Abstract
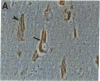
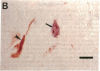
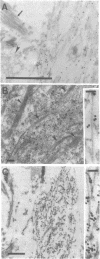
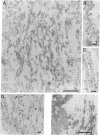
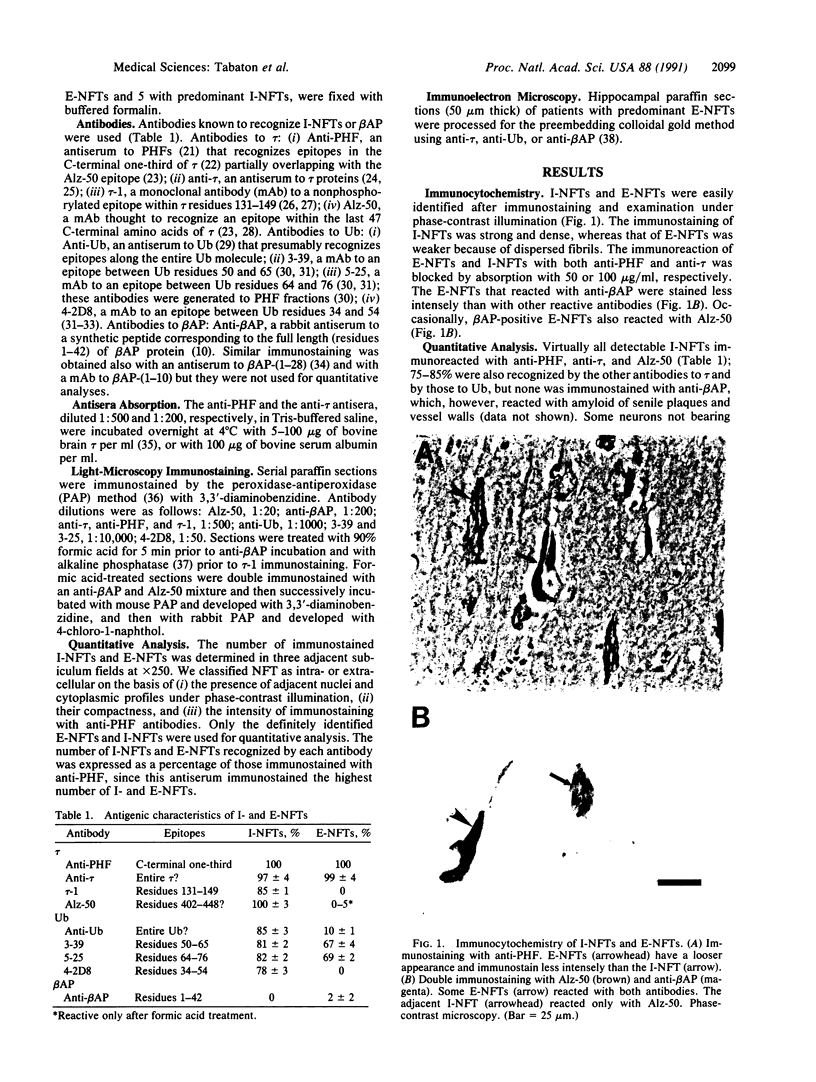
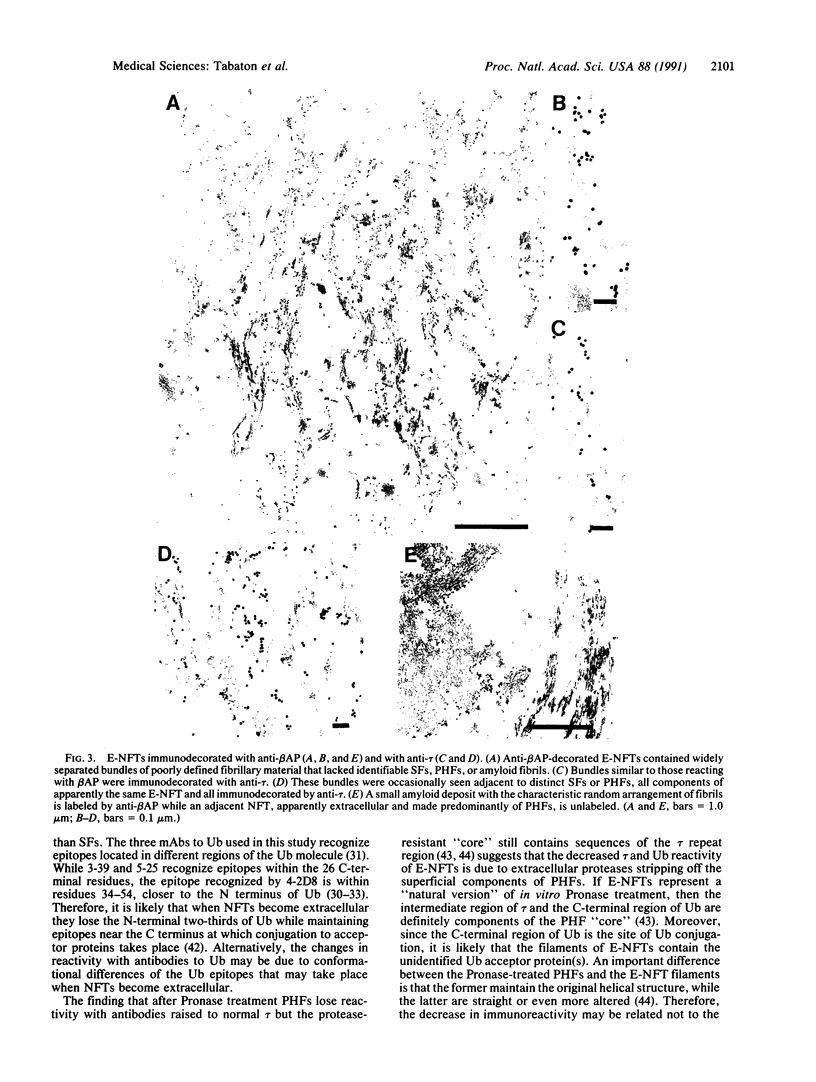
Neurofibrillary tangles (NFTs), a hallmark of Alzheimer disease, are commonly located in perikarya of neurons. In advanced cases of Alzheimer disease, however, NFTs are observed also in the extracellular space. As extracellular NFTs (E-NFTs), and occasionally intracellular NFTs (I-NFTs), are recognized by antibodies to beta-amyloid protein (beta AP), beta AP may be present not only in amyloid deposits but also in paired helical filaments (PHFs), the primary components of NFTs. We compared the antigenic characteristics of I-NFTs and E-NFTs with light- and electron-microscopic immunocytochemistry by using several antibodies to noncontiguous epitopes of the microtubule-associated protein tau and of ubiquitin (Ub) as well as an antiserum to beta AP. At variance with I-NFTs, E-NFTs were made predominantly of straight filaments (SFs), rather than PHFs, that were often separated by astroglial processes and in close association with small beta AP deposits. Occasionally, E-NFTs were made of bundles of amorphous material, which showed no resemblance to SFs, PHFs, or amyloid fibrils. The antigenic changes in E-NFTs suggest that when NFTs become extracellular they lose the N and, possibly, the C termini of tau while maintaining the intermediate region of the molecule; they also lose the N-terminal two-thirds of Ub while the C-terminal conjugation site of Ub is preserved. A small subset of E-NFTs reacted with antibodies to both beta AP and tau. Although in most E-NFTs, the epitopes recognized by tau and Ub antibodies were located in typical PHFs and SFs, the epitopes recognized in this subset of anti-beta AP and anti-tau-positive E-NFTs were located exclusively in the bundles of amorphous material. It is suggested that either beta AP epitopes are present but inaccessible in PHFs and SFs and become exposed after conformational changes occurring in the extracellular space or PHFs and SFs become closely associated with beta AP in the extracellular space.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Haga S., Bruton C., Ishii T., Roberts G. W. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer's disease. Am J Pathol. 1990 Feb;136(2):255–260. [PMC free article] [PubMed] [Google Scholar]
- Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989 Jan 16;477(1-2):90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W., Wischik C. M., Novak M., Amos W. B., Klug A., Roth M. Molecular analysis of neurofibrillary degeneration in Alzheimer's disease. An immunohistochemical study. Am J Pathol. 1990 Sep;137(3):711–723. [PMC free article] [PubMed] [Google Scholar]
- Castaño E. M., Ghiso J., Prelli F., Gorevic P. D., Migheli A., Frangione B. In vitro formation of amyloid fibrils from two synthetic peptides of different lengths homologous to Alzheimer's disease beta-protein. Biochem Biophys Res Commun. 1986 Dec 15;141(2):782–789. doi: 10.1016/s0006-291x(86)80241-8. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I. The distribution of tau and HMW microtubule-associated proteins in different cell types. Exp Cell Res. 1980 Jun;127(2):341–350. doi: 10.1016/0014-4827(80)90439-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Guiroy D. C., Miyazaki M., Multhaup G., Fischer P., Garruto R. M., Beyreuther K., Masters C. L., Simms G., Gibbs C. J., Jr, Gajdusek D. C. Amyloid of neurofibrillary tangles of Guamanian parkinsonism-dementia and Alzheimer disease share identical amino acid sequence. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2073–2077. doi: 10.1073/pnas.84.7.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Beyreuther K., Masters C. L. A4 amyloid protein immunoreactivity is present in Alzheimer's disease neurofibrillary tangles. Neurosci Lett. 1989 Jul 3;101(3):352–355. doi: 10.1016/0304-3940(89)90559-4. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J., Kosik K. S. Tau epitopes are incorporated into a range of lesions in Alzheimer's disease. J Neuropathol Exp Neurol. 1987 Nov;46(6):611–622. doi: 10.1097/00005072-198711000-00001. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988 Nov;1(9):827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Chien C. H., Lee V. M., Yen S. H. Mapping of the Alz 50 epitope in microtubule-associated proteins tau. J Neurosci Res. 1990 Mar;25(3):412–419. doi: 10.1002/jnr.490250319. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984 Oct 10;259(19):12241–12245. [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetto V., Sternberger N. H., Perry G., Sternberger L. A., Gambetti P. Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1988 Nov;47(6):642–653. doi: 10.1097/00005072-198811000-00007. [DOI] [PubMed] [Google Scholar]
- Mann D. M. The neuropathology of Alzheimer's disease: a review with pathogenetic, aetiological and therapeutic considerations. Mech Ageing Dev. 1985 Sep;31(3):213–255. doi: 10.1016/0047-6374(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K. G., Smith H. T., Fried V. A. Ubiquitin is a component of the microtubule network. Proc Natl Acad Sci U S A. 1988 May;85(9):3019–3023. doi: 10.1073/pnas.85.9.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Hirano A., Yamaguchi H., Hirai S. [The fine structure of eosinophilic stages of Alzheimer's neurofibrillary tangles]. Rinsho Shinkeigaku. 1982 Sep;22(9):840–846. [PubMed] [Google Scholar]
- Perry G., Mulvihill P., Fried V. A., Smith H. T., Grundke-Iqbal I., Iqbal K. Immunochemical properties of ubiquitin conjugates in the paired helical filaments of Alzheimer disease. J Neurochem. 1989 May;52(5):1523–1528. doi: 10.1111/j.1471-4159.1989.tb09203.x. [DOI] [PubMed] [Google Scholar]
- Probst A., Ulrich J., Heitz P. U. Senile dementia of Alzheimer type: astroglial reaction to extracellular neurofibrillary tangles in the hippocampus. An immunocytochemical and electron-microscopic study. Acta Neuropathol. 1982;57(1):75–79. doi: 10.1007/BF00688880. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. L., Gur R. E., Gur R. C., Trojanowski J. Q. Intraneuronal and extracellular neurofibrillary tangles exhibit mutually exclusive cytoskeletal antigens. Ann Neurol. 1988 Feb;23(2):184–189. doi: 10.1002/ana.410230212. [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M., Jakes R., Klug A. Different configurational states of beta-amyloid and their distributions relative to plaques and tangles in Alzheimer disease. Proc Natl Acad Sci U S A. 1990 May;87(10):3947–3951. doi: 10.1073/pnas.87.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M., Jakes R., Klug A. Topographical relationship between beta-amyloid and tau protein epitopes in tangle-bearing cells in Alzheimer disease. Proc Natl Acad Sci U S A. 1990 May;87(10):3952–3956. doi: 10.1073/pnas.87.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T., Gallatin W. M., Siegelman M., Smith H. T., Fried V. A., Weissman I. L. Expression cloning of a lymphocyte homing receptor cDNA: ubiquitin is the reactive species. Science. 1986 Feb 21;231(4740):845–850. doi: 10.1126/science.3003914. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Mandybur T. I., Perry G., Onorato M., Autilio-Gambetti L., Gambetti P. The widespread alteration of neurites in Alzheimer's disease may be unrelated to amyloid deposition. Ann Neurol. 1989 Dec;26(6):771–778. doi: 10.1002/ana.410260614. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Perry G., Autilio-Gambetti L., Manetto V., Gambetti P. Influence of neuronal location on antigenic properties of neurofibrillary tangles. Ann Neurol. 1988 Jun;23(6):604–610. doi: 10.1002/ana.410230613. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Whitehouse P. J., Perry G., Davies P., Autilio-Gambetti L., Gambetti P. Alz 50 recognizes abnormal filaments in Alzheimer's disease and progressive supranuclear palsy. Ann Neurol. 1988 Sep;24(3):407–413. doi: 10.1002/ana.410240309. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Uéda K., Masliah E., Saitoh T., Bakalis S. L., Scoble H., Kosik K. S. Alz-50 recognizes a phosphorylated epitope of tau protein. J Neurosci. 1990 Oct;10(10):3295–3304. doi: 10.1523/JNEUROSCI.10-10-03295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]