Abstract
Background
The association between leptin and complement in hepatitis C virus (HCV) infection remains unknown.
Methods
A prospective study was conducted including 474 (250 genotype 1, 224 genotype 2) consecutive chronic hepatitis C (CHC) patients who had completed an anti-HCV therapy course and undergone pre-therapy and 24-week post-therapy assessments of interferon λ3-rs12979860 and HCV RNA/genotypes, anthropometric measurements, metabolic and liver profiles, and complement component 3 (C3), C4, and leptin levels.
Results
Of the 474 patients, 395 had a sustained virological response (SVR). Pre-therapy leptin levels did not differ between patients with and without an SVR. Univariate and multivariate analyses showed that sex (pre- and post-therapy, p<0.001), body mass index (BMI) (pre- and post-therapy, p<0.001), and C3 levels (pre-therapy, p = 0.027; post-therapy, p = 0.02) were independently associated with leptin levels with or without HCV infection. Pre-therapy BMI, total cholesterol (TC), C4 levels, and the rs12979860 genotype were independently associated with pre-therapy C3 levels in all patients. Post-therapy BMI, alanine aminotransferase, TC, C4 levels, white blood cell counts, and hepatic steatosis were independently associated with the post-therapy C3 levels of SVR patients. Compared with pre-therapy levels, SVR patients showed higher 24-week post-therapy C4 (20.32+/-7.30 vs. 21.55+/-7.07 mg/dL, p<0.001) and TC (171.68+/-32.67 vs. 186.97+/-36.09 mg/dL, p<0.001) levels; however, leptin and C3 levels remained unchanged after therapy in patients with and without an SVR.
Conclusions
Leptin and C3 may maintain immune and metabolic homeostasis through association with C4 and TC. Positive alterations in C4 and TC levels reflect viral clearance after therapy in CHC patients.
Introduction
Hepatitis C virus (HCV), a human pathogen responsible for acute and chronic liver disease, has variants classified into 7 major genotypes and infects an estimated 170 million individuals worldwide [1]. It affects insulin signaling, and much of its life cycle is closely associated with lipid metabolism [2]. In addition to cirrhosis and hepatocellular carcinoma, HCV is thought to cause metabolic alterations, including steatosis, dyslipidemia, insulin resistance (IR), diabetes, obesity, and cardiovascular events [2–4]. Although most HCV infections are currently curable using potent direct-acting anti-viral agents, not all HCV-associated cardiometabolic complications are reversible after viral clearance [2]. Adipose tissue has emerged as an important endocrine organ that exerts vital endocrine and immune functions via adipokines [5]. Moreover, free fatty acids and glycerol derived from visceral adipose tissue reach the liver and stimulate the biosynthesis of lipoprotein and glucose, respectively [6]. Because adipose tissues and the liver are functionally linked, elucidating the relationship between adipokine alterations and HCV infection has the potential to reveal the basis of HCV-associated cardiometabolic complications and probe the therapeutic targets.
The adipokine leptin, a product of the obese gene, is primarily expressed in adipose tissue but is also expressed in other organs, including the liver [7]. Most of the circulating leptin originates from subcutaneous, but not visceral adipose tissue, which may reduce its biological activity [5]. Leptin is crucial for maintaining total body fat and glucose homeostasis as well as regulating food intake and energy expenditure through a complex central feedback mechanism [8]. Its secretion is influenced by numerous physiological and hormonal factors. The leptin receptor is expressed in hypothalamic neurons, T cells, and hepatic stellate cells [9]. Importantly, leptin promotes IR to increase intracellular fatty acids in hepatocytes, amplifies proinflammatory responses [10], and mediates hepatic fibrogenesis during chronic liver injury [11] through the activation of hepatic stellate cells [12]. Concordantly, leptin levels are elevated in patients with a higher fibrosis index [13]. Importantly, leptin is critical for the modulation of adaptive and innate immune responses, such as regulating T-cell-mediated immune responses [14] and natural killer cell activity [15], as well as increasing complement component 3 (C3) levels [16]. Because both HCV infection and leptin are critically involved in metabolism, inflammation, and immunity [5–16], their potential relationship has attracted attention; however, no definite conclusion regarding such a relationship has been drawn [16–21]. In addition to the multifaceted functions of leptin, this uncertainty is primarily due to variability among individuals, which is difficult to completely eliminate from case-control studies, retrospective studies, or prospective studies with inadequate confounder adjustments. Indeed, although the impact of HCV infection on alterations in leptin levels is unclear, even less is known regarding whether viral genotype-specific influences on these alterations exist [21–23]. Therefore, we sought to elucidate the aforementioned relationships by conducting a prospective study to analyze the leptin levels adjusting for viral, metabolic, and immune profiles in genotype 1 (G1) and genotype 2 (G2) CHC patients who completed anti-HCV therapy.
Methods
Patients
The study group comprised subjects 18 years or older with G1 or G2 CHC, which was defined as the presence of documented HCV antibody positivity and detectable HCV RNA for >24 weeks. Subjects with heavy alcohol consumption (alcohol consumption more than 10 g/day for women and 20 g/day for men [5]), human immunodeficiency virus infection, hepatitis B infection, hemochromatosis, coronary heart disease, renal insufficiency, or malignancy and recipients of solid organ transplants were excluded.
Methods
A total of 250 G1 and 224 G2 CHC patients were consecutively recruited at a tertiary referral center between July 2010 and June 2015. All patients received anti-HCV therapy with weight-based pegylated interferon-α-2b (1.5 μg/kg/week) and ribavirin (800–1400 mg/day) for either 24 or 48 weeks according to the therapeutic response-guided protocol [1,4]. The HCV RNA levels were assessed using a COBAS Amplicor (Roche Diagnostics, Tokyo, Japan). The HCV genotype was determined using the InoLipa method (Roche Diagnostics). Single nucleotide polymorphisms of interferon λ3 (IFNL3 or interleukin-28B) rs12979860 were assessed using genomic DNA, as previously described [1,4]. The patients were evaluated for HCV RNA to examine the therapeutic response 2 weeks prior to therapy, after 4, 12, and 24 weeks of therapy, at the end of therapy, and 12 and 24 weeks after the end of therapy. At 2 weeks prior to therapy and 24 weeks after the end of therapy, after fasting, the patients were evaluated for body mass index (BMI), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), triglycerides (TGs), uric acid, homeostasis model assessment-estimated insulin resistance (HOMA-IR) [fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5], alanine aminotransferase (ALT), aspartate aminotransferase (AST)-to-platelet ratio index (APRI), white blood cell (WBC) count, platelet count, and high-sensitivity C-reactive protein (hsCRP), C3, C4, and leptin (R&D Systems, MN, USA) levels. Abdominal ultrasound studies were performed in every patient prior to therapy and every 6 months thereafter to assess the presence and severity of fatty liver and cirrhosis. IR was defined as a HOMA-IR score ≥2.5. A sustained virological response (SVR) was defined as undetectable HCV RNA levels 24 weeks after the completion of therapy.
A liver biopsy was performed in every patient before anti-HCV therapy to assess the liver histology. The liver biopsy specimens were semi-quantitatively scored by an experienced hepatopathologist blinded to the clinical data. Histological scores for steatosis and fibrosis were reported using the criteria of Kleiner et al. [24] and staged according to the Metavir scoring system [25], respectively.
Statistics
All statistical analyses were performed using the Statistical Package for Social Science software (SPSS ver. 21.0, SPSS Inc., Chicago, IL, USA). Continuous variables were analyzed using Student’s t-test or the Mann-Whitney U test, and categorical variables were analyzed using the chi-square test or Fisher’s exact test, as appropriate. Univariate and multivariate linear regression models were used to assess the relationships between various pre-therapy dependent and independent variables. Paired t-tests were used to compare the variables prior to and 24 weeks after anti-HCV therapy within the same individuals. The variable values were logarithmically transformed and then used for the statistical analyses where indicated. Statistical significance was defined at the 5% level based on two-tailed tests of the null hypothesis.
Informed consent
Written informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Chang Gung Memorial Hospital institutional review board.
Results
Pre-therapy leptin levels did not differ between patients with and without an SVR
The baseline characteristics of the 474 CHC patients are listed in Table 1. Patients with an SVR (n = 395) had a lower BMI, lower HCV RNA and HOMA-IR levels, and a lower prevalence of G1 HCV infection and severe fibrosis (F3 and F4) but a higher prevalence of the IFNL3-rs12979860 CC genotype than patients without an SVR (n = 79). No differences in pre-therapy leptin levels were noted between the patients with and without an SVR. With regard to the genotype impact on leptin, no difference in the pre-therapy leptin levels was noted between the G1 and G2 patients after sex stratification (p = 0.328 for the males and p = 0.177 for the females).
Table 1. Baseline characteristics of CHC patients.
| All CHC patients (n = 474) | Patients with SVR (n = 395) | Patients without SVR (n = 79) | Student’s t-test p-values | |
|---|---|---|---|---|
| Male, n (%) | 270 (57.0) | 228 (57.7) | 42 (53.2) | 0.456 |
| Age (yr) | 55.18+/-11.92 | 53.72+/-11.61 | 55.99+/-10.59 | 0.109 |
| BMI | 24.89+/-3.78 | 24.79+/-3.59 | 25.77+/-4.10 | 0.035* |
| HCV RNA (Log10IU/ml) | 5.97+/-1.12 | 5.84+/-1.18 | 6.45+/-0.75 | <0.001* |
| HCV genotype [G1, n (%)] | 250 (52.7) | 187 (47.3) | 63 (79.7) | <0.001* |
| AST (U/L) | 75.06+/-68.55 | 73.06+/-61.63 | 73.74+/-63.90 | 0.933 |
| ALT (U/L) | 92.20+/-94.24 | 97.53+/-88.50 | 80.39+/-64.12 | 0.124 |
| TC (mg/dL) | 171.55+/-34.55 | 171.7+/-32.7 | 172.7+/-29.8 | 0.805 |
| HDL (mg/dL) | 42.82+/-13.81 | 48.22+/- 13.80 | 43.12+/-13.8 | 0.867 |
| TG (mg/dL) | 104.85+/-54.96 | 106.19+/-57.88 | 96.13+/-39.4 | 0.099 |
| HOMA-IR | 3.21+/-5.38 | 2.88+/-3.03 | 4.46 +/-9.42 | 0.039* |
| Uric acid (mg/dL) | 5.91+/-1.56 | 5.94+/-1.54 | 5.86+/-1.50 | 0.709 |
| WBC count (103/μL) | 5.72+/-3.23 | 5.82+/-1.91 | 5.63+/-1.73 | 0.433 |
| Platelets (103/μL) | 176.3+/-64.3 | 181.4+/-58.1 | 156.0+/-58.7 | 0.001* |
| hsCRP (mg/dL) | 1.87+/-3.72 | 1.68+/-3.13 | 1.56+/-1.95 | 0.774 |
| C3 (mg/dL) | 105.80+/-20.00 | 107.4+/-19.8 | 102.6+/-17.2 | 0.088 |
| C4 (mg/dL) | 20.42+/-7.89 | 20.56+/-7.70 | 19.25+/-7.28 | 0.238 |
| APRI | 1.65+/-2.05 | 1.47+/-1.62 | 1.75+/-1.83 | 0.195 |
| Hepatic steatosis | ||||
| None, n (%) | 166 (35) | 150 (33) | 16 (20.2) | 0.395 |
| Mild, n (%) | 213 (45) | 176 (44.6) | 37 (46.8) | 0.5 |
| Moderate, n (%) | 85 (17.9) | 62 (15.7) | 23 (29.1) | 0.49 |
| Severe, n (%) | 10 (2.1) | 7 (2.5) | 3 (3.7) | 0.48 |
| Fibrosis | ||||
| F0, n (%) | 76 (16) | 68 (17.2) | 8 (10.1) | 0.095 |
| F1, n (%) | 171 (36) | 151 (38.2) | 20 (25.3) | 0.17 |
| F2, n (%) | 166 (35) | 138 (34.9) | 28 (35.4) | 0.317 |
| F3, n (%) | 38 (8) | 25 (6.3) | 13 (16.4) | 0.049* |
| F4, n (%) | 23 (5) | 13 (3.3) | 10 (12.6) | 0.032* |
| Leptin (pg/ml) | 9748.6+/-9968.2 | 9332+/-9049 | 12168+/-14092 | 0.267 |
| Log leptin (Log10pg/ml) | 3.77+/-0.52 | 3.75+/-0.54 | 3.83+/-0.48 | 0.4 |
| SNP rs12979860 CC, n (%) | 401 (85) | 348 (88.1) | 53 (67.1) | 0.013* |
BMI: body mass index; G1: genotype 1; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TC: total cholesterol; HDL: high-density lipoprotein cholesterol; TGs: triglycerides; HOMA-IR: homeostasis model assessment-estimated insulin resistance; WBC: white blood cell; hsCRP: high-sensitivity C-reactive protein; C3: complement component 3; C4: complement component 4; APRI: AST to platelet ratio index; Log: logarithmic; SNP: single nucleotide polymorphism;
*: p<0.05.
Sex, BMI, and C3 levels were independently associated with leptin levels regardless of viral presence
The results of the univariate and multivariate analyses performed to determine the factors associated with the pre-therapy (for all patients, n = 474) and 24-week post-therapy (for patients with an SVR, n = 395) leptin levels are listed in Table 2. The univariate analyses revealed that sex, pre-therapy BMI, C3 levels, and hepatic steatosis were associated with pre-therapy leptin levels, whereas the multivariate analysis showed that sex, pre-therapy BMI, and C3 levels were independently associated with the pre-therapy leptin levels. Regarding the post-therapy leptin levels, the univariate analyses showed an association with sex, post-therapy BMI, HOMA-IR, C3 levels, and hepatic steatosis, whereas the multivariate analyses showed that sex, post-therapy BMI, and C3 levels were associated factors. Regarding the pre-therapy HCV RNA levels, age [estimated β: -0.054, 95% confidence interval (CI) of β: -0.105–0.004, p = 0.035] and pre-therapy TGs (estimated β: 0.021, 95% CI of β: 0.01–0.033, p<0.001) were independent factors.
Table 2. Univariate and multivariate analyses of factors associated with pre- and post-therapy leptin levels.
| Pre-therapy leptin (all patients) | Post-therapy leptin (patients with SVR) | |||
|---|---|---|---|---|
| Univariate analysis 95% CI of β [β](p value) | Multivariate analysis 95% CI of β [β](p value) | Univariate analysis 95% CI of β [β](p value) | Multivariate analysis 95% CI of β [β](p value) | |
| Sex (male) | -11325~-6839[–8857](<0.001*) | -12362.7~-8313.0[-10333.7](<0.001*) | -13016~-7235[–10126](<0.001*) | -39774.2~-18863.2[-12592.2](<0.001*) |
| Age (yr) | -108.9~138.9[11.48] (0.851) | -88.2~204.0[57.8](0.436) | ||
| BMI | 999.7~1649.7 [1324](<0.001*) | 1075.7~1679.3 [1377.5](<0.001*) | 729.3~1226.4[1210] (<0.001*) | 1013.8~1813.8[1422.8] (<0.001*) |
| HCV RNA (Log10IU/ml) | -493.8~2189.0[847.6](0.214) | NA | ||
| HCV genotype | -2133.3~456.3[-838.4] (0.203) | NA | ||
| AST (U/L) | -26.3~16.5[-4.81](0.653) | -227.0~109.2[-58.9](0.49) | ||
| ALT (U/L) | -22.8~8.8[-7.0](0.384) | -94.1~115.9[10.9](0.838) | ||
| TC (mg/dL) | -34.9~49.0[7.04](0.749) | -40.5~48.9[4.17](0.854) | ||
| HDL (mg/dL) | -115.7~96.4[-9.6](0.858) | -15.8~233.8 [109.0](0.087) | ||
| TG (mg/dL) | -10.1~43.3[16.6](0.223) | -21.4~25.8[2.22](0.853) | ||
| HOMA-IR | -108.7~348.7[119.9](0.302) | 32.8~935.6[484.2](0.036*) | -216.0~419.6[126.8](0.466) | |
| Uric acid (mg/dL) | -668.1~1113.9[222.9](0.622) | -1677.9~346.3[-665.8](0.196) | ||
| WBC count (103/μL) | -459.3~941.0[241.0](0.498) | -997.9~748.1[-124.9](0.778) | ||
| Platelets (103/μL) | -12.1~32.8[10.37](0.364) | -45.8~11.6[-17.1](0.242) | ||
| hsCRP (mg/dL) | -1.7~987.5[492.8](0.051) | -148.9~444.6[147.8](0.327) | ||
| C3 (mg/dL) | 72.1~207.5[139.8](<0.001*) | 7.2~116.3[61.7](0.027*) | 140.2~330.5[236.4](<0.001*) | 16.8~185.8[101.0](0.02*) |
| C4 (mg/dL) | -120.1~240.2[59.1](0.521) | -36.6~435.2[199.4](0.097) | ||
| APRI | -1183.1~348.7[-417.2](0.284) | -6173.4~4671.5[-740.8](0.788) | ||
| Hepatic steatosis (yes) | 2191.3~7591.7[4879](<0.001*) | -1527.6~2820.2[646.3] (0.558) | 2275.8~8578.8[5420.5](0.001*) | -501.9~4558.3 [2028.2](0.115) |
| SNP rs12979860 | -2359.9~4020.2[830.1](0.608) | -2893.1~4819.8[963.4] (0.622) | ||
BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TC: total cholesterol; HDL: high-density lipoprotein cholesterol; TGs: triglycerides; HOMA-IR: homeostasis model assessment-estimated insulin resistance; WBC: white blood cell; hsCRP: high-sensitivity C-reactive protein; C3: complement component 3; C4: complement component 4; APRI: AST to platelet ratio index; SNP: single nucleotide polymorphism;
*: p<0.05.
Because the HOMA-IR was reported to be positively correlated with the leptin serum levels in nondiabetic CHC patients [19,26], we stratified the patients by the presence of IR and performed univariate and multivariate analyses to determine the leptin levels. Pre-therapy HOMA-IR was an independent factor for pre-therapy leptin in patients without IR (estimated β: 3498, 95% CI: 1395–5600, p = 0.001) but not in patients with IR (p = 0.521).
TC and C4 levels were independently associated with C3 levels regardless of viral presence
Because sex, BMI, and C3 levels were associated with the leptin levels regardless of the presence of HCV, we investigated the factors associated with sex, BMI, and C3 using univariate and multivariate analyses (Table 3). Regarding the pre-therapy levels of all the patients, the univariate and multivariate analyses showed that uric acid and HDL-C were independently associated with sex; HDL-C, C3, and hepatic steatosis were independently associated with BMI; and BMI, TC, C4, and the IFNL3-rs12979860 CC genotype were independently associated with the C3 level. Regarding the post-therapy levels of the patients with an SVR, HDL-C, uric acid, and WBC count were independently associated with sex; HDL, HOMA-IR, uric acid, and C3 were independently associated with BMI; and BMI, ALT, TC, WBC count, hepatic steatosis, and C4 were independently associated with the C3 level. The associated leptin-centered relationships between the dependent and independent factors (pre-therapy and post-therapy) are summarized in Fig 1.
Table 3. Multivariate analyses of factors associated with pre- and post-therapy sex, BMI, and C3 levels.
| Pre-therapy dependent variables (all patients) | Post-therapy dependent variables (SVR patients) | |||||
|---|---|---|---|---|---|---|
| Sex | BMI | C3 | Sex | BMI | C3 | |
| Multivariate analysis, 95% CI of OD [OD], (p value) | Multivariate analysis, 95% CI of β [estimated β], (p value) | Multivariate analysis, 95% CI of OD [OD], (p value) | Multivariate analysis, 95% CI of β [estimated β], (p value) | |||
| Sex (male) | -0.701~1.075 [0.187](0. 679) | |||||
| BMI | 0.441~1.514 [0.978](<0.001*) | 0.893 ~1.070 [0.977](0.621) | 0.35~1.318 [0.834](0. 001*) | |||
| ALT (U/L) | 1.0~1.004[1.002](0.0059) | 0.0~0.06 [0.03](0.05) | 0.987~1.025 [1.006] (0.529) | -0.01~0.04 [0.015](0.239) | 0.092~0.296 [0.194] (<0.001*) | |
| TC (mg/dL) | 0.914~1.005 [1.0] (0.934) | 0.046~0.184 [0.115](0.001*) | 0.027~0.019 [0.073](0.002*) | |||
| HDL (mg/dL) | 0.934~0.965 [0.949](<0.001*) | -0.077~-0.018 [-0.048](0.002*) | -0.295~0.074 [-0.11](0.24) | 0.903~0.952 [0.927] (<0.001*) | -0.08~-0.011 [-0.046](0.009*) | -0.18~0.099 [-0.04](0.568) |
| TG (mg/dL) | -0.009~0.007 [-0.001](0. 778) | -0.006~0.085 [0.039](0.087) | -0.009~0.002 [-0.003](0.271) | -0.023~0.025 [0.001](0.936) | ||
| HOMA-IR | -0.018~0.137 [0.059](0.131) | -0.344~0.385 [0.02](0.912) | 0.077~0.315 [0.196](0.001*) | -0.102~0.898 [0.398](0.118) | ||
| Uric acid (mg/dL) | 1.462~1.919 [1.675] (<0.001*) | -0.443~2.305[0.931](0.183) | 1.41~2.233 [1.775] (<0.001*) | 0.188~0.745 [0.466](0.001*) | -1.128~1.090 [-0.019](0.973) | |
| WBC count (103/μL) | 0.975~1.184 [1.074](0.149) | -0.05~0.346 [0.148](0.141) | -0.779~1.452[0.337](0.553) | 1.011~1.411 [1.194](0.037*) | -0.156~0.33 [0.087](0.481) | 0.285~2.269 [1.277](0.012*) |
| Platelets (103/μL) | -0.028~0.061 [0.017](0.462) | -0.003~0.012 [0.004](0.277) | -0.013~0.049 [0.018](0.265) | |||
| hsCRP (mg/dL) | -0.082~0.208 [0.063] (0.39) | -0.261~0.188 [0.463](0.209) | -0.041~0.114[0.037](0.355) | -0.037~0.606 [0.285](0.082) | ||
| C3 (mg/dL) | 0.028~0.073 [0.05](<0.001*) | 0.019~0.075 [0.047](0.001*) | ||||
| C4 (mg/dL) | 0.987~1.034 [0.558](0.383) | -0.051~0.064 [0.007](0.815) | 0.74~1.302 [1.021](<0.001*) | -0.047~0.064[0.008](0.766) | 0.512~0.943 [0.728](<0.001*) | |
| APRI | -2.231~1.415 [-0.408](0.66) | |||||
| Hepatic steatosis (yes) | 0.354~1.885 [1.12](0.004*) | -2.4~5.7 [1.654](0.423) | -0.47~0.064 [0.298] (0.445) | 0.341~6.651 [3.496](0.03*) | ||
| SNP rs12979860 CC genotype | 1.233~9.747 [5.49](0.012*) | |||||
Only the variables significant in the univariate analyses (data not shown) were included in the multivariate analyses; OR: odds ratio; BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TC: total cholesterol; HDL: high-density lipoprotein cholesterol; TGs: triglycerides; HOMA-IR: homeostasis model assessment-estimated insulin resistance; WBC: white blood cell; hsCRP: high-sensitivity C-reactive protein; C3: complement component 3; C4: complement component 4; APRI: AST to platelet ratio index; SNP: single nucleotide polymorphism
*: p<0.05.
Fig 1. The leptin-centered associations between the dependent and independent factors before (pre-therapy) and 24 weeks after anti-hepatitis C therapy (post-therapy).
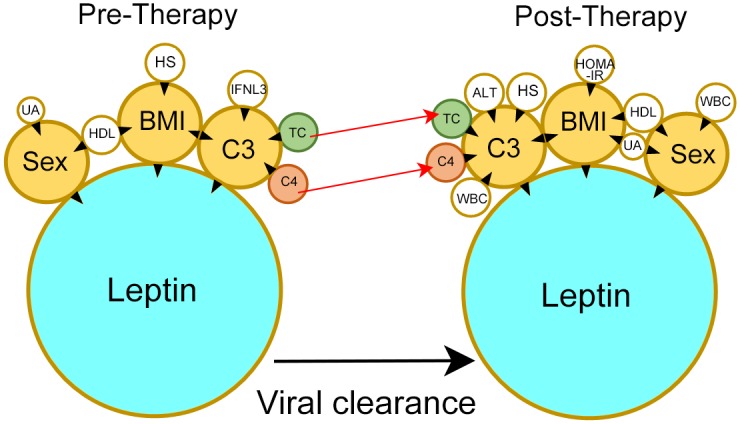
Tips of black arrowheads: dependent factors; Bases of black arrowheads: independent factors; UA: uric acid; HDL-C: high-density lipoprotein-cholesterol; BMI: body mass index; HS: hepatic steatosis; IFNL3: interferon, λ3; TC: total cholesterol; C3: complement component 3; complement component 4: C4; WBC: white blood cell; HOMA-IR: homeostasis model assessment-estimated insulin resistance; red arrows: post-therapeutic increases in the TC and C4 levels.
After anti-HCV therapy, the lipid profile and C4 levels increased, and the C3 and leptin levels remained unchanged
Although trivial, patients with and without an SVR had a decreased BMI after anti-HCV therapy. However, only patients with an SVR had decreased AST, ALT, and APRI levels but increased lipid profiles, uric acid, and C4 levels. The leptin and C3 levels remained unchanged regardless of the therapeutic response (Table 4).
Table 4. Comparison of the pre- and 24-week post-therapy variables in CHC patients stratified by the therapeutic response.
| SVR (+) (n = 395) | Paired t-test p-values | SVR (-) (n = 79) | Paired t-test p-values | |||
|---|---|---|---|---|---|---|
| Pre-therapy value | Post-therapy value | Pre-therapy value | Post-therapy value | |||
| BMI | 24.77+/-3.65 | 24.36+/-3.53 | <0.001* | 25.84+/-4.10 | 24.86+/-3.60 | <0.001* |
| AST (U/L) | 73.08+/-62.01 | 26.21+/-11.15 | <0.001* | 74.93+/-64.40 | 73.37+/-66.06 | 0.841 |
| ALT (U/L) | 97.40+/-88.67 | 21.64+/-14.70 | <0.001* | 81.60+/-64.52 | 71.20+/-67.80 | 0.799 |
| TC (mg/dL) | 171.68+/-32.67 | 186.97+/-36.09 | <0.001* | 173.54+/-29.79 | 171.85+/-32.81 | 0.615 |
| HDL (mg/dL) | 48.15+/-13.80 | 49.85+/-13.35 | <0.001* | 48.83+/-14.82 | 49.91+/-14.77 | 0.314 |
| TG (mg/dL) | 101.35+/-46.74 | 120.73+/-75.58 | <0.001* | 115.97+/-66.98 | 104.79+/-47.70 | 0.069 |
| HOMA-IR | 2.88+/-4.75 | 2.74+/-2.98 | 0.374 | 4.50+/-5.94 | 4.69+/-7.81 | 0.703 |
| Uric acid (mg/dL) | 5.89+/-1.52 | 6.13+/-1.54 | <0.001* | 5.88+/-1.48 | 5.89+/-1.46 | 0.9 |
| WBC count (103/μL) | 5.85+/-1.94 | 5.83+/-1.81 | 0.801 | 5.56+/-1.69 | 5.13+/-1.26 | 0.032* |
| Platelets (103/μL) | 182.3+/-58.78 | 184.53+/-56.51 | 0.239 | 154.9+/-58.73 | 148.9+/-54.2 | 0.203 |
| hsCRP (mg/dL) | 1.59+/-2.73 | 1.78+/-4.46 | 0.494 | 1.58+/-1.99 | 1.90+/-3.64 | 0.436 |
| C3 (mg/dL) | 107.2+/-19.74 | 108.6+/-17.39 | 0.169 | 101.9+/-16.66 | 102.6+/-17.58 | 0.677 |
| C4 (mg/dL) | 20.32+/-7.30 | 21.55+/-7.07 | <0.001* | 19.08+/-7.77 | 19.47+/-8.33 | 0.536 |
| APRI | 1.47+/-1.65 | 0.50+/-0.39 | <0.001* | 1.76+/-1.84 | 1.71+/-1.75 | 0.845 |
| Hepatic steatosis (yes) | (51) | (52) | 0.630 | (43) | (49) | 0.288 |
| Leptin (pg/mL) | 9441.4+/-8992.8 | 9809.4+/-9448.8 | 0.543 | 10229.7+/-10528.5 | 10821.7+/-12650.4 | 0.763 |
| Log leptin (Log10pg/mL) | 3.74+/-0.55 | 3.77+/-0.54 | 0.392 | 3.83+/-0.38 | 3.85+/-0.40 | 0.835 |
SVR: sustained virological response; Log: log transformation; BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TC: total cholesterol; HDL: high-density lipoprotein cholesterol; TGs: triglycerides; HOMA-IR: homeostasis model assessment-estimated insulin resistance; WBC: white blood cell; hsCRP: high-sensitivity C-reactive protein; C3: complement component 3; C4: complement component 4; APRI: AST to platelet ratio index; SNP: single nucleotide polymorphism
*: p<0.05.
Discussion
To the best of our knowledge, this prospective study is the first to demonstrate the relationship between leptin and complements in CHC patients. There were several compelling results. (1). No differences in pre-therapy leptin levels were noted between patients with and without an SVR or between G1 and G2 patients. (2). Leptin levels were not associated with HCV RNA levels. (3). Sex, BMI, and C3 levels were independently associated with the leptin levels regardless of the presence of HCV. (4). The IFNL3 genotype, pre-therapy BMI, TC, and C4 were associated with pre-therapy C3 levels, whereas post-therapy BMI, ALT, TC, WBC count, C4, and hepatic steatosis were associated with post-therapy C3 levels. (5). Although both the leptin and C3 levels remained unchanged after viral clearance, C4 and TC, which were independent factors for C3, were significantly increased 24 weeks post-therapy.
Because both fibrosis and the IFNL3 non-CC genotype are two well-documented negative factors for SVR in interferon-based therapy [2], the reliability of this prospective study of 474 CHC patients who had completed a course of anti-HCV therapy is assured by the fact that the non-SVR patients had significantly more advanced fibrosis but a lower prevalence of the IFNL3 CC genotype than the SVR patients. Whether hyperleptinemia is associated with HCV infection [17,18,23] and the pan-genotypic [27,28] or genotype-specific anti-HCV therapeutic responses [29] has remained a matter of debate. In this study, based on the lack of an association between the leptin and HCV RNA levels, and the fact that the leptin levels remained unchanged after an SVR, it is likely that any relationship between leptin alterations and HCV infections is indirect rather than direct. Furthermore, the role of leptin levels in predicting the anti-HCV therapeutic response is negligible, regardless of the HCV genotype, as the pre-therapy leptin levels between the SVR and non-SVR patients and between G1 and G2 patients were similar.
Due to the unavailability of immunoprecipitation which directly assesses the interaction between two proteins [30], it is truly difficult to elucidate the role of leptin in homeostasis upon viral clearance in clinical studies of CHC, especially as the pre- and post-therapy leptin levels were similar. Thus, we adopted the "concept" of popular software programs that organize high-throughput bioinformatic data, such as Metacore and IPA [31], to dissect the interactions between the independent and dependent factors based on the statistical results and the literature. Previous studies of 133~194 nondiabetic CHC patients [19, 26] showed a positive relationship between HOMA-IR and leptin. Consistently, we could only demonstrate this trend in the patients without IR but not in those with IR. All of the above confirmed that the connection between the HOMA-IR and leptin levels vanished with deteriorating glucose metabolism, which may be an HCV-associated sequela [2]. In contrast, sex, BMI, and C3 were independently associated with the leptin level regardless of the presence of HCV infection. With regard to sex and BMI, that female and obese patients have higher leptin levels than male and lean subjects, respectively, is a central dogma of leptin dynamics [5]. The positive association of leptin with C3 is likely due to the regulation of leptin in innate immunity [15] and the co-association of leptin with BMI [5], which was consistent with the results of previous non-HCV studies of the co-culture of adipocytes with macrophages [32] and of obese subjects [16]. The above findings seemed to indicate a non-HCV-specific phenomenon in a CHC cohort. However, the close association between leptin and C3 and the different trends in post-therapeutic changes in C3 and C4 suggest that HCV infection may affect leptin in a qualitative but not quantitative manner through leptin-associated factors. Both C3 and C4 are major proteins of the complement pathways [33], and their synthesis has been shown to be transcriptionally downregulated by the HCV core and NS5A proteins in in vitro studies [34,35]. However, this negative regulation is not compatible with the results of either our previous study on conditional HCV core-expressing mice, which demonstrated C3 up-regulation in inflamed liver samples via microarray analyses [36], or with studies of CHC patients who had higher C3 and C4 levels than the controls [37]. The discrepancy may result from the fundamental differences between in vitro and in vivo immunological studies. In this clinical prospective study, we would like to stress that the positive associations between leptin and C3 and among C3, C4, and TC were consistent regardless of HCV infection (Fig 1). Interestingly, only C4 and TC but not C3 or leptin levels increased after SVR. Additionally, the pre-therapy IFNL3 genotype, a strong determinant of SVR [1,2,4], was associated with the pre-therapy C3 level, although this association diminished after anti-HCV therapy. Instead, the post-therapy C3 levels were affected by the post-therapy WBC count and ALT after viral clearance (Fig 1). This evolution suggests that C3 probably plays a role in HCV clearance. However, the role becomes non-HCV-specific after viral clearance. In contrast to leptin, which is primarily expressed in subcutaneous adipose tissue [5], higher expression levels of C3 and C4 have been reported in visceral than in subcutaneous adipose tissue [38]. The close associations between leptin, C3, and C4 suggest the presence of a strong collaboration between visceral and subcutaneous adipose tissues, which may be essential for maintaining whole-body homeostasis. Collectively, these findings highlight the general importance of leptin in homeostasis, as it needs to remain stable during viral infection but may modulate the immune response through C3, whose levels also remain stable but seem to facilitate immunity and metabolism in conjunction with C4 and TC, respectively. After anti-HCV therapy, only SVR patients had decreased levels of transaminase and APRI but increased lipid profile levels, It is subsequent to the reversal of HCV-associated hepatic injury and hypolipidemia after viral clearance [2,4]. Similarly, the increase in C4 after viral clearance indicated the reversal of the HCV-associated down-regulation of the complement system [34,35].
Because adipose tissue is the major source of leptin [4], the main limitation of this study is the lack of a pathological study of adipose tissue. Moreover, making conclusions based on analyzing the associated factors is an imperfect way to build a complete picture of the leptin-associated pathways. Future studies of leptin in CHC patients with adipose tissue pathology surveys and associated fundamental cellular or animal models studies such as immunopreciptation [30] may be required to elucidate the genuine connection and molecular basis between leptin and C3.
Together, our results demonstrate that sex, BMI, and C3 levels are independently associated with leptin levels and that TC and C4 levels are independently associated with C3 levels regardless of the presence of HCV. Compared with the pre-therapy levels, leptin and C3 remained unchanged 24 weeks post-therapy regardless of the therapeutic response, whereas the levels of C4 and TC increased among patients with an SVR. During HCV infection, leptin and C3 may maintain the homeostasis of metabolism and immunity based on associations with C4 and TC, the positive post-therapy alterations of which reflect viral clearance. These findings will facilitate the development of agents or strategies to probe viral-related metabolic and immunity alterations.
Supporting Information
(XLS)
Acknowledgments
The authors thank Mr. Chun-Ming Fan from the Department of Biomedical Sciences, College of Medicine, Chang Gung University for his excellent figure generation; Ms. Shu-Chun Chen from the Liver Research Center, Chang Gung Memorial Hospital, Taiwan, for her data mining; and Mr. Yu-Jr Lin from the Resource Center of Clinical Research, Chang Gung Memorial Hospital, Linko, Taiwan, for his consultation regarding the statistical analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from the Chang Gung Medical Research Program (CMRPG3F0471, CRRPG3F0011, XMRPG3A0521 and CIRPG3D0121) and the National Science Council, Taiwan (102-2628-B-182-021-MY3, MOST 105-2314-B-182-023, and MOST 105-2629-B-182-001-) to Ming-Ling Chang. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chang ML, Liang KH, Ku CL, Lo CC, Cheng YT, Hsu CM, et al. Resistin reinforces interferon λ-3 to eliminate hepatitis C virus with fine-tuning from RETN single-nucleotide polymorphisms. Sci Rep. 2016;6: 30799 10.1038/srep30799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang ML. Metabolic alterations and hepatitis C: from bench to bedside. World J Gastroenterol. 2016;22: 1461–1476. 10.3748/wjg.v22.i4.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu JH, Chen MY, Yeh CT, Lin HS, Lin MS, Huang TJ, et al. Sexual dimorphic metabolic alterations in hepatitis C Virus-infected patients: A community-based study in a hepatitis B/hepatitis C Virus hyperendemic area. Medicine (Baltimore). 2016;95: e3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang ML, Tsou YK, Hu TH, Lin CH, Lin WR, Sung CM, et al. Distinct patterns of the lipid alterations between genotype 1 and 2 chronic hepatitis C patients after viral clearance. PLOS ONE. 2014;9: e104783 10.1371/journal.pone.0104783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, et al. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30: 329–336. 10.1111/jgh.12705 [DOI] [PubMed] [Google Scholar]
- 6.Funahashi T, Matsuzawa Y. Metabolic syndrome: clinical concept and molecular basis. Ann Med. 2007;39: 482–494. 10.1080/07853890701491026 [DOI] [PubMed] [Google Scholar]
- 7.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62: 413–437. 10.1146/annurev.physiol.62.1.413 [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76: 253–262. 10.1016/0092-8674(94)90333-6 [DOI] [PubMed] [Google Scholar]
- 9.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35: 762–771. 10.1053/jhep.2002.32029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12: 57–65 [PubMed] [Google Scholar]
- 11.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol 2002;37: 206–213. [DOI] [PubMed] [Google Scholar]
- 12.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122: 1399–1410. 10.1053/gast.2002.32995 [DOI] [PubMed] [Google Scholar]
- 13.Patel K, Muir A, McHutchison JG, Patton HM. A link between leptin and steatosis in chronic hepatitis C? Time to weigh up the fats. Am J Gastroenterol. 2003;98: 952–955. 10.1111/j.1572-0241.2003.07422.x [DOI] [PubMed] [Google Scholar]
- 14.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394: 897–901. 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- 15.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122: 2011–2025. 10.1053/gast.2002.33631 [DOI] [PubMed] [Google Scholar]
- 16.Nestvold TK, Nielsen EW, Ludviksen JK, Fure H, Landsem A, Lappegård KT. Lifestyle changes followed by bariatric surgery lower inflammatory markers and the cardiovascular risk factors C3 and C4. Metab Syndr Relat Disord. 2015;13: 29–35. 10.1089/met.2014.0099 [DOI] [PubMed] [Google Scholar]
- 17.Giannini E, Ceppa P, Botta F, Mastracci L, Romagnoli P, Comino I, et al. Leptin has no role in determining severity of steatosis and fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2000;95: 3211–3217. 10.1111/j.1572-0241.2000.03294.x [DOI] [PubMed] [Google Scholar]
- 18.Widjaja A, Wedemeyer H, Tillmann HL, Horn R, Ockenga J, Jaeckel E, et al. Hepatitis C and the leptin system: bound leptin levels are elevated in patients with hepatitis C and decrease during antiviral therapy. Scand J Gastroenterol. 2001;36: 426–431. [DOI] [PubMed] [Google Scholar]
- 19.Cua IH, Hui JM, Bandara P, Kench JG, Farrell GC, McCaughan GW, et al. Insulin resistance and liver injury in hepatitis C is not associated with virus-specific changes in adipocytokines. Hepatology. 2007;46: 66–73. 10.1002/hep.21703 [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, et al. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology. 2006;349: 347–358. 10.1016/j.virol.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 21.Romero-Gómez M, Castellano-Megias VM, Grande L, Irles JA, Cruz M, Nogales MC, et al. Serum leptin levels correlate with hepatic steatosis in chronic hepatitis C. Am J Gastroenterol. 2003;98: 1135–1141. 10.1111/j.1572-0241.2003.07450.x [DOI] [PubMed] [Google Scholar]
- 22.Petit JM, Minello A, Jooste V, Bour JB, Galland F, Duvillard L, et al. Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J Clin Endocrinol Metab. 2005;90: 2240–2243. 10.1210/jc.2004-1266 [DOI] [PubMed] [Google Scholar]
- 23.Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, et al. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39: 1042–1048. 10.1016/S0168-8278(03)00463-X [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41: 1313–1321. 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 25.Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20: 15–20. 10.1002/hep.1840200104 [DOI] [PubMed] [Google Scholar]
- 26.Lemoine M, Chevaliez S, Bastard JP, Fartoux L, Chazouillères O, Capeau J, et al. Association between IL28B polymorphism, TNFα and biomarkers of insulin resistance in chronic hepatitis C-related insulin resistance. J Viral Hepat. 2015;22: 890–896. 10.1111/jvh.12408 [DOI] [PubMed] [Google Scholar]
- 27.Nkontchou G, Bastard JP, Ziol M, Aout M, Cosson E, Ganne-Carrie N, et al. Insulin resistance, serum leptin, and adiponectin levels and outcomes of viral hepatitis C cirrhosis. J Hepatol. 2010;53: 827–833. 10.1016/j.jhep.2010.04.035 [DOI] [PubMed] [Google Scholar]
- 28.Eguchi Y, Mizuta T, Yasutake T, Hisatomi A, Iwakiri R, Ozaki I, et al. High serum leptin is an independent risk factor for non-response patients with low viremia to antiviral treatment in chronic hepatitis C. World J Gastroenterol. 2006;12: 556–560. 10.3748/wjg.v12.i4.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlidis C, Panoutsopoulos GI, Tiniakos D, Koutsounas S, Vlachogiannakos J, Zouboulis-Vafiadis I. Serum leptin and ghrelin in chronic hepatitis C patients with steatosis. World J Gastroenterol. 2011;17: 5097–5104. 10.3748/wjg.v17.i46.5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Song X, Nisbet R, Götz J. Co-immunoprecipitation with Tau isoform-specific antibodies reveals distinct protein interactions and highlights a putative role for 2N Tau in disease. J Biol Chem. 2016;291: 8173–8188. 10.1074/jbc.M115.641902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeGrand J, Park ES, Wang H, Gupta S, Owens JD Jr, Nelson PJ, et al. Global gene expression profiling in mouse plasma cell tumor precursor and bystander cells reveals potential intervention targets for plasma cell neoplasia. Blood. 2012;119: 1018–1028. 10.1182/blood-2011-06-363887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Gauvreau D, Tom FQ, Lapointe M, Luo XP, Cianflone K. Inflammatory markers and adipokines alter adipocyte-derived ASP production through direct and indirect immune interaction. Exp Clin Endocrinol Diabetes. 2013;121: 194–200. 10.1055/s-0032-1333231 [DOI] [PubMed] [Google Scholar]
- 33.Szabová M, Jahnová E, Horváthová M, Ilavská S, Pružincová V, Nemessányi T, et al. Changes in immunologic parameters of humoral immunity and adipocytokines in obese persons are gender dependent. Hum Immunol. 2012;73: 486–492. 10.1016/j.humimm.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 34.Kim H, Bose SK, Meyer K, Ray R. Hepatitis C virus impairs natural killer cell-mediated augmentation of complement synthesis. J Virol. 2014;88: 2564–2571. 10.1128/JVI.02988-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazumdar B, Kim H, Meyer K, Bose SK, Di Bisceglie AM, Ray RB, et al. Hepatitis C virus proteins inhibit C3 complement production. J Virol. 2012;86: 2221–2228. 10.1128/JVI.06577-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang ML, Yeh CT, Lin DY, Ho YP, Hsu CM, Bissell DM. Hepatic inflammation mediated by hepatitis C virus core protein is ameliorated by blocking complement activation. BMC Med Genomics. 2009;2: 51 10.1186/1755-8794-2-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali OS, Abo-Shadi MA, Hammad LN. The biological significance of serum complements C3 and C4 in HCV-related chronic liver diseases and hepatocellular carcinoma. Egypt J Immunol. 2005;12: 91–99. [PubMed] [Google Scholar]
- 38.Gabrielsson BG, Johansson JM, Lönn M, Jernås M, Olbers T, Peltonen M, et al. High expression of complement components in omental adipose tissue in obese men. Obes Res. 2003;11: 699–708. 10.1038/oby.2003.100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


