Abstract
INTRODUCTION
We evaluated the effect of Helicobacter pylori (HP) eradication on p53, cyclin D1 expression, and cell proliferation in gastric mucosa.
MATERIALS AND METHODS
We assessed p53, cyclin D1, and ki67 immunoexpression in gastric mucosa from 31 HP chronic gastritis patients and 12 controls. Reassessment was performed 6 months after successful HP eradication.
RESULTS
Successful eradication resulted in significant decrease of p53 (1.53 ± 0.16 vs 0.83 ± 0.19, P = 0.01) and ki67 (9.84 ± 0.96 vs 4.77 ± 0.27, P < 0.001) staining in the antrum. Similarly, p53 immunoreactivity significantly decreased in the corpus (1.27 ± 0.20 vs 0.46 ± 0.15, P = 0.02), while there was a trend for decreased corpus cyclin D1 and ki67 expression (0.17 ± 0.07 vs 0.0, P = 0.08 and 8.71 ± 1.24 vs 5.85 ± 0.54, P = 0.09, respectively). Importantly, after successful HP eradication, the immunoreactivity of the studied parameters was similar to that of controls.
CONCLUSION
Successful HP infection eradication restores p53, cyclin D1, and ki67 immunoreactivity in the gastric mucosa to the level of controls.
Keywords: Helicobacter pylori, eradication, p53, cyclin D1, ki67
Introduction
Helicobacter pylori is classified as class I carcinogen for gastric cancer and plays a pivotal role in the pathogenesis of gastric cancer, through different changes in the gastric epithelial cells, including DNA methylation1 and cell cycle dysregulation.2 Eradication of H. pylori is considered as a decisive step for the prevention of the gastric cancer in countries with high incidence.3
The p53 protein inhibits the cell cycle and induces apoptosis in cells with DNA damage, including gastric cancerous epithelial cells;4 however, there are conflicting data regarding the role of p53 on the regulation of the H. pylori-infected nondysplastic gastric epithelium.5 Cyclin D1 is member of the cyclin protein family and a regulator of the cell cycle. While overexpression of this protein has been observed during the process of carcinogenesis,6 recent studies suggest that H. pylori infection also causes abnormalities to various cell cycle inhibitors, such as cyclin D1,7 thus leading to dysregulation of cell cycle. Antigen ki67 is associated with ribosomal RNA transcription and cell proliferation. It has been widely used as a prognostic marker in various malignancies.8
Therefore, we conducted this study aiming to examine the expression of the aforementioned proteins in nondysplastic gastric mucosa and the effects of H. pylori eradication on their expression.
Materials and Methods
Patients
Outpatients aged >18 years suffering from dyspeptic symptoms9 were asked to participate in the study. Exclusion criteria comprised history of severe comorbidity preventing endoscopy, recent—within the previous 3 months—use of proton pump inhibitors (PPIs) and antibiotics, H. pylori eradication treatment in the past, inability to sign informed consent, and significant pathology (ulcers, esophagitis, varices, portal gastropathy, and neoplasms) detected during endoscopy.
Eligible patients underwent upper gastrointestinal endoscopy (T0), with biopsies obtained according to the Sydney protocol for gastritis.10 H. pylori-positive participants received first-line eradication treatment regimen (amoxicillin: 1,000 mg bid; clarithromycin: 500 mg bid; and PPI: bid).11 One month later, they underwent 13C-urea breath test (Helicobacter test INFAI®; Institut fur biomedizinische Analytik and NMR-Imaging GmbH, Bochum, Germany)11 to confirm successful H. pylori eradication. In case of treatment failure, second-line treatment regimen (amoxicillin: 1,000 mg bid; levofloxacin: 250 mg bid; and PPI: bid)11 was prescribed and eradication of H. pylori was once again evaluated following the aforementioned procedure. Successfully eradicated participants were asked to undergo a second upper gastrointestinal endoscopy with mucosal sampling, 6 months later (T1).
Attikon University General Hospital Institutional Review Board approved the study protocol (third meeting, March 9, 2006) and all participants provided written informed consent at enrollment. Our research complied with the principles of the Declaration of Helsinki.
Histology and immunochemistry
An experienced gastrointestinal pathologist examined all specimens for H. pylori gastritis and immunoexpression of p53, ki67, and cyclin D proteins. In case of uncertainty, an expert in gastrointestinal pathology decided. Using Giemsa staining, classification and grading of gastritis was performed according to the updated Sydney system.10 Activity and chronicity of gastritis, as well as H. pylori density, were evaluated using a scale of 0–3. Intestinal metaplasia and gastric atrophy were scored as present or absent.
Immunoexpression of p53, cyclin D1, and ki67 proteins was evaluated with the avidin-biotin complex method using p53 monoclonal mouse anti-human antibody (Clone PA6240; Dako, Glostrup, Denmark), cyclin D1 monoclonal rabbit anti-human antibody (Clone EP12; Dako), and ki67 mouse anti-human antibody (clone MIB-1; Dako). Expression of p53 protein was assessed with a semiquantitative score, for both staining intensity (0: no stain, 1: weak stain, 2: medium stain, and 3: strong stain) and distribution (0: no nuclear expression, 1: <10% nuclear expression, 2: 10%–50% nuclear expression, 3: >50% nuclear expression), with the overall score ranging from zero to six. A section of gastric adenocarcinoma tissue with high p53 immune-positivity was used as a positive control. Immunoexpression of cyclin D1 protein was evaluated using a semiquantitative score (0: no expression, 1: <5% expression, 2: 5%–30%, 3: 31%–60%, 4: >60%). A section of breast cancer tissue with intense immune-positivity for cyclin D1 was used as the positive control. Finally, cellular proliferation was assessed with ki67 labeling index (LI), which is the proportion of cells with ki67-positive nuclear immunostaining. A section of gastric adenocarcinoma tissue with high ki67 LI was used as a positive control. Consecutive paraffin sections, in which incubation with primary antibody was omitted, were used as negative control for p53, ki-67, and cyclin D.
Statistical analysis
Quantitative data are expressed as mean (SEM) or mean (SD), as appropriate, while categorical data are expressed as number (%).
Nonparametric tests for nonrelated and paired samples were performed to detect significance at the level of 0.05.
Study end points
Our study end points comprised the following: (1) evaluation of the effects of H. pylori eradication therapy on the immunoexpression of p53, cyclin D1, and ki67 proteins in the gastric mucosa; and (2) comparison of the expression of the aforementioned proteins in the gastric mucosa of the H. pylori-eradicated patients with that of the H. pylori-negative ones.
Results
Patients’ characteristics
As shown in study flowchart (Fig. 1), 43 of the 70 screened dyspeptic outpatients were enrolled. The study population consisted of middle-aged (mean age: 47.6 years) dyspeptic patients; 30 (69%) were female and 13 (31%) male. Among them, 31 patients were H. pylori positive at T0 histology. All of them received eradication therapy, and 28 (90%) were successfully eradicated of H. pylori (one patient required second-line therapy). However, two patients denied second endoscopy; therefore, biopsies were obtained from 26 patients at T1. Participants’ baseline demographic, histological, and immunohistochemical characteristics are shown in Table 1.
Figure 1.
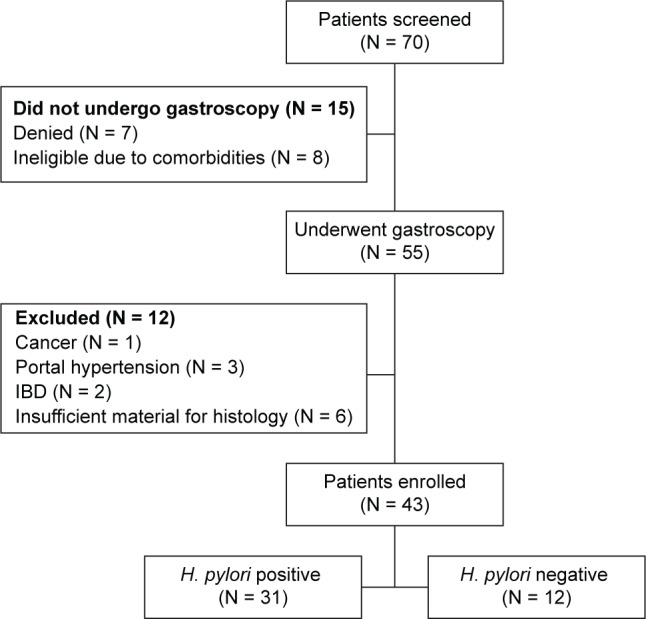
Flowchart of study.
Table 1.
Histological and immunohistochemical findings at enrollment (T0).
|
H. pylori POSITIVE N = 31 |
H. pylori NEGATIVE N = 12 |
P | |
|---|---|---|---|
| Male sex, n (%) | 10 (32.2) | 3 (25.0) | 0.73 |
| Age, mean (SD) | 49 (14) | 41 (13) | 0.1 |
| Gastritis activity,mean (SEM) | |||
| Antrum | 1.32 (0.12) | 0 | <0.0001 |
| Body | 0.94 (0.15) | 0 | 0.002 |
| Gastritis chronicity, mean (SEM) | |||
| Antrum | 1.81 (0.13) | 0.33 (0.14) | <0.0001 |
| Body | 1.42 (0.13) | 0.55 (0.15) | 0.02 |
| Gastric atrophy,n (%) | |||
| Antrum | 4 (13) | 1 (8.3) | 1 |
| Body | 4 (13) | 1 (8.3) | 1 |
| Intestinal metaplasia, n (%) | |||
| Antrum | 6 (19) | 0 | 0.16 |
| Body | 4 (13) | 0 | 0.55 |
| H. pylori density,median (SEM) | |||
| Antrum | 2.2 (0.13) | 0 | <0.0001 |
| Body | 1.7 (1.7) | 0 | <0.0001 |
| p53, mean (SEM) | |||
| Antrum | 1.53 (0.16) | 0.92 (0.26) | 0.065 |
| Body | 1.27 (0.19) | 0.75 (0.32) | 0.083 |
| Cyclin D1, mean (SEM) | |||
| Antrum | 0.27 (0.8) | 0.33 (0.14) | 0.669 |
| Body | 0.17 (0.07) | 0 | 0.12 9 |
| ki67 labeling index, mean (SEM) | |||
| Antrum | 9.84 (0.96) | 6.0 (1.16) | 0.008 |
| Body | 8.71 (1.24) | 5.25 (0.44) | 0.107 |
End points
As shown in Table 2, there was a statistically significant decline of p53 protein expression after the successful eradication of H. pylori, in both the gastric antrum (P = 0.01) and the body (P = 0.023) mucosa. There was also a reduction in cyclin D1 immunostaining after H. pylori eradication only in the gastric body mucosa, albeit this reduction did not reach statistical significance (P = 0.083). Finally, there was a significant reduction of ki67 antigen expression in the gastric antrum mucosa (P < 0.001) and a trend toward lower ki67 LI in the gastric body mucosa (P = 0.095) at T1. Representative pictures of p53, cyclin D1, and ki67 immunostaining in H. pylori-positive gastric tissue and after successful eradication of H. pylori are shown in Figures 2 and 3, respectively.
Table 3 and Figures 3 and 4 indicate that there was no difference in terms of p53, cyclin D1, and ki67 immunostaining in the gastric mucosa among the groups of successfully eradicated and the original H. pylori-negative patients.
Table 2.
Histological and immunohistochemical findings in the gastric mucosa of Helicobacter pylori-positive patients at enrollment (T0) and at 6 months after successful eradication (T1).
| T0 N = 31 |
T1 N = 26 |
P | |
|---|---|---|---|
| Gastritis activity, mean (SEM) | |||
| Antrum | 1.32 (0.12) | 0 | <0.0001 |
| Body | 0.94 (0.15) | 0 | 0.002 |
| Gastritis chronicity, mean (SEM) | |||
| Antrum | 1.81 (0.13) | 0.85 (0.07) | <0.0001 |
| Body | 1.42 (0.13) | 0.62 (0.10) | <0.0001 |
| Gastric atrophy, n (%) | |||
| Antrum | 4 (13) | 4 (15.4) | 1 |
| Body | 4 (13) | 0 | 0.05 |
| Intestinal metaplasia, n (%) | |||
| Antrum | 6 (19) | 4 (15.4) | 1 |
| Body | 4 (13) | 0 | 0.05 |
| H. pylori density, median (SEM) | |||
| Antrum | 2.2 (0.13) | 0 | <0.0001 |
| Body | 1.7 (1.7) | 0 | <0.0001 |
| p53, mean (SEM) | |||
| Antrum | 1.53 (0.16) | 0.83 (0.19) | 0.01 |
| Body | 1.27 (0.19) | 0.46 (0.15) | 0.02 |
| Cyclin D1,mean (SEM) | |||
| Antrum | 0.27 (0.8) | 0.32 (0.09) | 0.593 |
| Body | 0.17 (0.07) | 0 (0) | 0.083 |
| ki67 labeling index, mean (SEM) | |||
| Antrum | 9.84 (0.96) | 4.77 (0.27) | <0.001 |
| Body | 8.71 (1.24) | 5.85 (0.54) | 0.095 |
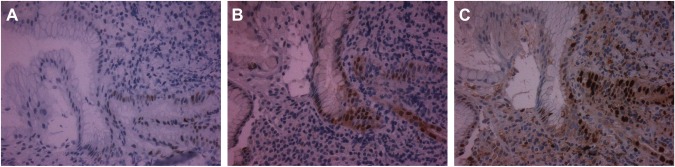
Figure 2.
Immunostaining for p53 (A), cyclin D1 (B), and ki67 (C) in H. pylori-associated gastritis section.
Note: Positively stained cells for each protein are brown.
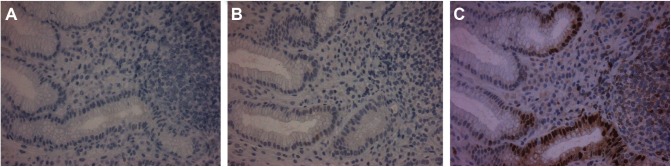
Figure 3.
Immunostaining for p53 (A), cyclin D1 (B), and ki67 (C) in gastric mucosa sections 6 months after successful H. pylori eradication therapy.
Notes: Positively stained cells for each protein are brown. There are only scarce p53 (A) and cyclin D1 (B) weakly stained cells, while ki67 immunostaining is limited to the regenerative part of the gastric pits (C).
Table 3.
Comparison of p53, cyclin D1, and ki67 immunostaining in the gastric mucosa of Helicobacter pylori-negative and H. pylori-eradicated patients.
|
H. pylori NEGATIVE (T0) N = 12 |
H. pylori ERADICATED (T1) N = 26 |
P | |
|---|---|---|---|
| p53, mean (SEM) | |||
| Antrum | 0.92 (0.26) | 0.83 (0.19) | 0.8 |
| Body | 0.75 (0.32) | 0.46 (0.15) | 0.7 |
| Cyclin D1,mean (SEM) | |||
| Antrum | 0.33 (0.14) | 0.32 (0.09) | 1 |
| Body | 0 | 0 (0) | 1 |
| ki67 labeling index, mean (SEM) | |||
| Antrum | 6.0 (1.16) | 4.77 (0.27) | 0.7 |
| Body | 5.25 (0.44) | 5.85 (0.54) | 0.9 |
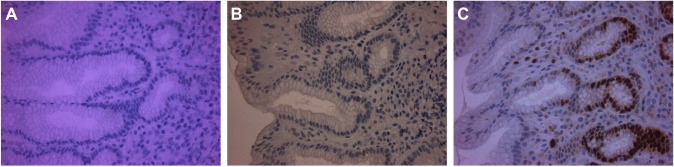
Figure 4.
Immunostaining for p53 (A), cyclin D1 (B), and ki67 (C) in H. pylori-negative gastric mucosa sections.
Notes: Positively stained cells for each protein are brown. There is absence of p53 immunostaining (A), weak cyclin D1 immunoexpression in a small amount of epithelial cells (B), and ki67 immunostaining is limited to the regenerative part of the gastric pits (C).
Discussion
Our study evaluated, for the first time, the effects of H. pylori eradication therapy on the expression of cell cycle-related genes using histological and immunohistochemical mapping of nonneoplastic gastric mucosa. We investigated, at the same time, the expression of three different proteins (p53, cyclin D1, and ki67) in H. pylori-infected patients, each one associated in a different way in cell cycle regulation—with no evidence that any of them predominates12—and consequently in gastric carcinogenesis.
In the past, several studies have investigated the immunoexpression of p53 in H. pylori-infected patients, alone or combined with the expression of ki67, which is the most commonly studied cell proliferation index in dysplastic and nondysplastic gastric mucosa.4,5 However, comparison of p53 expression before and in the long term after eradication treatment has been less studied, especially in nonneoplastic gastric mucosa. Cyclin D1 has been investigated only in neoplastic gastric lesions.13
Our study showed that eradication of H. pylori infection resulted in significantly lower immunostaining of the three studied proteins in the gastric nondysplastic mucosa, both in the antrum and in the corpus. More importantly, treatment of the infection restored mucosa immunoreactivity to the levels of the controls.
More precisely, while we observed higher expression of ki67 in the H. pylori-positive nondysplastic gastric mucosa (both in the antrum and in the body), as compared to the expression in the control gastric mucosa, successful H. pylori eradication statistically reduced cell proliferation in the antrum, this effect being less prominent in the gastric corpus. This finding probably correlates with the natural course of H. pylori infection because the bacterium colonizes the gastric antrum first and the gastric body later. Alternatively, the small study sample might have resulted in nonsignificant results. Immunoexpression of p53 was higher (albeit nonsignificant) in the gastric mucosa of infected individuals, compared to that of controls, and 6 months after eradication treatment, it was significantly reduced in the mucosa of the gastric antrum and body. Finally, we observed a weak expression of cyclin D1 in the gastric nondysplastic mucosa independently of H. pylori infection status. Apparently, there was no effect of eradication treatment on gastric mucosa cyclin D1 staining.
Earlier publications have shown similar findings regarding the expression of p53 and ki67 after eradication of H. pylori infection. Satoh et al14 have found a significant lowering of p53 expression in chronically infected H. pylori gastric mucosa, 1 month after successful eradication treatment. In contrast to our study evaluating the expression of the protein 6 months after successful eradication, early reexamination performed by Satoh et al14 precluded evaluation of a long-term result. Similar to our results, Hibi et al15 reported increased gastric cell proliferation and p53 accumulation in H. pylori-associated gastritis and showed that values returned to normal 6 months after eradication therapy. Moreover, a small study detected the persistent—up to 6 months—recovery of the normal rate of gastric antral epithelial proliferation following eradication treatment.16 Hsu et al17 approached the study of cell proliferation and p53 immunoexpression in H. pylori gastritis. They reported a significant reduction of p53 expression in the whole foveolar epithelium and in each segment (upper, middle, and lower) of the gastric pit after eradication treatment, while cell proliferation decreased only in the middle segment of the gastric pit. However, the effect of H. pylori infection on p53 protein expression and cell proliferation in gastritis is not unanimous in the literature. For example, Nardone et al18 reported weak expression of p53 and increased cell proliferation in chronic gastritis patients independently of the presence of H. pylori, and Unger et al19 showed that antral cell proliferation was higher in chronic gastritis than in normal gastric mucosa, independently of H. pylori infection, as well.
While cyclin D1 has been widely studied in gastric cancer20 and in gastric precancerous conditions,21 the influence of H. pylori on the expression of cyclin D1 has been studied previously only in in vitro models. Chang et al22 observed that H. pylori CagA strains increased cyclin D1 expression and host cell survival, raising the suspicion of increased risk for gastric oncogenesis. The weak expression of cyclin D1, irrespective of infection status, in our study may indicate that the contribution of cyclin D1 is a late event in gastric tumorigenesis.
No previous studies have investigated simultaneously the expression of these three proteins in relation to H. pylori infection in nonneoplastic gastric mucosa. However, two studies have examined this relation in cancerous or precancerous lesions. Polat et al23 have shown overexpression of p53, ki67, and cyclin D1 in gastric tissue from patients with gastric adenocarcinoma and chronic H. pylori gastritis but no overexpression in H. pylori-negative patients with nondysplastic gastric mucosa. Moreover, the effect of H. pylori eradication was not examined in their study. In contrast to our study where the prevalence of atrophy and intestinal metaplasia was low, Guarner et al24 studied biopsy samples from multifocal gastritis prior and 1 year after H. pylori eradication. They found that ki67 and p53 proteins were expressed more frequently in atrophic and metaplastic gastric mucosa compared to mucosa with gastritis only.
Beyond studying simultaneously the expression of three different proteins, each one related with a different pathway of cell cycle regulation in the gastric nondysplastic epithelium, another strength of our study is the evaluation of the expression of these proteins using histological and immunochemical mapping of the stomach, eg, in gastric mucosa from the antrum and the corpus. Moreover, we evaluated the expression of the studied proteins in gastric tissue with relatively mild abnormalities, thus giving us the opportunity to delineate the early appearance of p53 protein expression simultaneously with increased cell proliferation. However, there were also several drawbacks in our study. The small number of participants limits the strength of our findings and the potential generalization of our conclusions. Furthermore, H. pylori colonization can be patchy, leading to false-negative biopsy sampling. Therefore, inaccurate classification of infected patients to the control group cannot be ruled out. It is also impossible to obtain biopsy samples before and after the eradication treatment from the same site of the gastric mucosa exactly. Moreover, it is not clear if 6 months is an adequate period to detect sustained effects of eradication of H. pylori infection on the gastric epithelial cell kinetics. Finally, not using molecular studies in combination with the performed immunohistochemistry, as well as the inability to provide potential explanations regarding the regulatory mechanism of eradication of H. pylori infection on the expression of the studied proteins, is another drawback of the study.
In conclusion, successful H. pylori eradication significantly decreases the immunoexpression of p53 and cyclin D1, as well as cell proliferation, in nondysplastic gastric mucosa. Moreover, eradication therapy restores the expression of the studied proteins to levels similar to those in the gastric mucosa of the uninfected participants, indicating a potential cancer-preventive role of the eradication treatment when applied early during the disease process.
Ethics
The Ethics Committee of the Attikon University General Hospital has reviewed and approved the study protocol and all participants provided signed informed consent at enrollment.
Acknowledgments
We thank Dr. Kostis Papaxoinis for his valuable contribution to the immunohistochemistry experiments.
Footnotes
ACADEMIC EDITOR: Melpakkam Srinivas, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 687 words, excluding any confidential comments to the academic editor.
FUNDING: The study has been funded by the research grant “Kapodistrias” from the National and Kapodistrian University of Athens. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: KT, TE, SDL. Analyzed the data: KT, TE, PG, GT, IGP. Wrote the first draft of the manuscript: VP, PG, VD, GT. Contributed to the writing of the manuscript: KT, IGP. Agree with manuscript results and conclusions: KT, VP, TE, PG, VD, GT, IGP, IV, SDL. Jointly developed the structure and arguments for the paper: KT, TE, IV, SDL. Made critical revisions and approved final version: KT, IV, IGP, SDL. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Perrin D, Ruskin HJ, Niwa T. Cell type-dependent, infection-induced, aberrant DNA methylation in gastric cancer. J Theor Biol. 2010;264(2):570–577. doi: 10.1016/j.jtbi.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305(2):228–238. doi: 10.1016/j.canlet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Sipponen P, Naumann M, et al. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100(9):2100–2115. doi: 10.1111/j.1572-0241.2005.41688.x. [DOI] [PubMed] [Google Scholar]
- 4.Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A. TP53 and gastric carcinoma: a review. Hum Mutat. 2003;21(3):258–270. doi: 10.1002/humu.10180. [DOI] [PubMed] [Google Scholar]
- 5.Ozturk Y, Ozer E, Lebe B, Bekem O, Buyukgebiz B. Immunohistochemical evaluation of p53 expression and proliferative activity in children with Helicobacter pylori associated gastritis. J Pediatr Gastroenterol Nutr. 2005;40(4):467–470. doi: 10.1097/01.mpg.0000148832.22130.d7. [DOI] [PubMed] [Google Scholar]
- 6.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sougioultzis S, Foukas PG, Tzivras M, et al. Alterations in the proliferating compartment of gastric mucosa during Helicobacter pylori infection: the putative role of epithelial cells expressing p27(kip1) Mod Pathol. 2003;16(11):1076–1085. doi: 10.1097/01.MP.0000093626.15701.76. [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Moore DH, II, Vartanian R. Correlation of Ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum Pathol. 1994;25(4):337–342. doi: 10.1016/0046-8177(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 9.Committee ASoP, Shaukat A, Wang A, et al. The role of endoscopy in dyspepsia. Gastrointest Endosc. 2015;82(2):227–232. doi: 10.1016/j.gie.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56(6):772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumari S, Puneet, Prasad SB, et al. Cyclin D1 and cyclin E2 are differentially expressed in gastric cancer. Med Oncol. 2016;33(5):40. doi: 10.1007/s12032-016-0754-8. [DOI] [PubMed] [Google Scholar]
- 14.Satoh K, Kihira K, Kawata H, et al. p53 expression in the gastric mucosa before and after eradication of Helicobacter pylori. Helicobacter. 2001;6(1):31–36. doi: 10.1046/j.1523-5378.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 15.Hibi K, Mitomi H, Koizumi W, Tanabe S, Saigenji K, Okayasu I. Enhanced cellular proliferation and p53 accumulation in gastric mucosa chronically infected with Helicobacter pylori. Am J Clin Pathol. 1997;108(1):26–34. [PubMed] [Google Scholar]
- 16.Demiray M, Gulten M, Manavoglu O, et al. Evaluation of the effects of Helicobacter pylori eradication therapy on gastric antral epithelial hyperproliferation: a prospective six-month follow-up study. Hepatogastroenterology. 2004;51(59):1531–1535. [PubMed] [Google Scholar]
- 17.Hsu PI, Lai KH, Chien EJ, et al. Impact of bacterial eradication on the cell proliferation and p53 protein accumulation in Helicobacter pylori-associated gastritis. Anticancer Res. 2000;20(2B):1221–1228. [PubMed] [Google Scholar]
- 18.Nardone G, Staibano S, Rocco A, et al. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44(6):789–799. doi: 10.1136/gut.44.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger Z, Molnar B, Szaleczky E, et al. Effect of Helicobacter pylori infection and eradication on gastric epithelial cell proliferation and apoptosis. J Physiol Paris. 2001;95(1–6):355–360. doi: 10.1016/s0928-4257(01)00048-1. [DOI] [PubMed] [Google Scholar]
- 20.Gao P, Zhou GY, Liu Y, Li JS, Zhen JH, Yuan YP. Alteration of cyclin D1 in gastric carcinoma and its clinicopathologic significance. World J Gastroenterol. 2004;10(20):2936–2939. doi: 10.3748/wjg.v10.i20.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anagnostopoulos GK, Stefanou D, Arkoumani E, et al. Immunohistochemical expression of cell-cycle proteins in gastric precancerous lesions. J Gastroenterol Hepatol. 2008;23(4):626–631. doi: 10.1111/j.1440-1746.2007.05219.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang YJ, Wu MS, Lin JT, Pestell RG, Blaser MJ, Chen CC. Mechanisms for Helicobacter pylori CagA-induced cyclin D1 expression that affect cell cycle. Cell Microbiol. 2006;8(11):1740–1752. doi: 10.1111/j.1462-5822.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Polat A, Cinel L, Dusmez D, Aydin O, Egilmez R. Expression of cell-cycle related proteins in Helicobacter pylori gastritis and association with gastric carcinoma. Neoplasma. 2002;49(2):95–100. [PubMed] [Google Scholar]
- 24.Guarner J, Bartlett J, Seitz R, et al. Cell proliferation and inflammation on biopsy samples with multifocal atrophic gastritis before and 1 year after Helicobacter pylori eradication. Arch Pathol Lab Med. 2005;129(11):1451–1456. doi: 10.5858/2005-129-1451-CPAIOB. [DOI] [PubMed] [Google Scholar]