Hypertension affects 30% of the population and 70% of the elderly. Despite its frequency and decades of research, the etiology of most cases of adult hypertension remains undefined. Perturbations of the kidney, the vasculature and the central nervous system have all been implicated in the pathogenesis of hypertension. As examples, in most cases of adult hypertension, systemic vascular resistance is elevated and vasodilators lower blood pressure, supporting a vascular etiology. Alternatively, renal cross-transplantation studies have shown that hypertension follows the kidney and in most cases of experimental hypertension, pressure natriuresis is impaired. Likewise, most single gene mutations that cause hypertension affect sodium transport in the distal nephron. In keeping with a renal etiology, diuretics and sodium restriction are effective in lowering blood pressure. Finally, there is ample evidence to suggest a central-neural cause of hypertension. Sympathetic outflow is almost uniformly increased in experimental models of hypertension and in humans with this disease and renal denervation has been variably reported to reduce blood pressure. Adrenergic receptor antagonists are commonly used to treat hypertension, further supporting a neural etiology.
Is there a unifying mechanism that underlies abnormalities of the brain, kidneys, and the vasculature in hypertension? Accumulating evidence shows that immune cells infiltrate these organs in response to hypertensive stimuli, causing their dysfunction leading to blood pressure elevation and subsequent end-organ damage. Both innate and adaptive immune cells play a key role in this process. Monocyte/macrophages are increased in the kidney and vasculature of animal models of experimental hypertension and in humans with hypertension. Wenzel et al showed that deletion of monocytes/macrophages completely eliminates Ang II-induce hypertension and markedly improves vascular function.1 Monocytes have several fates upon tissue entry. One is to become inflammatory macrophages that can generate reactive oxygen species (ROS), release cytokines and produce matrix metalloproteinases. A second fate is to become monocyte-derived dendritic cells (DCs), which present antigen to T cells and guide T cell fate. Kirabo et al recently showed that such DCs play a major role in hypertension.2 They demonstrated that hypertension is associated with modification of self-proteins in DCs by oxidized lipid products known as isolevuloglandins, which are immunogenic and seem to act as neoantigens. Adoptive transfer of DCs from hypertensive mice predisposes to severe hypertension and promotes inflammation in the recipients. A third fate of monocytes is transformation to fibrocytes, which are fibroblast precursors that secrete collagen leading to fibrosis. Wu et al showed that these bone marrow-derived cells accumulate in the perivascular space during hypertension and are responsible for aortic stiffening.3 Closely related to monocytes are microglial cells of the brain. It has recently become evident that these are activated in hypertension and that there is an exchange of these cells with circulating monocytoid cells. Interventions that quiesce microglial cells reduce blood pressure.4
A key function of macrophages and DCs is to present antigen to T cells. Indeed, T cells are critical in the genesis of hypertension.5 T cells with an effector phenotype infiltrate the vasculature and kidneys and release cytokines that promote vasoconstriction, remodeling, sodium retention and fibrosis. Mice lacking T cells develop blunted hypertension and the organ damage caused by various hypertensive challenges. B cells have also been implicated in hypertension and clearing B cells has proven effective in reducing experimental hypertension.6 Ang II-infusion has been shown to activate human T cells in a humanized mouse model of hypertension.7 Moreover, hypertensive humans have increased levels of circulating IL-17 and have increased circulating immunosenescent pro-inflammatory cytotoxic CD8+ T cells.8
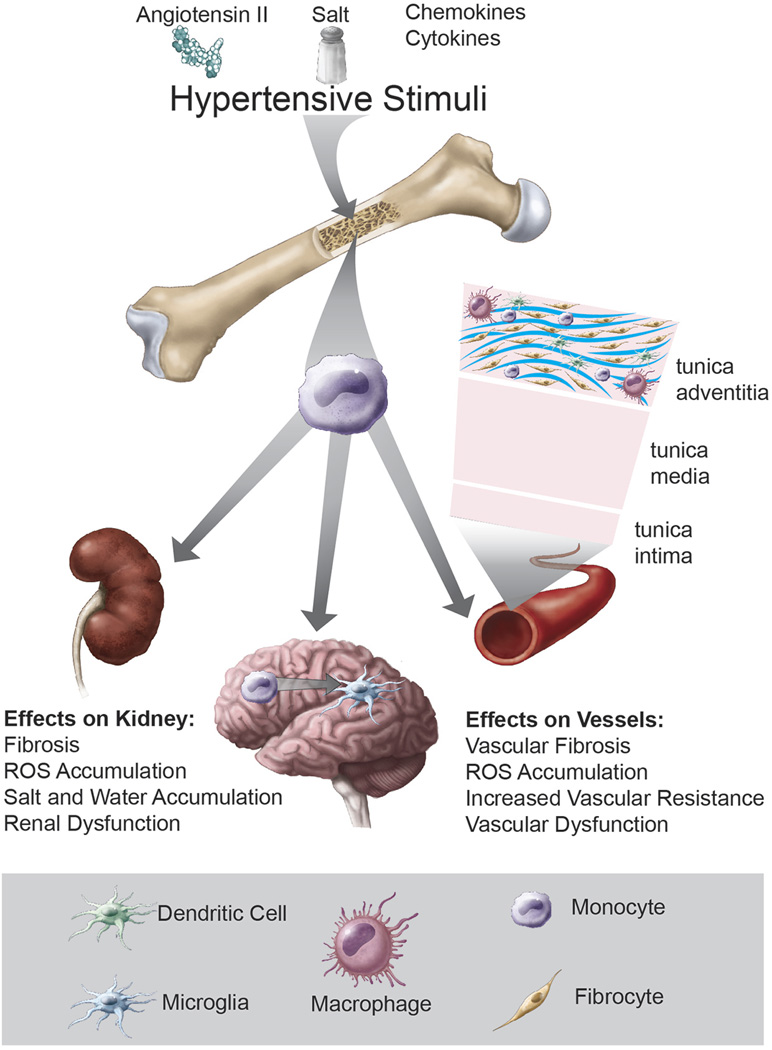
A common feature of all of the above mentioned immune cells is that they originate in the bone marrow (Figure). Monocytes and B cells directly originate from the bone marrow, while T cells are originally produced in the bone marrow but mature and differentiate in the thymus. For this reason, the bone marrow has become of substantial interest to investigators who study hypertension and a number of recent studies have shown that bone marrow transplant can either enhance or protect from hypertension, depending on the experimental condition. T cells require co-stimulation, often involving interaction of CD28 with B7 ligands on APCs, to become fully stimulated. Vinh et al showed that mice lacking B7 ligands exhibit blunted hypertension to both Ang II and DOCA salt challenges and that transplantation of bone marrow to these animals restores their blood pressure elevation.9 Santisteban et al showed that transplant of Wistar-Kyoto (WKY) bone marrow to spontaneously hypertensive rats (SHR) reduces mean arterial pressure and central and peripheral inflammation.4 Ahmari et al showed that transplantation of bone marrow lacking beta1 and beta2 adrenergic receptors reduces baseline blood pressure in wild-type recipients and decreases circulating CD4+ T cells, myeloid (CD11b+) cells and neutrophils in these animals, suggesting that signaling through adrenergic receptors may play a role in blood pressure regulation and bone marrow cell production or mobilization.10 The endogenous anti-inflammatory protein Suppressor of Cytokine Signaling 3 (SOCS3) inhibits cytokine signaling by binding to Janus kinases and cytokine receptors. Li et al showed that SOCS3+/− mice are protected from Ang II induced vascular dysfunction and that transplantation with wild type bone marrow restores the usual hypertensive effects of Ang II.11 Genome wide association studies have identified polymorphisms of SH2B3 (LNK) as being associated with hypertension. LNK is an intracellular docking protein that blunts T cell activation and studies of mice lacking this protein showed that they are predisposed to severe hypertension and peripheral inflammation in response to Ang II infusion. Bone marrow transplant studies showed loss of LNK in hematopoietic cells is almost entirely responsible for this phenotype.12
Figure.
Various factors common to the hypertensive milieu, including ang II, salt, catecholamines, chemokines and cytokines likely act on the bone marrow to mobilize monocytes and other inflammatory cells, some of which express the surface marker CXCR2. These cells enter the circulation and are attracted to peripheral sites of inflammation, guided by interactions with ligands such as CXCL1. These monocytes become activated and undergo transformation to inflammatory macrophages, dendritic cells and likely fibrocytes, leading to organ damage, dysfunction and fibrosis.
Thus, the above studies strongly indicate that the bone marrow plays a previously unappreciated role in the genesis of hypertension. In keeping with this concept, in the current issue of Circulation, Wang et al show that the chemokine receptor CXCR2, which is present on a subset of leukocytes, is critical in the development and maintenance of hypertension.13 These investigators found that aortic mRNA levels of CXCR2 and its ligand, CXCL1 are elevated in mice with hypertension. They elegantly demonstrated that mice lacking CXCR2 are protected from blood pressure elevation, vascular infiltration of inflammatory cells, fibrosis, ROS formation, NADPH activation, and vascular dysfunction in response to either Ang II or DOCA salt. These results were recapitulated using a novel allosteric inhibitor of CXCXR2, SB265610. Importantly, they also showed that hypertensive humans have increased numbers of circulating CXCR2+ cells and that there is a correlation between blood pressure and the number of CXCR2+ cells in the circulation.
CXCR2 is critical in the recruitment of neutrophils and other leukocytes to sites of inflammation. Its ligand CXCL1 is produced in sites of injury and hence, recruits CXCR2+ cells to these sites. CXCR2 activation causes downstream signaling including activation of the Akt and mitogen activated kinase pathways leading to cytokine production, cytoskeletal restructuring and chemotaxis.14 These events are important for the transmigration of leukocytes through activated endothelium – a key step in the initiation of inflammation. Often, there is substantial redundancy in the signaling between chemokine receptors and ligands and that lack of one is often compensated for by the presence of others.15 An important finding in this study is that CXCR2 seem to have a non-redundant role in experimental hypertension and that its loss has myriad beneficial effects.
An important future area of investigation is to determine if hypertension has a direct effect on the bone marrow to activate the production and mobilization of these cells or to determine if its major effect on recruitment of these cells to sites of injury. Findings like those made by Wang et al raise two possible scenarios. One is that hypertension increases vascular expression of adhesion molecules and chemokines so that pre-existing circulating cells are attracted to peripheral tissues. A second scenario is that hypertension stimulates production of these cells so that more enter the circulation and are available for recruitment to sites of inflammation. A remarkable and fundamental tenet of the immune response is that both of these phenomenon often occur together. In sites of inflammation, antigens are released which are taken up by antigen presenting cells that ultimately activate and mobilize T cells in secondary lymphoid organs and from the bone marrow. Simultaneously there is a local increase in chemokines and adhesion molecules that attract immune cells to the original site of injury. An important finding of the study by Wang et al is that hypertensive humans exhibit an increased circulating CXCR2+ cells, strongly suggesting that hypertension in some way stimulates mobilization of these cells, likely from the bone marrow.
The concept that hypertension has an effect on the bone marrow is paradigm shifting. How could a disease like hypertension activate the bone marrow? One possibility is related to sympathetic outflow. Bone marrow cells have adrenergic receptors and sympathetic nerves richly innervate the bone marrow. Thus, increased sympathetic nerve firing, which commonly accompanies hypertension, might activate and mobilize these cells. As mentioned above, chemokines and cytokines arising from peripheral tissues such as the kidney and vasculature might also signal bone marrow cells. Other factors in the hypertensive milieu such as sodium, Ang II, aldosterone, or other inflammatory cytokines might directly activate these cells. Indeed, Itani et al recently showed that memory T cells accumulate in the bone marrow in experimental hypertension, and are reactivated by salt feeding and subsequently emerge and enter the kidney, and produce renal dysfunction.16 The precise signals that affect homing of immune cells to the bone marrow and that recruit cells from the bone marrow is an area of future investigation that will be fascinating to pursue.
Finally, cardiovascular physicians and scientists are increasingly asked to understand organ systems outside of the cardiovascular system, including the liver, which produces lipids, the pancreas, which is dysfunctional in diabetics that commonly have cardiovascular disease and the kidney, which as discussed above is altered in hypertension. It is becoming increasingly apparent that cardiologists and cardiovascular researchers will need to understand dynamics of the bone marrow, and in the future perhaps to use therapeutic interventions to prevent production of injurious cells like those described by Wang et al.
Acknowledgments
Funding Sources: This work was supported by American Heart Association pre- doctoral fellowship 15PRE25780039 to Kim Montaniel, the American Heart AHA Strategically Focused Research Network Grants 14SFRN20420046 and 14SFRN20770001 and NIH grants R01HL039006, P01HL058000, P01GM015431, R01HL125865, P01HL129941.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-positive monocytes mediate angiotensin II- induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 2.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Montaniel KR, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Madhur MS, Harrison DG. Origin of Matrix-Producing Cells That Contribute to Aortic Fibrosis in Hypertension. Hypertension. 2016;67:461–468. doi: 10.1161/HYPERTENSIONAHA.115.06123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension. 2015;66:1023–1033. doi: 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
- 7.Itani HA, McMaster WG, Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68:123–132. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 9.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmari N, Schmidt JT, Krane GA, Malphurs W, Cunningham BE, Owen JL, Martyniuk CJ, Zubcevic J. Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiol Genomics. 2016;48:526–536. doi: 10.1152/physiolgenomics.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Kinzenbaw DA, Modrick ML, Pewe LL, Faraci FM. Context-dependent effects of SOCS3 in angiotensin II-induced vascular dysfunction and hypertension in mice: mechanisms and role of bone marrow-derived cells. Am J Physiol Heart Circ Physiol. 2016;311:H146–H156. doi: 10.1152/ajpheart.00204.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Zhao XC, Cui W, Ma YQ, Ren HL, Zhou X, Fassett J, Yang YZ, Chen Y, Xia YL, Du J, Li HH. Genetic and Pharmacologic Inhibition of the Chemokine Receptor CXCR2 Prevents Experimental Hypertension and Vascular Dysfunction. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.115.020754. XXX:XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 15.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 16.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 Exacerbates Blood Pressure Elevation and Renal Damage in Response to Repeated Hypertensive Stimuli. Circ Res. 2016;118:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]