Abstract
Purpose
Tyrosinase-related protein-1 (TYRP1) is a transmembrane glycoprotein that is specifically expressed in melanocytes and melanoma cells. Preclinical data suggest that monoclonal antibodies targeting TYRP1 confer anti-melanoma activity. IMC-20D7S is a recombinant human IgG1 mAb targeting TYPR1. Here we report the first-in-human phase 1/1b trial of IMC-20D7S.
Experimental Design
The primary objective of this study was to establish the safety profile and the maximum tolerated dose (MTD) of IMC-20D7S. Patients with advanced melanoma who progressed after or during at least one line of treatment or for whom standard therapy was not indicated enrolled in this standard 3 + 3 dose escalation, open-label study. IMC-20D7S was administered intravenously every 2 or 3 weeks.
Results
Twenty seven patients were enrolled. The most common adverse events were fatigue and constipation experienced by 9 (33%) and 8 (30%) patients respectively. There were no serious adverse events related to treatment, no discontinuations of treatment due to adverse events, and no treatment related deaths. Given the absence of dose-limiting toxicities, an MTD was not defined but a provisional MTD was established at the 20-mg/kg q2w dose based on serum concentration and safety data. One patient experienced a complete response (CR). A disease control rate, defined as stable disease or better, of 41% was observed.
Conclusion
IMC-20D7S is well tolerated among patients with advanced melanoma with evidence of anti-tumor activity. Further investigation of this agent as monotherapy in selected patients or as part of combination regimens is warranted.
Keywords: Melanoma, monoclonal antibody, ADCC, ADCP, immunotherapy
Introduction
The incidence of melanoma in the United States has increased over the last three decades, with an estimated 76,100 new cases diagnosed in 2014(1). Historically, treatment of unresectable melanoma has been challenging as cytotoxic chemotherapy has failed to improve overall survival in this patient population. More recently, immunotherapy (2,3) and small molecule inhibitors targeting BRAF and MEK (4,5) have been shown to improve outcomes among patients with advanced melanoma. Nevertheless, many patients will either be refractory to such treatment or ultimately develop resistance to therapy and succumb to their disease. There remains a need to develop efficacious treatment options for this group of patients.
TYRP1 is a transmembrane glycoprotein involved in melanin biosynthesis that is specifically expressed in melanocytes(6). Following protein translation, TYRP1 is trafficked from the endoplasmic reticulum through the Golgi apparatus to melanosomes; it is subsequently transferred to the melanocyte cell surface upon membrane fusion(7). TYRP1 is highly expressed in melanocytes and melanoma cells (8), and its expression is generally stable throughout melanoma progression(9). Given its expression pattern, TYRP1 is a promising and potentially safe therapeutic target for melanoma patients.
The ability of therapeutic IgG1 monoclonal antibodies (mAbs) to induce antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) on target cells has led to the successful development of multiple mAbs now in clinical use (10). Of note, successful targeting of cell surface proteins that appear to be uninvolved in growth signaling (e.g., CD20 in B-cell lymphomas) highlights the importance of ADCC and CDC, as opposed to the inhibition of signaling pathways, in the anti-cancer activity of some therapeutic mAbs (11).
IMC-20D7S is a recombinant human IgG1 mAb against TYRP1. Development of this clinical antibody is based on preclinical data showing that TA99, a murine IgG2a anti-TYRP1 mAb, localizes to subcutaneous melanoma xenografts (12), and inhibits syngeneic tumor growth in preclinical models (13). The antitumor effect was dependent on the intact antibody (7), the presence of Fc receptor(14), and natural killer (NK) cells (13) highlighting the importance of NK-mediated ADCC for this mAb.
Given the preclinical activity of TYRP1-directed mAb therapy, we conducted a phase 1/1b of IMC-20D7S in patients with advanced melanoma. The primary objective of this study was to assess the safety of IMC-20D7S and establish a maximum tolerated dose (MTD). Secondary objectives were to describe the pharmacokinetic profile of IMC-20D7S, to recommend doses for subsequent clinical trials, to evaluate the immunogenicity of IMC-20D7S, and to assess progression free survival (PFS).
Materials and Methods
Patient population
All enrolled patients were at least 18 years of age and had confirmed, previously treated unresectable stage III or IV melanoma with measurable disease as per the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Patients who progressed after or during at least one line of treatment or for whom standard therapy was not indicated were enrolled. Other inclusion criteria included a life expectancy of at least three months, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better and adequate hematologic, renal, and hepatic function. Key exclusion criteria included ongoing grade 2 or worse side effects from prior radiation or chemotherapy, symptomatic brain or leptomeningeal disease, and ongoing immunosuppressive therapy, including steroid use. Patients were enrolled at three academic centers, and the protocol was approved by the institutional review boards of the respective participating institutions. All patients provided written informed consent.
Study Design and Treatment
This was an open-label, dose-escalation phase 1/1b study. IMC-20D7S injection for intravenous infusion was provided by Eli Lilly and Company. An initial dose of 5 mg/kg, administered over 60 minutes, was selected based on preclinical toxicology studies. This clinical study consisted of evaluating escalating doses of IMC-20D7S in two different schedules: an every 2 week schedule (Arm A) with a cycle composing 4 weeks and an every 3 week schedule (Arm B) with a cycle composing 6 weeks. After starting treatment for the first patient in the initial cohort (1A), a minimum of 7 days observation period elapsed until the next patient started treatment within this cohort. No waiting period was mandated in other cohorts, and no intrapatient dose escalation was permitted.
This study was performed with a 3 + 3 dose escalation study design. Within Arm A, planned dosing levels in the absence of dose limiting toxicities (DLT) were 5, 10, 20, and 30 mg/kg. Arm B (q 3 week dosing) was opened after the cohort receiving 10mg/kg every 2 weeks was completed without any safety concerns. Planned dosing levels in Arm B were 10, 20, and 30 mg/kg. Patients were enrolled into both Arm A and Arm B in parallel. Cumulative DLTs across all dose levels in both arms were assessed on an ongoing basis, but dose escalation within each arm proceeded independently. Following completion of Arms A and B escalating-dose cohorts, a provisional MTD was to be defined. An expanded cohort was to be formed at the dose level defined at the MTD. At least six patients in total were to be treated at this dose level.
Patients in the escalating-dose cohorts were able to continue to receive IMC-20D7S in the absence of treatment failure, treatment intolerance, or consent withdrawal. Radiographic assessment of tumor response in both study arms was scheduled for every six weeks and evaluated as per RECIST v1.1. Additional imaging was performed if clinically indicated.
Tolerability and Safety
The incidence and severity of adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4.02. Treatment emergent adverse events (TEAEs) were defined as events that occurred or worsened after the first dose of study drug. Serious adverse events (SAEs) were defined as any untoward medical occurrence, at any dose, that was life threatening, resulted in death, significant incapacity, or congenital anomaly; or that required (or extended) hospitalization, intervention to prevent permanent impairment, or intervention to prevent one of the other listed serious outcomes. Dose limiting toxicities (DLTs) were defined as any Grade 3 or above toxicity that emerged during study treatment and was clearly not attributable to melanoma or co-medication and was possibly, probably, or definitely related to IMC-20D7S in the judgment of the investigator. If a patient experienced a DLT, the patient would not receive further IMC-20D7S.
Pharmacokinetics and biomarker studies
In Arm A, serial blood samples were collected prior to infusion and up to 2 weeks (336 hours) following the first (Cycle 1 Day 1) and fifth infusions (Cycle 3 Day 1). In Arm B, blood samples were collected prior to and up to 3 weeks (504 hours) following the first infusion (Cycle 1 Day 1), and up to two weeks following the fifth infusion (Cycle 3 Day 1). Two blood samples (pre- and 1 hour post end of infusion) were collected for the first infusion of cycles 2, 4, and subsequent cycles. Serum concentrations of IMC-20D7S were quantified using a non-validated enzyme-linked immunosorbent assay (ELISA) using human gp75 protein as the capture antigen and peroxidase-conjugated anti-human IgG Fcγ as the detector antibody. IMC-20D7S concentrations were derived using Softmax Pro software from a four-parameter logistic regression line taken off the standard curve. Serum concentration data were analyzed by standard non-compartmental analysis (NCA) using Phoenix WinNonlin 6.3.
A blood sample was also collected prior to study treatment to assess Fc-receptor polymorphism status. We used a linear regression model, adjusted for dosage, gender, and age, to ask whether polymorphisms correlated with treatment response or duration of treatment.
Statistical analysis
Efficacy and safety analyses were planned to be descriptive. The safety and efficacy population consisted of all patients exposed to any amount of study drug. Median PFS was determined by the Kaplan-Meier method. They were performed using SAS Version 9.2. Data from patients in the expanded cohort were included with those of patients initially treated at the same dose in the escalating-dose cohort. With regard to Fc-receptor polymorphism studies, we used linear regression models to associate duration of treatment with candidate single nucleotide polymorphisms (SNPs) with adjustment for covariates (age, gender, and treatment arms). Such analyses were done using R 3.0.2 software. Significance was defined as p < 0.05 for specific polymorphisms.
Results
Patient Population and Treatment
This study enrolled 27 patients between June 29, 2010 and August 20, 2012 of which 16 were men and 11 were women. Age ranged from 44 to 84 years with a median of 67 years. Each of the seven escalating-dose cohorts included three patients with the exception of Cohort 2B, which included four patients, one of whom had withdrawn prior to completing the first treatment cycle and had been replaced per protocol. An expanded cohort of 5 patients was treated at 20 mg/kg every two weeks (Cohort 3A dosing). Detailed patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics.
| Cohort | 1A | 2A | 3A | 4A | 1B | 2B | 3B | All treatment groups | |
| Dose (mg/kg) | 5 | 10 | 20 | 30 | 10 | 20 | 30 | ||
| schedule | q2w | q2w | q2w | q2w | q3w | q3w | q3w | ||
| N | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 | |
| Gender, n (%) | Male | 1 (33.3) | 2 (66.7) | 5 (62.5) | 2 (66.7) | 2 (66.7) | 3 (75.0) | 1 (33.3) | 16 (59.3) |
| Female | 2 (66.7) | 1 (33.3) | 3 (37.5) | 1 (33.3) | 1 (33.3) | 1 (25.0) | 2 (66.7) | 11 (40.7) | |
| Race, n (%) | White | 2 (66.7) | 2 (66.7) | 8 (100.0) | 3 (100.0) | 3 (100.0) | 4 (100.0) | 3 (100.0) | 25 (92.6) |
| Black/AA | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | |
| Asian | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | |
| Ethnicity, n (%) | Hispanic/Latino | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (3.7) |
| Not H/L | 3 (100.0) | 3 (100.0) | 7 (87.5) | 3 (100.0) | 3 (100.0) | 4 (100.0) | 3 (100.0) | 26 (96.3) | |
| Age (yrs) | N | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 |
| Mean | 77.7 | 64.9 | 69.8 | 61.1 | 66.6 | 69.1 | 65.4 | 68.2 | |
| SD | 5.5 | 4.92 | 8.37 | 11.7 | 4.41 | 5.53 | 18.9 | 9.26 | |
| Median | 75 | 67.2 | 71 | 65.7 | 64.6 | 67.9 | 72.1 | 68.6 | |
| Min | 74.1 | 59.2 | 58.3 | 47.8 | 63.6 | 63.8 | 44.1 | 44.1 | |
| Max | 84 | 68.2 | 82.7 | 69.7 | 71.6 | 76.8 | 80.1 | 84 | |
| Age group, n (%) | 18 to <65 | 0 | 1 (33.3) | 2 (25.0) | 1 (33.3) | 2 (66.7) | 1 (25.0) | 1 (33.3) | 8 (29.6) |
| >= 65 | 3 (100.0) | 2 (66.7) | 6 (75.0) | 2 (66.7) | 1 (33.3) | 3 (75.0) | 2 (66.7) | 19 (70.4) | |
| ECOG, n (%) | 0 | 3 (100.0) | 1 (33.3) | 5 (62.5) | 0 | 2 (66.7) | 2 (50.0) | 2 (66.7) | 15 (55.6) |
| 1 | 0 | 2 (66.7) | 3 (37.5) | 3 (100.0) | 0 | 2 (50.0) | 1 (33.3) | 11 (40.7) | |
| >=2 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.7) | |
| Prior Therapy | Systemic | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 |
| Radiotherapy | 2 | 2 | 3 | 2 | 1 | 2 | 1 | 13 | |
| Surgery | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 |
Abbreviations: q2w, every two weeks; q3w every three weeks; AA African American, H Hispanic, L Latino
Treatment duration ranged from 3 to 27 weeks with the highest number of treatment cycles (7) completed by one patient in Cohort 2A. Across all treatment groups, mean duration of treatment was 10.5 weeks. The cohort with the shortest mean treatment duration (5.3 weeks) was cohort 1A; the longest mean treatment duration (18.7 weeks) was in Cohort 2A.
Safety and tolerability
Fourteen patients (51.9%) experienced treatment-related adverse events. Most of these treatment-related adverse events were low grade, and no patients had grade 3 or greater treatment-related adverse events. Adverse events occurring in more than one patient in the study are shown in Table 2. The most frequent adverse events were fatigue and constipation, occurring in 9 and 8 patients respectively.
Table 2.
Safety
| Serious Adverse Events | ||||||||
| Cohort | 1A | 2A | 3A | 4A | 1B | 2B | 3B |
All treatment groups |
| N | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 |
|
No (%) of patients with SAE |
1 (33.3) | 1 (33.3) | 4 (50.0) | 2 (66.7) | 0 | 4 (100.0) | 0 | 12 (44.4) |
|
No of Treatment emergent SAEs |
1 | 3 | 6 | 4 | 0 | 7 | 0 | |
| SAEs | Syncope | Abdominal Pain, Pyrexia, Metastatic Pain |
Disease Progression, UTI, Failure to thrive, Hypoglycemia, Cerebral Hemorrhage, Mental Status Change |
Melena, Mouth hemorrhage, hematuria, urinary bladder hemorrhage |
Subdural hematoma, Hypophosphatemia, Metastases to CNS, Dyspnea, Hypoxia, Pleural effusion, DVT |
|||
| TEAE occurring in more than one patient per cohort | ||||||||
| Cohort | 1A | 2A | 3A | 4A | 1B | 2B | 3B | |
| N | 3 | 3 | 8b | 3 | 3 | 4 | 3 | |
| Event/n (%) | NA | night sweats / 2 (66.7) |
Constipation / 4 (50.0), Fatigue / 4 (50.0), Arthralgia / 2 (25.0), Musculoskele tal pain / 2 (25.0), Cough / 2 (25.0) |
Diarrhea / 2 (66.7), Dry mouth / 2 (66.7), Hyponatremia / 2 (66.7) |
NA | Hypophosphatem ia / 2 (50.0) |
Fatigue / 2 (66.7), Arthralgia / 2 (66.7) |
|
| TEAE Occurring in More than one patient per study | ||||||||
|
No. of patients (%) |
TEAE | |||||||
| 9 (33.3) | fatigue | |||||||
| 8 (29.6) | constipation | |||||||
| 5 (18.5) | hypophosphatemia, arthralgia | |||||||
| 4 (14.8) | headache, cough, diarrhea, decreased appetite, | |||||||
| 3 (11.1) | abdominal pain, hyponatremia, night sweats | |||||||
| 2 (7.4) | dry mouth, chills, upper respiratory tract infection, dehydration, back pain, muscle spasms, musculoskeletal pain, nodule on extremity, peripheral neuropathy, insomnia, dyspnea, pleural effusion, postnasal drip, hyperhidrosis, pruritis, deep vein thrombosis |
|||||||
A total of 12 patients experienced 21 treatment-emergent serious adverse events (SAEs) collectively (Table 2). Each treatment-emergent SAE occurred in one patient, and none were characterized as treatment-related. Furthermore, none of the SAEs led to death or discontinuation of study treatment. No deaths occurred during the study or during the protocol-required 30-day follow up period. There were no DLTs in this study (see supplementary Table 1 for a list of grade 3 or greater TEAE by system organ class).
Pharmacokinetics and biomarker studies
The pharmacokinetic parameters computed for IMC-20D7S, are shown in Table 3. The terminal elimination half-life (t1/2) could not be reliably estimated due to the limited sampling time. Clearance at steady state for IMC-20D7S was approximately 0.01L/hr.
Table 3.
Pharmacokinetic parameters following Single (Cycle 1 Day 1) and Multiple Administrations (Cycle 3 Day 1).
| Geometric Mean (CV%)a | |||||||
|---|---|---|---|---|---|---|---|
| Arm-A (q2w) | Arm-B (q3w) | ||||||
| 5 mg/kg q2w (N=3) |
10 mg/kg q2w (N=3) |
20 mg/kg q2w (N=8) |
30 mg/kg q2w (N=3) |
10 mg/kg q3w (N=3) |
20 mg/kg q3w (N=4) |
30 mg/kg q3w (N=3) |
|
| First Infusion (Cycle 1 Day1) | |||||||
|
Cmax (µg/mL) |
159 (30) | 609 (4) | 1228.893; 1450.289b |
1320 (64) | 580.517; 331.732b |
1290 (15)c | 1280 (21) |
|
tmax (hr)d |
1.50 (1.50–1.50) |
1.50 (1.00–2.00) |
9.00; 1.32b |
1.52 (1.52–4.43) |
2.00; 1.58b |
2.00 (1.00–2.00)c |
3.65 (1.83–8.63) |
|
AUC(0–336) (µg·hr/mL) |
20900e | 34200; 47600b |
153000; 135000b |
100000; 321000b |
64700e | 161000 (43)c | 152000 (23) |
| Fifth Infusion (Cycle 3 Day1) | |||||||
|
Cmax (µg/mL) |
NA | 505 (22) | NA | 2501.066e | 850.041e | 1159.235e | NA |
|
tmax (hr)d |
NA | 25.00 (1.00–49.00) |
NA | 2.63e | 2.03e | 3.67e | NA |
|
AUCτ (µg·hr/mL) |
NA | 59300; 87400b |
NA | 395000e | NC | NC | NA |
|
CLss (L/hr) |
NA | 0.0118; 0.00744b |
NA | 0.00922e | NC | NC | NA |
|
Vss (L) |
NA | 1.70e | NA | 2.51e | NC | NC | NA |
| RA,Cmax | NA | 0.829 (22) | NA | 0.981e | 1.46e | 0.808e | NA |
| RA,AUC | NA | 1.24; 2.56b |
NA | 1.23e | NC | NC | NA |
Abbreviations : AUC(0–336) = area under the concentration-time curve from time 0 to 336 hours; AUCτ = area under the concentration-time curve during 1 dose interval (336 hours for q2w and 504 hours for q3w); CLss = clearance at steady state (after intravenous administration); Cmax = maximum observed drug concentration; CV = coefficient of variation; N = number of patients dosed; n = number of observations; NA = not available; NC = not calculated; RA,AUC = accumulation ratio calculated using AUC; RA,Cmax = accumulation ratio calculated using Cmax; tmax = time of maximum observed drug concentration; Vss = volume of distribution at steady state.
Geometric mean and geometric CV% are provided for n ≥3; otherwise actual values are provided.
Values separated by semicolon are provided when n=2.
n=3.
Median (range) are provided for tmax.
The value is given when n=1.
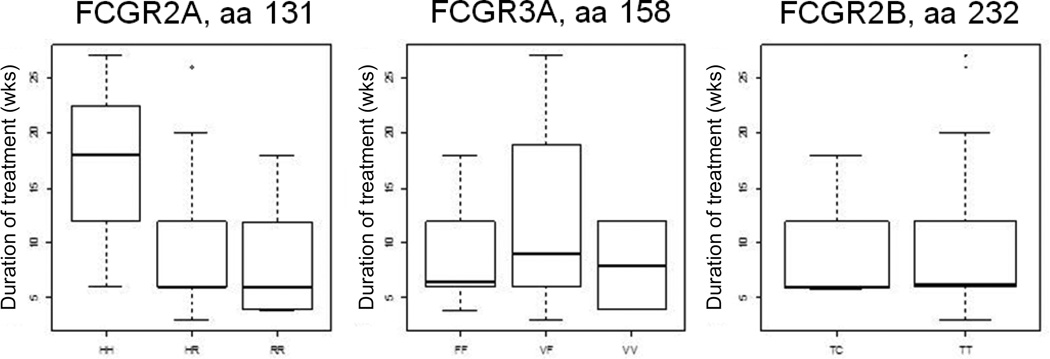
Consistent with recently published data on cetuximab (15), which carries the same IgG1 backbone as IMC-20D7S, there was a significant correlation between the duration of treatment and a polymorphism in the gene encoding FcγRIIa, namely FCGR2A (p < 0.05); while there was no discernible correlation between duration of treatment and genotype for FCGR3A or FCGR2B (Figure 1). The dose of drug administered positively correlated with duration of treatment (p < 0.05). There was no significant correlation between clinical outcome and genotype.
Figure 1. FCGR polymorphism status.
FCGR2A polymorphisms correlate with duration of treatment.
Efficacy
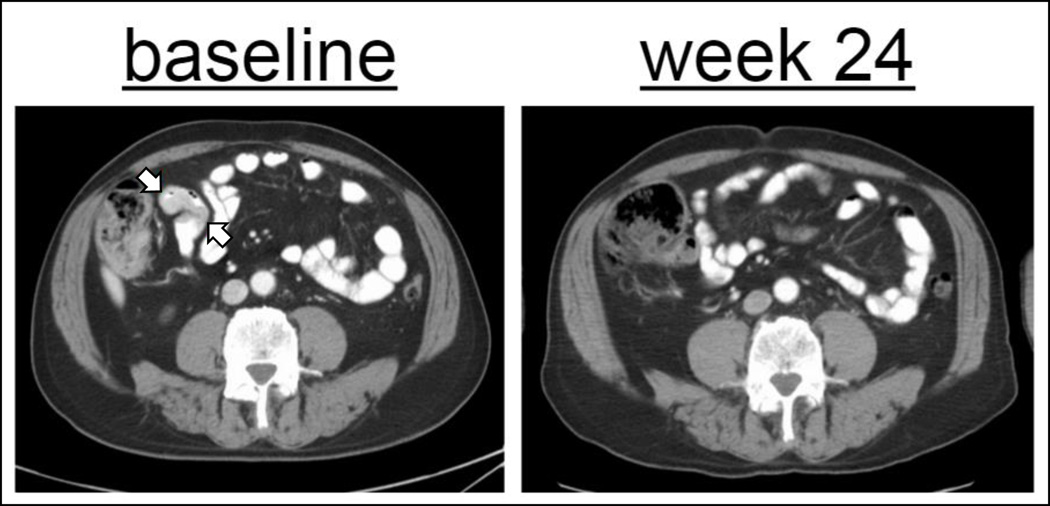
There was one patient in cohort 2A who had a complete response (CR) to IMC-20D7S at week 24 (Figure 2). At baseline, this 67 year old man had ileal metastases measuring 3.3 × 1.7 cm in conglomerate dimension. His first on-treatment CT showed regression, with a CR evident by week 24. His PFS was 5.95 months. No patients had a best response of partial response (PR). Ten patients (37%) had stable disease; their PFS values were as follows: 2.6, 3.98, 2.6, 4.4, 4.21, 2.1, 5.55, 4.3, 2.73, and 4.14 months. Twelve patients (44%) had progressive disease. Three patients (11%) were not evaluable. The disease control rate, defined as stable disease or better, was 41% (Table 4). Median PFS of pooled patients from all dose levels was 2.10 months (95% confidence interval, 1.22 to 2.73). Six patients had a PFS beyond 3 months (4.14, 4.21, 4.3, 4.4, 5.55 and 5.95 months respectively) with 6 to 13 infusions in total.
Figure 2. Patient achieving CR.
Resolution of ileal metastases measuring 3.3 × 1.7 (arrows) cm in conglomerate dimension.
Table 4.
Response
| Cohort | 1A | 2A | 3A | 4A | 1B | 2B | 3B | All treatment groups |
|
| N | 3 | 3 | 8 | 3 | 3 | 4 | 3 | 27 | |
| Best overall response, n (%) | CR | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (3.7) |
| PR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SD | 0 | 2 (66.7) | 2 (25.0) | 0 | 1 (33.3) | 2 (50.0) | 3 (100.0) | 10 (37.0) | |
| Non-CR/Non- PD* |
1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | |
| PD | 2 (66.7) | 0 | 5 (62.5) | 2 (66.7) | 2 (66.7) | 1 (25.0) | 0 | 12 (44.4) | |
| Not Evaluable | 0 | 0 | 1 (12.5) | 1 (33.3) | 0 | 1 (25.0) | 0 | 3 (11.1) | |
|
Disease control rate (CR+PR+SD) |
0.00% | 100.00% | 25.00% | 0 | 33.30% | 50 | 100.00% | 40.70% | |
| 95% CI (%) | 0.0, 70.8 |
29.2, 100.0 |
3.2, 65.1 |
0.0, 70.8 |
0.8, 90.6 |
6.8, 93.2 |
29.2, 100.0 |
22.4, 61.2 | |
| p-value | 0.1000 |
One patient in this cohort had nontarget disease only, and as per protocol was characterized as Non-CR/Non-PD
Discussion
Treatment with IMC-20D7S was well tolerated with doses safely escalated to 30mg/kg every 2 weeks (Arm A) and 30mg/kg every 3 weeks (Arm B). No MTD was determined since there were no treatment-related SAEs, DLTs, or grade 3 toxicities. The recommended dose for further evaluation was established at 20 mg/kg given every two weeks based on pharmacokinetic and safety data. While the overall objective response rate in this study was low, there was one patient with a complete response. Ten patients achieved stable disease.
There is consistent data showing an associated between FcR polymorphisms and function of tumor-targeting mAbs (16,17). Since FcγRIIa has been implicated in ADCC (18) and antibody-dependent cell-mediated phagocytosis (19) (ADCP) and the efficacy of the 20D7S preclinical analogue, TA99, was dependent upon FcγR interactions, we hypothesized that patients’ FcγRIIA polymorphisms would be relevant to clinical outcome with 20D7S. Due to the low response rate and patient numbers, however, we were unable to find an association between FcγRIIA polymorphisms and clinical response. Nevertheless, we found that FcγRIIA polymorphism status was correlated with treatment duration (Figure 1). Further exploration of Fcγ receptor polymorphisms, in larger cohorts, is warranted.
Although the efficacy of IMC-20D7S as a single agent was limited, IMC-20D7S may have greater clinical efficacy in combination with other immunotherapeutic approaches such as checkpoint (e.g., CTLA-4, PD-1, PD-L1) blockade. In principle, since tumor-targeted mAb therapeutics like IMC-20D7S can induce a tumor-specific T-cell response via ADCP (20–22), the induced T-cell response may theoretically be augmented with checkpoint blockade. The favorable toxicity profile of 20D7S makes it an attractive candidate for use in such combinations in subsequent clinical trials.
Supplementary Material
Statement of translational relevance.
IMC-20D7S is a monoclonal antibody targeting TYRP1 on melanoma cells. We find that IMC-20D7S is well tolerated among patients with advanced melanoma. Furthermore, there is evidence of anti-tumor activity including a patient who achieved a complete response. In light of the possibility that it triggers an anti-tumor T cell response via antibody-dependent cell-mediated phagocytosis, its efficacy may be augmented with immune checkpoint blockade. Given IMC-20D7S’s safety profile and its mechanism of action, there is strong rationale for testing it in combination with checkpoint blockade therapies such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies. Further investigation of IMC-20D7S in melanoma patients is thus warranted.
Acknowledgments
We are grateful for the support of patients and their families. Nicholas Cimaglia provided thorough research assistance. NIH support was provided through grant number P30CA008748. D. Khalil is currently supported by a fellowship in Clinical/Translational Cancer Research from the American Association for Cancer Research (AACR) and Amgen, in Clinical Investigation from the American Philosophical Society, and through a Young Investigator Award from the Conquer Cancer Foundation and the American Society of Clinical Oncology (ASCO).
Footnotes
Potential conflicts of interest:
M. Postow, Bristol-Myers Squibb (advisory board participation and research grant). J. Wolchok, Bristol Myers Squibb, Medimmune, Merck Pharmaceuticals, Genentech, Polynoma Pharmaceuticals (Grants/Research Support/Consultant).
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. [cited 2015 Apr 21];N Engl J Med [Internet] 2015 doi: 10.1056/NEJMoa1503093. 150419053123009. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25891173. [DOI] [PubMed]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. [cited 2015 Jun 2];N Engl J Med [Internet] 2015 doi: 10.1056/NEJMoa1504030. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26027431. [DOI] [PMC free article] [PubMed]
- 4.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. [cited 2015 Jun 7];Lancet [Internet] 2015 386:444–451. doi: 10.1016/S0140-6736(15)60898-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26037941. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. [cited 2015 Aug 20];N Engl J Med [Internet] 2014 371:1867–1876. doi: 10.1056/NEJMoa1408868. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25265494. [DOI] [PubMed] [Google Scholar]
- 6.Ghanem G, Fabrice J. Tyrosinase related protein 1 (TYRP1/gp75) in human cutaneous melanoma. [cited 2015 Aug 21];Mol Oncol [Internet] 2011 5:150–155. doi: 10.1016/j.molonc.2011.01.006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21324755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takechi Y, Hara I, Naftzger C, Xu Y, Houghton AN. A melanosomal membrane protein is a cell surface target for melanoma therapy. [cited 2015 Aug 26];Clin Cancer Res [Internet] 1996 2:1837–1842. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9816138. [PubMed] [Google Scholar]
- 8.Tai T, Eisinger M, Ogata S, Lloyd KO. Glycoproteins as differentiation markers in human malignant melanoma and melanocytes. [cited 2015 Aug 26];Cancer Res [Internet] 1983 43:2773–2779. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6850592. [PubMed] [Google Scholar]
- 9.Journe F, Id Boufker H, Van Kempen L, Galibert M-D, Wiedig M, Salès F, et al. TYRP1 mRNA expression in melanoma metastases correlates with clinical outcome. [cited 2015 Aug 21];Br J Cancer [Internet] 2011 105:1726–1732. doi: 10.1038/bjc.2011.451. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3242608&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science [Internet] 2013;341:1192–1198. doi: 10.1126/science.1241145. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24031011. [DOI] [PubMed] [Google Scholar]
- 11.Boross P, Leusen JHW. Mechanisms of action of CD20 antibodies. [cited 2015 Aug 20];Am J Cancer Res [Internet] 2012 2:676–690. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3512181&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 12.Welt S, Mattes MJ, Grando R, Thomson TM, Leonard RW, Zanzonico PB, et al. Monoclonal antibody to an intracellular antigen images human melanoma transplants in nu/nu mice. [cited 2015 Aug 26];Proc Natl Acad Sci U S A [Internet] 1987 84:4200–4204. doi: 10.1073/pnas.84.12.4200. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=305052&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. [cited 2015 Aug 26];J Exp Med [Internet] 1995 182:1609–1614. doi: 10.1084/jem.182.5.1609. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2192219&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clynes R, Takechi Y, Moroi Y, Houghton a, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. [cited 2015 Aug 26];Proc Natl Acad Sci U S A [Internet] 1998 95:652–656. doi: 10.1073/pnas.95.2.652. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=18475&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Tu D, Lewis M, Cheng D, Sullivan LA, Chen Z, et al. Fc-γ Receptor Polymorphisms, Cetuximab Therapy, and Survival in the NCIC CTG CO.17 Trial of Colorectal Cancer. [cited 2016 May 15];Clin Cancer Res [Internet] 2016 22:2435–2444. doi: 10.1158/1078-0432.CCR-15-0414. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27179112. [DOI] [PubMed] [Google Scholar]
- 16.Bekaii-Saab TS, Roda JM, Guenterberg KD, Ramaswamy B, Young DC, Ferketich AK, et al. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. [cited 2016 Jun 23];Mol Cancer Ther [Internet] 2009 8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. NIH Public Access; Available from: http://www.ncbi.nlm.nih.gov/pubmed/19887543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava RM, Lee SC, Andrade Filho Pa, Lord Ca, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derer S, Glorius P, Schlaeth M, Lohse S, Klausz K, Muchhal U, et al. Increasing FcγRIIa affinity of an FcγRIII-optimized anti-EGFR antibody restores neutrophil-mediated cytotoxicity. [cited 2015 Aug 27];MAbs [Internet] 6:409–421. doi: 10.4161/mabs.27457. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3984330&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. [cited 2015 Jul 31];J Transl Med [Internet] 2013 11:307. doi: 10.1186/1479-5876-11-307. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4029549&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Ravetch JV. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. [cited 2015 May 12];Cell [Internet] 2015 doi: 10.1016/j.cell.2015.04.016. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25976835. [DOI] [PMC free article] [PubMed]
- 21.Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: Support for a “vaccinal effect” of rituximab. Blood. 2009;113:3809–3812. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abès R, Gélizé E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.