Abstract
Introduction
Attention deficit/hyperactivity disorder (ADHD) is associated with health risks in adolescence which includes the potential for smoking cigarettes, early smoking initiation, and rapid progression to daily smoking. Much less is known, however, about prognostically-relevant smoking behaviors among individuals with childhood ADHD. Further research in this area is important for identifying individuals at pronounced risk for nicotine addiction, and for developing effective interventions for this population.
Method
This study examined initiation of cigarette smoking, progression to regular smoking, quantity of use, indicators of tobacco dependence, and quit rates among adolescents and young adults with (n=364) and without (n=240) childhood ADHD.
Results
Individuals with, versus without, ADHD histories were significantly more likely to become daily smokers independent of conduct disorder. They were also more likely to initiate smoking at younger ages and to progress to regular smoking more quickly. There were no significant group differences in cigarettes smoked per day, Fagerstrom Test of Nicotine Dependence or Nicotine Dependence Syndrome Scale scores or in smoking within 30 min of waking. However, smokers with ADHD reported more intense withdrawal and craving during periods of abstinence than nonADHD smokers. There were no significant group differences in number of quit attempts. Lastly, there were no significant differences among symptom persisters and desisters in daily smoking and amount.
Conclusions
Individuals with ADHD histories are at high risk for persistent smoking given their early onset, rapid course, and abstinence characteristics. Smoking cessation programs may need to be adapted or otherwise intensified for those with ADHD.
Keywords: ADHD, cigarette smoking, nicotine dependence, craving, withdrawal
Cigarette smoking is the leading cause of preventable death in the United States, accounting for approximately one of every five deaths each year (Rostron, 2013). The prevalence of smoking among individuals with a comorbid psychiatric diagnosis is significantly higher than cigarette smoking in the general population (Aubin, Rollema, Svensson, & Winterer, 2012; Lasser et al., 2000), and approximately 21–31% of smokers also exhibit psychiatric symptoms (e.g. mood, anxiety, substance use) (Aubin et al., 2012).
Cigarette smoking figures prominently among the most common substance use behaviors of individuals with Attention-Deficit/Hyperactivity Disorder (ADHD) histories (Barkley et al, 1990; Burke, Loeber & Lahey, 2001; Charach, Yeung, Climans & Lillie, 2011; Derefinko & Pelham, 2014; Hartsough & Lambert, 1987; Milberger et al., 1997; Molina & Pelham, 2003; Sibley et al., 2014). ADHD is among the most prevalent mental health disorders of childhood and is estimated to occur in approximately 5–8% of children and 4–5% of adults (Kessler et al., 2006; Visser et al., 2014). ADHD is characterized by symptoms of inattention, impulsivity, and hyperactivity, as well as impairments in activities of daily living (e.g., academic and social functioning) in both childhood (Bagwell, Molina, Pelham, & Hoza, 2001; Biederman et al., 2004; DuPaul et al., 2001; Kent et al., 2011) and adulthood (Babinski et al., 2010; Biederman et al., 2006; Kessler et al., 2006; Murphy & Barkley, 1996; Sibley et al., 2012). Childhood ADHD increases risk for multiple adverse outcomes, including alcohol and other drug use, abuse, and dependence (August et al., 2006; Barkley et al., 1990; Charach, 2011; Derefinko & Pelham, 2014; Ernst et al., 2006; King, Iacono & McGue, 2004; Lambert & Hartsough, 1998; Lee, Humphreys, Flory, Liu, & Glass, 2011; Molina & Pelham, 2003; Molina et al., 2007). Children with ADHD are also more likely to become regular cigarette smokers. They initiate smoking at earlier ages (Kollins et al., 2005; Milberger et al., 1997; Molina & Pelham, 2003; Sibley et al., 2014) and are more likely to continue smoking and to progress to regular smoking by adolescence or adulthood (Lambert & Hartsough, 1998; Molina & Pelham, 2003; Molina et al., 2013; Rohde et al., 2004, Sibley et al., 2014).
Converging lines of research suggest that cognitive (i.e. attention, Levin et al., 1996; inhibition, Levin et al., 2001, Potter & Newhouse, 2004, 2008; Ashare, Falcone, & Lerman, 2014), affective (i.e. emotion regulation; Kassel, Stroud, & Paronis, 2003), neurobiological (dopaminergic systems) and genetic factors (Faraone et al., 2005; Munafo et al., 2004; McClernon et al., 2008) likely play key roles in the ADHD-smoking comorbidity (McClernon & Kollins, 2008 and Modesto-Lowe et al., 2010). Additionally, psychological and social factors such as personality (e.g. impulsivity; Doran et al., 2004; Mitchell, 1999; Reynolds et al., 2004), deviant peer associations, ineffective coping strategies, and parenting (Harakeh et al., 2004; Laucht et al., 2007; Marshal & Molina, 2006; Simons-Morton, et al., 2001) have been implicated in the ADHD-smoking relationship. Although the prospective studies demonstrating higher rates of smoking, earlier initiation, and faster progression among ADHD smokers (Milberger et al., 1997; Molina & Pelham, 2003; Rohde et al., 2004; Sibley et al., 2014) represent important initial efforts toward understanding the ADHD-smoking relationship, other important smoking characteristics (i.e. heaviness of smoking, dependence, withdrawal, quit attempts) have been under-studied. These behaviors are known in the smoking literature to be important for prognosis during adolescence/young adulthood. Also, existing studies have, for the most part, been retrospective in nature (c.f. McClernon et al., 2008) and based on individuals with adult-diagnosed ADHD (Fuemmeler et al., 2007; Kollins et al., 2005; McClernon et al., 2008; Pomerleau et al., 2003).
Experimentation with smoking during adolescence is common and while most teen smokers do not progress to regular smoking, adolescent smoking is a risk factor for smoking in adulthood (Chassin, Presson, Sherman, & Edwards, 1990; Chassin, Presson, Rose & Sherman, 1996). Most adult daily smokers begin smoking during adolescence (U.S. Department of Health and Human Services, 2014). Initiation to smoking in adolescence is associated with a longer lifetime duration of smoking, heavier smoking rates, nicotine dependence and difficulty quitting (Breslau & Peterson, 1996; Hymowitz et al., 1997; Khuder, Dayal, & Mutgi, 1999). Adolescence and young adulthood are developmentally important periods for researching the initiation and progression of smoking behaviors. Thus, additional research is needed on initiation and rapidity of progression to daily smoking among young people with ADHD.
Factors that maintain smoking over time are prognostically-relevant because of their association with quitting and abstinence. Nicotine dependence (defined as chronic and repetitive use of nicotine products, tolerance for nicotine, experiencing withdrawal symptoms after abstaining, and difficulty quitting despite knowledge of the harmful health consequences of smoking (APA, 2013)), and quantity of cigarette consumption are relatively independent (Donny, Griffin, Shiffman & Sayette, 2008). Although the majority of daily smokers consume cigarettes at high rates each day (Shiffman, 1989), not all daily smokers are nicotine dependent (Donny & Dierker, 2007; Shiffman, Kassel, Paty, Gnys, & Zettler-Segal, 1994; Shiffman, Paty, Gnys, Kassel, & Elash, 1995). However, beginning in adolescence, each are prognostic of smoking behavior later in life (Breslau & Peterson, 1996; Chen & Kandel, 1995; Colby, Tiffany, Shiffman, & Niaura, 2000; Prokhorov et al., 2001; Sargent, Mott, & Stevens, 1998). The studies of dependence and quantity in relation to ADHD have largely focused on adult smokers cross-sectionally; findings are mixed, with some suggesting that adult ADHD is related to higher quantities (Kollins et al., 2005) and dependence (Fuemmeler et al., 2007), and others suggesting no difference (McClernon et al., 2008; Pomerleau et al., 2003). More research is needed to better understand the role these smoking maintenance factors play in the ADHD-smoking association and especially in young samples studied prospectively from ADHD in childhood.
Withdrawal symptom severity is a specific maintenance factor that may begin in adolescence (Colby, Tiffany, Shiffman, & Niaura, 2000) and is prognostic of future smoking behavior (Piasecki, 2006; Prokhorov et al., 2001). When smokers abstain, they typically experience a range of affective (e.g. irritability) and cognitive (e.g. difficulty concentrating) symptoms, and more severe withdrawal symptoms predict a faster return to smoking (Ferguson, Shiffman & Gwaltney, 2006; Piasecki at al., 2003a; 2003b; Javitz, Lerman, & Swan, 2012). Among adolescent smokers, craving (a strong desire to smoke) is the most commonly reported withdrawal symptom (Colby, Shiffman, Tiffany, & Niaura, 2000). There is preliminary evidence, based on retrospective recall, that adult smokers with ADHD experience more severe self-report and laboratory-based withdrawal symptoms than adult smokers without ADHD (McClernon et al., 2008; McClernon et al., 2011; Pomerleau et al., 2003). It has been posited that individuals with ADHD may smoke to alleviate their symptoms of ADHD (i.e. “self-medication” hypothesis) (e.g. Evans & Drobes, 2008; Gehricke et al., 2007) and there is a growing, albeit small, literature suggesting that smoking is negatively reinforcing by reducing ADHD symptoms, primarily inattention symptoms (Levin et al., 1996; Wilens et al., 1999). Either way, these findings suggest that during periods of abstinence, smokers with ADHD experience more withdrawal symptoms, particularly inattention, resulting in an increased vulnerability to smoking maintenance. This hypothesis has not been tested for adolescents. Given that a) withdrawal and craving may appear during adolescence and are predictive of cessation, and b) individuals with ADHD may exhibit more severe symptoms, more research is needed to better understand symptom severity among ADHD smokers during adolescence and young adulthood.
Finally, attempts to quit smoking in the general population of adult smokers are frequent (CDC, 2014), often unplanned (Larabie, 2005) and correlate with eventual long-term abstinence (Hymowitz et al., 1997). In adolescence, it is typical for smokers to alternate between periods of smoking and abstinence. While a small fraction of adolescent smokers achieve successful cessation (Burt & Peterson, 1998), length of previous quit attempts (i.e. longer duration) during adolescence predicts successful cessation (Zhu et al., 2009). Individuals with ADHD may be less likely to make serious, sustained quit attempts because of their longer smoking histories (and other vulnerabilities). To our knowledge, there is no research comparing quit rates and abstinence duration between individuals with and without childhood histories of ADHD.
The present study aimed to expand the characterization of these prognostic smoking behaviors in a sample of individuals with ADHD who were diagnosed in childhood and interviewed later as adolescents or young adults. The Pittsburgh ADHD Longitudinal Study (PALS) provides the opportunity to examine prognostically-relevant smoking behaviors. We hypothesized that individuals with ADHD, compared to demographically similar individuals without ADHD histories, would report a) higher quantities of consumption and stronger dependence, b) more severe withdrawal symptoms including craving, and c) a lower frequency and duration of quit attempts. In addition, we expected to replicate group differences in attainment of smoking milestones, including more experimental and daily smoking at younger ages, and more rapid progression to regular smoking, in the ADHD compared to the nonADHD group, as well as extend the examination of the contribution of ADHD symptom persistence to current smoking (Molina & Pelham, 2014). A more clear understanding of tobacco use among individuals with ADHD during this critical developmental window may lead to improved understanding of smoking risk for people with ADHD and, ultimately, more targeted and effective prevention and treatment strategies.
Methods
Participants
ADHD Probands
Screening and diagnostic procedures have been described previously (Molina et al., 2012; Pedersen et al., 2014; Sibley et al., 2012). Briefly, participants with childhood ADHD were diagnosed with DSM-III-R or DSM-IV ADHD at the ADD Clinic, Western Psychiatric Institute and Clinic, in Pittsburgh, PA between 1987 and 1996. The average age at initial evaluation was 9.40 years (SD = 2.27 years). Ninety percent of children were diagnosed between ages 5 and 12 years. ADHD probands were selected for follow-up interviews due to their diagnosis of ADHD and participation in a summer treatment program (STP) for children with ADHD (Pelham & Hoza, 1996). Diagnostic information was collected in childhood using standardized parent and teacher DSM-III-R and DSM-IV symptom rating scales (DBD; Pelham, Evans, Gnagy, & Greenslade, 1992) and a standardized semi-structured diagnostic interview administered to parents by a Ph.D. level clinician. Following DSM guidelines, diagnoses of ADHD were made if a sufficient number of symptoms were endorsed to result in diagnosis. Exclusion criteria for follow-up were assessed in childhood and included a full-scale IQ < 80, a history of seizures or other neurological problems, and/or a history of pervasive developmental disorder, schizophrenia, or other psychotic or organic mental disorders.
Of those eligible for follow-up in the PALS sample (n = 516), 70.5% (n = 364) participated for an average of M = 8.35 years after childhood diagnosis (S.D. = 2.79). A minority could not be located (n = 23); 129 refused or failed to participate. Participants were different (p < .05) from nonparticipants on only one (conduct disorder symptom ratings: participants M = .43, non-participants M = .53, Cohen's d = .30) of 14 comparisons on demographic (e.g. age at first treatment, race, parental education level, and marital status) and diagnostic (e.g., parent and teacher ratings of ADHD symptoms) variables from childhood. In childhood, average DSM-III-R ADHD symptom rating was 2.26, S.D. = .45, on a scale of 0 to 3; average number of DSM-III-R ADHD symptoms endorsed by parent or teacher was 12.56, S.D. = 1.78; percent with DSM-III-R ODD was 47%; percent with DSMIII-R CD was 36%. At the first PALS follow-up interview, when data used in this report were collected and which occurred on a rolling basis between 1999 and 2003, mean age was 17.75 years, S.D. = 3.39 years, range = 11 to 28 (three subjects were 26–28 years old), 89.6% were male, and 18.4% were racial/ethnic minority.
NonADHD comparison group
Individuals without ADHD were recruited into the PALS at the same time as the probands' recruitment into the follow-up study. NonADHD comparison participants were recruited on a rolling basis to ensure demographic similarity to the probands and were matched to probands (age within one year, sex, race, highest parental education). They were recruited from the greater Pittsburgh area from several sources including pediatric practices serving patients from diverse socioeconomic backgrounds, advertisements in local newspapers and the university hospital staff newsletter, local universities and colleges, and other miscellaneous sources. A telephone screening with parents gathered demographic characteristics, history of diagnosis and treatment for ADHD and other behavior problems, presence of exclusionary criteria as previously listed for ADHD probands, and a checklist of ADHD symptoms. Young adults (18+) also provided self-report. Individuals who met DSM-III-R criteria for ADHD were excluded. NonADHD comparison participants with subthreshold ADHD symptomatology, or with other psychiatric disorders other than those listed above as exclusionary, were retained. There were no statistically significant differences between the 364 ADHD probands and 240 nonADHD comparison participants on age, sex, ethnicity/racial minority status, or highest parental education. As with the ADHD probands, the nonADHD comparison participants were interviewed on an annual basis once recruited into the PALS.
Procedures
Interviews were conducted in the ADD Program offices by post-baccalaureate research staff. Data from the first follow-up interview of the children with ADHD, and the first interview of the nonADHD group, were used for this study. Informed consent was obtained and all participants were assured confidentiality of all disclosed material except in cases of impending danger or harm to self or others (reinforced with a DHHS Certificate of Confidentiality). In cases where distance prevented participant travel to WPIC, information was collected through a combination of mailed and telephone correspondence; home visits were offered as need dictated. Self-report questionnaires were completed either with pencil and paper or web-based versions.
Measures
Substance Use Questionnaire
Cigarette use was evaluated with a structured paper-and-pencil substance use questionnaire (SUQ) that is an adaptation of existing measures (e.g., Health Behavior Questionnaire, Jessor et al, 1989; National Household Survey of Drug Abuse, NHSDA 1992). The SUQ includes lifetime exposure questions (e.g., ever tried smoking; ever smoked a cigarette, more than just a puff; age started regular smoking; ever smoked on a daily basis), age of initiation, quantity of use, frequency of use, and number of quit attempts. Two items were added to assess craving (“If you had any craving, how severe was it, at its worst?”) and difficulty concentrating (“If you had any difficulty concentrating, how severe was it at its worst?”) during past periods of abstinence (i.e. “What is the longest period of time that you went without smoking after quitting or trying to quit?”). Participants rated their experience on a Likert scale of 1 (I had none) to 5 (Very Severe). Two-week test-retest reliability for the SUQ is good; for example, kappa for ever tried cigarettes = .87.
Fagerström Test for Nicotine Dependence (FTND)
The FTND is a widely used 6-item, self-report measure of nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). The FTND has adequate psychometric properties (Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994). Using a sum score, the possible range of scores is from 0–10, with 5 indicating moderate nicotine dependence. Research has demonstrated predictive validity (for cessation) of a single dependence item from the FTND indicating whether a smoker has their first cigarette within 30 minutes of waking (e.g. Baker et al., 2007). As such, this item was also examined separately.
Nicotine Dependence Syndrome Scale (NDSS)
The NDSS (Shiffman, Waters, & Hickcox, 2004) is a five-factor (drive, priority, tolerance, continuity, and stereotypy), nineteen-item scale that requires participants to rate features of nicotine dependence (e.g. I feel a sense of control over my smoking) on a 5-point Likert scale (1 = Extremely Untrue, 5 = Extremely True). A total score and individual scale scores were calculated following recommended scoring procedures (see Appendix in Shiffman, Waters, & Hickcox, 2004). The NDSS was included as a second measure of nicotine dependence because of its multidimensional assessment of dependence. It exhibits adequate psychometric properties (Shiffman & Sayette, 2005; Shiffman, Waters, Hickcox, 2004) and demonstrates sensitivity to individual differences across the continuum of nicotine dependence (Shiffman & Sayette, 2005).
ADHD Symptom Persistence
ADHD symptom persistence, defined as 4 or more symptoms (Sibley et al., 2012) was obtained for ADHD probands from follow-up measures of symptoms reported by participants, parents and teachers at the follow-up interview.
Results
Cox Regressions were performed to compare first trying a cigarette, first smoking a cigarette (more than a puff), and initiating daily smoking between the ADHD and nonADHD groups, as well as to compare the two groups on the time of progression to daily smoking from first trying a cigarette. Ordinal regression was used to examine group differences in cigarette consumption. ANOVAs were used to compare the two groups on dependence rates, withdrawal and craving, and quit attempts. Gender, childhood conduct disorder, race, and highest parental education were included as covariates in all analyses.
Before testing our hypotheses about ADHD and prognostically-relevant smoking behaviors, we first report smoking milestones and rates of initiation by ADHD/nonADHD group. For smoking milestones and for descriptive purposes, we report comparisons by age subgroups due to prior findings of age-specificity for other substance use (alcohol) outcomes (Molina et al., 2007). Binary logistic regressions were performed to compare smoking milestones among ADHD and nonADHD groups (see Table 1).
Table 1.
Rates of ever trying a cigarette, ever smoking a cigarette, and ever smoking daily, by diagnostic group and age group.
| Mean Age (SD) | ADHD | nonADHD | Wald | Exp(B) | |
|---|---|---|---|---|---|
| 11–14 year olds | 13.0 (1.0) | N = 69 | N = 56 | ||
| Tried a cigarette | 41% | 14% | 7.2** | 4.0 | |
| Smoked a cigarette | 19% | 5% | 3.1+ | 3.6 | |
| Daily smokinga | 9% | 0% | na | na | |
| 15–17 year olds | 16.0 (.8) | N = 94 | N = 64 | ||
| Tried a cigarette | 63% | 42% | 4.1* | 2.1 | |
| Smoked a cigarette | 54% | 36% | 3.6+ | 2.0 | |
| Daily smoking | 33% | 5% | 10.9*** | 8.7 | |
| 18–20 year olds | 18.8 (.8) | N = 124 | N = 84 | ||
| Tried a cigarette | 73% | 74% | .75 | .73 | |
| Smoked a cigarette | 65% | 66% | 1.1 | .71 | |
| Daily smoking | 48% | 32% | .46 | 1.3 | |
| 21+ year olds | 22.5 (1.5) | N = 73 | N = 34 | ||
| Tried a cigarette | 92% | 82% | .57 | 1.7 | |
| Smoked a cigarette | 85% | 79% | .12 | 1.3 | |
| Daily smoking | 68% | 41% | 5.9** | 3.6 |
Note.
Due to low sample size in nonADHD group, analyses were not conducted.
< .10
p < .05,
p < .01,
p < .001.
Smoking milestones
Table 1 displays percent of participants reaching smoking milestones (ever tried a cigarette, ever smoked a cigarette (more than a puff), and ever smoked on a daily basis) by ADHD/nonADHD group separately for each age subgroup. Individuals in the ADHD group were significantly more likely than those in the nonADHD group to have tried smoking, smoked more than a puff, and to have smoked daily in early and late adolescence. ADHD probands were also more likely to be daily smokers in the young adulthood groups.
Initiation of smoking behaviors
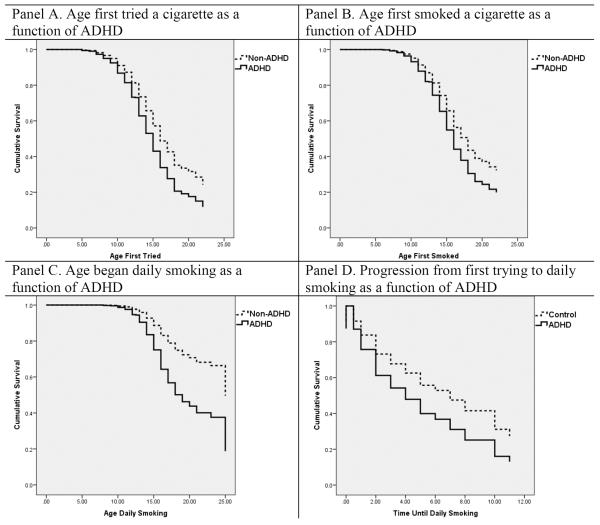
Compared to the nonADHD group, individuals in the ADHD group reported earlier onset of each milestone: first trying a cigarette (Wald statistic = 5.74, df = 1, p = .02, Hazard ratio = 1.35), first smoking a cigarette (Wald statistic = 2.76, df = 1, p = .10, Hazard ratio = 1.25), and first daily smoking (Wald statistic = 12.26, df = 1, p < .001, Hazard ratio = 2.02) (See Figure 1). The median ages for these behaviors (based on raw medians from participants who reported smoking) were as follows: ADHD probands first tried a cigarette on average at 13.00 years (SD = 3.20) vs. 14.00 years (SD = 2.56) for the nonADHD group, ADHD probands first smoked a cigarette on average at 14.00 years (SD = 3.00) vs. 15.00 years (SD = 2.29) for the nonADHD group, and ADHD probands first began daily smoking at 15.00 years (SD = 2.48) vs. 16.00 years (SD = 2.08) for the nonADHD group.
Figure 1.
Cox Regressions of Initiation and Progression
Speed of progression to daily smoking
Cox Regressions were performed on all participants who reported ever trying smoking to compare the two groups on the time of progression to daily smoking from first trying a cigarette (Figure 1). Compared to nonADHD participants, individuals with ADHD progressed more rapidly (Wald statistic = 5.30, df = 1, p = .02, Hazard ratio = 1.57) from first trying smoking to daily smoking, having a 57% greater risk of progression each year. The probands' model-estimated median rate of progression from first trying a cigarette to daily smoking was one year, whereas the median rate of progression for nonADHD smokers was two years.
Cigarette consumption
Quantity of cigarette use was examined among individuals who reported currently smoking. This subsample included 128 in the ADHD group (Mean age = 19.4, SD = 2.8; 87% male; 87% Caucasian) and 39 in the nonADHD group (Mean age = 20.0, SD = 1.8; 82% male; 92% Caucasian). Based on the distributions, responses were grouped for analysis into fewer than 5 cigarettes per day (44 ADHD vs 16 nonADHD), between 5 and ½-pack per day (35 ADHD vs. 8 nonADHD), and about a pack or more per day (49 ADHD vs. 15 nonADHD). Using ordinal regression, these categories were regressed on ADHD group and years smoking (because the latter is known to predict higher quantity). Although years smoking (beta estimate = .18, Wald=11.9 (1), p = .001) predicted higher quantities of cigarette consumption, ADHD group (beta estimate = −.24, Wald=..35 (1), p > .05) did not.
Dependence
The NDSS and FTND total scores were compared between ADHD and nonADHD daily smokers (n=95 and 29, respectively) using an ANOVA controlling for total years smoking. The results in Table 2 show that there was no significant group difference in nicotine dependence scores, F's <1, p's > .05, d's = .15, .18. Means of both scales indicate mild dependence in both groups (NDSS Range = −2.32 to .35; FTND Range = 0 to 9). Analyses of the NDSS subscale scores demonstrated that participants in the ADHD group did not significantly differ from participants without ADHD on any of the individual NDSS subscale scores, F's < 1.17, p's > .28, d's < .40.
Table 2.
Descriptive Information for Reported Smoking Behaviors by ADHD Group.
| ADHD | nonADHD | p value | Cohen's d | |
|---|---|---|---|---|
| Dependence | ||||
| NDSS | −1.2 (.6) | −1.1 (.5) | ns | .18 |
| FTND | 3.9 (1.9) | 3.6 (2.0) | ns | .15 |
| Withdrawal (Difficulty Concentrating) | 2.0 (1.1) | 1.5 (1.0) | .02 | .48 |
| Craving | 2.2 (1.1) | 1.8 (1.1) | .04 | .36 |
| Quit Attempts | ||||
| Number of Attempts | 3.5 (7.1) | 4.8 (16.9) | ns | .10 |
| Length of Attempt | 3.7 (1.6) | 4.1 (1.6) | ns | .25 |
Note. Values represent mean values, parenthesis reflect standard deviation. Sample sizes are as follows: Dependence ADHD n=111, nonADHD n=31; Withdrawal ADHD n=208, nonADHD n=109; Craving ADHD n=208, nonADHD n=109; Quit Attempts ADHD n=112, nonADHD n=34.
To examine differences in the time to first cigarette (within the day), responses were categorized into (1) less than 30 minutes after waking, and (2) more than 30 minutes after waking. There was a nonsignificant trend for the ADHD probands to be more likely to smoke within 30 minutes of waking (62%; n=65/105) compared to the nonADHD group (45%; n=14/31), X2 = 2.81, p = .09. Given that the present sample includes adolescents whose smoking behaviors may be restricted due to environmental contexts (e.g. living with parents), an ordinal regression was performed to explore the effects of age. Neither age (beta estimate = .08, Wald statistic = .964, p > .05) nor the group (ADHD vs. nonADHD) × age interaction (beta estimate = .21, Wald statistic = .60, p >.05) predicted smoking within 30 minutes of waking.
Withdrawal (Difficulty Concentrating) & Craving
Smokers with ADHD reported significantly greater difficulty concentrating (F(1, 288) = 5.36, p=..02) and craving (F(1, 290) = 4.44, p = .04) during periods of abstinence compared to smokers in the nonADHD group (see Table 2 for descriptive statistics). Effect sizes were medium (see Table 2) with all participants indicating on average mild difficulty.
Quit Attempts
The number of reported quit attempts (see Table 2 for descriptive statistics) was compared between the ADHD (n = 99) and nonADHD (n = 34) groups after controlling for number of years smoking. No significant group differences emerged, F=1.55, p=.22. Additionally, the length (lower values reflect shorter duration of quit attempt) of serious quit attempts was compared between the ADHD and nonADHD groups after controlling for number of years smoking; no significant group differences were observed, F<1, p=.59. Effect sizes were small (see Table 2).
ADHD Symptom Persistence
Finally, we examined whether the persistence of ADHD symptoms was associated with likelihood of being a daily smoker and with current smoking amount (none, <5 cigarettes, ½ pack, 1 or more packs per day) among those with ADHD. For daily smoking, 73% (n=62/85) of ADHD symptom desisters and 71% (n=83/117) of ADHD symptom persisters were daily smokers, X2 = .10, df(1), p = .76. For current smoking quantity among smokers, the association was also not statistically significant, X2 = 1.24, df(3), p = .74. For example, 48% (n=42/87) of ADHD symptom desisters and 45% (n=53/119) of ADHD symptom persisters smoked ½ pack a day or more.
Discussion
The primary aim of the present study was to examine smoking behaviors that are prognostically-relevant for long-term smoking in a sample of individuals diagnosed with ADHD in childhood and followed prospectively into adolescence and early adulthood. We found important replication of higher rates of daily smoking, earlier initiation, and faster progression to daily smoking among those with ADHD histories compared to those without1. However, when we compared prognostically-relevant smoking behaviors important for long-term smoking outcome, between ADHD and nonADHD smokers, our findings were mixed. We discuss below the implications of these varied findings and provide suggestions for future research.
Our replication of previous reports of early initiation and fast progression (Kollins et al., 2005; Milberger et al., 1997; Molina & Pelham, 2003; Rohde et al., 2004; Sibley et al., 2014) provides important additional support for a previously observed behavioral pattern among ADHD smokers. Replication was needed because of important differences in methodologies across these studies. Some have followed children prospectively into adolescence and young adulthood from childhood (Molina et al., 2007; 2013; Sibley et al., 2014), while others have assessed initiation and progression retrospectively (Kollins et al., 2005; Milberger et al., 1997; Rohde et al., 2004). In our study, some participants were recalling their initiation behaviors over multiple years (e.g., those who were in their 20s when they were first interviewed), but others (the adolescents) were younger. Given that self-reported age of onset tends to increase as participant age at the time of recall increases (O'Neil, Parra, & Sher, 2003), our findings are probably the most precise for those participants whose initiation occurred close-in-time to the first interview. Another methodologic consideration is that some studies (e.g. Kollins et al., 2005, Rohde et al., 2004) have relied on self-report of ADHD symptoms, which has been shown to be an underestimation of severity (e.g. Kooij et al., 2008). In the current study, ADHD was diagnosed in childhood using standardized interview and rating scale data from parents and teachers. Despite these differences, our findings and those of others consistently indicate that individuals with ADHD exhibit earlier and rapid uptake of smoking milestones.
Specific psychological, psychosocial, and biological vulnerabilities associated with ADHD may explain early experimentation with smoking and rapid progression to increasingly involved smoking behavior. For example, difficulties with delay discounting, impulsivity, novelty seeking, and associations with deviant peer groups are each considered risk factors for smoking (Doran et al., 2004; Marshal, Molina, & Pelham, 2003; Reynolds, et al., 2003; Shaw, Stringaris, Nigg & Leibenluft, 2014), and also characterize many individuals with ADHD (Hoza, 2007; Marshal & Molina, 2006; Marshal, Molina, & Pelham, 2003; Winstanley, Eagle & Robbins, 2006). The combination of an increased tendency to act impulsively, perhaps in the presence of strong rewards and/or new situations, and engagement with peers who are more likely to expose the individual to smoking, may contribute to smoking at a young age. Similarly, once initiated, individuals with ADHD may experience nicotine as more reinforcing than those without ADHD, due to an aberrant dopaminergic system (Sonuga-Barke, 2003; Tripp & Wickens, 2008; Volkow et al., 2009) that may result in a more rapid progression to regular smoking. While it is unclear exactly why individuals with a history of ADHD progress through the stages of smoking more quickly than those without ADHD, research that focuses on ADHD-related vulnerabilities, may highlight potential contributing factors.
Surprisingly we found no group differences in either quantity of cigarettes smoked or dependence scores. The lack of group differences may be due to our sample's relatively young age; their smoking behaviors may be developmentally “young” for this outcome and factors such as living at home with parents and smoking policies on school campuses, may affect consumption and dependence. If so, that may explain the equivocal nature of the literature on adolescents (Fuemmeler, et al., 2007; McClernon et al., 2008; Pomerleau et al., 2003). Lack of group differences in dependence scores may also be caused by under-reporting of impairment by individuals with ADHD histories (Sibley et al., 2012). Given that both lower consumption and dependence are associated with better success in quitting smoking, this pattern of results highlights the importance of future research prospectively following individuals with a childhood history of ADHD into adulthood to determine whether group differences emerge at older ages than in the current study. It is also possible that the absence of group differences is due to the small number of participants without ADHD who reported regular smoking; this small subsample may have limited our ability to observe group differences in dependence.
The ADHD group difference in timing of the first cigarette of the day was marginally statistically significant with those in the ADHD group slightly more likely to report smoking within the first 30 minutes of the day. Some research has demonstrated that this specific behavior reflects dependence and predicts cessation and relapse (Baker et al., 2007). Behaviors typical of heavy dependence (e.g. smoking more frequently during first hours of waking; difficulty refraining from smoking in places where it is forbidden; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) are often just beginning to emerge during adolescence (Wellman, DiFranza, Savageau & Dussault, 2004). Though not statistically significant, these results suggest that for individuals with a history of ADHD, emerging dependence in adolescence may be reflected in this behavior and pertinent to the development of long-term dependence.
An important finding of the current study was the ADHD group differences in difficulties with craving and concentration (medium effect sizes) when deprived of cigarettes. The literature examining differences in craving and other withdrawal symptoms is limited and equivocal, with some studies reporting ADHD group differences (McClernon et al., 2011, Pomerleau et al., 2003) and others not (McClernon et al., 2008; McClernon et al., 2011; Pomerleau et al., 2003. The methodological differences among these studies may be important. Our findings were similar to those of Pomerleau and colleagues (2003) who also used retrospective self-report. On the other hand, McClernon and colleagues implemented laboratory-based abstinence paradigms using overnight (2008) and longer-term (2011) abstinence manipulations to investigate prospective effects on self-report withdrawal, craving, and cognition (computerized assessments). The aggregated findings across research groups suggest the presence of some important differences, particularly in craving and concentration, for ADHD versus nonADHD smokers experiencing deprivation. There is a clear need for additional research to employ more comprehensive and consistent assessments of withdrawal and craving during actual periods of deprivation to further elucidate these highly prognostic processes among smokers with ADHD.
Individuals with a history of ADHD did not differ in quit attempts compared to nonADHD smokers regardless of a longer duration of smoking. The young age of our sample may be a contributing factor. Smokers typically make several quit attempts prior to successful cessation in adulthood, with previous attempts predicting cessation (Hymowitz et al., 1997). Also, the literature suggesting that individuals with ADHD have more difficulty achieving cessation is based solely in adult samples (Pomerleau et al., 1995; Humfleet et al., 2005). Thus, the present sample may not have captured the pertinent timeframe for evaluating this stage of smoking. There is a need for prospective studies throughout adolescence and adulthood that examine quit attempts and related processes that may precede successful cessation.
ADHD symptom persistence was not associated with likelihood of being a daily smoker or with smoking amount. Given that many individuals with ADHD report a persistence of symptoms over time (Barkley, Fischer, Smallish, & Fletcher, 2002; Sibley et al., 2012a; 2012b) and this persistence from childhood has been associated with substance use (Molina & Pelham, 2003; Molina et al., 2007; Sibley et al., 2012a,b) including tobacco use (Breyer et al., 2014; Chang, Lichtenstein & Larsson, 2012), our findings were contrary to expectation. Perhaps, when considering the ADHD-smoking relationship over time, other contributing factors ought to be considered. For instance, it is widely accepted that factors such as parenting and parental modeling, school functioning, and peer relationships drive a range of negative outcomes, including substance use (Derefinko & Pelham, 2014; Molina & Pelham, 2014). Additionally, the construct of impulsivity, known to influence substance use in general (Verdejo-Garcia, Lawrence, & Clark, 2008) and smoking specifically (Doran et al., 2004; VanderVeen et al., 2008), is not well captured by ADHD symptom reports which may partly explain inconsistent findings. Lastly, the present findings are intriguing in light of failure to consistently find beneficial effects of stimulant medication on smoking behavior among people with ADHD (e.g. Rush et al., 2005; Schoenfelder, Faraone, & Kollins, 2014; Winhusen et al., 2010). ADHD symptoms may have little to do with smoking-related behavior once the initial uptake process has crystallized into daily smoking.
Our findings of a much greater likelihood of smoking at young ages and increased craving and difficulty concentrating during abstinence highlight the need for prevention and treatment considerations in this population. Specifically, children with ADHD may benefit from preventative measures early in adolescence before smoking experience begins to accumulate. Smoking prevention programs have been developed for adolescents (e.g. Bruvold, 1993); however, there are no prevention programs to our knowledge specifically designed for smokers with a history of ADHD. Treatment/prevention efforts ought to target factors that may mediate the relationship between ADHD and smoking. For example, Molina and colleagues (2005) demonstrated that poor coping skills and low parent support partially mediated the relationship between ADHD and smoking. The authors suggest that these “problem-solving deficits” may partially explain why this group of individuals is at risk for smoking. As such, these deficits may be important, clinically malleable treatment targets in preventing and extinguishing early smoking behaviors (e.g. Evans, Axelrod & Langberg, 2003; Griffin, Botvin, Nichols, & Doyle, 2003). Additionally, parental smoking has been linked to adolescent smoking (Chassin et al., 2002). Parental cessation and antismoking attitudes may lower the risk of adolescent smoking. Focusing on maintenance factors (abstinence-related experiences) may be especially beneficial for smokers with a history of ADHD. For example, it has been suggested that withdrawal-related cognitive deficits should be targeted behaviorally and/or pharmacologically (e.g. Sofuoglu, 2013). Overall, it is clear that future research needs to better target both prevention and cessation strategies for smokers with a history of ADHD.
While the present findings replicate and extend the current literature on the ADHD-smoking relationship using a sample of participants across a variety of ages (some of which are recalling smoking behaviors relatively recently), there are important limitations to be considered. Small sample sizes for some of our questions limited our power to detect group differences. In addition, bioverification of smoking reports may have increased reports of smoking. Given that the ADHD sample was drawn from a tertiary care specialty treatment clinic, these participants may represent a more severe presentation than an epidemiological sample (although we note that partial indicators of severity, namely conduct disorder and ADHD symptom persistence, did not drive our main findings). Finally, there may be some error in the recall of smoking behaviors, such as age of initiation and quit attempts, especially given reporting challenges with this population (Sibley et al., 2012). Even with these limitations, the present study provides an important demonstration that ADHD is a significant risk factor for cigarette smoking across most phases of smoking behaviors, and as such it ought to be targeted in prevention and treatment efforts. Our findings point to smoking behaviors that may maintain smoking differentially for those with a history of ADHD; however, more research is needed to better understand the mechanisms underlying the vulnerability for early initiation and progression to smoking as well as the specific unfolding of dependence with age and smoking experience. The present study highlights how future research may better address the question of how and why individuals with ADHD are at risk for smoking. Identifying the mechanisms at the core of the ADHD-smoking association will better inform treatment for smokers with ADHD.
Acknowledgments
This research was supported by grant DA12414 from the National Institute on Drug Abuse awarded to W.P. and grants AA011873 and AA00202 from the National Institute of Alcohol Abuse and Alcoholism awarded to B.S.G.M. Research was also supported by grants from the National Institute of Mental Health (MH53554, MH069614, MH62946) and the Institute of Educational Sciences (R324B060045, LO3000665A) awarded to W.P., from the National Institute of Alcohol Abuse and Alcoholism (AA12342), the National Institute on Drug Abuse (DA016631, DA85553), and the National Institute of Mental Health (MH50467, MH12010, KAI-118-S1, MH065899, MH077676) awarded to B.S.G.M. Research was also supported by MH018269.
Footnotes
Our results reflect surprisingly high rates of daily smoking even among the nonADHD participants and wide confidence intervals among the youngest age groups. Pennsylvania ranks high among other states with regard to smoking prevalence (range of 10.3% to 27.3%) with approximately 21.3% of adults over the age of 18 years (CDC, 2010) and approximately 18.6% of high school students (CDC, 2010) reporting current smoking. Nevertheless, even when taken within this context, our findings suggest important group differences in the development of smoking behavior.
References
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76(B):581–591. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin, Rollema, Svesson, Winterer Smoking, quitting, and psychiatric disease: A review. Neuroscience and Biobehavioral Reviews. 2012;36:271–284. doi: 10.1016/j.neubiorev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and Non-ADHD participants. J Am Acad Child Adolesc Psychiatry. 2006;45(7):824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Fourth Edition. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Babinski DE, Pelham WE, Jr., Molina BSG, Gnagy EM, et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: An exploratory investigation. Journal of Attention Disorders. 2010 doi: 10.1177/1087054710361586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagwell CL, Molina BSG, Pelham WE, Jr., Hoza B. Attention-Deficit Hyperactivity Disorder and problems in peer relations: Predictions from childhood to adolescence. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1285–1292. doi: 10.1097/00004583-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kin S, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nic & Tobacco Research. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology. 2002;111(2):279–289. [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Siedman LJ, Wilens TE, Ferrero F, Morgan CL, Faraone SV. Impact of executive function deficits and Attention Deficit Hyperactivity Disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004 doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Wilens TE, Fontanella JA, Poetzl KM, et al. Is cigarette smoking a gateway to alcohol and illicit drug use disorders? A study of youths with and without attention deficit hyperactivity disorder. Biol Psychiatry. 2006;59(3) doi: 10.1016/j.biopsych.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86(2):214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychology of Addictive Behaviors. 2014;28(1):238–246. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruvold WH. A meta-analysis of adolescent smoking prevention programs. American Journal of Public Health. 1993;83(6):872–880. doi: 10.2105/ajph.83.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry. 2001;42(4):493–502. [PubMed] [Google Scholar]
- Burt RD, Peterson AV. Smoking cessation among high school seniors. Prev Med. 1998;27 doi: 10.1006/pmed.1998.0269. [DOI] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood Attention-Deficit/Hyperactivity Disorder and future substance use disorders: Comparative meta-analyses. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose JS, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood: Demographic predictors of continuity and change. Health Psychology. 1996;15(6):478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Rose J, Sherman SJ, Prost J. Parental smoking cessation and adolescent smoking. Journal of Pediatric Psychology. 2002;27(6):485–496. doi: 10.1093/jpepsy/27.6.485. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: Predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychology. 1990;9(6):701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85(1):41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug and Alcohol Dependence. 2000;59:83–95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Derefinko KJ, Pelham WE. ADHD and Substance Use. In: Sher KJ, editor. The oxford handbook of substance use disorders. Vol.2. Oxford University Press; New York: 2014. [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender on substance use and abuse in adolescence. Am J Psychiatry. 1999;156 doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug and Alcohol Dependence. 2007;89(1):93–96. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Griffin KM, Shiffman S, Sayette MA. The relationship between cigarette use, nicotine dependence, and craving in laboratory volunteers. Nicotine & Tobacco Research. 2008;10(3):447–455. doi: 10.1080/14622200801901906. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue DE, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine & Tobacco Research. 2004;6(4):641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Schaughency EA, Weyandt LL, Tripp G, Kiesner J, Ota K, Stanish H. Self-report of ADHD symptoms in university students: Cross-gender and cross-national prevalence. Journal of Learning Disabilities. 2001;34(4):370–379. doi: 10.1177/002221940103400412. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of Attention-Deficit/Hyperactivity Disorder, Conduct Disorder, and sex on adolescent substance use and abuse. JAMA Psychiatry. 2007;64(10):1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eschel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without Attention-Deficit/Hyperactivity Disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Evans SW, Axelrod J, Langberg JM. Efficacy of a school-based treatment program for middle school youth with ADHD. Behavior Modification. 2004;28(4):528–547. doi: 10.1177/0145445503259504. [DOI] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addiction Biology. 2008;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. BiolPsychiatry. 2005 doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2006;74(4):1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relationship between attention deficit hyperactivity disorder and substance abuse: what role does conduct disorder play? Clin Child Fam Psychol Rev. 2003;6(1):1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention Deficit Hyperactivity Disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. Journal of Pediatric Psychology. 2007;32(10):1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Gehricke J-G, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, Belluzzi JD, Leslie FM. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine & Tobacco Research. 2007;9:S523–S536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Griffin KW, Botvin GJ, Nichols TR, Doyle MM. Effectiveness of a universal drug abuse prevention approach for youth at high risk for substance use initiation. Preventive Medicine. 2003;36(1):1–7. doi: 10.1006/pmed.2002.1133. [DOI] [PubMed] [Google Scholar]
- Harakeh Z, Scholte RHJ, Vermulst AA, de Vries H, Engels RCME. Parental factors and adolescents' smoking behavior: an extension of The theory of planned behavior. Preventive Medicine. 2004;39(5):951–961. doi: 10.1016/j.ypmed.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Hartsough CS, Lambert NM. Pattern and progression of drug use among hyperactives and controls: A prospective short-term longitudinal study. J Child Psychology Psychiatry. 1987;28:543–553. doi: 10.1111/j.1469-7610.1987.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoza B. Peer functioning in children with ADHD. J Pediatr Psychol. 2007;32(6):655–663. doi: 10.1093/jpepsy/jsm024. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Prochaska JL, Mengis M, Cullen J, Munoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood Attention-Deficit/Hyperactivity Disorder and smoking treatment failure. Nicotine & Tobacco Research. 2005;7(3):453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco Control. 1997;6 doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitz HS, Lerman C, Swan GE. Comparative dynamics of four smoking withdrawal symptom scales. Addiction. 2012;107:1501–1511. doi: 10.1111/j.1360-0443.2012.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Health Behavior Questionnaire. Institute of Behavioral Sciences, University of Colorado; Boulder: 1989. [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2) doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kent KM, Pelham WE, Jr., Molina BSG, Sibley MH, et al. The academic experience of male high school students with ADHD. JACP. 2011;39(3):451–462. doi: 10.1007/s10802-010-9472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey replication. The American Journal of Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99(12) doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and Attention-Deficit/Hyperactivity Disorder symptoms in a population-based sample of young adults. Archives of General Psychiatry. 2005;62(10):1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kooij JJS, Boonstra AM, Swinkels SHN, Bekker EM, de Noord I, Buitelaar JK. Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008;11(4):445–458. doi: 10.1177/1087054707299367. [DOI] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addictive Behaviors. 1999;24:673–677. doi: 10.1016/s0306-4603(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31(6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Larabie LC. To what extent do smokers plan quit attempts? Tob Control. 2005;14:425–428. doi: 10.1136/tc.2005.013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and Mental Illness: A population-based prevalence study. The Journal of American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Laucht M, Hohm E, Esser G, Schmidt MH, Becker K. Association between ADHD and smoking in adolescence: shared genetic, environmental and psychopathological factors. Journal of Neural Transmission. 2007;114:1097–1104. doi: 10.1007/s00702-007-0703-y. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Liu R, Flory K, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention-deficit/hyperactivity disorder. Experimental & Clinical Psychopharmacology. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG. Antisocial behaviors moderate the deviant peer pathway to substance use in children with ADHD. J Clin Child Adol. 2006;35(2):216–226. doi: 10.1207/s15374424jccp3502_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG, Pelham WE., Jr. Childhood ADHD and adolescent substance use: An examination of deviant peer group affiliation as a risk factor. Psychology of Addictive Behavior. 2003;17(4):293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH. ADHD and Smoking: From genes to brain to behavior. Annals of the New York Academy of Sciences. 2008;1141(1) doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology. 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Fuemmeler BF, Kollins SH, Kail ME, Ashley-Koch AE. Interactions between genotype and retrospective ADHD symtpoms predict lifetime smoking risk in a sample of young adults. Nicotine & Tobacco Research. 2008;10:117–127. doi: 10.1080/14622200701704913. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Van Voorhees EE, English J, Hallyburton M, Holdaway A, Kollins SH. Smoking withdrawal symptoms are more severe among smokers with ADHD and independent of ADHD symptom change: Results from a 12-day contingency-managed abstinence trial. Nicotine & Tobacco Research. 2011;13(9):784–792. doi: 10.1093/ntr/ntr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. Journal of Academic Child Adolescent Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Danforth JS, Neering C, Easton C. Can we prevent smoking children with ADHD: a review of the literature. Connecticut Medicine. 2010;74(4):229–236. [PubMed] [Google Scholar]
- Molina BSG, Flory K, Hinshaw SP, Greiner AR, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. J Am Acad Child Adol Psychiatry. 2007;46(8):1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Arnold LE, Swanson JM, et al. the MTA Cooperative Group Adolescent Substance Use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a Function of Childhood ADHD, Random Assignment to Childhood Treatments, and Subsequent Medication. J Am Acad Child Adol Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Marshal MP, Pelham WE, Jr., Wirth RJ. Coping skills and parent support mediate the association between childhood Attention-Deficit/Hyperactivity Disorder and adolescent cigarette use. Journal of Pediatric Psychology. 2005;30(4):345–357. doi: 10.1093/jpepsy/jsi029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal Abnorm Psychol. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE., Jr. Attention-Deficit/Hyperactivity Disorder and Risk of Substance Use Disorder: Developmental Considerations, Potential Pathways, and Opportunities for Research. Annual Review of Clinical Psychology. 2014;10:607–639. doi: 10.1146/annurev-clinpsy-032813-153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Jr., Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood ADHD and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. Journal of Abnormal Psychology. 2012;121(4):922–935. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcoholism: Clinical and Experimental Research. 2007;31(4):643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Clark TG, Johnstone EC, Murphy MFG, Walton RT. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6:583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Murphy K, Barkley RA. Attention Deficit Hyperactivity Disorder Adults: Comorbidities and Adaptive Impairments. Comprehensive Psychiatry. 1996;37(6):393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- O'Neil S, Parra G, Sher K. Clinical relevance of heavy drinking during college years: cross sectional and prospective perspective. Psychology of Addictive Behavior. 2001;15 doi: 10.1037//0893-164x.15.4.350. [DOI] [PubMed] [Google Scholar]
- Pedersen SL, Harty SC, Pelham WE, Jr., Gnagy EM, Molina BSG. Differential associations between alcohol expectancies and adolescent alcohol use as a function of childhood ADHD. Journal of Studies on Alcohol and Drugs. 2014;75(1):145–152. doi: 10.15288/jsad.2014.75.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr., Evans SW, Gnagy EM, Greenslade KE. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders: Prevalence, factor analyses, and conditional probabilities in a special education sample. School Psych Rev. 1992;21(2) doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Hoza B. Intensive treatment: A summer treatment program for children with ADHD. In: Hibbs E, Jensen PS, editors. Psychosocial treatments for child and adolescent disorders: Empirically based strategies for clinical practice. American Psychological Association; Washington, DC: 1996. pp. 311–340. [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology. 2003a;112(1):3–13. doi: 10.1037/0021-843X.112.1.3. [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Experimental and Clinical Psychopharmacology. 2003b;11(4):276–285. doi: 10.1037/1064-1297.11.4.276. doi:10.1037/1064-1297.11.4.276. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addictive Behaviors. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of Substance Abuse. 1995;7(3):373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Downey KK, Snedecor SM, Mehringer AM, Marks JL, Pomerleau OF. Smoking patterns and abstinence effects in smokers with no ADHD, childhood ADHD, and adult ADHD symptomatology. Addict Behav. 2003;28(6):1149–1157. doi: 10.1016/s0306-4603(02)00223-x. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology Biochemistry & Behavior. 2008;88:407–411. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, de Moor CA, Hudmon KS, Kelder SH, Conroy JL, Ordway N. Nicotine dependence, withdrawal symptoms, and adolescents' readiness to quit smoking. Nicotine & Tobacco Research. 2001;3(2):151–155. doi: 10.1080/14622200110043068. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Karraker K, Horn K, Richards JB. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behavioural Processes. 2003;64(3):333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Rohde P, Kahler CW, Lewinsohn PM, Brown RA. Psychiatric disorders, familial factors, and cigarette smoking: II. Associations with progression to daily smoking. Nicotine & Tobacco Research. 2004;6(1):119–132. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- Rostron B. Smoking-attributable mortality by cause in the United States: revising the CDC's data and estimates. Nicotine & Tobacco Research. 2013;15(1):238–246. doi: 10.1093/ntr/nts120. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PEA. Methylphenidate increases cigarette smoking. Psychopharmacology. 2005;181:781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Sargent JD, Mott LA, Stevens M. Predictors of smoking cessation in adolescents. Archives of Pediatric Adolescent Medicine. 1998;152(4):388–393. doi: 10.1001/archpedi.152.4.388. [DOI] [PubMed] [Google Scholar]
- Schoenfelder EN, Faraone SV, Kollins SH. Stimulant treatment of ADHD and cigarette smoking: A meta-analysis. Pediatrics. 2014;133(6) doi: 10.1542/peds.2014-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in Attention Deficit Hyperactivity Disorder. The American Journal of Psychiatry. 2014;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers” – individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M. Smoking typology profiles of chippers and regular smokers. Journal of Substance Abuse. 1994;6(1):21–35. doi: 10.1016/s0899-3289(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel JD, Elash G. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14(4):301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sayette MA. Validation of the nicotine dependence syndrome scale (NDSS): a criterion-group design contrasting chippers and regular smokers. Drug and Alcohol Dependence. 2005;79(1):45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004;6(2):327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Jr., Molina BSG, Cox S, et al. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. Journal of Abnormal Psychology. 2014;123(2):362–374. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, et al. When Diagnosing ADHD in Young Adults Emphasize Informant Reports, DSM Items, and Impairment. J Consult Clin Psychol. 2012a;80(6):1052–1061. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, Kuriyan AB, Babinski DE, Karch KM. Diagnosing ADHD in Adolescence. J Consult Clin Psychol. 2012b;80(1):139–150. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons-Morton B, Haynie DL, Crump AD, Eitel P, Saylor KE. Peer and parent influences on smoking and drinking among early adolescents. Health Educ Behav. 2001;28(1) doi: 10.1177/109019810102800109. [DOI] [PubMed] [Google Scholar]
- Smith BH, Pelham WE, Gnagy E, Molina B, Evans S. The reliability, validity, and unique contributions of self-report by adolescents receiving treatment for attention-deficit/hyperactivity disorder. J Consult Clin Psychol. 2000;68(3):489–499. doi: 10.1037/0022-006X.68.3.489. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience & Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Audrain J. Association of Attention-Deficit/Hyperactivity Disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41(7):799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research Review: Dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. The Journal of Child Psychology and Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [Google Scholar]
- Vanderveen JW, Cohen LM, Cukrowicz KC, Trotter DRM. The role of impulsivity on smoking maintenance. Nicotine & Tobacco Research. 2008;10(8):1397–1404. doi: 10.1080/14622200802239330. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(1):34–46. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Savageau JA, Dussault GF. Short term patterns of early smoking acquisition. Tobacco Control. 2004;13:251–257. doi: 10.1136/tc.2003.005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J. Psychopathology in preadolescent children at high risk for substance abuse: a review of the literature. Harvard Review of Psychiatry. 1993;1:207–218. doi: 10.3109/10673229309017081. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418. A cholinergic agonist, in the treatment of adults with Attention Deficit Hyperactivity Disorder. The American Journal of Psychiatry. 1999;156(12):1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Brigham GS, Liu DS, et al. Impact of Attention-Deficit/Hyperactivity Disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2010;71(12) doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WH, Yang L, Jiang CQ, Deng LZ, Lam TH, Zhang JY, Chan SSC. Characteristics of smokers and predictors of quitting in a smoking cessation clinic in Guangzhou, China. Journal of Public Health. 2009:1–10. doi: 10.1093/pubmed/fdp107. [DOI] [PubMed] [Google Scholar]