YH, Camarero JA. In vivo biosynthesis of an Ala-scan library based on the cyclic peptide SFTI-1. Amino Acids. 2010;38:1313–1322. doi: 10.1007/s00726-009-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YH, Colgrave ML, Clark RJ, Kotze AC, Craik DJ. Lysine-scanning mutagenesis reveals an amendable face of the cyclotide kalata B1 for the optimization of nematocidal activity. J Biol Chem. 2010;285:10797–10805. doi: 10.1074/jbc.M109.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonsen SM, Sando L, Rosengren KJ, Wang CK, Colgrave ML, Daly NL, Craik DJ. Alanine scanning mutagenesis of the prototypic cyclotide reveals a cluster of residues essential for bioactivity. J Biol Chem. 2008;283:9805–9813. doi: 10.1074/jbc.M709303200. [DOI] [PubMed] [Google Scholar]
- 10.Garcia AE, Camarero JA. Biological activities of natural and engineered cyclotides, a novel molecular scaffold for peptide-based therapeutics. Curr Mol Pharmacol. 2010;3:153–163. doi: 10.2174/1874467211003030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aboye TL, Ha H, Majumder S, Christ F, Debyser Z, Shekhtman A, Neamati N, Camarero JA. Design of a novel cyclotide-based CXCR4 antagonist with anti-human immunodeficiency virus (HIV)-1 activity. J Med Chem. 2012;55:10729–10734. doi: 10.1021/jm301468k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CT, Rowlands DK, Wong CH, Lo TW, Nguyen GK, Li HY, Tam JP. Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew Chem Int Ed Engl. 2012;51:5620–5624. doi: 10.1002/anie.201200984. [DOI] [PubMed] [Google Scholar]
- 13.Chan LY, Gunasekera S, Henriques ST, Worth NF, Le SJ, Clark RJ, Campbell JH, Craik DJ, Daly NL. Engineering pro-angiogenic peptides using stable, disulfide-rich cyclic scaffolds. Blood. 2011;118:6709–6717. doi: 10.1182/blood-2011-06-359141. [DOI] [PubMed] [Google Scholar]
- 14.Ji Y, Majumder S, Millard M, Borra R, Bi T, Elnagar AY, Neamati N, Shekhtman A, Camarero JA. In Vivo Activation of the p53 Tumor Suppressor Pathway by an Engineered Cyclotide. J Am Chem Soc. 2013;135:11623–11633. doi: 10.1021/ja405108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras J, Elnagar AY, Hamm-Alvarez SF, Camarero JA. Cellular uptake of cyclotide MCoTI-I follows multiple endocytic pathways. J Control Release. 2011;155:134–143. doi: 10.1016/j.jconrel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cascales L, Henriques ST, Kerr MC, Huang YH, Sweet MJ, Daly NL, Craik DJ. Identification and characterization of a new family of cell-penetrating peptides: cyclic cell-penetrating peptides. J Biol Chem. 2011;286:36932–36943. doi: 10.1074/jbc.M111.264424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriques ST, Craik DJ. Cyclotides as templates in drug design. Drug Discov Today. 2010;15:57–64. doi: 10.1016/j.drudis.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Mylne JS, Chan LY, Chanson AH, Daly NL, Schaefer H, Bailey TL, Nguyencong P, Cascales L, Craik DJ. Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell. 2012;24:2765–2778. doi: 10.1105/tpc.112.099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poth AG, Mylne JS, Grassl J, Lyons RE, Millar AH, Colgrave ML, Craik DJ. Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae) J Biol Chem. 2012;287:27033–27046. doi: 10.1074/jbc.M112.370841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poth AG, Colgrave ML, Lyons RE, Daly NL, Craik DJ. Discovery of an unusual biosynthetic origin for circular proteins in legumes. Proc Natl Acad Sci U S A. 2011;108:1027–1032. doi: 10.1073/pnas.1103660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings C, West J, Waine C, Craik D, Anderson M. Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis. Proc Natl Acad Sci U S A. 2001;98:10614–10619. doi: 10.1073/pnas.191366898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillon AD, Saska I, Jennings CV, Guarino RF, Craik DJ, Anderson MA. Biosynthesis of circular proteins in plants. Plant J. 2008;53:505–515. doi: 10.1111/j.1365-313X.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- 23.Saska I, Gillon AD, Hatsugai N, Dietzgen RG, Hara-Nishimura I, Anderson MA, Craik DJ. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J Biol Chem. 2007;282:29721–29728. doi: 10.1074/jbc.M705185200. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GK, Wang S, Qiu Y, Hemu X, Lian Y, Tam JP. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat Chem Biol. 2014;10:732–738. doi: 10.1038/nchembio.1586. [DOI] [PubMed] [Google Scholar]
- 25.Jagadish K, Borra R, Lacey V, Majumder S, Shekhtman A, Wang L, Camarero JA. Expression of fluorescent cyclotides using protein trans-splicing for easy monitoring of cyclotide-protein interactions. Angew Chem Int Ed Engl. 2013;52:3126–3131. doi: 10.1002/anie.201209219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin J, Wang W, Puttamadappa S, Shekhtman A, Camarero JA. Biosynthesis and biological screening of a genetically encoded library based on the cyclotide MCoTI-I. Chembiochem. 2009;10:2663–2670. doi: 10.1002/cbic.200900534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camarero JA, Kimura RH, Woo YH, Shekhtman A, Cantor J. Biosynthesis of a fully functional cyclotide inside living bacterial cells. Chembiochem. 2007;8:1363–1366. doi: 10.1002/cbic.200700183. [DOI] [PubMed] [Google Scholar]
- 28.Kimura RH, Tran AT, Camarero JA. Biosynthesis of the cyclotide kalata B1 by using protein splicing. Angew Chem Int Ed. 2006;45:973–976. doi: 10.1002/anie.200503882. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Trinh Hong T, Pham TT, Le Nguyen D. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry. 2000;39:5722–5730. doi: 10.1021/bi9929756. [DOI] [PubMed] [Google Scholar]
- 30.Sancheti H, Camarero JA. “Splicing up” drug discovery. Cell-based expression and screening of genetically-encoded libraries of backbone-cyclized polypeptides. Adv Drug Deliv Rev. 2009;61:908–917. doi: 10.1016/j.addr.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zettler J, Schutz V, Mootz HD. The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans-splicing reaction. FEBS Lett. 2009;583:909–914. doi: 10.1016/j.febslet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Iwai H, Zuger S, Jin J, Tam PH. Highly efficient protein trans-splicing by a naturally split DnaE intein from Nostoc punctiforme. FEBS Lett. 2006;580:1853–1858. doi: 10.1016/j.febslet.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 33.Jagadish K, Gould A, Borra R, Majumder S, Mushtaq Z, Shekhtman A, Camarero JA. Recombinant Expression and Phenotypic Screening of a Bioactive Cyclotide Against alpha-Synuclein-Induced Cytotoxicity in Baker's Yeast. Angew Chem Int Ed Engl. 2015;54:8390–8394. doi: 10.1002/anie.201501186. [DOI] [PMC free article] [PubMed] [Google Scholar]