Abstract
The functions of basic helix-loop-helix (bHLH) transcription factor-differentiated embryonic chondrocyte (DEC)1 (BHLHE40) and 2 (BHLHE41) are involved in various fields such as circadian rhythms, immune responses, cell proliferation, hypoxia reaction as well as malignant tumors. Previous findings showed that DEC served as apoptosis regulators of various cancer cell lines. However, little is known regarding the expression of DEC1 and DEC2 in prostate cancer cells. The present study aimed to examine the roles of DEC1 and DEC2 in human prostate cancer DU145 and PC-3 cells that were treated with paclitaxel. The expression of DEC1 and DEC2 was decreased in DU145 cells but was increased in PC-3 cells when treated with paclitaxel. DU145 cells were more sensitive to paclitaxel than PC-3 cells since the amount of cleaved poly(ADP-ribose) polymerase (PARP) reached its peak at 50 μM of paclitaxel in DU145 cells but at 100 μM in PC-3 cells. In addition, the amount of cleaved PARP was decreased by DEC1 siRNA, while it was increased by DEC2 siRNA in the presence of paclitaxel. Although DEC2 overexpression slightly inhibited cleaved PARP in the two cell lines, the effects of DEC1 overexpression on apoptosis remain to be determined. In conclusion, DEC1, at least partly, exerted a pro-apoptotic effect, whereas DEC2 exerted an anti-apoptotic effect in paclitaxel-induced apoptosis of human prostate cancer cells.
Keywords: DEC1, DEC2, paclitaxel, prostate cancer, DU145 cells, PC-3 cells
Introduction
Prostate cancer remains among the most frequently diagnosed solid tumors in men. According to the latest cancer statistics, prostate cancer accounts for 27% of all cancer incidences and ranks the second leading cause of cancer-related mortality among men in the United States (1). Although androgen deprivation therapy is initially effective for treating localized and androgen responsive prostate cancer, most patients relapse due to the development of castration-resistant prostate cancer (CRPC) after 2–3 years (2). Treatment options for castration-resistant prostate cancer are limited and often associated with significant morbidity and mortality. Mitoxantrone plus prednisone were initially identified as the standard chemotherapy for hormone-refractory prostate cancers until the drug taxane was applied for treatment. Based on a simultaneous publication of two large randomized control trials, Tannock et al showed that combined taxane drugs and prednisone can significantly prolong survival in men with hormone-refractory prostate cancer (3,4).
Paclitaxel is one of the typical taxane drugs and is also a well-studied chemotherapeutic agent. Paclitaxel stabilizes guanosine diphosphate (GDP)-bound tubulin to prevent the depolymerization of microtubules, thereby terminating cell division. Paclitaxel has clinical efficacy in various types of cancer, including several refractory tumors such as ovarian carcinoma, acute myeloblastic leukemia, and CRPC (5–7). Specific mechanisms for paclitaxel inducing inhibition in cancer cells are considered to be mediated by the activation of c-Jun N-terminal kinase (JNK), downregulation of Bcl-2/Bcl-xL and the activation of caspases and poly(ADP-ribose) polymerase PARP (8–11), resulting in the induction of apoptosis. It can also cause growth arrest at the G2/M phase of the cell cycle, leading to the promotion of cell apoptosis (11,12).
Differentiated embryonic chondrocyte gene (DEC)1 and 2 are members of the basic helix-loop-helix (bHLH) superfamily of transcription factors that have been reported to be associated with cell proliferation, circadian rhythms, tumor progression, as well as the response to hypoxia (13–16). In a previous study, we showed that DEC1 and DEC2 have opposite properties in regulating apoptosis, i.e., DEC2 has anti-apoptotic, whereas DEC1 has pro-apoptotic effects on an estrogen receptor-positive cell line, MCF-7, when treated with paclitaxel (17). However, the roles of DEC1 and DEC2 in apoptosis induced by paclitaxel in CRPC are unknown.
In the present study, we investigated the effects of DEC1 and DEC2 on paclitaxel-induced apoptosis of DU145 and PC-3 cells. The results demonstrated that DEC1 has pro-apoptotic effects, and DEC2 has anti-apoptotic effects on paclitaxel-treated DU145 and PC-3 cells.
Materials and methods
Cell culture and treatment
The DU145 and PC-3 human prostate cancer cells were purchased from RIKEN BRC through the National Bio-Resource Project of the MEXT (Japan). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 95% air and 5% CO2. In some experiments, the cells were incubated with various concentrations of paclitaxel (Calbiochem, San Diego, CA, USA) for 24, 48 or 72 h.
Knockdown of DEC1 or DEC2 by RNA interference
Short interference RNA (siRNA) against DEC1 or DEC2 were synthesized by Qiagen (Mississauga, ON, Canada). The sequences of DEC1, DEC2, and the negative control siRNA were described previously (18). For the siRNA transfection experiments, 5×104 cells of DU145 or PC-3 cells were seeded per 35-mm well. SiRNAs were transfected into the cells 24 h later using the Lipofectamine RNA iMAX reagent (Invitrogen, Carlsbad, CA, USA). After transfection, the cells were incubated for another 24 h and subjected to western blot analysis.
DEC1 and DEC2 overexpression
Human DEC1 and DEC2 plasmids were a kind gift from Dr Katsumi Fujimoto (Hiroshima University) (14). DU145 or PC-3 cells (5×104) were seeded per 35-mm well. DEC1 or DEC2 plasmid was transiently transfected into the cells 24 h later using the Lipofectamine LTX reagent (Invitrogen). Following transfection, the cells were incubated with paclitaxel for another 24 h and then subjected to western blot analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Three independent RNA samples (n=3) from DU145 and PC-3 cells were prepared for RT-PCR. Total RNA was isolated using an RNeasy RNA isolation kit (Qiagen). First-strand cDNA was synthesized from 1 μg of total RNA using ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan). PCR was carried out using Taq PCR Master mix (Qiagen). The primer sequences used were: DEC1 F, 5′-GTCTGTGAGTCACTCTTCAG-3′ and R, 5′-GAGTCTAGTTCTGTTTGAAGG-3′; DEC2 F, 5′-CACCTTTGACGTCTTTGGAG-3′ and R, 5′-GAGAGTGGGAATAGATGCAC-3′ and GAPDH F, 5′-CCACCCATGGCAAATTCCATGGCA-3′ and R, 5′-AGACCACCTGGTGCTCAGTGTAGC-3′. The amplified products of DEC1, DEC2 and GAPDH were 534, 502 and 696 bp in length, respectively. The cDNAs for DEC1 and DEC2 were amplified for >27 cycles, and those for GAPDH were amplified at 20 cycles. The PCR products were separated on 1.5% (w/v) agarose gels.
Western blot analysis
Cells were lysed using M-PER lysis buffer (Thermo Scientific, Waltham, MA, USA), and their protein concentrations were determined using the bicinchoninic acid (BCA) assay. The obtained lysates (5 μg protein) were subjected to SDS-PAGE, and the separated proteins were transferred to PVDF membranes (Immobilon P, Millipore, Billerica, MA, USA), followed by immunoblot analysis utilizing the indicated antibodies. Signals were detected using Bio-Rad western blotting systems (Bio-Rad, Hercules, CA, USA) with the ECL-prime or ECL-select western blot analysis detection systems (GE Healthcare, Little Chalfont, UK).
Cell viability assay
The cells seeded in 96-well plate were cultured with different concentrations of paclitaxel for 24, 48 and 72 h. In another experiment, the cells were initially transfected with the DEC2 overexpression plasmid and culture was continued in medium with 50 μM paclitaxel for an additional 24 h; the cells transfected with pcDNA without paclitaxel treatment were used as the control. Cell viability analysis was performed using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay as described previously (19).
Statistical analysis
The results are presented as the means ± standard error of the mean (SEM). Statistical analysis was performed using the Student's t-test. A value of P<0.05 was considered to indicate statistically significant results.
Results
Effects of paclitaxel on DEC1 and DEC2 expression in DU145 and PC-3 cells
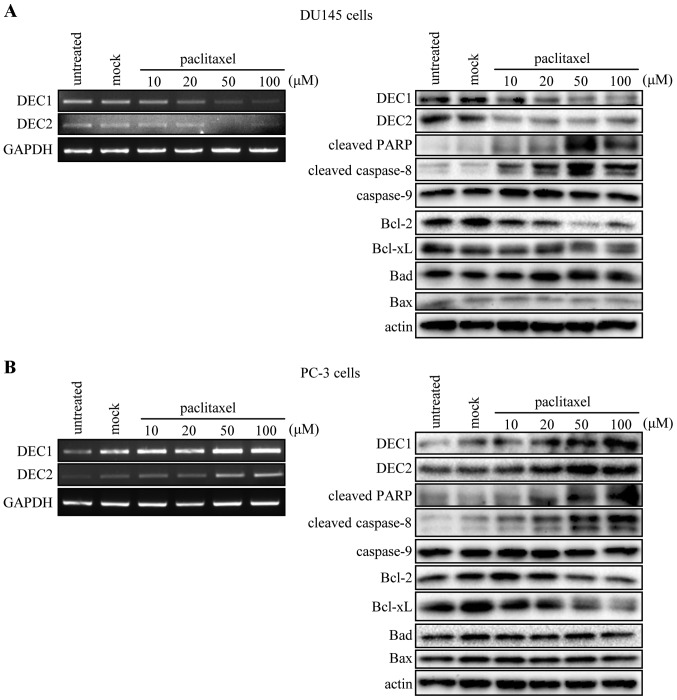
It is known that paclitaxel induces the apoptosis of various cancer cells. Thus, we examined the expression of apoptotic markers, as well as that of DEC1 and DEC2 in DU145 and PC-3 cells treated with 10, 20, 50 and 100 μM of paclitaxel. The mRNA and protein levels of DEC1 and DEC2 were decreased in a dose-dependent manner in DU145 cells when treated with paclitaxel for 24 h (Fig. 1A, left panel), but were increased in PC-3 cells (Fig. 1B, left panel). In addition, paclitaxel increased the amount of cleaved PARP and cleaved caspase-8, but decreased that of Bcl-2 and Bcl-xL. However, the level of caspase-9, Bad, and Bax was not affected by paclitaxel in the two cell lines (Fig. 1A and B right panel).
Figure 1.
Paclitaxel affects the expression of DEC1 and DEC2. DU145 and PC-3 cells were treated with various concentrations of paclitaxel. After 24 h, total RNA was prepared and subjected to RT-PCR analysis of DEC1, DEC2 and GAPDH (A and B, left panel). DU145 and PC-3 cells were treated with paclitaxel for 24 h. The cells were lysed, and the lysates were subjected to western blot analysis of DEC1, DEC2, cleaved PARP, cleaved caspase-8, caspase-9, Bcl-2, Bcl-xL, Bad, Bax, and actin (A and B, right panel). DEC, differentiated embryonic chondrocyte ; PARP, poly (ADP-ribose) polymerase.
Knockdown of DEC1 and DEC2 in the presence of paclitaxel has opposite effects on apoptotic markers in DU145 and PC-3 cells
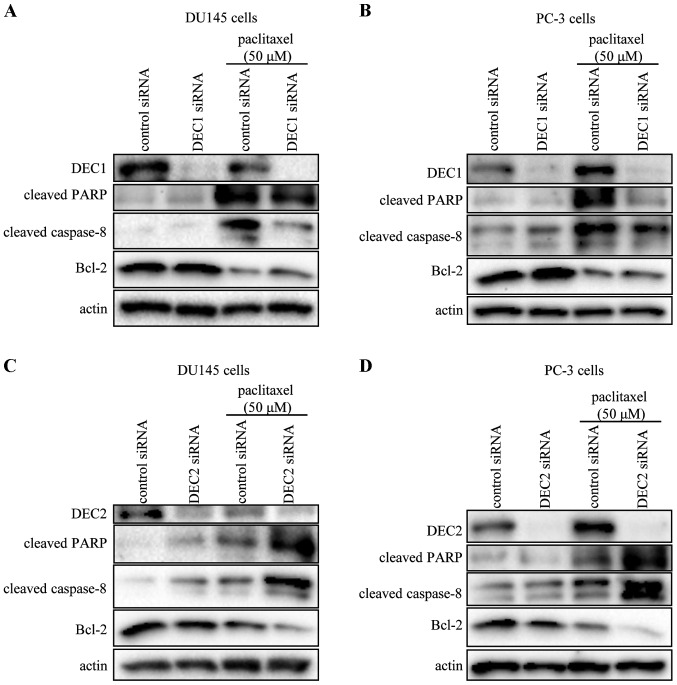
DEC1 or DEC2 knockdown by siRNA significantly reduced the expression of DEC1 or DEC2, respectively (Fig. 2). Compared with DU145 or PC-3 cells transfected with control siRNA, paclitaxel-combined control siRNA induced the amount of cleaved PARP and cleaved caspase-8, which was decreased by DEC1 siRNA. On the other hand, the expression of Bcl-2 was downregulated by paclitaxel treatment, but was upregulated when DEC1 was knocked down (Fig. 2A and B). By contrast, DEC2 knockdown increased the amount of cleaved PARP and caspase-8, whether with or without paclitaxel (Fig. 2C and D). The expression of Bcl-2, which was downregulated by paclitaxel treatment, was further inhibited when DEC2 was knocked down.
Figure 2.
Analysis of apoptotic marker expression in DU145 and PC-3 cells by DEC1 or DEC2 knockdown. DU145 and PC-3 cells were transfected with DEC1 siRNA (A and B) or DEC2 siRNA (C and D) and incubated for 24 h. Subsequently, the cells were incubated with or without paclitaxel (50 μM) for another 24 h. Cell lysates were prepared and subjected to western blot analysis of DEC1, DEC2, cleaved PARP, cleaved caspase-8, Bcl-2 and actin. One representative of at least three independent experiments with similar results is shown. DEC, differentiated embryonic chondrocyte; PARP, poly (ADP-ribose) polymerase.
Overexpression of DEC1 or DEC2 has no effect on apoptotic markers in DU145 and PC-3 cells
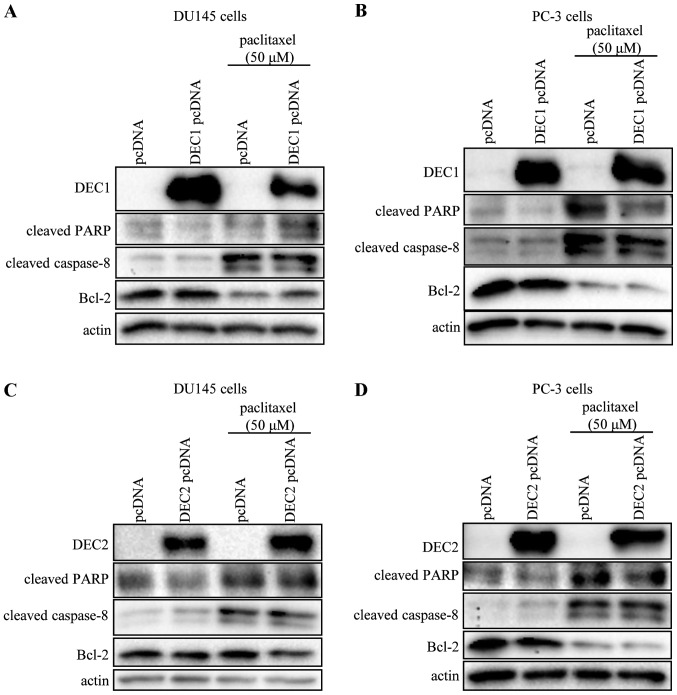
Using transient transfection, we examined whether the overexpression of DEC1 or DEC2 affected the expression of apoptotic markers in DU145 and PC-3 cells. DEC1 and DEC2 overexpression in the presence or absence of paclitaxel (50 μM) showed little effect on the expression of cleaved PARP, cleaved caspase-8, and Bcl-2 (Fig. 3).
Figure 3.
Overexpression of DEC1 or DEC2 has no effect on the expression of apoptotic markers in DU145 and PC-3 cells. DU145 and PC-3 cells were transfected with DEC1 pcDNA (A and B) or DEC2 pcDNA (C and D) and incubated for 24 h. The cells were treated with paclitaxel (50 μM) and incubated for an additional 24 h, before being lysed. The lysates were subjected to western blot analysis of DEC1, DEC2, cleaved PARP, cleaved caspase-8, Bcl-2 and actin. One representative of at least three independent experiments with similar results is shown. DEC, differentiated embryonic chondrocyte; PARP, poly (ADP-ribose) polymerase.
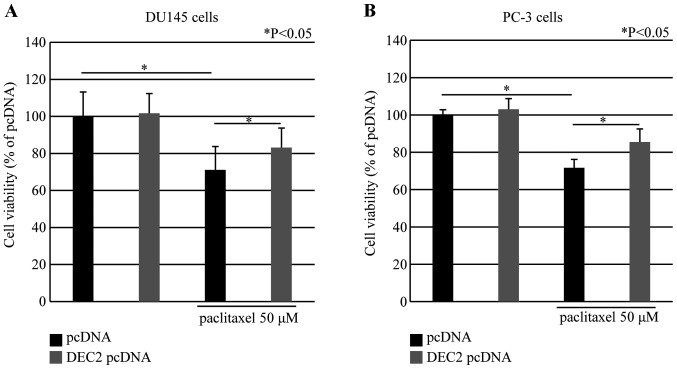
DEC2 overexpression inhibits cell death in DU145 and PC-3 cells
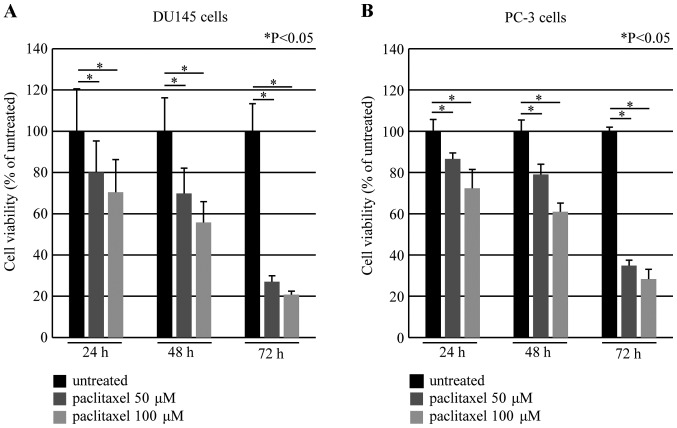
Paclitaxel induced cell death in a time- and dose-dependent manner in DU145 and PC-3 cells using the MTS assay (Fig. 4). We also investigated whether DEC2 overexpression affected cell viability in these cells. DEC2 overexpression in the presence of paclitaxel (50 μM) significantly increased the cell viability compared with that in paclitaxel-treated control cells. In addition, DEC2 overexpression in the absence of paclitaxel had little effect on the viability in these cells (Fig. 5).
Figure 4.
Paclitaxel-induced time- and dose-dependent cell death in DU145 and PC-3 cells. The cells were treated with 50 or 100 μM of paclitaxel for 24, 48 and 72 h, and cell viability was measured using the MTS-assay in (A) DU145 and (B) PC-3 cells. The values are shown as a percentage of each control. Each value represents the mean ± SEM (bars) of three independent experiments (*P<0.05, compared with the untreated). MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium].
Figure 5.
DEC2 overexpression inhibits cell death induced by paclitaxel in DU145 and PC-3 cells. DU145 or PC-3 cells transfected with DEC2 pcDNA were treated with or without 50 μM of paclitaxel for 24 h. Cell viability was measured using the MTS assay in (A) DU145 and (B) PC-3 cells. The values are shown as a percentage of the control. Each value is the mean ± SEM (bars) of three independent experiments (*P<0.05, compared with the control pcDNA). DEC2, differentiated embryonic chondrocyte 2; MTS, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium].
Discussion
In the present study, we examined how DEC1 or DEC2 functions during apoptosis caused by the anti-cancer drug paclitaxel. Two of the well-characterized androgen-independent prostate cancer cell lines DU145 and PC-3, were selected in the present study as many prostate cancer patients showed androgen-independent growth. Paclitaxel-induced apoptosis in the two cells, however, differential chemotherapeutic response was found between the two cells. Paclitaxel treatment (50 μM) produced the largest amount of cleaved PARP in DU145 cells and 100 μM was needed in PC-3 cells. Additionally, >50% of DU145 cells died when cultured in 100 μM of paclitaxel, which explains the relatively weaker band of cleaved-PARP obtained in these cells. Moreover, the viability of DU145 cells following paclitaxel treatment showed a more significant reduction than that of PC-3 cells. This difference may be caused by the status of a tumor suppressor gene, PTEN. Mutations or loss of expression of this gene was found in a high percentage of prostate cancer patients. Lee et al showed that PC-3 was a PTEN-negative cell line, while DU145 was a PTEN-positive cell line (20). On the other hand, the expression of apoptotic markers, as well as DEC was differentially regulated in the two cell lines. Thus, DEC1 and DEC2 were decreased by paclitaxel in DU145 cells but increased in PC-3 cells. Previous studies showed that DEC1 and DEC2 were regulated by many environmental and endogenous factors in various cell lines (21–24). Changes of DECs were dependent on the cell lines and the types of reagents, or even the amount of the reagents applied. The function of paclitaxel on DEC in the breast MCF-7 adeno-carcinoma cell line has been previously discussed (17). In MCF-7 cells, the expression of DEC1 and DEC2 was increased by paclitaxel in a dose-dependent manner. Additionally, the extent of DEC2 upregulation was much higher than that of DEC1. In another report, we analyzed the effect of DEC during cisplatin-induced apoptosis (25). Cisplatin decreased DEC1 and DEC2 in an oral squamous carcinoma CA9-22 cell line, but DEC1 and DEC2 were not directly associated with apoptosis in this cell line. By contrast, in HSC-3, another oral squamous carcinoma cell line, cisplatin increased DEC2, which inhibited apoptosis by regulating Bim protein. Although a similar protein structure exists in DEC1 and DEC2, it is better to consider them distinctly when referring to their functions. For example, DEC2 was usually identified as an apoptosis inhibitor whereas the role of DEC1 in apoptosis is under debate (16,17). Another report suggested that DEC1 was induced but DEC2 was reduced when PANC-1 cells were exposed to transforming growth factor (TGF)-β (26). In addition, DEC1 exerted its effect on transcription factors that subsequently regulated the epithelial-mesenchymal molecules to progress the epithelial-mesenchymal transition (EMT) of carcinoma cells, whereas DEC2 did not exert such an effect. As effectors of hypoxia, DEC1 response was more rapid to hypoxic stress than DEC2. Previous findings showed that the upregulation of DEC2 in the latter stage of hypoxia accompanied with the increase in cell proliferation led to oxygen-deprived stress (19).
In the present study, DU145 and PC-3 cells transfected with DEC1 siRNA under paclitaxel treatment showed decreased levels of cleaved PARP and cleaved caspase-8, but an increased level of anti-apoptotic factor Bcl-2 when compared with the control siRNA-transfected cells. However, DEC2 siRNA-transfected cells exhibited opposite results to those mentioned above. Since DEC1 or DEC2 functioned similarly in regulating apoptosis but showed a distinct response to paclitaxel in DU145 and PC-3 cells, the key factor in modulating apoptosis was considered to be different in the two cell lines. In addition to the knockdown analyses, overexpression experiments were also applied in the present study and a weak downregulation of cleaved PARP as well as a slight upregulation in cell viability by DEC2 overexpression were obtained in the two cell lines, although significant changes of cleaved caspase-8 and Bcl-2 were not found. On the other hand, DEC1 overexpression had an unstable effect on the expression of apoptotic markers. Since proteins exert their functions only at optimal concentrations, this may provide a more plausible explanation for the fact that the results of overexpression treatment were not consistent with those of the knockdown treatment.
In conclusion, the results of the present study confirm the anti-apoptotic character of DEC2 in paclitaxel-induced apoptosis, in two prostate adenocarcinoma cell lines. In addition to DU145 and PC-3 cell lines, DEC2 has been shown to inhibit apoptosis in breast carcinoma MCF-7 cells (17) oral squamous cell carcinoma HSC-3 cells (25), and esophageal squamous cell carcinoma (ESCC) TE-11 cells (article accepted). Inhibitor of DEC2 may be developed and be used as one of the choices when conferring CRPC in clinical chemotherapy in the future.
Acknowledgments
The present study was supported by Grants-in-Aid for Science from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant for Hirosaki University Institutional Research; and the Fund for the Promotion of International Scientific Research.
Abbreviations
- DEC1
differentiated embryonic chondrocyte 1
- DEC2
differentiated embryonic chondrocyte 2
- PARP
poly (ADP-ribose) polymerase
- CRPC
castration-resistant prostate cancer
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP1, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Kelly WK, Curley T, Slovin S, Heller G, McCaffrey J, Bajorin D, Ciolino A, Regan K, Schwartz M, Kantoff P, et al. Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol. 2001;19:44–53. doi: 10.1200/JCO.2001.19.1.44. [DOI] [PubMed] [Google Scholar]
- 6.McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC. Taxol: A unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111:273–279. doi: 10.7326/0003-4819-111-4-273. [DOI] [PubMed] [Google Scholar]
- 7.Rowinsky EK, Burke PJ, Karp JE, Tucker RW, Ettinger DS, Donehower RC. Phase I and pharmacodynamic study of taxol in refractory acute leukemias. Cancer Res. 1989;49:4640–4647. [PubMed] [Google Scholar]
- 8.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC, Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 9.Kolomeichuk SN, Terrano DT, Lyle CS, Sabapathy K, Chambers TC. Distinct signaling pathways of microtubule inhibitors-vinblastine and Taxol induce JNK-dependent cell death but through AP-1-dependent and AP-1-independent mechanisms, respectively. FEBS J. 2008;275:1889–1899. doi: 10.1111/j.1742-4658.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- 10.Tudor G, Aguilera A, Halverson DO, Laing ND, Sausville EA. Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000;7:574–586. doi: 10.1038/sj.cdd.4400688. [DOI] [PubMed] [Google Scholar]
- 11.Lim SJ, Choi MK, Kim MJ, Kim JK. Alphatocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells. Exp Mol Med. 2009;41:737–745. doi: 10.3858/emm.2009.41.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders DE, Lawrence WD, Christensen C, Wappler NL, Ruan H, Deppe G. Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. Int J Cancer. 1997;70:214–220. doi: 10.1002/(SICI)1097-0215(19970117)70:2<214::AID-IJC13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA. 2000;97:4058–4063. doi: 10.1073/pnas.070526297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti J, Turley H, Campo L, Han C, Harris AL, Gatter KC, Fox SB. The transcription factor DEC1 (stra13, SHARP2) is associated with the hypoxic response and high tumour grade in human breast cancers. Br J Cancer. 2004;91:954–958. doi: 10.1038/sj.bjc.6602059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y, Yan B. The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter. Oncogene. 2006;25:3296–3306. doi: 10.1038/sj.onc.1209363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Morohashi S, Kato Y, Kijima H. Basic helix-loop-helix transcription factors DEC1 and DEC2 regulate the paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer cells. Int J Mol Med. 2011;27:491–495. doi: 10.3892/ijmm.2011.617. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Sato F, Kawamoto T, Fujimoto K, Morohashi S, Akasaka H, Kondo J, Wu Y, Noshiro M, Kato Y, et al. Anti-apoptotic effect of the basic helix-loop-helix (bHLH) transcription factor DEC2 in human breast cancer cells. Genes Cells. 2010;15:315–325. doi: 10.1111/j.1365-2443.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Sato H, Suzuki T, Yoshizawa T, Morohashi S, Seino H, Kawamoto T, Fujimoto K, Kato Y, Kijima H. Involvement of c-Myc in the proliferation of MCF-7 human breast cancer cells induced by bHLH transcription factor DEC2. Int J Mol Med. 2015;35:815–820. doi: 10.3892/ijmm.2014.2042. [DOI] [PubMed] [Google Scholar]
- 20.Lee JT, Jr, Steelman LS, McCubrey JA. Phosphatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 21.Thin TH, Li L, Chung TK, Sun H, Taneja R. Stra13 is induced by genotoxic stress and regulates ionizing-radiation-induced apoptosis. EMBO Rep. 2007;8:401–407. doi: 10.1038/sj.embor.7400912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki K, Kawamoto T, Tanimoto K, Nishiyama M, Honda H, Kato Y. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J Biol Chem. 2002;277:47014–47021. doi: 10.1074/jbc.M204938200. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Kawata H, Shou Z, Mizutani T, Noguchi T, Miyamoto K. Insulin induces the expression of the SHARP-2/Stra13/DEC1 gene via a phosphoinositide 3-kinase pathway. J Biol Chem. 2003;278:30719–30724. doi: 10.1074/jbc.M301597200. [DOI] [PubMed] [Google Scholar]
- 24.Kon N, Hirota T, Kawamoto T, Kato Y, Tsubota T, Fukada Y. Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol. 2008;10:1463–1469. doi: 10.1038/ncb1806. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Sato F, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Kato Y, Kijima H. BHLH transcription factor DEC2 regulates pro-apoptotic factor Bim in human oral cancer HSC-3 cells. Biomed Res. 2012;33:75–82. doi: 10.2220/biomedres.33.75. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada K, et al. The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol. 2012;41:1337–1346. doi: 10.3892/ijo.2012.1559. [DOI] [PubMed] [Google Scholar]