Abstract
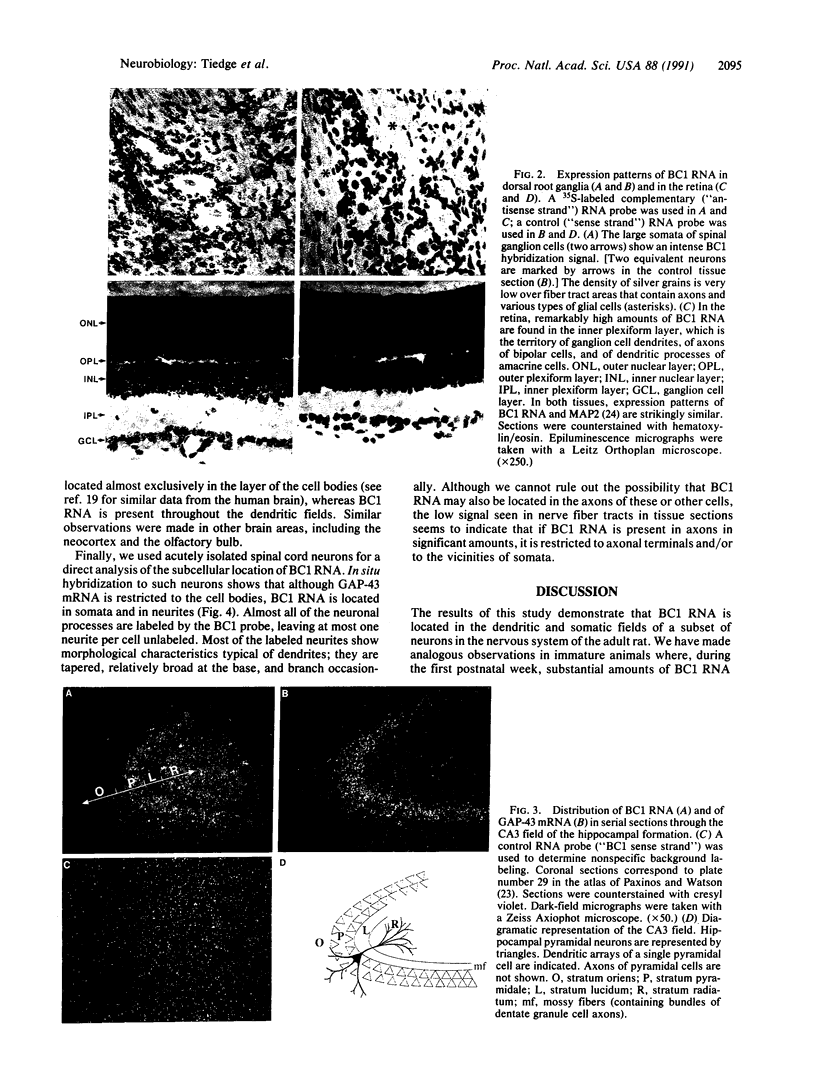
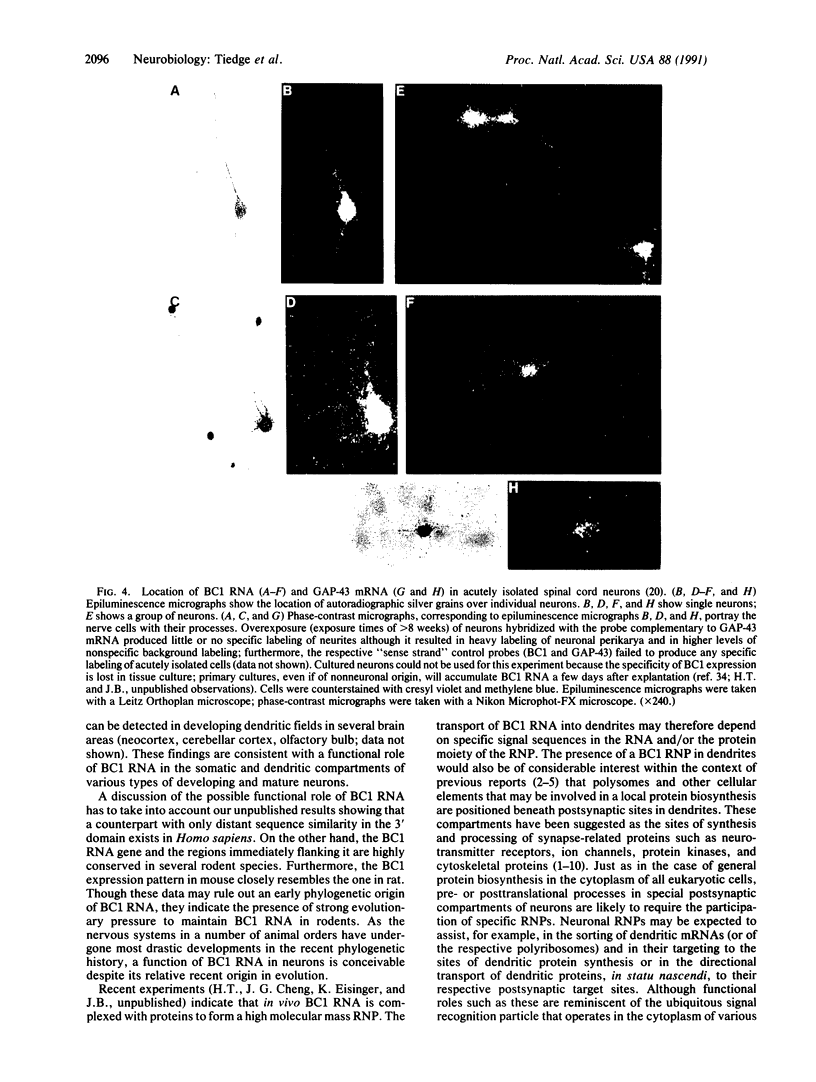
In nerve cells, a specialized protein synthetic machinery is thought to operate in local compartments of dendrites, in particular beneath synaptic junctions, and thereby to facilitate swift adjustments of the postsynaptic protein repertoire in situ. This notion has been supported by the identification of polyribosomes and selected mRNAs in those compartments. In this study, we report the discovery of a specific RNA polymerase III transcript in dendrites. This RNA, a noncoding, 152-nucleotide-long, single-gene transcript known as BC1 RNA, is expressed almost exclusively in the nervous system. In adult rats as well as in immature rats in late developmental stages, BC1 RNA has been located in the dendrites and somata of a subset of neurons in the central and peripheral nervous system. The colocalization of BC1 RNA with dendritic mRNAs and polyribosomes may indicate a role--possibly within the functional unit of a high molecular mass ribonucleoprotein particle--in specific pre- or posttranslational processes in postsynaptic compartments of neurons.
Full text
PDF
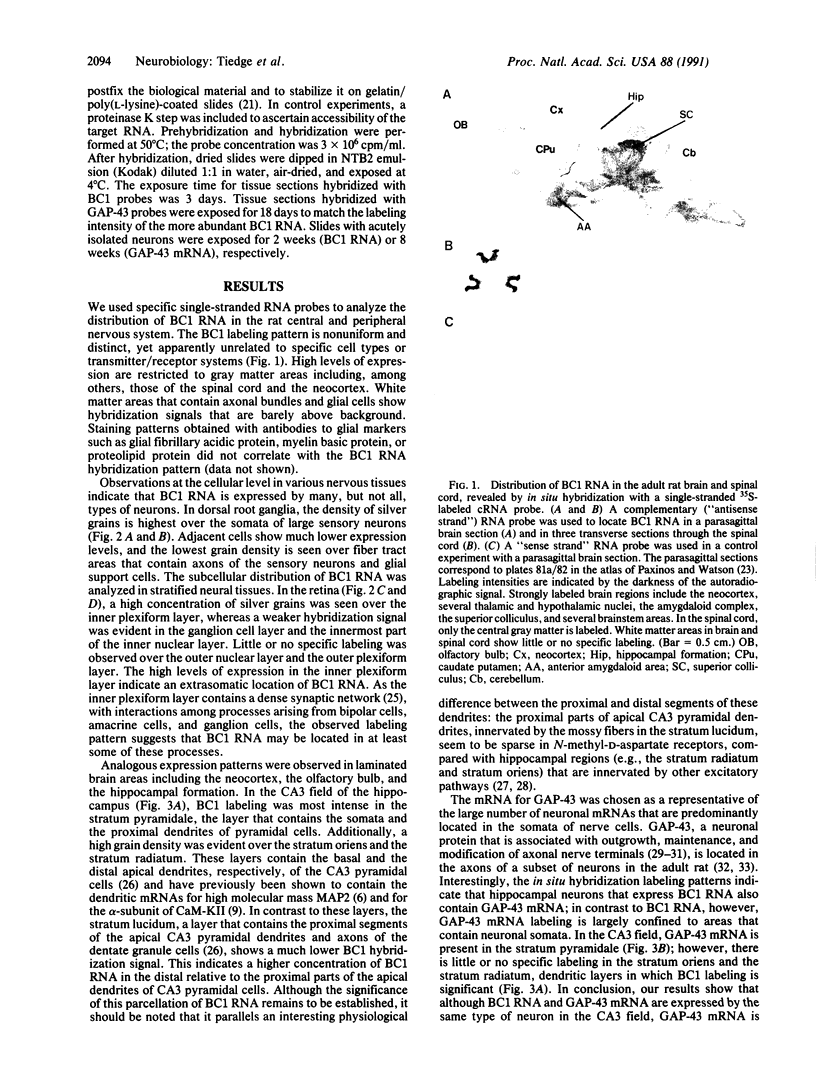

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Fremeau R. T., Jr, Tiedge H., Wilcox J., Bovolin P., Brosius J., Roberts J. L., Costa E. Diazepam binding inhibitor gene expression: location in brain and peripheral tissues of rat. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7018–7022. doi: 10.1073/pnas.85.18.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N., Finklestein S. P., Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988 Jan;8(1):339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M., Baas P. W. The basis of polarity in neurons. Trends Neurosci. 1989 Jun;12(6):211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Bruckenstein D. A., Lein P. J., Higgins D., Fremeau R. T., Jr Distinct spatial localization of specific mRNAs in cultured sympathetic neurons. Neuron. 1990 Dec;5(6):809–819. doi: 10.1016/0896-6273(90)90340-l. [DOI] [PubMed] [Google Scholar]
- Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J Neurosci. 1990 Jun;10(6):1788–1798. doi: 10.1523/JNEUROSCI.10-06-01788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Microtubule MAPping. Cell. 1990 Mar 9;60(5):701–702. doi: 10.1016/0092-8674(90)90083-q. [DOI] [PubMed] [Google Scholar]
- Davis L., Banker G. A., Steward O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature. 1987 Dec 3;330(6147):477–479. doi: 10.1038/330477a0. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Miller P. E., Navone F., Theurkauf W. E., Vallee R. B. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984 Apr;11(4):817–846. [PubMed] [Google Scholar]
- DeChiara T. M., Brosius J. Neural BC1 RNA: cDNA clones reveal nonrepetitive sequence content. Proc Natl Acad Sci U S A. 1987 May;84(9):2624–2628. doi: 10.1073/pnas.84.9.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R. RNA transport in dendrites. Trends Neurosci. 1988 Aug;11(8):342–343. doi: 10.1016/0166-2236(88)90054-9. [DOI] [PubMed] [Google Scholar]
- Goslin K., Schreyer D. J., Skene J. H., Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988 Dec 15;336(6200):672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- Jones O. T., Kunze D. L., Angelides K. J. Localization and mobility of omega-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989 Jun 9;244(4909):1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Kleiman R., Banker G., Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990 Dec;5(6):821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Shinnick T. M., Sutcliffe J. G. The neuronal identifier element is a cis-acting positive regulator of gene expression. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3751–3755. doi: 10.1073/pnas.83.11.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Holets V. R., Toy D. W., Cotman C. W. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989 Aug 14;103(1):56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Bird E. D., Benowitz L. I. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3638–3642. doi: 10.1073/pnas.85.10.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher A. B., Gispen W. H. Comparison of the immunocytochemical distribution of the phosphoprotein B-50 in the cerebellum and hippocampus of immature and adult rat brain. Brain Res. 1986 Jun 11;375(2):267–279. doi: 10.1016/0006-8993(86)90747-x. [DOI] [PubMed] [Google Scholar]
- Owens G. P., Chaudhari N., Hahn W. E. Brain "identifier sequence" is not restricted to brain: similar abundance in nuclear RNA of other organs. Science. 1985 Sep 20;229(4719):1263–1265. doi: 10.1126/science.2412293. [DOI] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Steward O. Alterations in polyribosomes associated with dendritic spines during the reinnervation of the dentate gyrus of the adult rat. J Neurosci. 1983 Jan;3(1):177–188. doi: 10.1523/JNEUROSCI.03-01-00177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Falk P. M. Protein-synthetic machinery at postsynaptic sites during synaptogenesis: a quantitative study of the association between polyribosomes and developing synapses. J Neurosci. 1986 Feb;6(2):412–423. doi: 10.1523/JNEUROSCI.06-02-00412.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Levy W. B. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982 Mar;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Reeves T. M. Protein-synthetic machinery beneath postsynaptic sites on CNS neurons: association between polyribosomes and other organelles at the synaptic site. J Neurosci. 1988 Jan;8(1):176–184. doi: 10.1523/JNEUROSCI.08-01-00176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Gottesfeld J. M., Reynolds W. Control of neuronal gene expression. Science. 1984 Sep 21;225(4668):1308–1315. doi: 10.1126/science.6474179. [DOI] [PubMed] [Google Scholar]
- Tiedge H. The use of UV light as a cross-linking agent for cells and tissue sections in in situ hybridization. DNA Cell Biol. 1991 Mar;10(2):143–147. doi: 10.1089/dna.1991.10.143. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]