Abstract
Objectives
Prostate cancer treatment is a significant source of morbidity and healthcare spending. Evolving clinical data have supported expanding surveillance as means to “right-size” treatment. Integrated delivery systems afford the possibility of hastening this objective.
Study Design
Retrospective cohort study of Medicare beneficiaries.
Methods
We used a 20% sample of national Medicare claims to assess the impact of healthcare integration on rates of treatment and potential overtreatment in newly diagnosed men with prostate cancer between 2007 and 2011. Rates were measured according to the extent of integration within a market (i.e., none, low, intermediate, high). Generalized estimating equations were used to assess the relationship between integration and utilization, adjusting for confounders.
Results
Rates of treatment declined across all markets (p < 0.01 for overall time trend) but the rate of decline was similar for the four market types (p = 0.27). In the most integrated markets, the rate decreased by 28.8%, or from 55.5 per 10,000 in 2007 to 39.5 per 10,000 in 2011. After adjusting for confounders, men residing in the most integrated markets were 2.1% less likely to be treated with curative intent compared to those living in areas without integrated delivery systems (p = 0.04). However, rates of potential overtreatment were similar across all markets regardless of the level of integration (p = 0.21).
Conclusion
Healthcare integration was associated with small declines in prostate cancer treatment in newly diagnosed men, but not potential overtreatment. Integrated care alone may be insufficient to curtail potential overtreatment of prostate cancer.
Graphical Abstract
Precis: Healthcare integration was associated with small declines in treatment but no change in overtreatment of prostate cancer. Integrated care delivery alone may be insufficient to curtail overtreatment.
Introduction
Prostate cancer is among the most common malignancies in U.S. men.1 Ongoing uncertainties about how best to treat the disease coupled with the availability of multiple options have led to wide variations in both the quantity and quality of care.2, 3 Prostate cancer spending has increased by 11% annually over the last decade, outpacing rates for other common conditions such as cardiovascular and pulmonary diseases and resulting in $12 billion in yearly expenditures.4, 5 While the merits of screening are a subject of ongoing debate in the field, there is growing consensus that some newly diagnosed men with prostate cancer stand little to gain from treatment.6-8
Improving the efficiency of the delivery system and eliminating wasteful spending have long been priorities for payers and policymakers. Many hope that accountable care organizations (ACOs) and related components of healthcare reform will do just that. By encouraging closer alignment between hospitals and caregivers, ACOs aim to focus on improving quality and cutting costs, both of which may affect prostate cancer care. To a large extent, ACOs are extensions of integrated delivery systems that, due to their emphasis on evidence-based medicine and minimizing unnecessary healthcare, are associated with providing higher quality.9-12 Thus, understanding the implications of integrated delivery systems for prostate cancer care will help us to anticipate the likely effect of evolving reforms of the Affordable Care Act.
For this reason, we performed a national study to determine the impact of health care integration on the treatment of prostate cancer. We hypothesize that the most integrated markets will be more selective in the use of curative treatment for prostate cancer, particularly among those unlikely to benefit from intervention.
Methods
Using a 20% sample of Medicare claims, we performed a retrospective cohort study of fee-for-service beneficiaries with newly diagnosed prostate cancer between 2007 and 2011. We limited our study to men continuously enrolled in Parts A and B for at least the 12-month period prior to and after the prostate cancer diagnosis. To ensure that we have complete claims on all patients, we excluded patients in risk-bearing Medicare managed care plans. Patients were followed though December 31, 2012.
To identify incident prostate cancer cases in national Medicare claims, we developed an algorithm and validated it using Surveillance Epidemiology and End Results (SEER) cancer registry data. Briefly, we used a 5% sample of Medicare beneficiaries residing in a catchment area of a SEER registry in the years 2003 to 2005. We selected men with at least two ‘Evaluation and Management’ visit codes where the line diagnosis ICD-9 code was ‘185’ for “prostate cancer”. We further required that all incident cases undergo prostate biopsy within 180 days of the first visit code associated with a prostate cancer diagnosis. We excluded men with any claim in the preceding 12-month period that was associated with a ICD-9 diagnosis code of ‘185’ (prostate cancer) or ‘V10.46’ (personal history of malignant neoplasm of prostate). Finally, we validated this approach against the Patient Entitlement Denominator Summary File, which identifies all incident cases in SEER regions, and found our algorithm to have a specificity and positive predictive value of 99.8% and 88.7%, respectively. We then implemented this algorithm in our 20% national sample of Medicare claims to identify incident cases, who comprise our study population.
We used hospital referral regions (HRRs), as described by the Dartmouth Atlas,13 to reflect distinct healthcare markets. There are 306 HRRs in the US, each of which represents a collection of zip codes where Medicare patients receive the bulk of their healthcare. We determined each market's level of integration by measuring the proportion of hospital discharges occurring from an integrated delivery system, which were identified from public reports based on data from IMS Health.14 These data provide information on delivery system relationships, including affiliations between hospitals and physician practices. Each health system is rated for 33 attributes in eight domains: overall integration, integrated technology, hospital utilization, financial stability, services, access, contract capabilities, and physicians. Domain-specific scores are added together to yield an overall score for the delivery system with higher scores reflecting a greater degree of integration.
All HRRs where characterized by the proportion of hospital discharges occurring from a top 100 integrated delivery system.15 Of these, 127 HRRs had no discharges from a top integrated delivery system. The remaining 179 HRRs were sorted into three equal groups (i.e., terciles) ranging from least (a mean of 14% of discharges from an integrated delivery system) to most (a mean of 71% of discharges from an integrated delivery system) integrated. For the purpose of analysis, our market level of integration exposure was treated as a categorical variable with four levels (none, low, intermediate and high).
Outcomes
Our primary outcome was the rate of treatment with curative intent (i.e., surgery, external beam radiation therapy, brachytherapy, cryotherapy) within 12 months of diagnosis, measured at the HRR level. For this calculation, the numerator was determined by the annual count of newly diagnosed patients undergoing any of the aforementioned treatments in a given HRR; the denominator was determined by the eligible male Medicare population residing in an HRR in a given year.
We also measured potential overtreatment. Because of their emphasis on quality and cost containment, integrated delivery systems, and presumably the markets where they dominate, may be more selective in the services they provide. In accordance with clinical guidelines, we would expect more integrated markets to have lower rates of treatment among those with a high risk of non-cancer mortality within 10 years of diagnosis. These patients typically have a low probability of dying from the disease, even absent treatment.16, 17 We assessed treatment among this population of patients (i.e., the quartile of men with the highest risk of dying from non-cancer causes within 10 years) implementing methods developed by Gross and colleagues.18 Briefly, we used a 5% sample of Medicare beneficiaries without cancer to build a robust patient-level model to predict mortality (C-index 0.91). This enabled us to estimate the 10-year mortality risk of patients in our prostate cancer cohort absent their cancer diagnosis. Those patients in the top quartile had a 78% risk of non-prostate cancer mortality within 10 years of their diagnosis. Potential overtreatment was assessed at the patient level, and population-based rates were calculated as described above (i.e., numerator was the number of potentially overtreated newly diagnosed patients with prostate cancer and the denominator was the number of eligible Medicare beneficiaries).
Analysis
We first contrasted patient and regional characteristics according to the healthcare integration exposure (i.e., none, low, intermediate or high). Statistical inference was made using the chi-square for categorical data and t-tests for continuous data. We then fit a Poisson model with an offset term for the population denominator to assess a trend in population-based rates of curative treatment over time across the integration groups.
To test the independent effect of healthcare integration on rates of prostate cancer treatment, we fit a multivariable logistic regression model using patient level treatment as the outcome. Our healthcare integration exposure was incorporated into the model at the HRR-level. To account for the nested nature of the data (i.e., patients within HRRs), generalized estimating equations were used. We adjusted the model for patient-level differences, including age, race, comorbidity and socioeconomic class. Comorbidity was calculated with patient claims for the 12-month window prior to diagnosis using established methods.19 Socioeconomic class was estimated using a composite measure developed at the zip code level, as described by Diez-Roux.20 We used the Area Resource File to derive several market level variables to include in the model, including a measure of social capital (% female head-of-household), education (% with a high school degree or more), and two supply side variables (urologists and hospital beds per capita). We computed adjusted percentages for the use of treatment for each level of our market integration exposure by back-transforming the predicted use from the model. A separate model was then fit to derive adjusted percentages of potential overtreatment using similar methods.
All analyses were carried out using computerized software (SAS, Cary, NC). All tests were two-tailed and the probability of Type 1 error was set at 0.05. The study protocol was judged to be exempt by our institutional review board.
Results
Patient and regional characteristics were contrasted according to the market level of integration in Table 1. While statistically significant differences between market types were evident for some variables (e.g., age), the magnitudes of the absolute differences were small, with one exception. Among markets with at least some discharges from integrated delivery systems, patients in markets with the lowest level of integration were more affluent than those in more integrated ones. For instance, 28.8% of patients in the least integrated markets resided in the highest quartile of socioeconomic class compared with 21.9% of patients in the most integrated ones (p < 0.01).
Table 1.
Differences in patient and regional characteristics according to market-level degree of healthcare integration
| Characteristic | Degree of Healthcare Integration | p-value | |||
|---|---|---|---|---|---|
| None (n=17,4985) | Low (n=19,415) | Intermediate (n=17,494) | High (n=12,797) | ||
| % discharges from IDN | - | 14 | 37 | 71 | - |
| Patient-level | |||||
| Age Mean (SD) |
70.5 (5.1) | 68.6 (4.0) | 69.3 (5.7) | 69.4 (5.3) | 0.12 |
| Race % non-white |
10.6 | 12.0 | 10.5 | 11.3 | <0.01 |
| Comorbidity % 2 or higher |
15.3 | 15.4 | 14.9 | 15.4 | 0.48 |
| Socioeconomic class % highest quartile |
19.0 | 28.8 | 29.1 | 21.9 | <0.01 |
| HRR-level | |||||
| Social capital % female head-of-household |
12.7 (3.2) | 12.6 (2.2) | 11.9 (2.6) | 11.7 (3.7) | 0.15 |
| Education % high school degree |
84.0 (6.0) | 84.7 (4.0) | 87.1 (3.7) | 87.3 (4.3) | <0.01 |
| Supply Urologists per 10K |
0.3 (0.1) | 0.3 (0.1) | 0.3 (0.1) | 0.3 (0.04) | 0.33 |
| Supply Hospital beds per 100K |
308.1 (111.4) | 296.8 (76.7) | 272.8 (82.1) | 284.7 (102.1) | 0.11 |
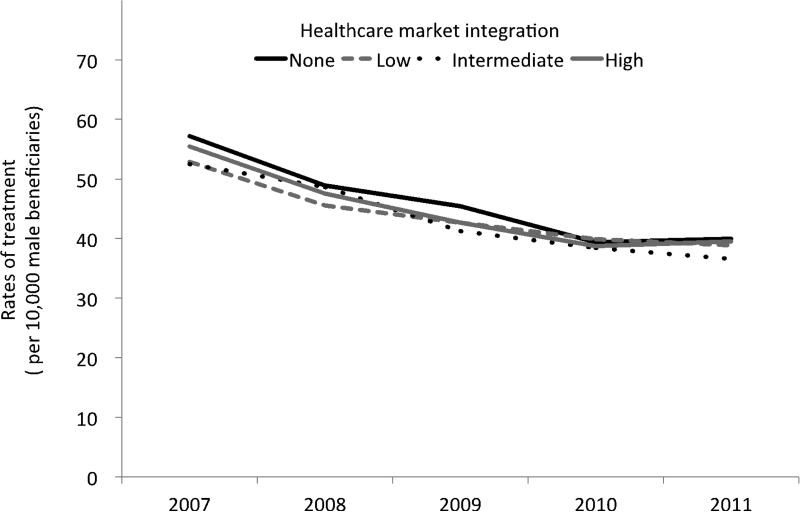
Between 2007 and 2011, population-based rates of curative treatment among newly diagnosed men with prostate cancer declined across all markets (Figure 1), irrespective of the level of integration (p < 0.01 for overall time trend). For example, in the most integrated markets, rates of treatment decreased from 55.5 per 10,000 male beneficiaries in 2007 to 39.5 per 10,000 in 2011, a relative decrease of 28.8%. In markets without integrated delivery systems (i.e., no discharges from these facilities), rates of treatment declined by 30.1%, or from 57.2 per 10,000 to 40.0 per 10,000 over the same period. The rate of decline in treatment over time was similar for the four market types (p = 0.27).
Figure 1.
Rates of treatment (prostatectomy, brachytherapy, cryotherapy and radiation) for prostate cancer between 2007 and 2011. There was a significant decline in rates of treatment over time (p < 0.01 for overall trend); however, the rate of decline was similar with respect to the level of market integration (p = 0.27).
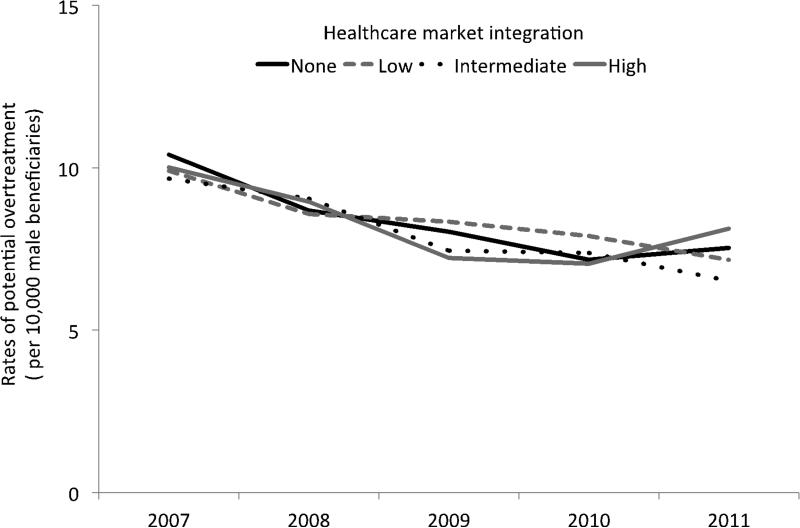
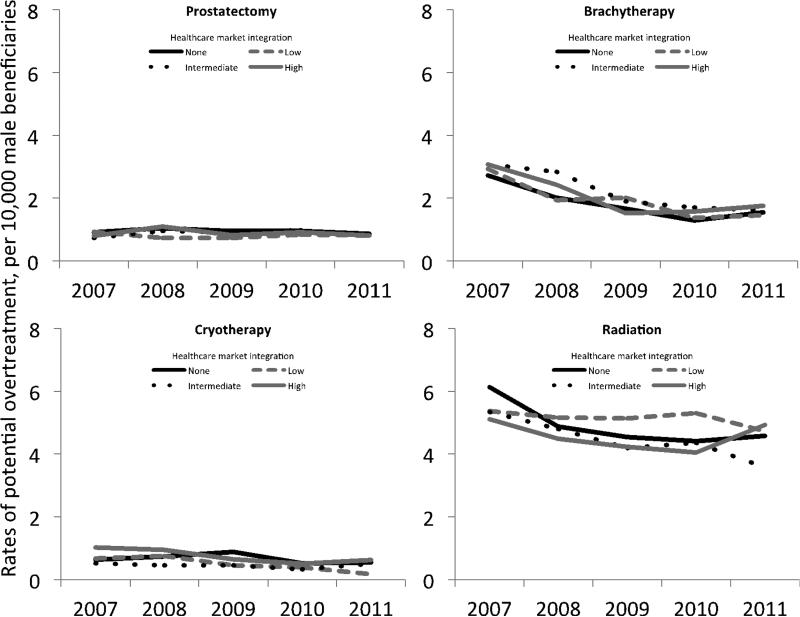
As illustrated in Figure 2, rates of potential overtreatment, or among those with the highest probability of death from non-cancer causes, also decreased, albeit to a lesser extent (p < 0.01 for overall time trend). Curative treatment in this population decreased by 19%, from 10.0 per 10,000 to 8.1 per 10,000 in the most integrated markets. Rates of decline were similar for the less integrated markets (p = 0.98). We next explored changes in potential overtreatment by modality (Figure 3). Rates of radical prostatectomy in these patients were low but remained stable over time (p = 0.99). Conversely, rates of brachytherapy, cryotherapy and external beam radiation therapy decreased during the course of the study (each p < 0.01 for overall time trend) but these declines did not vary with respect to the market level of integration (each p > 0.20).
Figure 2.
Rates of treatment (prostatectomy, brachytherapy, cryotherapy and radiation) for prostate cancer between 2007 and 2011 among men with the highest risk of non-cancer mortality (i.e., potential overtreatment). There was a significant decline in rates of potential overtreatment over time (p < 0.01 for overall trend); however, the rate of decline was similar with respect to the level of market integration (p = 0.98).
Figure 3.
Rates of potential overtreatment for prostate cancer by treatment modality between 2007 and 2011. Rates of prostatectomy remained flat over time (p = 0.99 for overall trend) while rates of brachytherapy, cryotherapy and radiation declined over time (each p < 0.01 for overall trend). Trends over time by treatment modality were independent of the level of market integration (each p > 0.20).
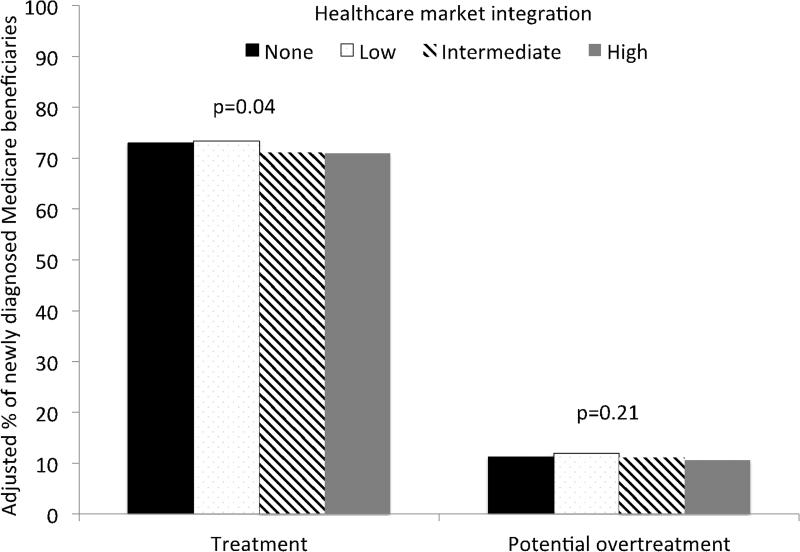
Finally, we used multivariable modeling to account for the observed subtle differences between both patients and healthcare markets (Figure 4). Among beneficiaries with newly diagnosed prostate cancer, we found that men residing in the most integrated markets were 2.1% less likely to be treated with curative intent compared to those living in areas without integrated delivery systems (p = 0.04). However, rates of potential overtreatment (i.e., among men with a high probability of death from non-cancer causes) were similar across all markets regardless of the level of integration (p = 0.21).
Figure 4.
Among newly diagnosed prostate cancer patients, market level integration was associated with lower rates of treatment (p = 0.04) but not overtreatment (p = 0.21) of prostate cancer. Models were adjusted for treatment year, patient characteristics (age, race, comorbidity, socioeconomic class) and market characteristics (% female head-of-household, % high school education or higher, urologists per 10,000 population, hospital beds per 10,000 population).
Discussion
Rates of treatment for prostate cancer among Medicare beneficiaries declined significantly among diagnosed with prostate cancer between 2007 and 2011. Similarly, treatment among men with a high risk of non-cancer mortality (i.e., potential overtreatment) decreased over the same period, albeit to a lesser extent. Among those treated with curative intent, the use of non-surgical approaches in men with the highest probability of non-cancer death within ten years decreased significantly, while rates of surgery remained stable in this population. Although patients residing in markets with the highest level of integration were less likely to undergo curative treatment for their cancer, the difference in magnitude relative to less integrated markets was small and of likely of limited clinical significance. Notwithstanding the advantages of highly integrated markets to deliver better evidence-driven health care, we found that the use of treatment among those least likely to benefit did not vary significantly according to the degree of integration in a market.
While prostate cancer remains a common cause of cancer-related death in the U.S., there is a growing consensus about the potential pitfalls of screening and the indolent nature of some cancers.6-8 Approaches to management of prostate cancer have evolved considerably over the last decade and surveillance has increasingly been recognized as a strategy in some men to prevent overtreatment. Despite this recognition, there is little evidence that the use of treatment in men unlikely to benefit is decreasing; rather, population-based data suggests that it is increasing.21 In our study of national Medicare beneficiaries, we noted a significant decline in potential overtreatment across all markets irrespective of their level of integration. However, these declines were relatively modest given the nature of the population (i.e., a very high risk of non-cancer mortality within 10 years of diagnosis).
That healthcare integration was associated with small declines in treatment but not with potential overtreatment is surprising. Indeed, integrated delivery systems are more apt to follow evidence-based practice guidelines, adopt electronic health information systems, implement strategies for performance improvement and, ultimately, associated with providing higher quality healthcare.9-12 Because of these qualities, we would expect healthcare markets dominated by these systems to be more selective in whom they treat for prostate cancer, particularly those who are unlikely to benefit.
With recent health reforms toward more accountable care, collections of providers, and in some cases hospitals, are evolving to become better stewards of population health. To some extent, accountable care organizations are the natural evolution of high performing integrated delivery systems. However, in addition to sharing a focus on care coordination and other aspects of integrated care, accountable care organizations imply a level of financial risk that likely surpasses that assumed by integrated delivery systems in the era prior to health reform. Perhaps, it is this added to pressure to realize savings at the beneficiary level that will promote stewardship of preference-sensitive conditions, such as prostate cancer, where there are clear tradeoffs with treatment.
One potential limitation of our findings is the absence of measures of disease severity (i.e., cancer grade, stage and prostate specific antigen levels) in national Medicare claims. Although we classify potential overtreatment using the probability of non-cancer death, our findings may underestimate the scope of treatment in the population, as patients with low-risk prostate cancer, irrespective of life expectancy, are well-accepted candidates for surveillance.6 Further, the absence of disease severity measures has little implication for our comparison of treatment rates across health care markets, as the distribution of cancer grade and stage tends to be similar across geographic regions.22
This limitation notwithstanding, our findings have important implications for evolving reforms aimed at improving the efficiency of healthcare delivery. We observed a small, albeit significant, association between higher levels integration and more constrained use of prostate cancer treatment. However, rates of potential overtreatment of men with prostate cancer were similar regardless of the extent of market integration. Collectively, these findings suggest that integration alone may be insufficient at optimizing management for conditions such as prostate cancer, where considerable uncertainty exists about the tradeoffs between treatment and observation. Future research should explore how the added financial risk implied by accountable care organizations is able to modulate the management of preference-sensitive diseases such as prostate cancer.
Take-Away Points.
Integration was associated with a small decrease in the treatment of prostate cancer.
Integration was not associated with treatment among men least likely to benefit (i.e., those with a high risk of non-cancer mortality).
Integration alone may be insufficient to promote optimal care for prostate cancer.
Financial risk, implied by evolving delivery system reforms, may help to promote better stewardship of preference-sensitive diseases, such as prostate cancer.
Acknowledgments
Funding: This work was supported by a Research Scholar Grant RSGI-13-323-01-CPHPS to BKH from the American Cancer Society. VBS is supported by funding from the NCI (R01 CA168691).
Footnotes
Publisher's Disclaimer: “This is the pre-publication version of a manuscript that has been accepted for publication in The American Journal of Managed Care (AJMC). This version does not include post-acceptance editing and formatting. The editors and publisher of AJMC are not responsible for the content or presentation of the prepublication version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to it (eg, correspondence, corrections, editorials, etc) should go to www.ajmc.com or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Disclosures: The authors have no conflicts of interest related to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol. 2008;26:3735–42. doi: 10.1200/JCO.2007.13.2555. [DOI] [PubMed] [Google Scholar]
- 4.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996-2005. Health Aff (Millwood) 2009;28(2):w358–67. doi: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 5.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. JNCI. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 7.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63(1):101–7. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 8.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Sterns JB. Quality, efficiency, and organizational structure. J Health Care Finance. 2007;34(1):100–7. [PubMed] [Google Scholar]
- 10.Casalino LP. Which type of medical group provides higher-quality care? Ann Intern Med. 2006:860–1. doi: 10.7326/0003-4819-145-11-200612050-00012. [DOI] [PubMed] [Google Scholar]
- 11.Tollen L. [September 21, 2011];Physician Organization in Relation to Quality and Efficiency of Care: A Synthesis of Recent Literature. 2008 Available from URL: http://www.commonwealthfund.org/usr_doc/Tollen_physician_org_quality_efficiency_1121.pdf.
- 12.Solberg LI, Asche SE, Shortell SM, et al. Is integration in large medical groups associated with quality? Am J Manag Care. 2009;15(6):e34–41. [PubMed] [Google Scholar]
- 13.Wennberg JE. The Dartmouth Atlas of Health Care in the United States. AHA Press; Chicago, IL: 1999. [Google Scholar]
- 14.Health I. [May 13, 2012];IMS IHN Rating System Description & Methodology. Available from URL: http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press Room/Top-Line Market Data & Trends/SDI Reports/2012_IHN_Rating_Methodology_.pdf.
- 15.Rodak S. [April 13, 2015];100 Integrated Health Systems to Know. Available from URL: http://www.beckershospitalreview.com/lists/100-integrated-health-systems-to-know.html.
- 16.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. JNCCN. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 17.Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(646-53):646. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. New Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of Advanced Treatment Technologies Among Men at Low Risk of Dying From Prostate Cancer. JAMA. 2013;309(24):2587–95. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S-Y, Wang R, Yu JB, et al. Understanding regional variation in Medicare expenditures for initial episodes of prostate cancer care. Med Care. 2014;52(8):680–7. doi: 10.1097/MLR.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]