Abstract
Background
The ideal timing of post-mastectomy radiation therapy (PMRT) in the setting of two-staged implant-based breast reconstruction remains unclear. In this cohort study, we sought to determine whether complication rates differed between patients who received PMRT following tissue expander placement (TE-XRT) and those who received PMRT after exchange for permanent implant (Implant-XRT) utilizing prospective, multicenter data.
Methods
Eligible patients in the Mastectomy Reconstruction Outcomes Consortium (MROC) study from 11 institutions across North America were included in the analysis. All patients had at least six-month follow-up after their last intervention (i.e. implant exchange for TE-XRT patients and radiation for Implant-XRT patients). Complications including seroma, hematoma, infection, wound dehiscence, capsular contracture, and implant loss were recorded.
Results
We identified a total of 150 patients who underwent immediate, two-staged implant-based breast reconstruction and received PMRT. Of these, there were 104 (69.3%) TE-XRT and 46 (30.7%) Implant-XRT patients. There were no differences in the incidence of any complications or complications leading to reconstructive failure between the two cohorts. After adjusting for patient characteristics and site effect, the timing of PMRT (i.e. TE-XRT versus Implant-XRT) was not a significant predictor in the development of any complication, a major complication, or reconstructive failure.
Conclusions
In the setting of PMRT and two-staged implant-based reconstruction, patients who received PMRT after expander placement (TE-XRT) did not have a higher incidence or increased odds of developing complications than those who received PMRT after exchange for a permanent implant (Implant-XRT).
Keywords: Breast reconstruction, breast cancer, radiation therapy, MROC, outcomes, complication
Introduction
Post-mastectomy radiation therapy (PMRT) is an integral component of oncologic management for breast cancer patients at high risk of developing locoregional recurrence. Since PMRT was first described in 1997 by Overgaard and colleagues, and demonstrated a survival benefit, use of this treatment intervention has become more widespread.1–7 As a consequence, more patients presenting for breast reconstruction after mastectomy will receive PMRT.1, 8 As reflected in the overall population of women who undergo breast reconstruction, an increasing proportion of those women who face PMRT will elect to undergo immediate implant-based reconstruction with a two-staged approach.8, 9 In light of these recent trends, it is important for plastic surgeons and oncologists to better understand the impact of PMRT on immediate two-staged breast reconstruction outcomes.
The adverse effects of radiation therapy on breast reconstruction, including decreased mastectomy flap perfusion, wound healing complications, and infection, are well known.10–13 What remains unclear however, is whether the timing of PMRT with respect to two-staged breast reconstruction affects post-operative complication rates. In addition, while previous studies have attempted to answer this important clinical question, they have been limited by retrospective, single-center designs.14–18
In our study, we evaluated patients recruited as part of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. This five-year prospective, multicenter cohort study funded by the National Cancer Institute enrolled patients from February 2012 to July 2015. Using this database that includes patients treated by 57 plastic surgeons in 11 centers, we sought to determine whether the timing of PMRT before or after exchange of a tissue expander for a permanent implant had an effect on short-term post-operative complication rates.
Methods
Study Population
Patients were recruited as part of the MROC Study, from 11 centers in Michigan, New York, Illinois, Ohio, Massachusetts, Washington, D.C., Georgia, Texas, British Columbia, and Manitoba. Eligible patients included women 18 years or older undergoing breast reconstruction after mastectomy. Participants were assessed preoperatively and were followed for two years post-reconstruction. We obtained approval from the Institutional Review Boards (IRBs) of all participating sites. The electronic medical record (EMR) for each patient enrolled was reviewed to obtain clinical data. All data were collected via Velos (Velos Inc., Fremont, CA), a web-based clinical trial management system.
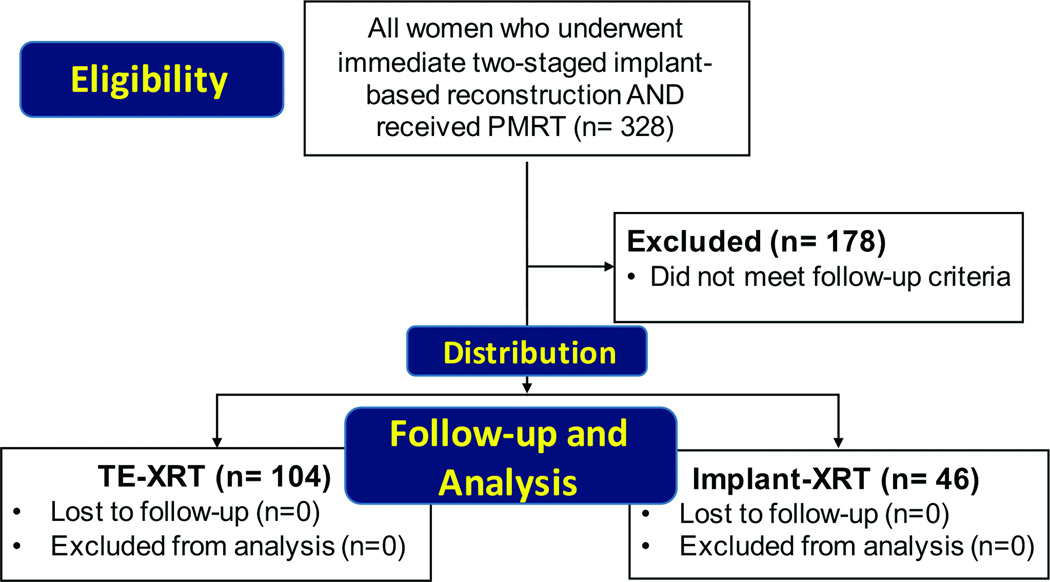
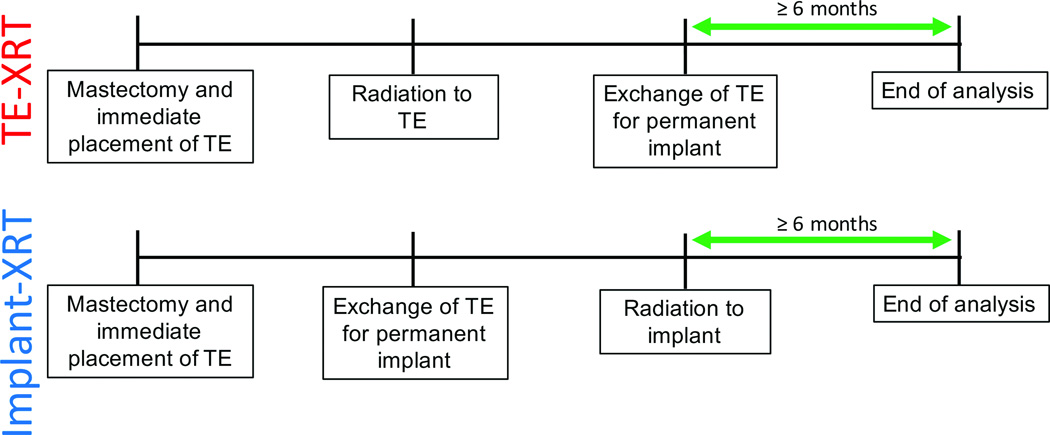
This study was a secondary analysis of patients who participated in the MROC study from February 2012 to December 2015. We included any woman who underwent immediate two-staged implant-based breast reconstruction and received PMRT. These patients were divided into two cohorts: 1) those receiving radiation to their expander, prior to exchange of the expander for a permanent reconstructive implant (TE-XRT); and 2) women undergoing radiation following expander exchange for a permanent implant (Implant-XRT). To allow for comparison of this study to others in the literature, we utilized these designations for the two study cohorts that were first described by Cordeiro and coworkers.15 We excluded patients who did not have at least six months of follow-up after their last intervention (Figure 1). In the TE-XRT cohort, patients completed reconstruction and had at least six months of follow-up from the date of their implant exchange procedure. Women in the Implant-XRT cohort had at least six months of follow-up from the completion date of radiation (Figures 1 and 2). Complication data were obtained from EMRs for all patients.
Figure 1.
Diagram showing flow of patients in the study.
Figure 2.
Follow-up for the different cohorts. For TE-XRT patients (i.e. those who received radiation to their expander), complication data was recorded from the time of initial mastectomy and immediate placement of TE (tissue expander) to at least six months after exchange of the expander for permanent implant. Complication data for Implant-XRT patients (i.e. those who received radiation to their permanent implant), was recorded from the time of initial mastectomy and immediate placement of TE to at least six months after radiation to the implant had occurred.
The dependent variables of interest were post-operative complications, including seroma, hematoma, surgical site infection, wound dehiscence, capsular contracture, implant loss, and any complication that resulted in complete reconstructive failure. Any complication that occurred between the time of the initial surgery to six months after their last intervention (i.e. implant exchange for TE-XRT patients and radiation for Implant-XRT patients) was recorded for each patient (Figure 2).
We used Centers for Disease Control and Prevention criteria for identification of post-operative infections in this study: 1) presence of purulent drainage; 2) positive aseptically obtained culture; 3) peri-incisional erythema and incision opened by surgeon; 4) physician diagnosis of infection, such as cellulitis, for which antibiotics were prescribed. Minor infections were defined as those treated with oral antibiotics, and major infections as those requiring treatment with intravenous antibiotics with or without surgical exploration. We defined implant loss as removal of an expander or permanent implant with replacement, while complete reconstructive failure was defined as removal of a tissue expander or implant without subsequent replacement. Finally, we also analyzed major complications, defined as those necessitating inpatient admission or surgical exploration.
Self-reported patient variables included age, race, and smoking status at the time of reconstruction. Categories for race were “White,” “Black,” and “Other” (i.e. American Indians, Asians, Hawaiians, and Pacific Islanders), in accordance with NIH standards. We also included general clinical variables such as body mass index (BMI, kg/m2) and presence of diabetes. In addition to general clinical variables, oncologic and treatment variables were collected. These included use of acellular dermal matrix (ADM), adjuvant chemotherapy, extent of disease, and mastectomy type. With respect to extent of disease, women were classified into two groups: 1) patients with local disease only (i.e. disease confined to the breast only), or 2) those with regional disease (i.e. disease in axillary or internal mammary lymph nodes). Mastectomy type was subdivided into three groups: 1) nipple-sparing, 2) simple or modified radical, or 3) in cases of bilateral procedures, a combination of the previous two.
Statistical Analysis
Patient characteristics and complications were compared between the two cohorts using t-tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. To compare risks for complications while adjusting for demographic and clinical characteristics and accounting for potential between-hospital differences, we utilized a generalized linear mixed model. In this analysis, any complication, major complication, and reconstructive failure were the dependent variables, with logit link and random intercepts to account for hospital clustering effects. The logit link allowed the generalized linear model to fit a logistic regression model, but was extended to account for between-hospital differences. Variables found to be significantly different on bivariate analysis with a p-value of < 0.05, and those known to be predictors of post-operative complications in breast reconstruction based on previous literature (i.e. age, BMI, laterality, and smoking status) were included in the logistic regression models.19 We reported adjusted odds ratios (OR) for developing any type of complication, with 95% confidence intervals (CI) based on the model. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC), and statistical significance was set at 0.05.
Results
Summary of Demographic Data
From February 2012 to December 2015, we identified a total of 150 patients who underwent two-staged implant-based breast reconstruction, received PMRT, and had at least six months of follow-up after their last intervention (i.e. implant exchange for TE-XRT patients and radiation for Implant-XRT patients). Of these 150 patients, 104 women (69.3%) underwent TE-XRT while 46 (30.7%) received Implant-XRT. Other than TE-XRT patients having somewhat longer lengths of follow-up (p<0.0001), the two study groups were similar with respect to age, BMI, race, smoking status, and the presence of diabetes (Table 1).
Table 1.
Patient Demographics for Total Cohort and by Timing of PMRT
| Variable | Total (%) | TE-XRT (%) | Implant-XRT (%) | p-value* |
|---|---|---|---|---|
| N = 150 | N = 104 (69.3%) | N = 46 (30.7%) | ||
| Mean age ± SD, yr | 46.9 ± 10.4 | 47.7 ± 10.5 | 45.0 ± 10.1 | 0.141 |
| Mean BMI ± SD, kg/m2 | 25.8 ± 5.3 | 25.9 ± 4.9 | 25.5 ± 6.3 | 0.695 |
| Mean follow-up ±, days | 480 ± 96.4 | 504 ± 101.9 | 423 ± 48.3 | <0.0001 |
| Race# | 0.961 | |||
| White | 137 (93.2%) | 96 (93.2%) | 41 (93.2%) | |
| Black | 4 (2.7%) | 3 (2.9%) | 1 (2.3%) | |
| Other | 6 (4.1%) | 4 (3.9%) | 2 (4.6%) | |
| Smokingδ | 0.538 | |||
| Never | 105 (70.5%) | 71 (68.9%) | 34 (73.9%) | |
| Current or Former | 44 (29.5%) | 32 (31.1%) | 12 (26.1%) | |
| Diabetes | 6 (4.0%) | 4 (3.9%) | 2 (4.4%) | 1.00 |
Abbreviation: TE-XRT, patients receiving post-mastectomy radiation therapy to a tissue expander; Implant-XRT, patients receiving post-mastectomy radiation therapy to an implant following exchange; BMI, body mass index
Based on χ2 test of independence or Fisher’s exact test. The t-test was used for age and BMI.
Data missing for three patients.
Data missing for one patient.
With regards to treatment characteristics, 102 patients (68%) underwent bilateral reconstruction whereas 48 patients (32%) had unilateral reconstruction. Between the study groups, there were no significant differences with respect to laterality or mastectomy type (Table 2). However, ADM was used more frequently in TE-XRT patients than Implant-XRT patients (55.8% vs. 32.6%, p=0.009). Patients with TE-XRT were also less likely to undergo adjuvant chemotherapy (55.8% vs. 87%, p<0.0001), and were less likely to have regional disease at the time of reconstruction as compared to Implant-XRT patients (73.1% vs. 93.5%, p=0.004) (Table 2).
Table 2.
Oncologic and Treatment Characteristics for Total Cohort and by Timing of PMRT
| Variable | Total (%) | TE-XRT (%) | Implant-XRT (%) | p-value* |
|---|---|---|---|---|
| N = 150 | N = 104 (69.3%) | N = 46 (30.7%) | ||
| Unilateral | 48 (32.0%) | 32 (30.8%) | 16 (34.8%) | 0.627 |
| Use of ADM | 73 (48.7%) | 58 (55.8%) | 15 (32.6%) | 0.009 |
| Chemotherapy | <0.0001 | |||
| During or after reconstruction | 98 (65.3%) | 58 (55.8%) | 40 (87.0%) | |
| No chemotherapy during or after reconstruction# |
52 (34.7%) | 46 (44.2%) | 6 (13.0%) | |
| Extent of Disease | 0.004 | |||
| Local (i.e. disease confined to breast only) |
31 (20.7%) | 28 (26.9%) | 3 (6.5%) | |
| Regional (i.e. disease in axillary or internal mammary lymph nodes) |
119 (79.3%) | 76 (73.1%) | 43 (93.5%) | |
| Mastectomy type | 0.786 | |||
| Nipple-sparing | 12 (8.0%) | 8 (7.7%) | 4 (8.7%) | |
| Simplified or modified radical | 137 (91.3%) | 95 (91.4%) | 42 (91.3%) | |
| Combination of above two | 1 (0.7%) | 1 (1.0%) | 0 |
Abbreviation: ADM, acellular dermal matrix
Based on χ2 test or Fisher’s exact test.
Designation of “no chemotherapy during or after reconstruction” could indicate that patients either did not receive chemotherapy altogether or that they received chemotherapy prior to surgery (i.e. neoadjuvant chemotherapy).
Expander/Implant Complications
Among all patients, over one-quarter of all patients (28.7%) experienced a complication. Overall, the most common complication was surgical site infection (either major or minor) (22 patients, 14.7%). Complications resulted in reconstructive failure in 16 women (10.7%). A summary of complications is reported in Table 3.
Table 3.
Summary of Post-Operative Complications
| Type of Complication | Total (%) |
|---|---|
| Seroma | 10 (6.7%) |
| Hematoma | 5 (3.3%) |
| Surgical site infection | 22 (14.7%) |
| Minor (oral antibiotics) | 10 (6.7%) |
| Major (IV antibiotics ± OR) | 12 (8.0%) |
| Wound dehiscence | 5 (3.3%) |
| Capsular contracture | 4 (2.7%) |
| Implant loss | 3 (2.0%) |
| Any complication | 43 (28.7%) |
| Reconstructive failure | 16 (10.7%) |
Abbreviation: OR, operating room.
PMRT before Exchange (TE-XRT) versus PMRT after Exchange (Implant-XRT)
There were no differences in the incidences of any type of complication or complications resulting in reconstructive failure between TE-XRT and Implant-XRT patients. Moreover, for specific complication types (i.e., seroma, hematoma, surgical site infection, wound dehiscence, capsular contracture, and implant loss), no significant differences between groups were found, though each complication type generally occurred more frequently in TE-XRT than Implant-XRT, with the exception of surgical site infections (Table 4).
Table 4.
Post-Operative Complications between PMRT before exchange (TE-XRT) versus PMRT after exchange (Implant-XRT)
| Complications | TE-XRT (%) | Implant-XRT (%) | p-value* |
|---|---|---|---|
| N = 104 (69.3%) | N = 46 (30.7%) | ||
| Seroma | 8 (7.7%) | 2 (4.4%) | 0.456 |
| Hematoma | 4 (3.9%) | 1 (2.2%) | 0.632 |
| Surgical site infection | |||
| Minor (oral antibiotics) | 7 (6.7%) | 3 (6.5%) | 0.962 |
| Major (IV antibiotics ± OR) | 7 (6.7%) | 5 (10.9%) | 0.395 |
| Wound dehiscence | 5 (4.8%) | 0 | 0.324 |
| Capsular contracture | 3 (2.9%) | 1 (2.2%) | 0.804 |
| Implant loss | 3 (2.9%) | 0 | 0.553 |
| Any complication | 32 (30.8%) | 11 (23.9%) | 0.395 |
| Reconstructive failure | 12 (11.5%) | 4 (8.7%) | 0.900 |
Abbreviation: OR, operating room.
Based on a global test of radiation timing from separate mixed-effects logistic regression models for each complication outcome adjusting for center or Fisher’s exact test.
Results from our mixed effects logistic regression models with logit link controlling for hospital clustering effects and other covariates are shown in Table 5. After controlling for covariates, timing of PMRT (i.e. TE-XRT versus Implant-XRT) was not a significant predictor for any complication, major complications, or reconstructive failure. Significantly higher odds of developing a complication however, were found with increased age (OR=1.046; 95% CI 1.003–1.091, p= 0.0367). Additionally, being a current or former smoker was also a significant predictor of reconstructive failure (OR=3.677; 95% CI 1.205–11.220, p=0.023).
Table 5.
Generalized Linear Mixed Models Predicting Post-Operative Complications
| Any Complication | Major Complication | Reconstructive Failure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AOR | 95% CI | p | AOR | 95% CI | p | AOR | 95% CI | p | |
| Age | 1.046 | (1.003, 1.091) | 0.036 | 1.022 | (0.977, 1.069) | 0.348 | 1.015 | (0.954, 1.079) | 0.639 |
| BMI | 1.026 | (0.953, 1.105) | 0.490 | 1.074 | (0.991, 1.165) | 0.081 | 0.997 | (0.896, 1.109) | 0.956 |
| Length of follow-up (days) |
1.000 | (0.995, 1.004) | 0.902 | 1.003 | (0.998, 1.008) | 0.310 | 1.002 | (0.996, 1.009) | 0.487 |
| Timing of radiation (reference: Implant-XRT) |
|||||||||
| TE-XRT | 1.249 | (0.444, 3.510) | 0.671 | 0.924 | (0.268, 3.181) | 0.900 | 0.792 | (0.154, 4.063) | 0.778 |
| Laterality (reference: unilateral) |
|||||||||
| Bilateral | 2.271 | (0.903, 5.711) | 0.081 | 1.821 | (0.655, 5.058) | 0.248 | 0.736 | (0.199, 2.729) | 0.645 |
| Use of ADM (reference: ADM not used) |
|||||||||
| ADM was used |
0.802 | (0.368, 1.745) | 0.575 | 0.908 | (0.365, 2.262) | 0.835 | 0.964 | (0.281, 3.312) | 0.954 |
| Smoking status (reference: non- smoker) |
|||||||||
| Current or previous |
2.196 | (0.996, 4.843) | 0.051 | 1.901 | (0.804, 4.491) | 0.142 | 3.677 | (1.205, 11.220) | 0.023 |
| Chemotherapy during or after |
0.825 | (0.344, 1.976) | 0.664 | 1.530 | (0.554, 4.4225) | 0.409 | 1.125 | (0.97, 4.264) | 0.861 |
| Extent of disease (reference: local disease) |
|||||||||
| Regional | 1.109 | (0.427, 2.879) | 0.831 | 1.081 | (0.366, 3.192) | 0.887 | 2.260 | (0.425, 12.017) | 0.336 |
Abbreviations: AOR, adjusted odds ratio; BMI, body mass index; ADM, acellular dermal matrix
Discussion
As the indications for PMRT expand over time, an increasing number of women presenting for breast reconstruction will receive PMRT as part of their oncologic management.1, 20, 21 According to a large database study analyzing trends among women who underwent radiation therapy and breast reconstruction by Agarwal and coworkers, the number of women requiring radiation and opting for immediate breast reconstruction techniques has increased over time. In addition, Agarwal’s analysis also demonstrated an increase in the percentage of women choosing implant-only reconstruction contrary to traditional recommendations.8 Given these trends and evolution in oncologic management, it is imperative for reconstructive surgeons to understand the effects of PMRT timing on breast reconstruction outcomes.
To date, several studies have attempted to address this question. Nava and coworkers performed a retrospective single-center cohort study with 109 patients undergoing PMRT to permanent implants and 50 patients treated with PMRT to their tissue expanders. The investigators found that patients who had PMRT to their expanders had a higher rate of reconstructive failures (40%), compared with those who receiving PMRT to their permanent implants (6.4%).22 Similarly, Cordeiro reported his single surgeon experience, analyzing 94 patients who underwent PMRT prior to implant exchange and 210 patients who underwent PMRT after their implant exchange. In this study, patients receiving PMRT to their tissue expanders experienced higher rates of reconstructive failure than those patients undergoing PMRT following their exchange procedure.15 In contrast, other studies by Lentz and colleagues and Anderson and colleagues, noted no significant differences in overall post-operative complication rates between patients undergoing PMRT prior to implant exchange and patients receiving PMRT after implant exchange.17, 18
Interestingly, the use of ADM differed between our cohorts, in that TE-XRT patients were more likely to be reconstructed with ADM than Implant-XRT patients (55.8% vs. 32.6% p=0.009). Interestingly, while prior studies have shown the use of ADM in breast reconstruction to be associated with higher complication risks,23, 24 we did not find ADM effects on any complication, major complications, or reconstructive failure in TE patients after adjusting for PMRT timing. Further evaluation of what explains the higher rate of ADM use in TE-XRT patients is beyond the scope of this study, but we speculate ADM use to vary greatly by surgeon preference.
Another interesting observation in our study was the difference in the proportion of patients receiving adjuvant chemotherapy between the two cohorts. A higher proportion of Implant-XRT patients received adjuvant chemotherapy, compared with that of TE-XRT patients (87% vs. 55.8%, p<0.0001). This finding may reflect a center (or site) effect in our analysis. Patients from the Implant-XRT group originated mainly from one center, where adjuvant chemotherapy is the norm. By contrast, patients from the TE-XRT cohort were drawn from the 10 other MROC centers, where chemotherapy protocols are more varied.
The reconstructive surgeon may face practical challenges in caring for this complex patient population, mainly due to recommendations from medical and radiation oncologists to administer PMRT as soon after chemotherapy as possible. For example, in Implant-XRT protocols, the coordination of timing the exchange procedure approximately four weeks after chemotherapy and four weeks before radiation can be challenging. This is in contrast to TE-XRT, in which the decision of when to perform the exchange procedure after radiation therapy is largely up to the patient and reconstructive surgeon. While some may choose to perform the exchange procedure shortly after PMRT in these patients, others may choose to wait several months after radiation to avoid the acute inflammatory changes associated with radiotherapy.15 The increased flexibility of when to perform implant exchange for TE-XRT patients compared to Implant-XRT likely explains the longer follow-up we observed in our cohort (18 months for TE-XRT vs. 14 months for Implant-XRT, p<0.0001).
While timing of PMRT did not affect the development of complications in this study, our findings highlight the importance of counseling patients pre-operatively about potential outcomes following reconstruction and PMRT. Our results reinforce the notion that complications are not unusual following breast reconstruction.10, 19 Over one-quarter of all patients, regardless of timing of PMRT, developed some type of complication. Moreover, as always, it is important to counsel smokers that they are at significantly increased risk of suffering reconstructive failure with their reconstructions.
The strengths of our current study include its multicenter design and use of prospectively collected data. There are also several notable limitations to our study. Despite our data set encompassing 11 centers across North America over a three-year period, a larger sample size would strengthen our findings, particularly for multivariate analyses. Our relatively small study population likely reflects current practice patterns among reconstructive surgeons, and the general avoidance of implant-only techniques in patients who plan to undergo PMRT. Additionally, as this study was a subgroup analysis of the larger MROC study, details regarding radiation protocols, inflation/deflation of tissue expanders at the time of radiation, and exact timing of radiation with respect to surgical procedures were not collected; future studies evaluating the impact of these variables on post-operative complication rates are certainly warranted. Longer follow-up is needed to fully evaluate the effects of PMRT timing on capsular contracture and reconstructive failure. And while our study did not control for individual surgeon factors, our findings are based on the collective data from 57 plastic surgeons and are likely generalizable to other populations, given variations in surgical technique and practice patterns.
Although the goal of this particular study was to determine whether timing of PMRT had an effect on the development of post-operative complications in implant-based breast reconstruction, assessment of other outcomes of these procedures require further investigation—specifically, effects on quality of life, cancer recurrence, and overall survival. While the primary concern of the reconstructive surgeon may be to minimize post-operative complications in this population, it is important to keep in mind that reconstruction patients who receive PMRT are by definition at high-risk for recurrence, and that oncologic priorities must take precedence over reconstructive considerations in these patients. After all, immediate reconstruction of any type- autologous or implant-based-- may prolong oncologic treatment time or complicate radiation treatment planning.21, 25, 26 Moreover, further research is needed to determine if radiation accessibility of lymph nodes, particularly the internal mammary chain, is affected by having a deflatable expander versus permanent implant in place, which is an area of active controversy. Thus, managing these patients in collaboration with surgical, medical, and radiation oncologists is essential to providing the best reconstructive and oncologic management. With increasing numbers of women undergoing implant-based reconstruction and PMRT, this team-based approach can limit complications while optimizing oncologic outcomes, survival, and quality of life.
Conclusion
Analyzing patient data from 11 centers across North America, we found no significant differences in the incidence of post-operative complications between patients receiving radiation after expander-implant exchange, compared with patients undergoing radiation prior to exchange. Additionally, timing of PMRT was not a predictor of overall complications, major complications, or reconstructive failure. Additional research is needed to assess patient-reported outcomes, cancer recurrence, and survival, to determine the optimal timing of PMRT in this special population of breast reconstruction patients.
Acknowledgments
Research reported in this study was supported by the National Institutes of Health (NIH) National Cancer Institute (NCI) (1R01CA152192). Dr. Santosa received support in part from a grant from the Washington University Institute of Clinical and Translational Sciences (UL1TR000448) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. Dr. Ballard received support in part from the Plastic Surgery Foundation Research Fellowship. The authors gratefully acknowledge the contributions of our colleagues at the following centers who contributed their expertise to this multicenter trial: University of Michigan Health System, Ann Arbor, MI; Memorial Sloan-Kettering Cancer Center, New York City, NY; St. Joseph Mercy Hospital, Ypsilanti, MI; Northwestern Memorial Hospital, Chicago, IL; Ohio State Medical Center, Columbus, OH; Brigham and Women’s Hospital, Boston, MA; Georgetown University Medical Center, Washington, D.C.; Georgia Institute of Plastic Surgery, Savannah, GA; M.D. Anderson Cancer Center, Houston, TX; University of Manitoba, Winnipeg, MB; University of British Columbia, Vancouver, BC.
Footnotes
Author Contributions
K.B.S., X.C., T.N.S.B., A.L.P. and E.G.W. conceived and designed the study, X.C., J.Q. and H.M.K. performed statistical analysis, K.B.S., J.B.H., J.M.B. and E.G.W analyzed data, and K.B.S. wrote the manuscript with feedback from all study authors.
Financial Disclosure
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in the manuscript
References
- 1.Frasier LL, Holden S, Holden T, et al. Temporal Trends in Postmastectomy Radiation Therapy and Breast Reconstruction Associated With Changes in National Comprehensive Cancer Network Guidelines. JAMA oncology. 2016;2:95–101. doi: 10.1001/jamaoncol.2015.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. The New England journal of medicine. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. The New England journal of medicine. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 6.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SM, Lowery AJ, Prichard RS, McDermott EW, Evoy D, Geraghty J. Postmastectomy radiotherapy: indications and implications. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2014;12:310–315. doi: 10.1016/j.surge.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall SD, Anderson LA, Ying J, et al. The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plastic and reconstructive surgery. 2015;135:29e–42e. doi: 10.1097/PRS.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagsi R, Jiang J, Momoh AO, et al. Complications After Mastectomy and Immediate Breast Reconstruction for Breast Cancer: A Claims-Based Analysis. Annals of surgery. 2016;263:219–227. doi: 10.1097/SLA.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. International journal of radiation oncology, biology, physics. 2001;49:713–721. doi: 10.1016/s0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro PG. Breast reconstruction after surgery for breast cancer. The New England journal of medicine. 2008;359:1590–1601. doi: 10.1056/NEJMct0802899. [DOI] [PubMed] [Google Scholar]
- 13.Vandeweyer E, Deraemaecker R. Radiation therapy after immediate breast reconstruction with implants. Plastic and reconstructive surgery. 2000;106:56–58. doi: 10.1097/00006534-200007000-00009. discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plastic and reconstructive surgery. 2014;134:588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 15.Cordeiro PG, Albornoz CR, McCormick B, et al. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plastic and reconstructive surgery. 2015;135:1509–1517. doi: 10.1097/PRS.0000000000001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2012;118:2552–2559. doi: 10.1002/cncr.26521. [DOI] [PubMed] [Google Scholar]
- 17.Lentz R, Ng R, Higgins SA, Fusi S, Matthew M, Kwei SL. Radiation therapy and expander-implant breast reconstruction: an analysis of timing and comparison of complications. Annals of plastic surgery. 2013;71:269–273. doi: 10.1097/SAP.0b013e3182834b63. [DOI] [PubMed] [Google Scholar]
- 18.Anderson PR, Freedman G, Nicolaou N, et al. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant--is there a difference in complication rates? International journal of radiation oncology, biology, physics. 2009;74:81–85. doi: 10.1016/j.ijrobp.2008.06.1940. [DOI] [PubMed] [Google Scholar]
- 19.Alderman AK, Wilkins EG, Kim HM, Lowery JC. Complications in postmastectomy breast reconstruction: two-year results of the Michigan Breast Reconstruction Outcome Study. Plastic and reconstructive surgery. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Farhangkhoee H, Matros E, Disa J. Trends and concepts in post-mastectomy breast reconstruction. Journal of surgical oncology. 2016 doi: 10.1002/jso.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagsi R. Postmastectomy radiation therapy: an overview for the practicing surgeon. ISRN Surg. 2013;2013:212979. doi: 10.1155/2013/212979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plastic and reconstructive surgery. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 23.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Annals of plastic surgery. 2010;64:674–678. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 24.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plastic and reconstructive surgery. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 25.Prabhu R, Godette K, Carlson G, et al. The impact of skin-sparing mastectomy with immediate reconstruction in patients with Stage III breast cancer treated with neoadjuvant chemotherapy and postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2012;82:e587–e593. doi: 10.1016/j.ijrobp.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Trombetta DM, Cardoso SC, Alves VG, Facure A, Batista DV, da Silva AX. Evaluation of the radiotherapy treatment planning in the presence of a magnetic valve tissue expander. PloS one. 2015;10:e0117548. doi: 10.1371/journal.pone.0117548. [DOI] [PMC free article] [PubMed] [Google Scholar]