Abstract
Background
Our objective was to estimate associations between gestational weight gain z-scores and preterm birth, neonatal intensive care unit admission, large- and small-for-gestational age birth (LGA, SGA), and cesarean delivery among grade 1, 2, and 3 obese women.
Methods
Singleton infants born in Pennsylvania (2003–2011) to grade 1 (body mass index (BMI) 30–34.9 kg/m2, n=148,335), grade 2 (35–39.9 kg/m2, n=72,032), or grade 3 (≥40 kg/m2, n=47,494) obese mothers were included. Total pregnancy weight gain (kg) was converted to gestational age-standardized z-scores. Multivariable Poisson regression models stratified by obesity grade were used to estimate associations between z-scores and outcomes. A probabilistic bias analysis, informed by an internal validation study, evaluated the impact of BMI and weight gain misclassification.
Results
Risks of adverse outcomes did not substantially vary within the range of z-scores equivalent to 40-week weight gains of −4.3 to 9 kg for grade 1 obese, −8.2 to 5.6 kg for grade 2 obese, and −12 to −2.3 kg for grade 3 obese women. As gestational weight gain increased beyond these z-score ranges, there were slight declines in risk of SGA but rapid rises in cesarean delivery and LGA. Risks of preterm birth and neonatal intensive care unit admission were weakly associated with weight gain. The bias analysis supported the validity of the conventional analysis.
Conclusions
Gestational weight gain below national recommendations for obese mothers (5–9 kg) may not adversely affect fetal growth, gestational age at delivery, or mode of delivery.
One in 12 U.S. women aged 20–39 years has a body mass index (BMI) of 40 kg/m2 or more.1 The prevalence of grade 3 obesity has more than tripled since 1970,1 and forecasts suggest it will continue to increase in the next decade.2 This upsurge poses significant concerns for the health of pregnant women—300,000 of whom begin pregnancy with a BMI ≥40 each year. As obesity becomes more severe, the risk of many adverse short- and long-term maternal and child outcomes rises.3,4 Reducing body weight before conception is likely the most effective intervention for lessening sequelae of severe maternal obesity,4 but few women seek preconception care5 or are aware of the adverse reproductive consequences of obesity.6,7
Optimizing weight gain during pregnancy may help to reduce risks of poor birth outcomes among severely obese women. In 2009 the National Academy of Sciences/Institute of Medicine (IOM) recommended that all obese women gain 5–9 kg (11–20 lb) to balance the risks associated with high weight gain (e.g., postpartum weight retention, child obesity) with the risks of low weight gain (e.g., fetal growth restriction, preterm birth).8 They based this guideline on data from studies of mostly grade 1 obese women (BMI 30–34.9 kg/m2), and acknowledged that these ranges may not apply to heavier women.8 The guidelines sparked debate, including calls for abandoning the recommendations in favor of restricting weight gain among obese women.9,10
Recently, empirical evidence has been published to inform this discussion. Researchers used large, administrative databases (to ensure adequate numbers of severely obese women and women with low weight gains or weight losses) that relied on self-reported weight.11–18 Despite wide recognition that self-reported weight is measured with error19—especially at the extremes of BMI and gestational weight gain20—no study validated BMI or weight gain or quantified the impact of the misclassification on results. Additionally, several previous studies evaluated preterm birth or preterm-related outcomes (e.g., neonatal intensive care unit [NICU] admission) in relation to a measure of gestational weight gain that is correlated with pregnancy duration,11,21,22 which prevents the effects of weight gain on the outcome to be disentangled from the effects of gestational age.23
Our objectives were twofold. First, we estimated associations between gestational weight gain z-scores (a measure that is uncorrelated with gestational age)24 and preterm birth, NICU admission, large- and small-for-gestational age birth (LGA, SGA), and cesarean delivery among grade 1, 2, and 3 obese women. Second, we performed a probabilistic bias analysis informed by an internal validation study to evaluate the impact of prepregnancy BMI and gestational weight gain misclassification on our findings.
Materials and Methods
Penn MOMS is a population-based cohort study designed to assess relations between gestational weight gain and adverse birth outcomes using infant birth records in Pennsylvania (2003–2011).20 We extensively cleaned the vital records database, including using maternal and infant identifiers to check for duplicate records, confirm or modify plurality, and identify successive deliveries to the same mother. The study was approved by our institutional review board.
Of the 1,274,772 available singleton births, we retained 1,247,047 (98%) with a gestational age ≤42 weeks and complete data on birth weight, infant sex, and birth facility. There were 469,421 records with missing data on prepregnancy weight (n=73,027), height (n=10,295), maternal weight at delivery (n=83,614), geocoded address (n=182,436), or other covariates in the final model (n=65,993). To address the missing data, we used multiple imputation, as described below.
The 2003 revised version of the U.S. birth certificate collects information on prepregnancy weight and height via maternal interview before hospital discharge.25 We calculated prepregnancy BMI [weight (kg)/ height (m2)] and categorized women as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), grade 1 obese (30–34.9 kg/m2), grade 2 obese (35–39.9 kg/m2), or grade 3 obese (≥40 kg/m2).26
Maternal weight at delivery on the birth certificate is ascertained by a hospital staff member, who uses either the last measured weight in the prenatal records or the weight upon admission to labor and delivery (which may be measured or self-reported).25 Total gestational weight gain was calculated as maternal weight at delivery minus the prepregnancy weight. Maternal weight gain measurements were standardized for gestational age by converting them to gestational age-specific z-scores.27 Z-scores were calculating by comparing a woman's weight gain to the gestational age-specific mean and standard deviation of weight gain in the population, obtained from BMI-specific pregnancy weight gain charts produced for Magee-Womens Hospital in Pittsburgh, Pennsylvania. Z-scores were calculated as: (observed weight gain – mean weight gain)/standard deviation of weight gain.
We defined preterm birth as <37 weeks of gestation. Gestational age was based on the best obstetric estimate at delivery,25 which we have shown to have high agreement with ultrasound-confirmed gestational age from medical records in a subset of 1204 births.20 SGA and LGA births were classified using ultrasound-based intrauterine fetal weight standards at <10th or >90th percentile, respectively.28
Maternal interview before discharge provided birth certificate data on maternal race/ethnicity, maternal education, marital status, and smoking during pregnancy. Medical records are used to ascertain birth certificate data on parity, source of payment at delivery, preexisting conditions, and address of primary residence at delivery. The Pennsylvania Bureau of Health Statistics and Research geocodes addresses of primary residence and provides census tracts and block groups, which we merged with U.S. Department of Agriculture Economic Research Service Urban-Rural Continuum Codes29 to define urban residence. Using the 2000 United States Census data, we also created a census-tract level measure of the percentage of Black residents.30 We classified the neonatal care of the birth facility as level I, II, or III.31
Statistical analysis
We used multiple imputation to address missing data. We imputed 10 datasets with 500 iterations using a multivariate normal imputation model.32 Missing variables were log-transformed. We jointly imputed prepregnancy weight, height, weight at delivery, insurance, race/ethnicity, age, parity, urban residence, smoking, education, marital status, percent Black residents in census tract, WIC use, and preexisting conditions by including infant vital status, gestational age, infant birth weight, infant sex, mode of delivery, infant malformations, hospital neonatal level of care, year of birth, and NICU admission in the imputation model. All variables were back-transformed before inclusion in analyses. Counts of subjects in each BMI group were averaged over the 10 imputed datasets. We compared the results generated from the imputation with the results from the complete case dataset (n=831,682).
Multivariable Poisson regression models stratified by obesity grade were used to estimate associations between weight gain z-score and each adverse outcome. To allow for flexible, non-linear relations, we modeled gestational weight gain z-score as a restricted cubic spline with 4 knots at the 5th, 35th, 65th, and 95th percentiles of the distribution.33 We selected this specification based on Akaike information criterion model comparison criteria.33 For ease of interpretation, we also categorized z-score as <−1 standard deviation (SD), −1 to +1 SD, >+1 SD. We included in all models the potential confounders identified through directed acyclic graphs:34 maternal age, maternal race/ethnicity, maternal education, marital status, parity, smoking during pregnancy, pre-existing diabetes or hypertension, height, Black racial composition of neighborhood, payment source, urban residence, neonatal care level of the birth facility, and year of birth.
After model estimation, we calculated risk differences and 95% confidence intervals (CI) for selected z-score values from −3 SD to +2 SD (approximately 1.5th to 99th percentiles of weight gain z-scores observed in our cohort) compared with the z-score that corresponded to a total weight gain of 5 kg at 40 weeks gestation (i.e., the lower limit of the IOM’s recommended range). We calculated the excess or prevented number of cases per 100 births by multiplying the risk difference and 95% CI by 100. We plotted the adjusted risks and 95% CI for each outcome by obesity grade with all covariates set to population means.
We conducted a probabilistic bias analysis to evaluate the impact of BMI and gestational weight gain misclassification on our conventional results. Validation data came from a subset of Pennsylvania births delivered at Magee-Womens Hospital that had BMI and gestational weight gain abstracted from medical records (n=4855). The validation study methodology and analytic approach have been described elsewhere.20,35 Briefly, we classified each validation record into 1 of 36 strata created by simultaneous stratification on 3 birth certificate variables: prepregnancy BMI category (underweight, normal weight, overweight, grade 1 obese, grade 2 obese, grade 3 obese), gestational weight gain category (<−1 SD, −1 to +1 SD, >+1 SD), and adverse outcome (yes, no). We performed this stratification for all five outcomes (SGA, LGA, preterm birth, cesarean delivery, and NICU admission). We then used medical chart-derived data on prepregnancy weight, height, and last measured weight before delivery to calculate and categorize BMI and weight gain z-score. We cross-classified the birth certificate-based strata with the medical record-based strata and calculated predictive values (see eTables 1–5). These were used in a Monte Carlo simulation of 100,000 datasets, each one yielding estimates of association that were then summarized by extracting the median, 2.5th percentile, and 97.5th percentile. These are analogous to the point estimate and limits of the 95% confidence interval of the conventional analysis, which treats all variables as if they were perfectly measured. Due to the computational intensity of the bias analysis,35 we performed this analysis using one randomly-selected multiply-imputed dataset.
Results
After imputation, there were 59,318 underweight (4.8%), 613,697 normal weight (50%), 298,335 overweight (24%), 148,335 grade 1 obese (12%), 72,032 grade 2 obese (5.8%), and 47,494 (3.9%) grade 3 obese mothers. All obese women were retained for further analyses.
The majority of the obese women were Non-Hispanic White, 20–29 years of age, college-educated, married, multiparous, and non-smokers during pregnancy (Table 1). More than half of women held private insurance, delivered in a birth facility with level III neonatal care, and lived in a large metropolitan area. Grade 3 obese women were more likely than grade 1 and 2 obese women to have a preexisting medical condition, and were slightly more likely to be non-Hispanic Black, older, and unmarried, and were slightly less likely to have private insurance.
Table 1.
Maternal characteristics by grade of obesity, Pennsylvania singleton births, 2003–2011
| Grade 1a obesity n=148,335 %, median (IQR) or mean (SD) |
Grade 2 obesity n=72,032 %, median (IQR) or mean (SD) |
Grade 3 obesity n=47,494 %, median (IQR) or mean (SD) |
|
|---|---|---|---|
| Race/ethnicity | |||
| Non-Hispanic White | 69 | 69 | 67 |
| Non-Hispanic Black | 18 | 20 | 23 |
| Hispanic | 10 | 9 | 8 |
| Non-Hispanic Other | 3 | 2 | 2 |
| Age | |||
| < 20 years | 7 | 6 | 4 |
| 20–29 years | 52 | 53 | 54 |
| ≥ 30 years | 41 | 41 | 42 |
| Mother’s education | |||
| Less than high school | 16 | 14 | 14 |
| High school or equivalent | 31 | 33 | 35 |
| Some college | 30 | 32 | 34 |
| College graduate | 23 | 21 | 17 |
| Marital Status | |||
| Unmarried | 42 | 42 | 44 |
| Married | 58 | 58 | 56 |
| Parity at index birth | |||
| 1 | 35 | 35 | 34 |
| 2 or more | 65 | 65 | 66 |
| Smoking during pregnancy | |||
| No | 82 | 82 | 82 |
| Yes | 18 | 18 | 18 |
| Preexisting diabetes or hypertension | |||
| No | 97 | 94 | 91 |
| Yes | 3 | 6 | 9 |
| Insurance | |||
| Private | 57 | 57 | 54 |
| Medicaid | 36 | 38 | 41 |
| Other | 7 | 5 | 5 |
| Neonatal care level of birth facility | |||
| Level I | 23 | 24 | 24 |
| Level II | 15 | 15 | 15 |
| Level III | 62 | 61 | 61 |
| Urban residence | |||
| Metropolitan, ≥1 million | 50 | 49 | 48 |
| Metropolitan, 250,000–<1 million | 29 | 29 | 29 |
| Metropolitan, <250,000 | 9 | 9 | 10 |
| Non-metropolitan | 12 | 13 | 13 |
| Census tract racial distribution | |||
| % Black residents (median, IQR) | 2.6 (0.6, 12.6) | 2.7 (0.6, 14) | 3.1 (0.6, 18) |
| Gestational weight gain (mean, SD), kg | 12.4 (8.4) | 10.0 (8.9) | 7.4 (9.7) |
| Gestational weight gain | |||
| Below recommended rangeb | 18 | 26 | 39 |
| Within recommended range | 14 | 17 | 17 |
| Above recommended range | 68 | 57 | 44 |
| Cesarean delivery | 36 | 42 | 50 |
| Preterm birth <37 weeks | 8.6 | 9.1 | 9.8 |
| Infant birth weight, g | 3371 (607) | 3386 (637) | 3408 (652) |
| Small-for-gestational-age birth | 8.7 | 8.6 | 7.9 |
| Large-for-gestational-age birth | 12 | 14 | 17 |
| Neonatal intensive care unit admission | 7.1 | 7.9 | 8.9 |
Grade 1 obesity, body mass index 30–34.9 kg/m2; grade 2 obesity, body mass index 35–39.9 kg/m2; grade 3 obesity, body mass index ≥40 kg/m2
Institute of Medicine recommended weight gain ranges are 5 to 9 kg at term (40 weeks), which corresponds to weight gain z-scores of −1.1 to −0.5 SD for grade 1 obese women, −0.6 to −0.1 SD for grade 2 obese women, and −0.2 to 0.2 SD for grade 3 obese women.
The mean (SD) gestational weight gain and the percent of women gaining below the recommended weight gain range increased as obesity became more severe. Approximately 8.2%, 14%, and 23% of grade 1, 2, and 3 obese women gained no weight or lost weight during pregnancy. As obesity became more severe, the incidence of cesarean delivery rose dramatically, while preterm birth, LGA, and NICU admission increased moderately and SGA decreased slightly.
Bivariate relations between gestational weight gain z-score and birth outcomes were consistent across the 3 grades of obesity (Table 2). Gestational weight gain z-score category was positively associated with cesarean delivery and LGA risk, and inversely associated with SGA (Table 2). Preterm birth and NICU admission tended to be lowest among women whose z-scores were −1 to +1 SD.
Table 2.
Unadjusted incidence of selected adverse birth outcome according to gestational weight gain z-score category and grade of obesity, Pennsylvania singleton births, 2003–2011.
| Gestational weight gain z-score a |
Births, n | Cesarean delivery |
Large-for- gestational-age birth |
Small-for- gestational-age birth |
Preterm birth <37 weeks |
Neonatal intensive care unit admission |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n |
% | Cases, n |
% | Cases, n |
% | Cases, n |
% | Cases, n |
% | |||
| Grade 1b obesity | < −1 SD | 28,061 | 8,051 | 29 | 1,958 | 7 | 3,597 | 13 | 2,678 | 9.5 | 1,988 | 7.1 |
| −1 to +1 SD | 100,858 | 36,199 | 36 | 12,177 | 12 | 8,067 | 8 | 8,129 | 8.1 | 6,882 | 6.8 | |
| >+1 SD | 19,690 | 8,951 | 46 | 3,957 | 20 | 1,206 | 6.2 | 2,074 | 11 | 1,728 | 8.8 | |
| Grade 2 obesity | < −1 SD | 12,364 | 4,174 | 34 | 1,074 | 8.7 | 1,519 | 12 | 1,140 | 9.2 | 870 | 6.9 |
| −1 to +1 SD | 50,786 | 21,452 | 42 | 7,112 | 14 | 4,069 | 8 | 4,313 | 8.5 | 3,840 | 7.5 | |
| >+1 SD | 8,941 | 4,731 | 53 | 2,117 | 24 | 619 | 6.9 | 1,158 | 13 | 1,027 | 12 | |
| Grade 3 obesity | < −1 SD | 5,542 | 2,306 | 42 | 529 | 9.6 | 666 | 12 | 631 | 11 | 505 | 9.1 |
| −1 to +1 SD | 37,608 | 18,802 | 50 | 6,248 | 17 | 2,816 | 7.5 | 3,414 | 8.5 | 3,157 | 8.4 | |
| >+1 SD | 4,549 | 2,730 | 60 | 1,243 | 27 | 316 | 6.9 | 658 | 15 | 604 | 13 | |
SD, standard deviation
Gestational weight gain z-scores < −1, −1 to +1, and >+1 SD correspond to total gestational weight gains at 40 weeks of <5.6, 5.6 to 22.3, >22.3 kg among grade 1 obese, <1.8, 1.8 to 21.1, >21.1 kg among grade 2 obese, and < −2.3, −2.3 to 20.8, >20.8 kg among grade 3 obese.
Grade 1 obesity, body mass index 30–34.9 kg/m2; grade 2 obesity, body mass index 35–39.9 kg/m2; grade 3 obesity, body mass index ≥40 kg/m2
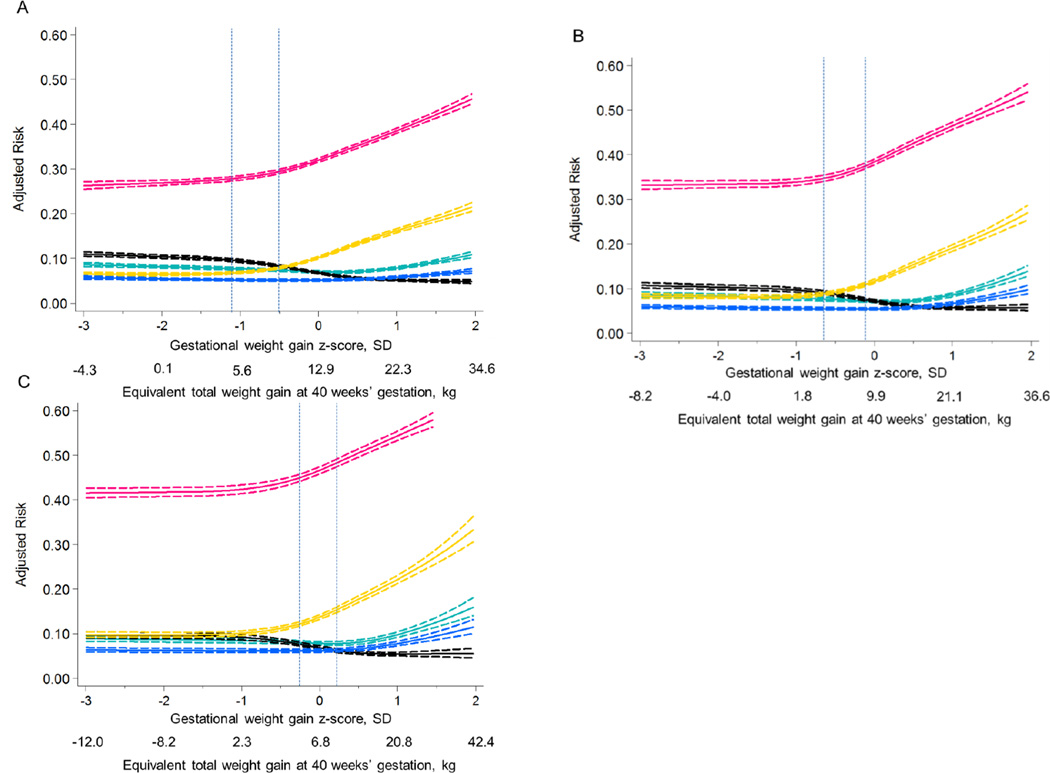
Figure 1 illustrates the non-linear associations between gestational weight gain z-score and the confounder-adjusted risk of each outcome (see eTable 6 for the adjusted predicted risks (95% CI)). Across the 3 grades of obesity, risk curves were similar in shape. From z-scores −3 SD to −1 SD, risk of all adverse outcomes were nearly uniform. Risks of cesarean delivery and LGA rose rapidly above z-scores of −0.5 SD. This rapid increase occurred for grade 1 obese women at weight gains greater than the IOM-recommended weight gain range of 5–9 kg at term (delineated with vertical lines in Figure 1) and for grades 2 and 3 obese women at weight gains within or above the recommended range. Risk of SGA declined slowly as z-score increased above −1 SD. Risks of preterm birth and NICU admission did not increase until z-scores increased above +1 SD.
Figure 1.
Adjusted predicted risks of 5 adverse perinatal outcomes by gestational weight gain z-score among grade 1 obese (Panel A, n=148,335), grade 2 obese (Panel B, n=72,032), and grade 3 obese (Panel C, n=47,494) mothers, Pennsylvania singleton birth certificates, 2003–2011.
The solid lines represent the point estimate and dashed lines represent its 95% confidence bands. Outcomes are preterm birth (green lines), small-for-gestational-age birth (black lines), large-for-gestational-age birth (gold lines), cesarean delivery (pink lines), and neonatal intensive care unit admission (blue lines). The dotted vertical lines represent the range of weight gain currently recommended by the Institute of Medicine.8 Risks were estimated using log-binomial regression and were set at the population average for maternal age, maternal race/ethnicity, maternal education, marital status, parity, smoking during pregnancy, pre-existing diabetes or hypertension, height, Black racial composition of census tract, payment source, urban residence, neonatal care level of the birth facility, and year of birth.
Table 3 shows the differences in risk of each adverse outcome per 100 births comparing a range of weight gain z-scores relative to the z-score equivalent of the lower limit of the IOM recommended weight gain (5 kg at 40 weeks). Among women with grade 1 obesity, compared with gaining 5 kg (z-score −1.1 SD), there were <2% differences in risk of any adverse outcome associated with gaining as low as −4.3 kg (−3 SD) or up to 9 kg (−0.5 SD). Similar to grade 1 obese women, risk differences of adverse outcomes among grade 2 obese women comparing those who gained 5 kg at 40 weeks with those who gained as low as −8.2 kg (−3 SD) were small. However, by 9.9 kg (0 SD), there were 3.5% (95% CI: 2.9%, 4.1%) and 2.7% (95% CI: 2.5%, 3.1%) differences in risk of cesarean delivery and LGA, respectively. Grade 3 obese women who gained 2.3 kg (−1 SD) or who lost up to 12 kg (−3 SD) of weight at 40 weeks had a 2.5% or greater reduction in risk of cesarean delivery or LGA compared with grade 3 obese women who gained 5 kg at term. There were 2.1% to 2.5% increases in risk of SGA associated with weight gains ≤2.3 kg compared with 5 kg at term among women with grade 3 obesity.
Table 3.
Associations between gestational age z-score and selected adverse perinatal outcomes among women with grade 1 obesity (n=148,335), grade 2 obesity (n=72,032), or grade 3 obesity (n=47,494), Pennsylvania singleton birth certificates, 2003–2011.
| Gestational weight gain z-score |
Total gestational weight gain equivalent at 40 weeks, kg |
Cesarean delivery | Adjusted a differences in risk per 100 births (95% CI) | Neonatal intensive care unit admission |
||
|---|---|---|---|---|---|---|
| Large-for- gestational-age birth |
Small-for- gestational-age birth |
Preterm birth | ||||
| Grade 1 obesityb | ||||||
| −3 SD | −4.3 | −1.6 (−2.5, −0.64) | −0.32 (−0.73, −0.09) | 1.4 (0.97, 1.8) | 0.89 (0.47, 1.32) | 0.52 (0.21, 0.83) |
| −2 SD | 0.05 | −0.92 (−1.3, −0.51) | −0.30 (−0.48, −0.12) | 0.81 (0.63, 0.99) | 0.43 (0.26, 0.61) | 0.23 (0.09, 0.36) |
| −1.1 SD | 5.0c | Ref | Ref | Ref | Ref | Ref |
| −1 SD | 5.6 | 0.19 (0.16, 0.22) | 0.11 (0.96, 0.12) | −0.16 (−0.18, −0.14) | −0.57(−0.07, −0.04) | −0.02 (−0.03, −0.01) |
| −0.5 SD | 9.0 | 1.6 (1.5, 1.8) | 1.2 (1.1, 1.2) | −1.3 (−1.5, −1.2) | −0.38 (−0.49, −0.27) | −0.08 (−0.16, −0.003 |
| 0 | 12.9 | 4.2 (3.7, 4.7) | 3.5 (3.2, 3.7) | −2.8 (−3.2, −2.6) | −0.74 (−1.0, −0.48) | −0.68 (−0.29, 0.13) |
| 0.5 SD | 17.3 | 7.4 (6.7, 8.2) | 6.6 (6.1, 7.0) | −4.1 (−4.4, −3.7) | −0.67 (−1.1, −3.0) | 0.13 (−0.15, 0.43) |
| 1 SD | 22.3 | 11 (10, 12) | 9.4 (8.9, 9.9) | −4.5 (−4.9, −4.1) | 0.14 (−0.24, 0.53) | 0.62 (0.29, 0.93) |
| 2 SD | 34.6 | 18 (17, 20) | 15 (14, 16) | −4.8 (−5.2, −4.3) | 3.3 (2.6, 3.9) | 2.1 (1.5, 2.6) |
| Grade 2 obesity | ||||||
| −3 SD | −8.2 | −1.7 (−2.7, −0.68) | −0.71 (−1.2, −0.24) | 1.8 (1.4, 2.1) | 1.1 (0.70, 1.5) | 0.49 (0.19, 0.78) |
| −2 SD | −4.0 | −1.5 (−2.1, −0.96) | −0.81 (−1.1, −0.55) | 1.3 (1.1, 1.6) | 0.69 (0.47, 0.90) | 0.27 (0.10, 0.43) |
| −1 SD | 1.8 | −1.01 (−1.2, −0.84) | −0.65 (0.84, 1.03) | 0.69 (0.58, 0.80) | 0.24 (0.16, 0.34) | 0.06 (−0.01, 0.12) |
| −0.6 SD | 5.0c | Ref | Ref | Ref | Ref | Ref |
| −0.5 SD | 5.6 | 0.39 (0.33, 0.47) | 0.28 (0.25, 0.31) | −0.23 (−0.28, −0.19) | −0.07 (−0.11, −0.04) | −0.01 (−0.04, 0.01) |
| 0 | 9.9 | 3.5 (2.9, 4.1) | 2.7 (2.5, 3.1) | −1.8 (−2.1, −1.5) | −0.48 (−0.74, −0.22) | −0.022 (−0.24, 0.19) |
| 0.5 SD | 15.1 | 7.7 (6.6, 8.8) | 6.6 (5.9, 7.2) | −2.9 (−3.3, −2.5) | −0.34 (−0.78, 0.10) | 0.25 (−0.096, 0.68) |
| 1 SD | 21.1 | 12 (10, 12) | 10 (9, 11) | −3.3 (−3.7, −2.8) | 1.01 (0.51, 1.51) | 1.2 (0.77, 1.7) |
| 2 SD | 36.6 | 19 (16, 22) | 18 (17, 21) | −3.2 (−3.9, −2.4) | 6.6 (5.2, 7.9) | 4.5 (3.4, 5.5) |
| Grade 3 obesity | ||||||
| −3 SD | −12.0 | −3.4 (−4.5, −2.3) | −2.8 (−3.3, −2.3) | 2.5 (1.9, 3.1) | 1.2 (0.71, 1.7) | 0.44 (0.08, 0.79) |
| −2 SD | −8.2 | −3.2 (−4.1, −2.3) | −2.8 (−3.2, −2.4) | 2.4 (1.9, 2.9) | 0.99 (0.55, 1.4) | 0.32 (−0.001, 0.63) |
| −1 SD | −2.3 | −2.7 (−3.5, −1.9) | −2.6 (−2.9, −2.3) | 2.1 (1.6, 2.6) | 0.76 (0.37, 1.2) | 0.20 (−0.079, 0.48) |
| −0.5 SD | 1.8 | −1.1 (−1.5, −0.74) | −1.3 (−1.5, −1.2) | 1.1 (0.89, 1.3) | 0.49 (0.29, 0.68) | 0.19 (0.049, 0.34) |
| −0.2 SD | 5.0c | Ref | Ref | Ref | Ref | Ref |
| 0 | 6.8 | 0.68 (0.53, 0.81) | 0.16 (0.01, 0.24) | 0.15 (0.098, 0.20) | 0.26 (0.21, 0.31) | 0.22 (0.17, 0.25) |
| 0.5 SD | 13.1 | 5.7 (4.7, 6.7) | 5.3 (4.7, 5.9) | −1.5 (−1.8, −1.2) | 0.39 (0.009, 0.78) | 0.66 (0.34, 0.99) |
| 1 SD | 20.8 | 9.6 (8.2, 11) | 9.8 (8.9, 11) | −1.8 (−2.2, −1.4) | 2.1 (1.6, 2.6) | 1.9 (1.4, 2.3) |
| 2 SD | 42.4 | 18 (13, 22) | 22 (18, 25) | −1.7 (−2.9, −0.45) | 8.6 (6.3, 11) | 5.7 (3.9, 7.6) |
Adjusted for maternal age, maternal race/ethnicity, maternal education, marital status, parity, smoking during pregnancy, pre-existing diabetes or hypertension, height, Black racial composition of neighborhood, payment source, urban residence, neonatal care level of the birth facility, and year of birth.
Grade 1 obesity, body mass index 30–34.9 kg/m2; grade 2 obesity, body mass index 35−39.9 kg/m2; grade 3 obesity, body mass index ≥40 kg/m2
The reference group for each body mass index group was the z-score equivalent to 5 kg, the lower limit of the 2009 Institute of Medicine recommended weight gain range for obese women.8
Results were not meaningfully different when we restricted to the sample with complete data, births at >24 weeks, or observations with GWG z-scores between 0.5th and 99.5th percentiles) (eFigures 1–3). Inclusion of prepregnancy BMI as a continuous confounder in the final obesity-stratified models had no meaningful impact on the results (data not shown).
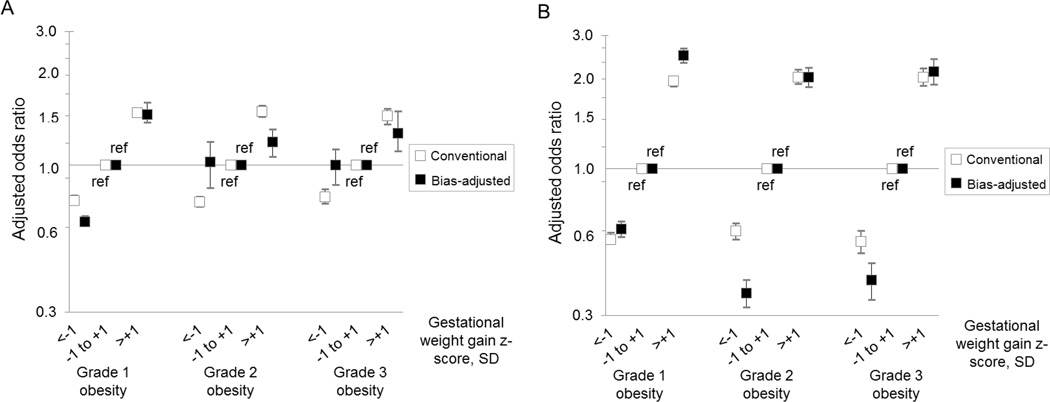
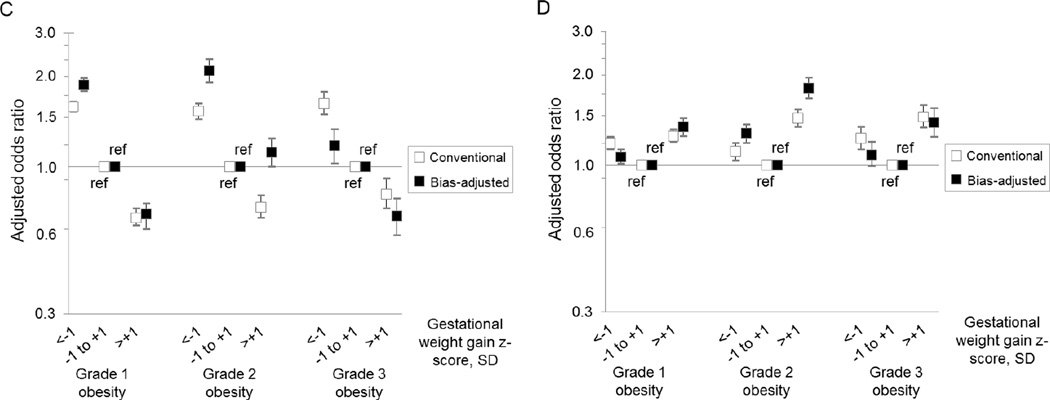
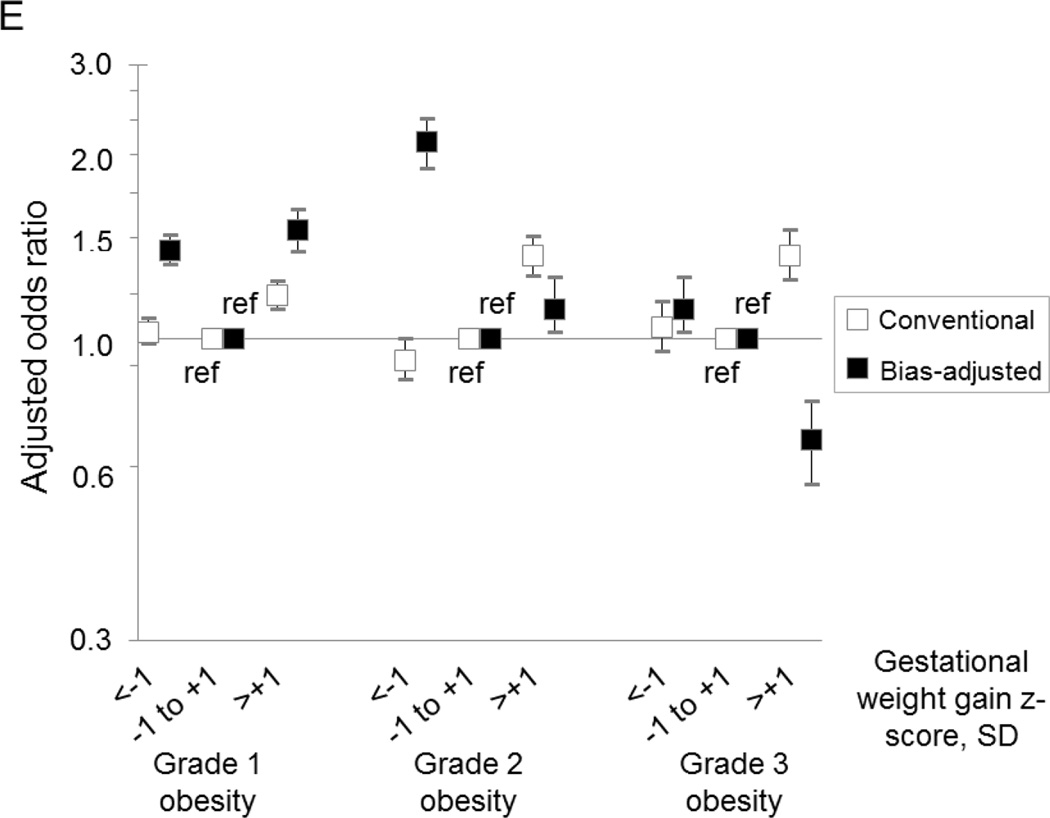
Our probabilistic bias analysis suggested that misclassification of prepregnancy BMI and gestational weight gain z-score biased our conventional results both towards and away from the null, depending on outcome (Figure 2). However, after bias analysis, the overall patterns of results and concomitant inferences were similar to those obtained from the conventional analysis.
Figure 2.
Comparison of estimates yielded by the conventional analysis (white boxes) and the probabilistic bias analysis (black boxes) of the association between gestational weight gain z-score and risk of cesarean delivery (Panel A), large-for-gestational-age birth (Panel B), small-for-gestational-age birth (Panel C), preterm birth (Panel D), and neonatal intensive care unit admission (Panel E) among women with grade 1 obesity (n=148,335), grade 2 obesity (n=72,032), or grade 3 obesity (n=47,494), Pennsylvania singleton birth certificates, 2003–2011.
All results were adjusted for maternal age, maternal race/ethnicity, maternal education, marital status, parity, smoking during pregnancy, pre-existing diabetes or hypertension, height, Black racial composition of census tract, payment source, urban residence, neonatal care level of the birth facility, and year of birth.
SD, standard deviation
Discussion
There is wide agreement that excessive gestational weight gain among obese women should be avoided to reduce risks of childhood obesity, postpartum weight retention, and later-life metabolic and cardiovascular diseases,8 but major gaps remain in our understanding of the safety of very low weight gain or weight loss in these high-risk pregnancies. Data from our population-based study, which is the largest to-date on weight gain among severely obese women, illustrated no meaningful difference in risk of cesarean delivery, LGA, preterm birth, or NICU admission from weight gains of −4.3 to 9 kg at 40 weeks for grade 1 obese women, −8.2 to 5.6 kg at 40 weeks for grade 2 obese women and −12 to −2.3 kg at 40 weeks for grade 3 obese women. Within these weight gain ranges, there were small increases in the risk of SGA. In all groups of obese women, weight gain beyond recommended ranges was associated with slight declines in risk of SGA and rapid rises in LGA and cesarean delivery. Preterm birth and NICU admission were not strongly associated with weight gain.
A recent systematic review of 10 weight gain studies in severely obese women concluded that the lowest combined risk of SGA, LGA, and cesarean delivery was observed at gestational weight gains 5–9 kg, 1–4.9 kg, and 0 kg for grades 1, 2, and 3 obese women, respectively.36 While our results generally agree with these conclusions, we extended previous research by evaluating dose-response associations across a wide distribution of gestational weight gain. Our finding that risk of these outcomes is essentially uniform until a weight gain threshold is reached may be important for determining safe lower limits of gestational weight gain. Notably, optimal gestational weight gain ranges for obese women may be substantially wider than currently recommended. Our study is also the first that we are aware of to examine the associations between gestational weight gain and preterm birth by severity of obesity using a measure of weight gain that is independent of length of pregnancy. Although we observed that weight gain z-scores were only weakly related to preterm birth and NICU admission, these outcomes were important to evaluate when considering safety of weight restriction because low weight gain increases their risk in non-obese samples.8
Analyses of large administrative databases with self-reported maternal weight are the source of our current knowledge on gestational weight gain in severely obese women. A major concern is the accuracy of these data, particularly for high BMI values, very low weight gains, and weight losses.20 Our probabilistic bias analysis, which was informed by a large internal validation study that accounted for the correlated errors in BMI and weight gain, was intended to overcome this limitation. We observed that the errors in the birth certificate-derived BMI and gestational weight gain categories depended on birth outcome, resulting in misclassification of conventional results. The direction of the bias varied, but its magnitude was not large enough to alter conclusions from our conventional analysis, conditional on the accuracy of our bias model. Our correction for misclassification assumes that the positive predictive values from our internal validation study were valid. These parameters were derived from a single hospital in Pennsylvania, which shares many characteristics of births statewide, but does not generalize perfectly.20 Nevertheless, this quantitative estimate of bias due to misclassification is superior to a solely qualitative evaluation of data quality.37
Although our study and others11–18 contribute evidence that will help to inform future gestational weight gain guidelines for severely obese women, fully understanding the safety of low weight gain or weight loss requires a wider exploration of other outcomes that may be associated with weight restriction, including stillbirth, infant death, and child neurocognitive development and behavior, as well as how weight gain-related outcomes should be weighted relative to one another according to their health impacts.8 Information is also needed on whether diet, activity, and provider oversight modify risk of poor maternal and child outcomes among obese women who gain little weight during pregnancy. Our study lacked data on important maternal and child outcomes that are likely related to high weight gain including later-life maternal and child obesity and its sequelae.8 We did not evaluate gestational diabetes or preeclampsia as outcomes because weight before diagnosis (which is needed to establish temporality)8 was not available. Exploring dose-dependent associations between these outcomes and gestational weight gain will aid in determining the trade-offs between high and low weight gain in severely obese mothers.
Gestational weight gain z-scores, like other measures of total weight gain, are based on one weight measurement, which is compared to other weight measurements of similar gestational age (expressed as a z-score). Therefore, z-scores do not provide information on the weight gain trajectory across pregnancy. Our z-score charts were developed in a large sample of obese women’s deliveries at a single tertiary care center in Pennsylvania. While the distribution of weight gain in this center is comparable to the distribution of weight gain across the state, it is possible that the percentiles in the chart may differ from true values in the underlying population. SGA is a commonly used surrogate for fetal growth restriction, but not all SGA infants have failed to reach their growth potential and are at risk for poor perinatal and long-term health consequences.38 Future weight gain studies with more sensitive measures of morbidity related to sequelae of fetal growth restriction at birth are needed. SGA and preterm birth rely on accurate pregnancy dating. Although we found high agreement between the birth certificate-derived and ultrasound-confirmed gestational age in a subsample of births, misclassification may remain because ultrasound has reduced effectiveness in dating pregnancies among obese women.39
Our study described associations between gestational weight gain and adverse pregnancy outcomes, but cannot determine causality. A recent randomized trial of lifestyle modification to reduce excessive gestational weight gain among obese women found that the intervention reduced mean weight gain and LGA births, but had too few cases of SGA or preterm birth to consider these endpoints.40
All levels of obesity complicate pregnancy, but the greatest concern is for women with severe obesity.3,4 Our results join others11–18 in suggesting that there is a range of healthy gestational weight gain in obese women that is associated with better pregnancy outcomes. Our findings support the view that gestational weight gain below national recommendations for obese mothers may not adversely affect fetal growth, gestational age at delivery, or mode of delivery and provide data that can help to establish evidence-based guidelines for these high-risk pregnancies.
Supplementary Material
Acknowledgments
The authors thank Melissa Papic, Suzanne Rich, Radkha Kerpedjieva, and Nancy Wolf for collecting and managing data for this study.
sources of funding: This project was supported by Thrasher Research Fund (#9181) and NIH grants R21 HD065807 and R01 HD072008.
Footnotes
Conflicts of interest:
The authors declare no conflicts of interest.
References
- 1.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. National Center for Health Statistics Health E-Stats. 2014 [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. American journal of preventive medicine. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Poston L, Harthoorn LF, Van Der Beek EM. Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatric research. 2011;69(2):175–180. doi: 10.1203/PDR.0b013e3182055ede. [DOI] [PubMed] [Google Scholar]
- 4.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125(1):133–143. doi: 10.1097/AOG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins CL, Zapata LB, Farr SL, Kroelinger CD, Morrow B, Ahluwalia I, D'Angelo DV, Barradas D, Cox S, Goodman D, Williams L, Grigorescu V, Barfield WD. Core state preconception health indicators - pregnancy risk assessment monitoring system and behavioral risk factor surveillance system, 2009. MMWR Surveill Summ. 2014;63(3):1–62. [PubMed] [Google Scholar]
- 6.Cardozo ER, Dune TJ, Neff LM, Brocks ME, Ekpo GE, Barnes RB, Marsh EE. Knowledge of obesity and its impact on reproductive health outcomes among urban women. J Community Health. 2013;38(2):261–267. doi: 10.1007/s10900-012-9609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitert MD, Foxcroft KF, Lust K, Fagermo N, Lawlor DA, O'Callaghan M, McIntyre HD, Callaway LK. Overweight and obesity knowledge prior to pregnancy: a survey study. BMC Pregnancy Childbirth. 2011;11:96. doi: 10.1186/1471-2393-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 9.Artal R, Lockwood CJ, Brown HL. Weight gain recommendations in pregnancy and the obesity epidemic. Obstetrics & Gynecology. 2010;115(1):152–155. doi: 10.1097/AOG.0b013e3181c51908. [DOI] [PubMed] [Google Scholar]
- 10.Barbour LA. Weight gain in pregnancy: is less truly more for mother and infant? Obstetric Medicine. 2012;5:58–64. doi: 10.1258/om.2012.120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyerlein A, Schiessl B, Lack N, von Kries R. Associations of gestational weight loss with birth-related outcome: a retrospective cohort study. BJOG. 2011;118(1):55–61. doi: 10.1111/j.1471-0528.2010.02761.x. [DOI] [PubMed] [Google Scholar]
- 12.Durie DE, Thornburg LL, Glantz JC. Effect of second-trimester and third-trimester rate of gestational weight gain on maternal and neonatal outcomes. Obstet Gynecol. 2011;118(3):569–575. doi: 10.1097/AOG.0b013e3182289f42. [DOI] [PubMed] [Google Scholar]
- 13.Duthie EA, Drew EM, Flynn KE. Patient-provider communication about gestational weight gain among nulliparous women: a qualitative study of the views of obstetricians and first-time pregnant women. BMC Pregnancy Childbirth. 2013;13:231. doi: 10.1186/1471-2393-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavard JA, Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern Child Health J. 2014;18(4):1038–1047. doi: 10.1007/s10995-013-1356-0. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle SN, Sharma AJ, Dietz PM. Gestational weight gain in obese mothers and associations with fetal growth. Am J Clin Nutr. 2010;92(3):644–651. doi: 10.3945/ajcn.2010.29726. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123(4):737–744. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oza-Frank R, Keim SA. Should obese women gain less weight in pregnancy than recommended? Birth. 2013;40(2):107–114. doi: 10.1111/birt.12037. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Sappenfield WM, Bish C, Salihu H, Goodman D, Bensyl DM. Assessment of the Institute of Medicine recommendations for weight gain during pregnancy: Florida, 2004–2007. Matern Child Health J. 2011;15(3):289–301. doi: 10.1007/s10995-010-0596-5. [DOI] [PubMed] [Google Scholar]
- 19.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8(4):307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 20.Bodnar LM, Abrams B, Bertolet M, Gernand AD, Parisi SM, Himes KP, Lash TL. Validity of birth certificate-derived maternal weight data. Paediatric and Perinatal Epidemiology. 2014;28(3):203–212. doi: 10.1111/ppe.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. American Journal of Clinical Nutrition. 2010;91(6):1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kominiarek MA, Seligman NS, Dolin C, Gao W, Berghella V, Hoffman M, Hibbard JU. Gestational weight gain and obesity: is 20 pounds too much? American Journal of Obstetrics & Gynecology. 2013 doi: 10.1016/j.ajog.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Paediatric and Perinatal Epidemiology. 2012;26:109–116. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) 2015;23(3):532–535. doi: 10.1002/oby.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC National Center for Health Statistics. Birth Edit Specifications for the 2003 Proposed Revision of the U.S. Standard Certificate of Birth. 2003 http://www.cdc.gov/nchs/data/dvs/birth_edit_specifications.pdf.
- 26.WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 27.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) doi: 10.1002/oby.21011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 29.USDA Economic Research Service. Rural-Urban Comtinuum Codes. 2013 [Google Scholar]
- 30.US Census Bureau. Census 2000, Summary File 3, Table P006, generated by Sarah Pugh using American FactFinder. [13 February 2015]; < http://factfinder2.census.gov/>>;
- 31.Stark AR. Levels of neonatal care. Pediatrics. 2004;114(5):1341–1347. doi: 10.1542/peds.2004-1697. [DOI] [PubMed] [Google Scholar]
- 32.Royston P. Multiple imputation of missing values: Further update of ice, with an emphasis on categorical variables. Stata Journal. 2009;9(3):466–477. [Google Scholar]
- 33.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. Journal of the National Cancer Institute. 1988;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 34.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lash TL, Abrams B, Bodnar LM. Comparison of bias analysis strategies applied to a large data set. Epidemiology. 2014;25(4):576–582. doi: 10.1097/EDE.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faucher MA, Barger MK. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: A systematic review. Women Birth. 2015 doi: 10.1016/j.wombi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Lash TL. Heuristic thinking and inference from observational epidemiology. Epidemiology. 2007;18(1):67–72. doi: 10.1097/01.ede.0000249522.75868.16. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg RL, Cliver SP. Small for gestational age and intrauterine growth restriction: definitions and standards. Clin Obstet Gynecol. 1997;40(4):704–714. doi: 10.1097/00003081-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Best KE, Tennant PW, Bell R, Rankin J. Impact of maternal body mass index on the antenatal detection of congenital anomalies. BJOG. 2012;119(12):1503–1511. doi: 10.1111/j.1471-0528.2012.03462.x. [DOI] [PubMed] [Google Scholar]
- 40.Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, McEvoy CT, Eckhardt CL, Smith KS, Stevens VJ. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity (Silver Spring) 2014;22(9):1989–1996. doi: 10.1002/oby.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.