Abstract
Purpose
Pharmacogenomics is an important component of precision medicine. Informatics, especially clinical decision support (CDS) in the electronic health record (EHR), is a critical tool for the integration of pharmacogenomics into routine patient care. The purpose of this paper is to describe existing pharmacogenomic informatics models, identify key implementation steps, and discuss emerging resources to facilitate the development of pharmacogenomic clinical decision support in the EHR.
Summary
Effective integration of pharmacogenomic CDS into the EHR can address implementation barriers, including the increasing volume of pharmacogenomic clinical knowledge, the enduring nature of pharmacogenetic results, and the complexity of interpreting results. Both passive and active CDS provide point-of-care information to clinicians that can guide the systematic use of pharmacogenomics to proactively optimize pharmacotherapy. Key considerations for a successful implementation have been identified including clinical workflows, identification of alert triggers, and tools to guide interpretation of results. These considerations are described along with emerging resources from the Clinical Pharmacogenetics Implementation Consortium (CPIC) and National Academy of Medicine.
Conclusion
Pharmacogenomic CDS in the EHR is essential to curate pharmacogenomic data and disseminate patient-specific information at the point of care. These tools facilitate prescribing optimal drug therapy based on a patient’s inherited genetic profile and ensure that genotype-guided therapy is used whenever available. Multiple model practices have demonstrated the feasibility of developing pharmacogenomic CDS within commercially available EHRs. In some situations, ancillary systems and applications outside the EHR may be integrated to augment the capabilities of the EHR.
Keywords: Pharmacogenomics, electronic health records, clinical decision support systems, precision medicine, Clinical Pharmacogenetic Implementation Consortium (CPIC)
Introduction
Pharmacogenomics is an important component of precision medicine.1 Though various barriers to implementation remain, there are an increasing number of examples demonstrating the utility of pharmacogenomic orientated electronic health record (EHR) informatics, using computerized health records to maintain awareness of pharmacogenomic results to guide drug selection and dosing. Particularly, clinical decision support (CDS) has been identified as a critical tool for the implementation of pharmacogenomics into routine patient care.1–6
The volume, evolving, and enduring nature of pharmacogenomic knowledge that must be applied during a patient encounter presents challenges to implementing pharmacogenomics into routine care. The Clinical Pharmacogenetics Implementation Consortium (CPIC) has published clinical guidelines for 13 genes including therapeutic recommendations for over 30 drugs.7–22 Further, over 120 drugs contain genomic information in their product label.23,24 Complexity will increase as additional guidelines and clinically important pharmacogenomic relationships are discovered, including scenarios where multiple genes influence drug therapy.20,21 Moreover, pharmacogenomic results can have clinical utility throughout a patient’s life. Test results in the distant past could still influence drug selection and dosing years later, and should be used to optimize drug therapy. It may be difficult for clinicians to remember both pertinent gene-drug interactions and any previous pharmacogenomic test results for a specific patient during the demanding workflow of patient care. Fortunately, the increased use of EHRs across health care facilitates provides solutions to these knowledge management challenges.
Even when clinicians have pharmacogenomic results readily available their interpretations can be complex. They may not be interested in the intricate details of a pharmacogenomic result, instead they primarily need evidence based therapeutic recommendations that are consistent with guidelines and health system policies to optimize drug therapy.2 Informatics tools provide a solution since aspects of the interpretation process can be automated and resources are emerging to organize the underlying knowledge required for automation.24,25
It has been suggested that implementing pharmacogenomic CDS presents challenges consistent with other sizable informatics efforts.26 However, EHR vendor support for pharmacogenomics is still emerging and is currently limited, which results in substantial local institutional effort compared to some other types of CDS. This is especially a problem for organizations without substantial genomics expertise or clinical informatics support. Challenges include storing pharmacogenomic data, which is valuable over a patient’s lifetime, presenting recommendations to clinicians in a timely manner that is seamlessly integrated with clinical workflows, and updating CDS recommendations as the knowledge base changes. The purpose of this paper is to describe existing pharmacogenomic informatics models, identify key implementation steps, and discuss emerging resources to facilitate the development of pharmacogenomic clinical decision support in the EHR.
Integrating Pharmacogenomics into the EHR with CDS: Model Practices
There are a growing number of health care systems incorporating pharmacogenomic information into the EHR with CDS. In contrast to other types of CDS where vendors supply databases that form the foundation of the CDS27 (e.g. various databases for drug-drug interactions); pharmacogenomic implementations to date have consisted of customized rule built by the institutions themselves.4,24,26,28–40 These initial pharmacogenomic CDS models illustrate the feasibility of establishing procedures to 1) translate pharmacogenomic data into a predicted phenotype and clinical recommendation, 2) represent these data discretely in the EHR to allow pharmacogenomic knowledge to be presented as both passive (e.g. patient data reports, clinical consult notes) and active CDS (e.g. interruptive alerts that notify clinicians to modify drug therapy).27,28 In addition, through its informatics working group CPIC provides model practices through vendor agnostic implementation resources, including descriptions of clinical workflow and sample CDS text.18,41
Pharmacogenomic information can be presented passively such as through interpretive notes or displaying results in the drug order screen. Pharmacogenomic interpretations entered into the EHR as static notes can provide a summary of test results along with other relevant information. Such notes are particularly useful in selected situations where it is difficult to assign a phenotype (such as when CYP2D6 allele duplications occur).
Active or interruptive CDS alerts are another well-established approach to presenting pharmacogenetic information.28,32,37 In the field of pharmacogenomics, two main types of interruptive alerts should be considered for clinical implementation. Pre-test (no genotype results available at time of prescribing) alerts inform prescribers they are attempting to order a medication affected by pharmacogenomic variation and the associated genotype test is not in the EHR. The alert can provide the clinician with the potential risks associated with prescribing the medicine to a patient with a high-risk phenotype. It can also provide a list of alternative agents to use instead of the high-risk medicine, as well as allow the ordering of the genotype test from the alert window itself.28 Post-test (high-risk genotype) alerts fire when a patient with a high-risk (or actionable) genotype is being prescribed a therapy that needs to be modified based on the patient’s genetics. This type of alert explains to the clinician the potential problems that might occur if the patient were to receive the medication at standard doses and can also provide a list of alternative medications. Incorporating these two types of active decision support CDS into the EHR facilitates prescribing the most appropriate drug at optimal starting doses for individual patients based on their inherited genetic profile thus limiting the occurrence of side effects, optimizing pharmacotherapy and ensures that genotype-guided therapy is utilized when available on every patient.
A systematic process for gathering, evaluating, and translating evidence into clinically useful CDS language and obtaining necessary approvals to implement the CDS can be a significant undertaking. Developing a systematic process with standard procedures is necessary. Across the model implementations to date, most have leveraged the existing institutional infrastructure for medication use and CDS deployment. The health-system’s Pharmacy and Therapeutics (P&T) committee has commonly been used for review of CDS recommendations including the identification of alternative therapies or dosages that complement existing pharmacotherapy and financial policies. P & T oversight may either be direct or by forming a subcommittee specifically focused on pharmacogenomics.3,28,32,33 Additionally, health system informatics integration committees are optimal resources for implementing pharmacogenomic CDS within clinical workflows.
Developing and implementing pharmacogenomic CDS
A successful implementation requires an institution-wide engagement and contributions across multiple disciplines.3 For example, while pharmacists are well positioned to lead the implementation of pharmacogenomics, pharmacists will likely have to facilitate institutional collaboration between clinical informatics and the clinical laboratories to ensure data entered into the EHR are discrete elements that can be queried by CDS rules. Additionally, engaging clinicians, who are most likely to prescribe the target drug, can be helpful to ensure the most desired information is delivered in a CDS alert. For most practice sites, once the alerts are created, their wording is approved by the P&T committee thus providing oversight for which genetic test results are placed in the EHR and which drugs are the subject of CDS alerts.28,33
Based on the work of early adopters and CPIC, 5 key concepts to integrate pharmacogenomics into the EHR have emerged (Table 1) including: 1) documenting pharmacogenomic results in the EHR in a patient centric and time independent manner to facilitate convenient access over a patient’s life, 2) providing a clinical interpretation (e.g., predicted phenotype) of test results and clinical recommendations, 3) entering genetic results and interpretations in discrete EHR fields to facilitate CDS and future retrieval as needed, 4) providing drug-specific pharmacotherapy recommendations based on resulted tests and the clinical interpretation, and 5) deploying CDS to guide the application of pharmacogenomics at the point of prescribing and dispensing. Other organizations with experience in this area have also published considerations for implementing pharmacogenomics in the EHR.32 Broader technical desiderata for the implementation of all types of genomic data into the EHR have also been published and subsequently updated.42,43
Table 1.
Five Key Concepts to Integrating Pharmacogenomics into the EHR
|
An important initial step to developing CDS is determining how test results will be placed in the EHR. Traditionally, genomic results were often reported as scanned documents or PDFs, making it impossible to drive CDS from results that are not represented in a discrete manner. To maximize the potential of pharmacogenomic CDS, test results need to be entered as discrete EHR data fields. This step may require significant collaboration with the clinical laboratories that report the genomic results. However, once achieved, discrete data facilitates the development of decision support rules and customized alerts. Because pharmacogenomic data is useful over a patient’s lifetime, results should be displayed in a time independent manner thus allowing clinicians an easy way to view and access pharmacogenomic results at any time, which is different from the typical approach to presenting the most recent laboratory values. Interpretative reports should also be readily available.
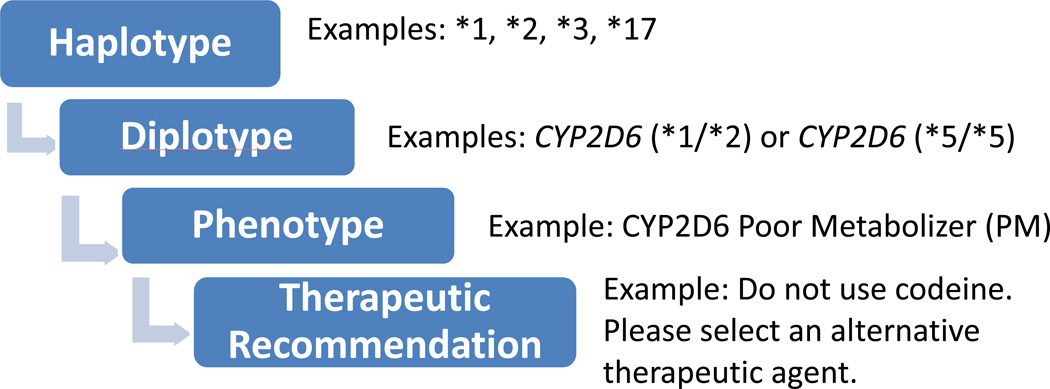
Depending on the practice site and the clinical laboratory involved, the genomic result may be displayed at different levels of interpretation in the EHR. Pharmacogenomic test results may be reported by reference laboratories as genetic variants, haplotypes (i.e., the summary of all genetic variants inherited on a single gene), diplotypes (i.e., summary of genetic variants inherited from both mother and father), presence of genetic variants (e.g., positive or negative), or predicted phenotypes (e.g., poor metabolizer). Figure 1 illustrates the relationship between the different levels of interpretations: alleles, diplotypes, phenotype and therapeutic recommendation. Certain sites have circumvented the need to discretely report the diplotype by adding custom built phenotype terminologies (e.g. CYP2D6 ultra-rapid metabolizer) into a problem list or genomic tab.28 The discrete entry of phenotypes into the EHR can serve as a trigger for interruptive CDS alerts. Some institutions heavily favor the therapeutic recommendation as the main way to display pharmacogenomic results.38 Given the ever changing and evolving nature of genomic data, the informatics team needs to build CDS rules in a way that can be updated and modified when new clinically actionable evidence emerges. Ideally, this process should be fully automated to allow for scalability and sustainability.
Figure 1.
Pharmacogenomics clinical decision support interpretation process.
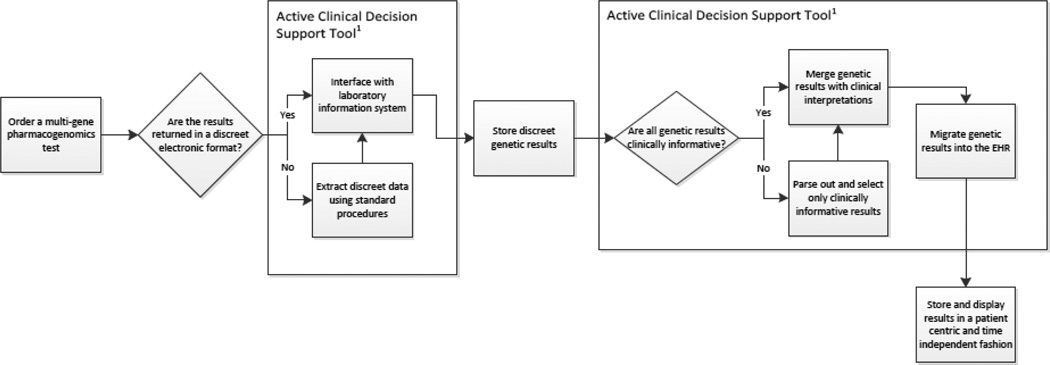
Once the first two steps of capturing discrete results and determining the level of interpretation to display are completed, the next step is to decide where to display the results (Figure 2). There are multiple solutions for this step and a single site might use several approaches. A driving principle behind this decision must be maximizing the clinical value of the genomic result. Because pharmacogenomic data is useful over a patient’s lifetime, results should be displayed in a time independent manner thus allowing clinicians an easy way to view and access the result at any time. The data must be displayed in a location commonly accessed in a provider’s routine workflow. For example a genetic test result might appear in an order entry or verification screen, as well as in a laboratory result section. Interpretative reports should also be readily available; to ensure the potential clinical impact of the result is known by the clinician.
Figure 2.
General overview of steps needed to successfully incorporate pharmacogenomic test results into the electronic health record (EHR) for routine clinical use.
Clinical recommendations to modify drug therapy are based on the patient’s predicted phenotype. Because there is not a standardized process for assigning a predicted phenotype based on genotype results, there may be rare instances where reference laboratory interpretations differ. For those using whole genome/exome sequencing, rare alleles with little associated phenotypic data may be observed. These examples highlight the importance of developing a systematic process of genotype to phenotype assignment to ensure consistent interpretations and associated CDS across the health system. Pharmacogenomic translation tables have been used as a guide to translate genotype data to phenotypes and associated CDS.24 Links to these tables consisting of thousands of genotype to phenotype translations can be found in CPIC guidelines.
Because the variability of pharmacogenomic terms can create confusion, CPIC conducted a consensus process to standardize terms for clinical pharmacogenetic results.44 This process has led to the creation of standardized pharmacogenetic terms for phenotypic and allele functional status terms that will be understood across the pharmacogenetic community.45 Using standard terms will help faciliate sharing data across diverse EHRs, and institutions that have implemented pharmacogenetic testing as well as laboratories that perfom pharmacogenetic analyses are now beginning to use these new standardized terms.
Optimal active CDS, should be gene-drug specific and consider all aspects of the clinical scenario. For example, drug-specific alerts must consider all formulations of a drug. For example, oral dapsone should be avoided in patients who have a G6PD deficiency but topical dapsone may be given to such patients without the occurrence of hemolytic anemia.46 To develop CDS for this gene/drug combination, the ideal design would have to integrate the route of administration of a drug and only interrupt prescribers when oral dapsone is used in patients with G6PD deficiency.
A common concern with creating any new CDS alert is that prescribers will be presented with more interruptive alerts, which could lead to alert fatigue where the clinician might ignore clinically important messages being presented to them.47,48 It is important to involve the prescribing clinicians in the CDS design process to ensure that information displayed is useful for the patient’s care. Given the custom nature of these alerts various opportunities may exist to design highly specific alerts that can limit the potential for alert fatigue. For example, a well-established pharmacogenomic relationship exists between HLA-B and carbamazepine, which is noted in the FDA label as a boxed warning.49 Individuals with the variant allele HLA-B*15:02 who receive carbamazepine are at an increased risk for serious adverse events, including Stevens-Johnson Syndrome and toxic epidermal necrolysis.11 The variant allele is most common in patients with Asian ancestry and this higher frequency is noted in the FDA boxed warning. When designing CDS for this pharmacogenomic relationship, Asian ancestry could be leveraged to improve the alert specificity, such as prompting HLA testing when carbamazepine is ordered but only presenting the alert for patients of Asian descent.50 This however could pose a problem because race and ancestry data may not always be reported in the EHR.51
Another consideration in developing pharmacogenomic CDS is to determine if all aspects of the CDS should be built by the health system or if consultants and additional vendors should be engaged. As published models have illustrated, select hospitals have the informatics infrastructure necessary to develop and maintain pharmacogenomic CDS. However, as the use of pharmacogenomics expands, this option might become harder to sustain. New pharmacogenomic knowledge and evidence is emerging constantly, which may prompt changes in custom CDS, and with time the ongoing maintenance may become overwhelming. Ancillary systems integrated with the health-systems EHR could help minimize the time required to create, store and maintain CDS rules. In addition, software designed specifically for pharmacogenomics may offer additional features and increased flexibility, including patient specific portals to review results and genetic specific CDS rules engines. Also, by executing complex rules outside of the primary EHR, pharmacogenomic CDS does not become detrimental to the performance of the health-system’s primary EHR. Taking this approach does not eliminate all the challenges pharmacogenomic CDS. For example, these systems do not eliminate the complexities that result from the different ways clinical laboratories provide results and organize data in the EHR. Further, if these ancillary systems are not fully integrated with the EHR the process of patient care is slowed since the clinicians will have to access another resource to retrieve the pharmacogenomic information of patients. Additional processes may be needed to make certain clinicians reliably access pharmacogenomic information. Finally, like any additional clinical software the benefits must be weighed against challenges, including additional cost, security, and the development of interfaces to integrate with the EHR.
Resources to support the development of pharmacogenomic CDS
As we have described, individual health systems develop custom, institution-specific pharmacogenomic CDS because database vendors that support other types of CDS in widely used commercial EHRs do not currently provide this content for pharmacogenomic CDS. While standard tools for developing rule based CDS within commercial EHRs have been successfully used, substantial site effort is required at each health system to organize relevant medication and genomic knowledge, define the clinical workflow, and prepare alert text. To reduce the burden on individual sites, vendor agnostic resources are emerging from the CPIC and the Displaying and Integrating Genetic Information Through the EHR Action Collaborative (DIGITizE AC), sponsored by the National Academy of Medicine (formerly the Institute of Medicine-IOM).
Established in 2009, CPIC provides clinical practice guidelines that enable the translation of genetic laboratory test results into actionable prescribing decisions for specific drugs.52 CPIC guidelines closely follow the IOM’s Standards for Developing Trustworthy Clinical Practice guidelines.23 Members have expertise in various aspects of pharmacogenetics, and many are involved in the implementation of clinical pharmacogenomics. CPIC recognized that successful adoption of pharmacogenetics into routine clinical care requires a curated and machine-readable database of pharmacogenetic knowledge suitable for use in an EHR with CDS. In 2013, CPIC formed the CPIC Informatics Working Group to support the adoption of CPIC guidelines into the clinical electronic environment. Starting with HLA-B genotype and abacavir use, a primary strategy has been systematically incorporating a set of implementation resources into all CPIC guidelines that are intended to apply to any EHR.25
By developing comprehensive tables that translate genotype information to phenotype to clinical recommendation for CPIC guidelines, using human readable and structured text, CPIC provides a foundational resource for all EHR implementation steps. The translation tables relate pharmacogene star allele nomenclature, allele function and phenotype, which allow results from multiple formats to be translated into a uniform and manageable data set. The CPIC guideline documents include diplotypes of known functional significance as a supplementary table, and a complete table with all possible diplotypes is posted to PharmGKB, which in one case includes over 700 diplotypes.12 The functional effect on the protein, the representation of gene and drug in standard vocabularies, and clinical workflows incorporating test results are presented. Suggested consultation text for each diplotype prior to prescription and post-prescription is presented, which may be used within CDS alerts. The standardized nomenclature tables help with potential data transfer both inside an EHR and outside to additional resources. The implementation workflows suggest best practices and facilitate a common understanding of the process across the multidisciplinary involved in implementation.
IOM’s DIGITizE effort has provided a forum for EHR vendors, clinical laboratories, and academic centers with experience implementing clinical genomics to collaborate on how genomic data can be consistently represented and integrated in the EHR.53 The group has focused extensively on pharmacogenomics. In 2015, this group published an implementation guide for HLA-B*57:01 and abacavir as well as for TPMT variants for thiopurines. The guide outlines a framework with specific details to implement these drug/gene pairs into the EHR. The guide reviews using standard clinical vocabularies including Logical Observation Identifiers Names and Codes (LOINC®) and Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT®) to represent pharmacogenomic results and provide active CDS.
Future Directions
Although precision medicine is often thought of as a genomics-based model, the concept of precision medicine goes well beyond an individual’s genomic profile to include family history, environmental exposures, lifestyle, and many other factors. To fully embrace the power of precision medicine, genomic knowledge needs to be integrated with other knowledge types to optimize pharmacotherapy. Pharmacists already seek to combine all their patient specific knowledge (e.g. various laboratory values, renal function, liver function, etc.) and CDS provides a framework to integrate and present all relevant patient specific factors to optimize pharmacotherapy. Combining all drug interaction knowledge (e.g., gene-drug, drug-drug) may be an initial step to integrate different knowledge types. Pharmacogenomic knowledge could be integrated with existing commercial knowledge bases already widely used to provide drug-drug interaction alerts in EHRs. Ideally, CDS should provide clinicians single clinical recommendation that considers all drug interactions and patient factors to optimize drug therapy.
EHR genomic infrastructure development efforts have mostly focused on building decision support tools that take into consideration single gene data. As our knowledge of genomics improves, it is clear multiple genes can influence the metabolism of certain drugs and the electronic infrastructure development must move towards more complex decision support algorithms capable of providing a summative clinical recommendation that takes into consideration multiple genetic results as well as other clinical factors. In instances where 2 genes influence prescribing practices, such as CYP2C9/VKORC1-warfarin, decision support triggers often consist of a summative phenotype (e.g., warfarin sensitive) that describes the impact of both genes. However, it is unlikely that a single summative phenotype can adequately represent all combinatorial gene effects in the future. The scenario of one drug being affected by multiple genes is likely to become more common as next generation sequencing becomes the norm for genotyping patients, and decision support rules will need to evolve in order to accommodate this new type of data.54
St. Jude Children’s Research Hospital has implemented CDS alerts using a 2 gene-one drug model using amitriptyline and the phenotypes for CYP2C19 and CYP2D6. Rules were created to build a CDS alert that takes into account all of the possible CYP2D6 and CYP2C19 phenotype combinations and clinical recommendations were incorporated into each possible combinatorial alert. We expect our understanding of how different combinations of variants present in drug transporter, drug target, and metabolizer genes influence pharmacotherapy will grow.
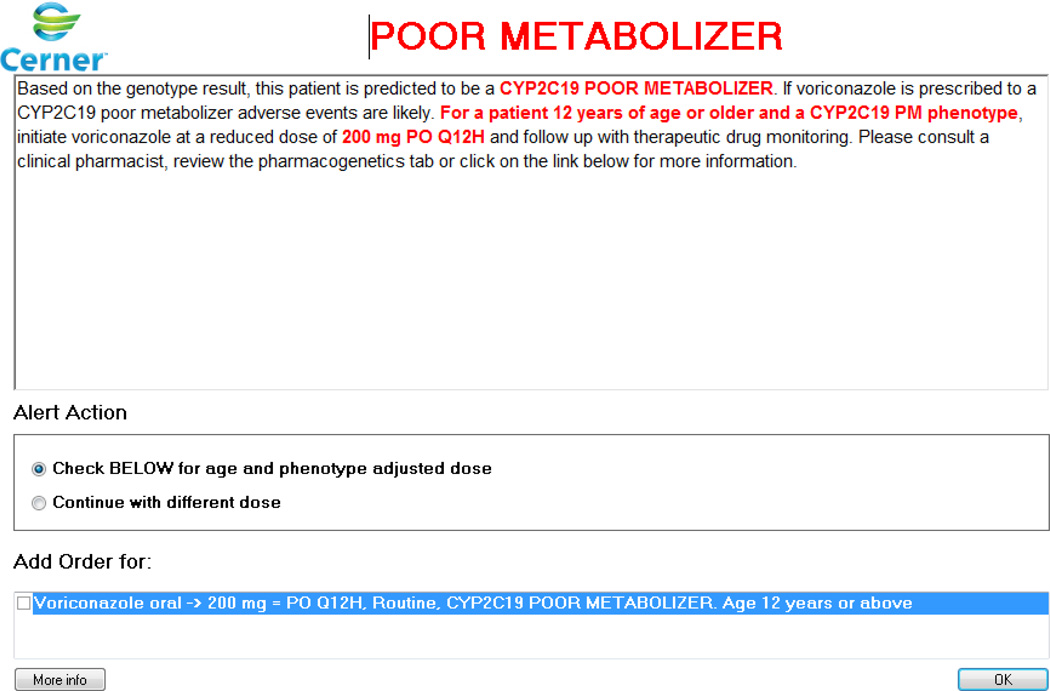
As an example of integrating non-genetic factors into pharmacogenomic CDS rules, recent post-test alerts developed at St. Jude for CYP2C19 and voriconazole take into account a patient’s age and the route of administration of this antifungal agent. When voriconazole is ordered on a patient who is a CYP2C19 poor metabolizer, the rules engine also searches for the patient’s age and the route of administration. The clinician is then presented with a dosing recommendation that takes into account all genetics, age, and route of administration (Figure 3). Because voriconazole therapeutic drug monitoring is used frequently in St. Jude patients, the rules were also customized to be presented to clinicians only with a new voriconazole order prior to therapeutic drug monitoring. The alert also does not fire if voriconazole has been ordered in the past 30 days or if voriconazole serum concentrations were obtained for therapeutic drug monitoring purposes in the month prior to ordering the drug because dosing decisions should be based on the serum concentrations. These additional limits to the alert were an attempt to limit unnecessary alerts as part of our ongoing efforts to understand the risks for alert fatigue in our setting and make corresponding refinements.55,56 The implementation of pharmacogenomic data into the EHR with CDS must also be considered in the broader context of an organization’s overall approach to clinical genomics and the EHR, and the core requirements for genomics in the EHR that are being further defined.42,43
Figure 3.
Example of a pharmacogenomic clinical decision support alert that takes into account non genetic risk factors. In this case, the recommendation displayed to clinicians is age, route and CYP2C19 phenotype specific. Used with Permission of Cerner.
Given the large size of genomic data, it is unlikely these data will be stored in the EHR, and ancillary systems that would be integrated with the EHR have been suggested as a solution.57–59 A common analogy is picture archiving and communication systems (PACS) in radiology where the raw files are outside of the EHR and accessed by experts, but summary information to guide patient care is readily available in the EHR. These ancillary systems may summarize and store pharmacogenomic data at the phenotype level, allele level, or variant level. Pharmacogenomics may serve as an initial use case for health systems working to integrate genomic data into routine clinical care and solving problems likely to be encountered in broader clinical genomic informatics implementation programs. Ideally solutions developed for pharmacogenomic CDS should be capable of incorporating different types of genomic data, and expandable to larger clinical genomic implementation projects, especially considering an organization’s approach to storing and presenting genomic data may change over time.
In conclusion, the EHR with CDS is essential to curate pharmacogenomic data and disseminate patient-specific information at the point of care. As part of the successful implementation of pharmacogenomics into clinical settings, all relevant gene-drug clinical recommendations must be summarized and presented to clinicians in a manner that seamlessly integrates into the clinical workflow of the EHR. In some situations, ancillary systems and applications outside the EHR may be integrated to augment the capabilities of the EHR.
Acknowledgments
C.E.H. and J. M. H. are funded by NIH grant R24GM115264 for CPIC and ALSAC. The authors thank Kelly E. Caudle, PharmD, PhD (CPIC Coordinator) and Donald K. Baker, PharmD, MBA (St. Jude Clinical Decision Support Officer) for their guidance and feedback on this paper.
References
- 1.Johnson JA, Weitzel KW. Advancing pharmacogenomics as a component of precision medicine: How, where and who? Clin Pharmacol Ther. 2016;99:154–156. doi: 10.1002/cpt.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owusu-Obeng A, Weitzel KW, Hatton RC, et al. Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy. 2014;34:1102–1112. doi: 10.1002/phar.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SG. Leading clinical pharmacogenomics implementation: Advancing pharmacy practice. Am J Health-Syst Pharm. 2015;72:1324–1328. doi: 10.2146/ajhp140613. [DOI] [PubMed] [Google Scholar]
- 6.Wilke RA, Xu H, Denny JC, et al. The emerging role of electronic medical records in pharmacogenomics. Clin Pharmacol Ther. 2011;89:379–386. doi: 10.1038/clpt.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gammal RS, Court MH, Haidar CE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin Pharmacol Ther. 2016;99:363–369. doi: 10.1002/cpt.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito Y, Stamp LK, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharmacol Ther. 2015;99:36–37. doi: 10.1002/cpt.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther. 2015;98:127–134. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birdwell KA, Decker B, Barbarino JM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015;98:19–24. doi: 10.1002/cpt.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leckband SG, Kelsoe JR, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Carbamazepine Dosing. Clin Pharmacol Ther. 2013;94:324–328. doi: 10.1038/clpt.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey LB, Johnson SG, Caudle KE, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014;96:423–428. doi: 10.1038/clpt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Relling MV, McDonagh EM, Chang T, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clin Pharmacol Ther. 2014;96:169–174. doi: 10.1038/clpt.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy JP, Johnson SG, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for ivacaftor therapy in the context of CFTR genotype. Clin Pharmacol Ther. 2014;95:592–597. doi: 10.1038/clpt.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95:376–382. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muir AJ, Gong L, Johnson SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon-alpha-based regimens. Clin Pharmacol Ther. 2014;95:141–146. doi: 10.1038/clpt.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin MA, Hoffman JM, Freimuth RR, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin Pharmacol Ther. 2014;95:499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90:625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks JK, Swen JJ, Thorn CF, et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther. 2013;93:402–408. doi: 10.1038/clpt.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clin Pharmacol Ther. 2011;89:662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 23.Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15:209–217. doi: 10.2174/1389200215666140130124910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks JK, Crews KR, Hoffman JM, et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clin Pharmacol Ther. 2012;92:563–566. doi: 10.1038/clpt.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin MA, Hoffman JM, Freimuth RR, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin Pharmacol Ther. 2014;95:499–500. doi: 10.1038/clpt.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herr TM, Bielinski SJ, Bottinger E, et al. Practical considerations in genomic decision support: The eMERGE experience. J Pathol Inform. 2015;6:50. doi: 10.4103/2153-3539.165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troiano D, Jones MA, Smith AH, et al. ASHP Guidelines on the Design of Database-Driven Clinical Decision Support: Strategic Directions for Drug Database and Electronic Health Records Vendors. Am J Health-Syst Pharm. 2015;72:1499–1505. doi: 10.2146/sp150014. [DOI] [PubMed] [Google Scholar]
- 28.Bell GC, Crews KR, Wilkinson MR, et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc. 2014;21:e93–e99. doi: 10.1136/amiajnl-2013-001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012;92:87–95. doi: 10.1038/clpt.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazaridis KN, McAllister TM, Babovic-Vuksanovic D, et al. Implementing individualized medicine into the medical practice. Am J Med Genet C Semin Med Genet. 2014;166C:15–23. doi: 10.1002/ajmg.c.31387. [DOI] [PubMed] [Google Scholar]
- 31.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166C:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson JF, Bowton E, Field JR, et al. Electronic health record design and implementation for pharmacogenomics: a local perspective. Genet. Med. 2013;15:833–841. doi: 10.1038/gim.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C:45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuldiner AR, Relling MV, Peterson JF, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: overcoming challenges of real-world implementation. Clin Pharmacol Ther. 2013;94:207–210. doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95:423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc. 2014;89:25–33. doi: 10.1016/j.mayocp.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldspiel BR, Flegel WA, DiPatrizio G, et al. Integrating pharmacogenetic information and clinical decision support into the electronic health record. J Am Med Inform Assoc. 2014;21:522–528. doi: 10.1136/amiajnl-2013-001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92:446–449. doi: 10.1038/clpt.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health-Syst Pharm. 2011;68:143–150. doi: 10.2146/ajhp100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014;96:482–489. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC) J Am Med Inform Assoc. 2016;23:796–801. doi: 10.1093/jamia/ocw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masys DR, Jarvik GP, Abernethy NF, et al. Technical desiderata for the integration of genomic data into Electronic Health Records. J Biomed Inform. 2012;45:419–422. doi: 10.1016/j.jbi.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch BM, Eilbeck K, Del Fiol G, et al. Technical desiderata for the integration of genomic data with clinical decision support. J Biomed Inform. 2014;51:3–7. doi: 10.1016/j.jbi.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Devine EB, Lee CJ, Overby CL, et al. Usability evaluation of pharmacogenomics clinical decision support aids and clinical knowledge resources in a computerized provider order entry system: a mixed methods approach. Int J Med Inform. 2014;83:473–483. doi: 10.1016/j.ijmedinf.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet. Med. 2016 Jul 21; doi: 10.1038/gim.2016.87. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pamba A, Richardson ND, Carter N, et al. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120:4123–4133. doi: 10.1182/blood-2012-03-416032. [DOI] [PubMed] [Google Scholar]
- 47.Carspecken CW, Sharek PJ, Longhurst C, Pageler NM. A clinical case of electronic health record drug alert fatigue: consequences for patient outcome. Pediatrics. 2013;131:e1970–e1973. doi: 10.1542/peds.2012-3252. [DOI] [PubMed] [Google Scholar]
- 48.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McDaniel RB, Burlison JD, Baker DK, et al. Alert dwell time: introduction of a measure to evaluate interruptive clinical decision support alerts. J Am Med Inform Assoc. 2006;13:138–147. doi: 10.1093/jamia/ocv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sherry ST, Ward MH, Kholodov M, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids. Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman MA, Williams MS. Electronic medical records and personalized medicine. Hum Genet. 2011;130:33–39. doi: 10.1007/s00439-011-0992-y. [DOI] [PubMed] [Google Scholar]
- 51.Liao KP, Cai T, Gainer V, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care. Res. 2010;62:1120–1127. doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DIGITizE. Displaying and Integrating Genetic Information Through the EHR. [(accessed 2016 Jan 12)];2016 http://iom.nationalacademies.org/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/EHR.aspx. [Google Scholar]
- 54.Yang W, Wu G, Broeckel U, et al. Comparison of genome sequencing and clinical genotyping for pharmacogenes. Clin Pharmacol Ther. 2016 Jun 17; doi: 10.1002/cpt.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman JM, Baker DK, Howard SC, et al. Safe and successful implementation of CPOE for chemotherapy at a children's cancer center. J Natl Compr Canc Netw. 2011;9:S36–S50. doi: 10.6004/jnccn.2011.0131. [DOI] [PubMed] [Google Scholar]
- 56.McDaniel RB, Burlison JD, Baker DK, et al. Alert dwell time: introduction of a measure to evaluate interruptive clinical decision support alerts. J Am Med Inform Assoc. 2015 Oct 24; doi: 10.1093/jamia/ocv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starren J, Williams MS, Bottinger EP. Crossing the omic chasm: A time for omic ancillary systems. JAMA. 2013;309:1237–1238. doi: 10.1001/jama.2013.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chute CG, Kohane IS. Genomic medicine, health information technology, and patient care. JAMA. 2013;309:1467–1468. doi: 10.1001/jama.2013.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirts BH, Salama JS, Aronson SJ, et al. CSER and eMERGE: current and potential state of the display of genetic information in the electronic health record. J Am Med Inform Assoc. 2015 Jul 3; doi: 10.1093/jamia/ocv065. [DOI] [PMC free article] [PubMed] [Google Scholar]