Abstract
Background
Herpes simplex virus type 1 (HSV-1) is prevalent worldwide and causes mucocutaneous infections of the oral area. We aimed to define the frequency and anatomic distribution of HSV-1 reactivation in the facial area in persons with a history of oral herpes.
Methods
Eight immunocompetent HSV-1 seropositive adults were evaluated for shedding of HSV-1 from 12 separate oro-facial sites (8 from oral mucosa, 2 from nose, and 2 from conjunctiva) five days a week and from the oral cavity seven days a week for approximately 5 consecutive weeks by a HSV DNA PCR assay. Symptoms and lesions were recorded by participants.
Results
HSV-1 was detected at least from one site on 77 of 291 (26.5%) days. The most frequent site of shedding was the oral mucosa, with widespread shedding throughout the oral cavity. Lesional shedding rate was 36.4% (4 of 11 days with lesions) and the asymptomatic rate was 27.1% (65 of 240 non-lesional days). In individual participants, the median rate of HSV shedding by HSV PCR was 19.7% of days (range 11%-63%).
Conclusions
Reactivation of HSV-1 on the oral mucosa is common and usually asymptomatic. However, HSV-1 is rarely found in tears and nasal mucosa. Frequent oral shedding of HSV-1 may increase the risk for transmitting the virus to both oral and genital mucosa of sexual partners.
Keywords: Cold sore(s), Herpes Labialis, Herpes Simplex-1 (HSV-1), HSV
Introduction
Herpes simplex virus type 1 (HSV-1) is a common mucocutaneous infection of the mouth. Most infections are mild, but life-threatening complications such as adult encephalitis or neonatal infection can occur. Seroprevalence of HSV-1 infection among 0-49 year olds is high, estimated at 67% (3.7 billion individuals) with almost universal infection in many parts of the world. The incidence of HSV-1 infection in 2012 was estimated at 118 million, making it an important global public health issue1.
Transmission of HSV-1 to susceptible persons can occur during direct contact with lesions, or with infected oral or genital secretions during asymptomatic shedding. While historically HSV-1 was usually acquired in early childhood, the seroprevalence among 14-19 year olds in the United States has declined significantly by 23%, from 39% to 30%, during the past decade2. Furthermore, HSV-1 has become an increasing cause of first episode genital herpes in developed countries3,4. An estimated 140 million people ages 15-49 years old are calculated to have prevalent genital HSV-1 infection1. Few studies detail the anatomic location of viral reactivation in people with HSV-1. Estimates on the frequency of HSV-1 shedding in immunocompetent individuals range widely depending on the assay used for viral detection and frequency of sampling. In this study, we detail the anatomic distribution of HSV-1 reactivation in a cohort of 8 immunocompetent persons with a history of symptomatic recurrent herpes labialis (RHL) both by a sensitive and specific HSV polymerase chain reaction (PCR) assay and viral culture.
Methods
Study population and data collection
Healthy immunocompetent HSV-1 seropositive, HIV-1 seronegative men and women with a history of symptomatic oral herpes were recruited at the University of Washington Virology Research Clinic in Seattle. All participants had standardized demographic and clinical information obtained and underwent a detailed examination by an experienced clinician 5 days a week for approximately 5 consecutive weeks. None of the participants had a history of herpetic eye disease or were taking antiviral therapy during the study. Antivirals were stopped at least a week prior to study start. At each clinic visit, samples were obtained from 12 separate oro-facial sites. Samples from 10 sites used Dacron swabs: left nares, right nares, pharynx, tongue, left palate, right palate, left upper lip, right upper lip, left lower lip and right lower lip. Samples of tears were obtained using sno-strips (Bausch & Lomb, Rochester, NY, USA) placed on conjunctiva. Each site was sampled with 2 swabs: one was placed in viral culture media and one in 1-mL PCR tubes for laboratory processing5. Care was taken to avoid contamination from other anatomic areas during sampling. Lesions were recorded on oral cavity diagrams.
Participants also obtained swabs of buccal and gingival surfaces of the mouth in the morning while at home, prior to showering or brushing their teeth 7 days a week for approximately 5 consecutive weeks and brought the swabs to clinic at the next visit. Each participant maintained a diary of oral symptoms and lesions.
Laboratory methods
HSV western blot was used to detect antibodies to HSV-1 and HSV-26. HSV DNA was detected using a real time fluorescent probe based PCR assay (Taqman; Applied Biosystems, Foster City, CA, USA)7. Samples were positive for HSV if ≥150 copies (c) of HSV DNA per mL of transport media was detected8. Specimens for viral culture were inoculated onto tissue culture. All isolates were defined with monoclonal antibodies as described previously9.
Definitions and Statistical Methods
Viral shedding was defined as detection of HSV from orofacial sites. Shedding rates were computed as proportion of days with detectable virus. Participants noted if they had prodromal symptoms during the study period, defined as itching, tingling or burning sensation on the lips without evidence of herpetic lesions. Viral shedding was categorized as either lesional (with lesions) or asymptomatic (without lesions).
To compare PCR and culture shedding rates, we used Poisson regression with a scale parameter to address overdispersion. For 3 persons who did not shed by culture, their shedding rate was assigned 0.5/n, where n was the number of samples obtained in order to assign a finite value to log(0).
Results
Eight persons with a history of cold sores were recruited. The median age was 37 (range 24-43); 6 participants were women. Of the 8 participants, 6 were seropositive for HSV-1 only, and 2 were both HSV-1 and HSV-2 seropositive. The median time since HSV-1 acquisition was 21 years (range 1-30) with a median of 6 recurrences (range 2-16) in the last year. Participants obtained swabs on a median of 36 days (range 32-42). Participant 1 did not provide a symptom diary. Participant 2 found conjunctival sampling uncomfortable so tears were collected on 2 of 40 study days.
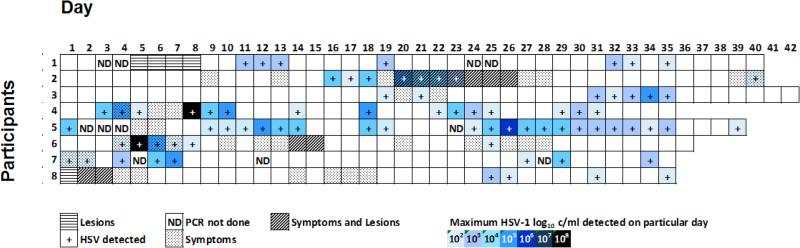
Overall, 2626 swabs for HSV DNA were collected on 291 days with a median of 334 swabs (range 283-360) per person. All 8 participants had HSV DNA detected at least once during the study period. HSV was detected by PCR on 77 of 291 (26.5%) days. Lesional shedding rate was 36.4% (4 of 11 days with lesions) and the asymptomatic rate was 27.1% (65 of 240 non-lesional days). Within the 240 non-lesional days, 34 involved prodromal symptoms with a calculated shedding rate of 23.5% (8 of 34 days). Considering only positive days, of the 69 days with shedding detected by PCR and valid diaries, 4 (5.8%) were collected on days on which participants had lesions and 65 (94.2%) were during asymptomatic periods (Figure 1).
Figure 1.
Maximum HSV DNA (log10 c/ml) detected per day, with presence or absence of lesions and symptoms for each participant. Participant 1 did not provide a diary.
There were 2622 swabs collected for viral culture on 290 days. HSV was isolated in culture on 22 of 290 (7.6%) days. Of the 22 days with viral shedding isolated by culture, 2 (9.1%) were positive when participants had lesions and 20 (90.9%) were collected when persons were asymptomatic.
With regards to swab-specific rates, HSV was detected by PCR in 271 of 2626 (10.3%) swabs. Of swabs with HSV detected by PCR, 23 (8.5%) were collected on days with lesions and 248 (91.5%) were collected on days when participants were asymptomatic. Of those non-lesion days, 44 swabs were collected on days with prodromal symptoms.
The median log10 c/ml of HSV DNA was 3.7 (range 2.2-8.3) for swabs with HSV DNA. On days without lesions, the median log10 c/ml of DNA detected was the same at 3.7 (range 2.2-8.3). The log10 c/ml for culture positive, PCR positive swabs was 4.2 (range 2.2-8.8) and the log10 c/ml for swabs that were culture negative but PCR positive was 3.2 (range 2.2-5.9).
The rate of HSV DNA found by PCR varied widely between individual participants, with the median rate of detection of 19.7% of days (range 11-63%) and 6.1% of swabs (range 2-30%). In contrast, the median rate of HSV isolated by culture was 5.1% of days (range 0-31%) per person. Viral shedding detected by PCR clustered together on consecutive days with the median duration of shedding episodes of 3.25 days (range 1-12).
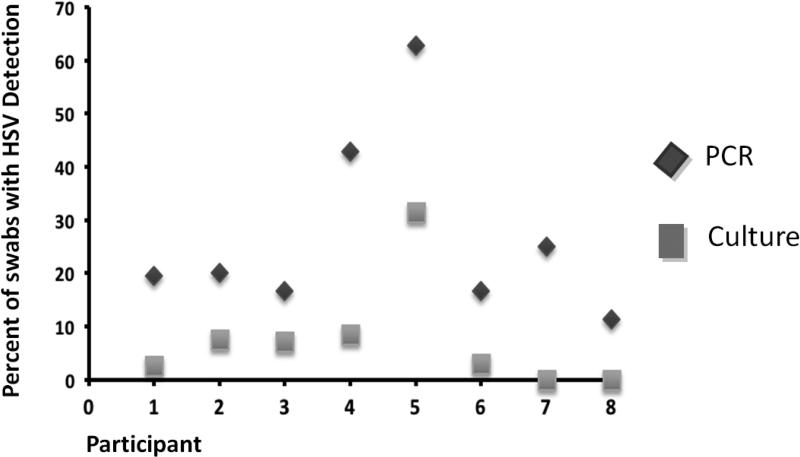
Of the swabs that had both culture and PCR data (2622 swabs), there were 269 (10.1%) swabs that were PCR positive and 79 (3.0%) swabs that were culture positive. Of these 269 PCR positive swabs, 73 (27.1%) swabs were also culture positive (Figure 2). All but 6 swabs (92.4%) that had HSV isolated by culture also had HSV DNA detected by PCR. The participants were 3.3 times as likely to have HSV DNA detected by PCR as by culture (95% CI 1.8, 6.1, p<0.0001).
Figure 2.
Rate of HSV detection by PCR and viral culture in 8 participants with history of oral herpes. Of 269 PCR positive swabs, 73 (27.1%) swabs were both PCR and culture positive.
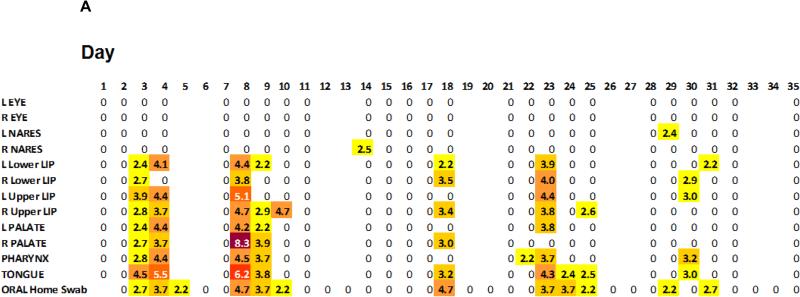
We observed distinct anatomic HSV-1 shedding patterns. The oral area was the most frequent site of HSV reactivation, occurring in all 8 participants. The mouth was positive on 76 (26.1%) days by PCR. Of 271 swabs that detected HSV by PCR, 256 (94.5%) were from the oral area (Table 1). We had detailed anatomical distribution of viral shedding on swabs from the oral region on 53 days. When HSV was detected in the mouth, all oral swabs were positive on 10 (18.9%) days, some swabs on 26 (49.1%) days and only a single oral swab on 17 (32.1%) days (Figure 3A). Out of 26 shedding episodes, 23 were identified in the mouth. Of these 23 oral episodes, 17 were bilateral and 6 were unilateral (3 on the right and 3 on the left). Shedding at a single oral site occurred in 2 out of 26 episodes while the other 24 episodes included shedding at multiple sites in the oral area.
Table 1.
Detection of HSV-1 by PCR at different facial sites
| Facial Site | Rate of HSV DNA detected n/n (%) swabs |
|---|---|
| Eye | 3/354 (0.85) |
| L eye | 3/177 (1.7) |
| R eye | 0/177 (0) |
| Nares | 12/398 (3.0) |
| L Nares | 5/199 (2.5) |
| R Nares | 7/199 (3.5) |
| Oral | 256/1874 (13.7) |
| L Lower Lip | 23/199 (11.6) |
| R Lower Lip | 24/199 (12.1) |
| L Upper Lip | 20/198 (10.1) |
| R Upper Lip | 32/199 (16.1) |
| L Palate | 22/199 (11.1) |
| R Palate | 21/199 (10.6) |
| Pharynx | 28/199 (14.1) |
| Tongue | 32/198 (16.2) |
| Oral home swab | 54/284 (19.0) |
Figure 3A.
Illustrative single participant depicting widespread shedding throughout the oral mucosa on days with viral reactivation. The number indicates the maximum HSV DNA (log10 c/ml) detected at each facial site. Blank spaces indicated days without sampling.
All 7 participants with diary results available experienced a viral prodrome of tingling, burning or redness during the study period. The number of diary days differed between participants, therefore the median proportion of days with symptoms per person was calculated at 9% (range 5-31). Excluding observations where either diary results, clinician assessment or HSV positive results were missing, prodromal symptoms were present on 34 out of 251 days. HSV was detected from any facial site on 8 out of 34 (24%) days when prodromal symptoms were present.
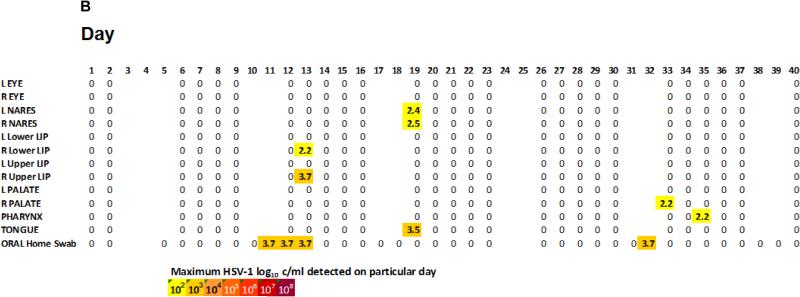
In contrast to the high rate of shedding from the mouth, HSV DNA was detected from tear samples in 3 (0.85%) swabs and nares in 12 (3%) swabs (Table 1). The 3 HSV DNA positive samples from the tears were low level (2.3-3.8 log10 c/ml), and all occurred in one eye from 2 different participants. Swabs from the mouth were also positive on those days. Of 6 participants who had positive HSV DNA from the nares, all but one participant had a positive oral swab on those days (Figure 3B). This participant had low HSV DNA copy level at 2.54 log10 c/ml on that day. The HSV DNA copy levels from the rest of the participants with HSV detected from the nares ranged from 2.39 to 3.62 log10 c/ml.
Figure 3B.
Illustrative single participant depicting several single site reactivation of HSV.
Four participants experienced clinical recurrence of oral herpes with 4 lesional episodes for a total of 16 days. The duration of each episode varied between 2 and 7 days. Of the 16 days with lesions, HSV was detected by PCR on 4 days and isolated in culture on 2 days from one herpetic episode in one participant. This particular episode lasted 7 days. Of the other 3 episodes, HSV DNA was not detected or isolated from culture.
Discussion
Our intensive study to characterize the patterns of oral HSV-1 shedding demonstrated widespread viral reactivation throughout the oral mucosa but little shedding from nasal and conjunctival surfaces. All 8 participants in our study shed virus at some point during the study period at an overall rate of 26.5% of days. We found that most HSV shedding occurred on the lips and in the oral cavity and was not associated with lesions or prodrome.
Our observation that oral HSV-1 reactivation is common is consistent with other studies in the literature. However, a limiting factor in past analysis of oral HSV-1 shedding has been inconsistency in the frequency and duration of sampling with many studies obtaining samples at only one time or irregular intervals. A review by Miller and Danaher10 to evaluate asymptomatic shedding of oral HSV-1 pooled results of multiple studies and found the viral shedding rate in each study was quite variable, ranging from 1.3-33% of swabs11-14, and depended on the frequency of sampling, time course of monitoring, and the diagnostic assay used. HSV-1 shedding was highest when samples were taken at least weekly, for at least 3 weeks and used PCR based assays15. Studies that meet these requirements are limited in the literature with only 4 studies that examined shedding on a daily basis16-19, only 1 of which evaluated participants for as long as 60 days.
The spatial patterns of oral HSV shedding we observed in this study are similar to widespread genital reactivation found in HSV-2 disease detailed by Johnston et al20. These shedding patterns may reflect reactivation in multiple neurons at once or could be due to contamination by saliva from a different site. While immunologic evaluation was not performed in the present study, Johnston et al. found a localized HSV-specific T cell infiltrate in association with HSV-2 shedding in the genital area, offering evidence that shedding patterns may not be an artifact of contamination. Our findings are consistent with one previous study showing HSV-1 shedding at multiple intraoral sites. Da Sliva et al.21 swabbed different areas in the oral cavity of participants periodically between 8 and 11.5 months and found the frequency of HSV-1 DNA was 29.5% on the buccal mucosa, 30.7% on the lower lip and 33.1% on the dorsum of the tongue. However, is not clear how often a positive swab in one part of the oral cavity resulted in HSV-1 DNA found at other oral sites simultaneously in that study. A pattern of diffuse reactivation could reflect a loss of control of latency at multiple neurons or failure in immunological control in the local epithelium. We have shown that HSV-2 reactivation in the genital track involves more than the area with recurrent lesions20, and our findings suggest a similar phenomenon occurs during oral HSV-1 reactivation.
Frequent shedding of HSV-1 in the oral cavity is an important public health concern given the potential for transmission to uninfected individuals. HSV-1 is an increasing cause of genital herpes in the developed world2,22, leading to a higher proportion of neonatal HSV cases caused by HSV-123,24. Neonatal HSV infection can also occur as a consequence of transmission of virus from oral secretions to infant during asymptomatic shedding23. The current assumption is that most genital HSV-1 is acquired from exposure to the source partner's oral secretions via oro-genital contact as receptive oral sex is a risk factor for first episode genital HSV-1 infection25,26.
Widespread shedding in other sites sampled, was not observed despite these regions also being innervated by the trigeminal ganglion. The rate of shedding was much lower in these regions (0.85% eyes, 3% nares) than the oral mucosa. The lower rates of reactivation are similar to several previous studies that evaluate HSV-1 in tears of asymptomatic individuals. For example, Kaye et al.27 found no shedding and Okinaga et al.14 found 0.05% shedding in the ocular area using culture based assays. In contrast to these low rates of HSV-1 reactivation, Kaufman et al.18 found 33% of eye swabs positive for HSV-1 in the tears of healthy adults. It is surprising that patients who were seronegative for HSV also demonstrated HSV-1 reactivation in the Kaufman study. Evaluation of HSV-1 shedding in the nares is limited in the literature and our study is the first to estimate the frequency of HSV-1 reactivation in the nasal mucosa of asymptomatic individuals using a PCR assay. Our results suggest that tears or the nasal mucosa are not major sites of HSV shedding and therefore unlikely to play a major role in transmission of the virus.
Strengths of our study include a PCR-based assay to detect viral shedding, excellent adherence to study visits and careful sampling of 12 facial regions 5 times a week with additional oral sampling 7 days a week. We followed participants for approximately 5 weeks to include episodes of reactivation that might be missed when swabbing at one time point. Limitations of our study include inability to capture episodes shorter than 24 hours. However, when oral swabs were collected four times a day in a study by Mark et al.19, the majority of reactivation episodes are detected with daily sampling. We chose a sample of persons with a history of oral herpes. It is possible that HSV-1 seropositive persons without a history of cold sores may have lower shedding rates, in parallel to what has been observed in HSV-2 infection28. However, even among persons with a history of clinically evident disease, we observed the bulk of viral reactivation was not accompanied by observable lesions or symptoms. We observed a low rate of HSV detected from lesional episodes. The low rate of HSV detection during the days with lesions could reflect either non-herpetic etiology of the lesion, or very rapid cessation of viral shedding in oral herpes, as has been noted by Spruance et al.29
Clinically, our study has implications for counseling of patients with cold sores who should be informed that shedding of oral HSV-1 is not confined to the site or time of cold sores but usually occurs without visible lesions or prodromal symptoms, and has the potential to transmit to the genital area of sexual partners. It raises the question whether disclosure of oral herpes to sexual partners should be advocated, in parallel to the counseling guidelines for genital HSV infection30. Further studies are needed to address widespread reactivation of HSV-1 in the oral cavity with transmission to a partner in the genital area or to neonates postnatally with the potential of causing disseminated disease.
Short Summary.
A study of HSV-1 shedding from 12 oro-facial sites daily for approximately 5 weeks using PCR found reactivation of HSV-1 throughout the oral mucosa is common and usually asymptomatic. Viral shedding in tears and the nasal mucosa is rare.
Acknowledgments
Source of Funding: T32 AI007044-38 (MR); P01 AI030731 (AW, SS, MLH, CJ, LC); K24 AI071113 (AW).
Footnotes
Conflict of interest: Dr. Anna Wald received research grants from Agenus, Gilead, Genocea and Vical and is a consultant for Aicuris, Amgen and GSK. Dr. Lawrence Corey holds stock in Immune Design and is a co-inventor on several patents associated with the development of an HSV-2 vaccine. Dr. Christine Johnston has a research contract with Sanofi and has been a co-investigator on research contracts with Agenus, Genocea and Vical. Dr. Amalia Magaret is a consultant for Aicuris and Immune Design. For the remaining authors, none were declared.
References
- 1.Looker KJ, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PloS one. 2015;10:e0140765. doi: 10.1371/journal.pone.0140765. doi:10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999-2010. The Journal of infectious diseases. 2014;209:325–333. doi: 10.1093/infdis/jit458. doi:10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 3.Tuokko H, Bloigu R, Hukkanen V. Herpes simplex virus type 1 genital herpes in young women: current trend in Northern Finland. Sexually transmitted infections. 2014;90:160. doi: 10.1136/sextrans-2013-051453. doi:10.1136/sextrans-2013-051453. [DOI] [PubMed] [Google Scholar]
- 4.Coyle PV, et al. Emergence of herpes simplex type 1 as the main cause of recurrent genital ulcerative disease in women in Northern Ireland. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2003;27:22–29. doi: 10.1016/s1386-6532(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 5.Mark KE, et al. Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. Journal of acquired immune deficiency syndromes. 2010;54:482–488. doi: 10.1097/QAI.0b013e3181d91322. doi:10.1097/QAI.0b013e3181d91322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. Journal of clinical microbiology. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. Journal of clinical microbiology. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magaret AS, Wald A, Huang ML, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. Journal of clinical microbiology. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. doi:10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langenberg A, Zbanyszek R, Dragavon J, Ashley R, Corey L. Comparison of diploid fibroblast and rabbit kidney tissue cultures and a diploid fibroblast microtiter plate system for the isolation of herpes simplex virus. Journal of clinical microbiology. 1988;26:1772–1774. doi: 10.1128/jcm.26.9.1772-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2008;105:43–50. doi: 10.1016/j.tripleo.2007.06.011. doi:10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Spruance SL. Pathogenesis of herpes simplex labialis: excretion of virus in the oral cavity. Journal of clinical microbiology. 1984;19:675–679. doi: 10.1128/jcm.19.5.675-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman HE, Brown DC, Ellison EM. Recurrent herpes in the rabbit and man. Science (New York, N.Y.) 1967;156:1628–1629. doi: 10.1126/science.156.3782.1628. [DOI] [PubMed] [Google Scholar]
- 13.Kameyama T, Sujaku C, Yamamoto S, Hwang CB, Shillitoe EJ. Shedding of herpes simplex virus type 1 into saliva. Journal of oral pathology. 1988;17:478–481. doi: 10.1111/j.1600-0714.1988.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 14.Okinaga S. Shedding of herpes simplex virus type 1 into tears and saliva in healthy Japanese adults. The Kurume medical journal. 2000;47:273–277. doi: 10.2739/kurumemedj.47.273. [DOI] [PubMed] [Google Scholar]
- 15.Scott DA, Coulter WA, Biagioni PA, O'Neill HO, Lamey PJ. Detection of herpes simplex virus type 1 shedding in the oral cavity by polymerase chain reaction and enzyme-linked immunosorbent assay at the prodromal stage of recrudescent herpes labialis. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1997;26:305–309. doi: 10.1111/j.1600-0714.1997.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert SC. Oral shedding of herpes simplex virus type 1 in immunocompetent persons. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2006;35:548–553. doi: 10.1111/j.1600-0714.2006.00461.x. doi:10.1111/j.1600-0714.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 17.Liljeqvist JA, Tunback P, Norberg P. Asymptomatically shed recombinant herpes simplex virus type 1 strains detected in saliva. The Journal of general virology. 2009;90:559–566. doi: 10.1099/vir.0.007070-0. doi:10.1099/vir.0.007070-0. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman HE, et al. HSV-1 DNA in tears and saliva of normal adults. Investigative ophthalmology & visual science. 2005;46:241–247. doi: 10.1167/iovs.04-0614. doi:10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. The Journal of infectious diseases. 2008;198:1141–1149. doi: 10.1086/591913. doi:10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston C, et al. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. Journal of virology. 2014;88:4921–4931. doi: 10.1128/JVI.03285-13. doi:10.1128/jvi.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva LM, Guimaraes AL, Victoria JM, Gomes CC, Gomez RS. Herpes simplex virus type 1 shedding in the oral cavity of seropositive patients. Oral diseases. 2005;11:13–16. doi: 10.1111/j.1601-0825.2004.01058.x. doi:10.1111/j.1601-0825.2004.01058.x. [DOI] [PubMed] [Google Scholar]
- 22.Vyse AJ, et al. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sexually transmitted infections. 2000;76:183–187. doi: 10.1136/sti.76.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kropp RY, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117:1955–1962. doi: 10.1542/peds.2005-1778. doi:10.1542/peds.2005-1778. [DOI] [PubMed] [Google Scholar]
- 24.Brown ZA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA : the journal of the American Medical Association. 2003;289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 25.Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. The Journal of infectious diseases. 2000;181:1454–1457. doi: 10.1086/315395. doi:10.1086/315395. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwenhuis RF, van Doornum GJ, Mulder PG, Neumann HA, van der Meijden WI. Importance of herpes simplex virus type-1 (HSV-1) in primary genital herpes. Acta dermatovenereologica. 2006;86:129–134. doi: 10.2340/00015555-0029. doi:10.2340/00015555-0029. [DOI] [PubMed] [Google Scholar]
- 27.Kaye SB, et al. Ocular shedding of herpes simplex virus. The British journal of ophthalmology. 1990;74:114–116. doi: 10.1136/bjo.74.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tronstein E, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA : the journal of the American Medical Association. 2011;305:1441–1449. doi: 10.1001/jama.2011.420. doi:10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spruance SL, et al. The natural history of recurrent herpes simplex labialis: implications for antiviral therapy. The New England journal of medicine. 1977;297:69–75. doi: 10.1056/NEJM197707142970201. doi:10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-3):1–137. [PMC free article] [PubMed] [Google Scholar]