Abstract
Context
Few studies have examined the associations between sleep duration, shiftwork, and exercise to the infrequent menstruation, hyperandrogenism, and ovarian morphological changes observed in women with polycystic ovarian syndrome (PCOS).
Objective
To examine whether lifestyle factors, including short sleep duration, insufficient exercise, and shiftwork, alone or in combination, are associated with the reproductive and metabolic abnormalities typical of PCOS in a healthy population.
Study Design, Size, Duration
Prospective cross-sectional study of 231 women, including healthcare workers recruited for an annual health screen, healthy referral patients from the Women’s Clinic and volunteers from the university community at the National University Hospital, Singapore, from 2011 to 2015.
Main Outcome Measures
The women completed a questionnaire, including their menstrual cycle length, sleep length, frequency of exercise and shift work. Hyperandrogenism (hirsutism score, testosterone, sex hormone binding globulin (SHBG)), ovarian morphology and function (anthral follicle count, ovarian volume, anti-mullerian hormone (AMH)), and metabolic measures (body mass index (BMI), waist hip ratio (WHR), blood pressure, fasting glucose, fasting insulin and fasting lipids) were examined through anthropometric measurements, transvaginal ultrasound scans, and blood tests.
Results
No significant associations were observed between shift work, exercise or sleep duration and the androgenic and ovarian measures that define PCOS. However, women reporting fewer than 6 hours of sleep were more likely to report abnormal (short or long) menstrual cycle lengths (OR = 2.1; 95% CI, 1.1 to 4.2). Women who reported fewer than 6 hours of sleep had increased fasting insulin levels (difference in means = 2.13; 95% CI, 0.27 to 3.99 mU/L) and higher odds of insulin resistance (OR = 2.58; CI, 1.16 to 5.76). Lack of regular exercise was associated with higher mean fasting insulin (difference in means = 2.3 mU/L; 95% CI, 0.5 to 4.1) and HOMA-IR (difference in means = 0.49; 95% CI, 0.09 to 0.90) levels.
Conclusions
Women with insufficient sleep are at increased risk of menstrual disturbances and insulin resistance, but do not have the hyperandrogenism and polycystic ovarian morphology typical of PCOS.
Wider Implications of the Findings
Improved sleep duration may help reduce the risks of diabetes or infertility. Shift work, exercise or sleep duration appear not to impact the androgenic and ovarian measures that define PCOS.
Introduction
The typical phenotype of polycystic ovarian syndrome (PCOS) is characterized by the presence of infrequent menstruation (>35 days for cycle length, or less than 8 cycles a year), hyperandrogenism (manifesting as hirsutism and/or elevated serum androgens), and polycystic ovarian morphology (PCOM) on ultrasound assessment. However as defined by the current diagnostic criteria for PCOS [1], numerous sub-phenotypes of the full syndrome are possible. At least three sub-phenotypes are generally recognized—oligomenorrhea and hyperandrogenism; PCOM and oligomenorrhea; PCOM and hyperandrogenism [2]. It is unclear whether these sub-phenotypes have distinct etiologies, or are merely precursors to the complete phenotype comprising of all three abnormalities.
Despite numerous studies, the etiology of PCOS remains elusive. Insulin resistance is considered important to the pathophysiology of PCOS [3]. Some 50% to 70% of women with PCOS are insulin-resistant, suggesting that insulin resistance may even be an etiological factor for PCOS [4, 5]. Insulin resistance can be the result of lifestyle factors such as shiftwork, insufficient sleep or lack of exercise [6]. In particular, nurses working on night shifts are at risk of insulin resistance [7]. Lifestyle factors associated with insulin resistance can be interrelated, as shiftwork can lead to reduced time available for sleep and exercise [6]. The question arises as to whether these lifestyle factors, acting alone or in combination, can lead to changes in menstrual cycle length, clinical or biochemical hyperandrogenism and abnormal ovarian morphology, thereby resulting in the sub-phenotypes of PCOS.
Although shift work has been reported to be associated with menstrual cycle abnormalities, its relevance to the pathophysiology of PCOS is difficult to interpret, because both long and short menstrual cycle lengths can be induced by shiftwork. Studies involving USA nurses [8], Canadian poultry workers [9], and Taiwanese factory workers [10] suggest that women subjected to rotating shift work are more likely to have shorter or longer menstrual cycle lengths than those without shift work. Nurses working only fixed night shifts have been reported to have shorter menstrual cycle lengths (<25 days) [11]. Several studies have examined shift work in relation to reproductive hormone levels [12, 13], but little attention has been given to the associations of shiftwork, insufficient sleep or exercise (alone or in combination) with hyperandrogenism and ovarian morphology characteristic of PCOS. In particular, it is unknown whether these lifestyle factors can result in the pathophysiological changes of PCOS in healthy women. To fill these gaps, we examined associations of sleep length, lack of regular exercise, and shiftwork with clinical and the biochemical abnormalities typical of PCOS in a cohort of healthy women.
Materials and Methods
Study design, setting, and population
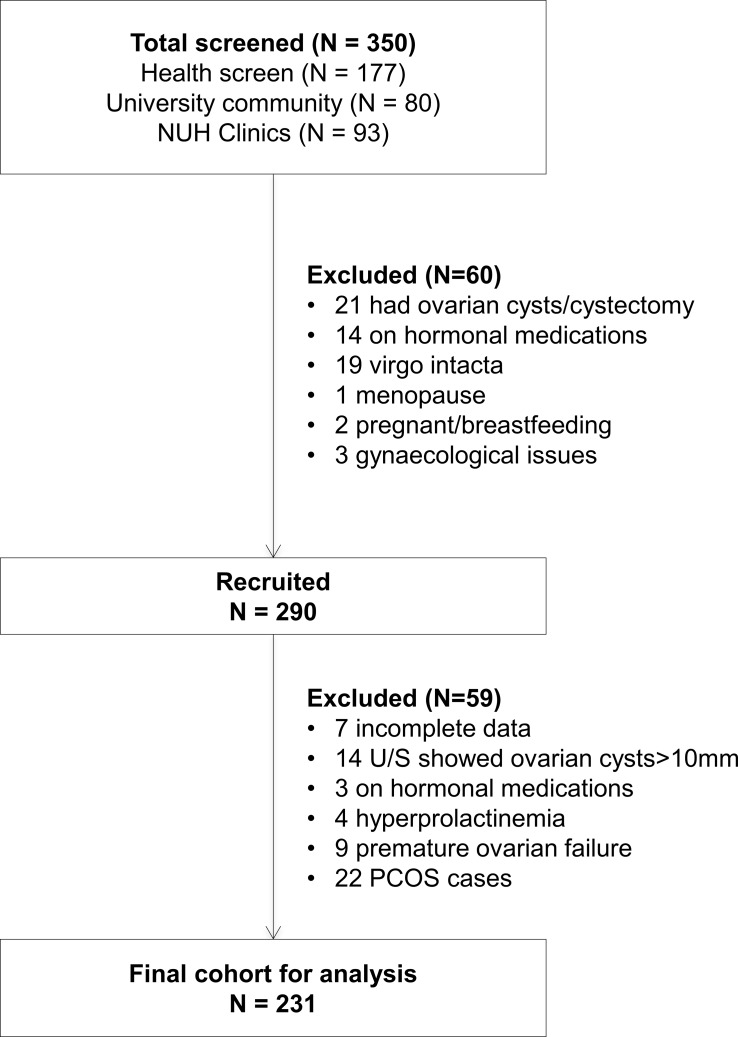
We carried out a prospective cross-sectional study at the National University Hospital (NUH), Singapore, from 2011 to 2015. Our study recruited healthy women 21–45 years of age who participated in an annual health screen offered to all employees of the hospital (53%), plus healthy volunteers from the university community (28%) and healthy referral cases from the NUH Women’s Clinic (19%). Exclusion criteria were virgo intacta, breastfeeding, pregnancy, menopause or use of medications known to influence reproductive or metabolic function (such as lipid lowering and diabetic drugs) within 60 days of study entry. Women who self-reported having congenital adrenal hyperplasia, adrenal tumors, androgen-secreting tumors, thyroid disease, established diabetes, severe cardiovascular disease, hysterectomy and/or oophorectomy or established diagnosis of PCOS were excluded. In addition, women with hyperprolactinemia (>1000 mIU/L), ovarian cysts larger than 10mm, premature ovarian failure (anti-mullerian hormone (AMH) below 0.6 pmol/L, estradiol (E2) below 70 pmol/L or follicle stimulating hormone above 25.8 IU/L) and incomplete data were excluded from the final cohort for analysis (Fig 1). Biochemical assays on E2, FSH and prolactin to determine inclusion/exclusion was performed using assays and instruments from Beckman Coulter Inc. (Brea, CA, USA). Between menstrual cycle days 2 to 5 at 7:30–8:30 AM after an overnight fast, participants completed a health and lifestyle questionnaire, underwent physical examination (including assessment of hirsutism and measurements of height, weight, waist and hip circumferences), transvaginal ultrasound scan of the ovaries, and plasma sampling for androgenic and metabolic biomarkers. The study and consent procedure was approved by the National Health Group Domain Specific Review Board. Informed written consent was obtained from all participants.
Fig 1. Flow chart showing study cohort selection.
Lifestyle factors
Participants were asked to complete a questionnaire on lifestyle patterns that may affect reproductive function. Shift work, exercise and sleep duration were selected as the primary lifestyle factors in this study. We hypothesized that these factors would be associated with one another and would affect reproductive and metabolic outcomes. The frequency of shift work was recorded as no shift work, <3 times /month, between 4–10 times /month and >10 times /month. Sleep duration was recorded as <4 hours, between 4–6 hours, between 6–8 hours and >8 hours. Exercise was recorded as never, rarely, sometimes, regularly or competitively. Other lifestyle factors recorded were smoking (yes or no), alcohol consumption (never, occasionally, once a week, 2 to 3 times a week or every day) and stress levels reported on a scale of 1 (low stress) to 10 (extreme stress). In order to have sufficient numbers in each group for meaningful analysis, lifestyle factors were subsequently regrouped for analysis as shift work (yes/no), sleep length (<6 hours/ ≥ 6 hours), and alcohol consumption (never or occasionally/once a week or more). Short sleep is generally defined as < 6 hours [14]. For analysis of exercise, study women were regrouped into those who reported regular exercise (regularly or competitively) and those who did not (i.e., reported exercising never, rarely or sometimes). Dietary intake, current depression status, and sleep comorbidities (such as sleep apnea, restless legs syndrome, and difficulty falling or staying asleep) was not assessed.
Menstrual cycle length
Study women were grouped into three categories of menstrual cycle lengths: <25 days, 25–34 days and ≥35 days. 25–34 day cycles were considered normal, <25 day cycles were considered short and ≥35 day cycles were considered long [15].
Hyperandrogenism
Clinical
Modified Ferriman–Gallwey scoring (mFG) for hirsutism was performed by two clinicians, using printed standard photographs as reference [16]. To standardize results, initial assessments were made independently, and the scores were then compared for inter-reliability. This process was repeated for 15 study women until a consensus in scoring was reached between the two clinicians.
Biochemical
Testosterone was detected by competitive binding immunoassays and sex hormone binding globulin (SHBG) was measured by a sandwich immunoassay (Beckman Coulter Inc). Free Androgen Index (FAI) was calculated as the percentage ratio of total testosterone to SHBG values.
Ovarian morphology and function
Transvaginal ultrasound measurements were performed in real time with a Voluson E8 machine and a 6–12 MHz transducer as described previously [15]. Measurements were performed in real time. Subjects with ovarian follicles or cysts >10 mm were asked to return in the next cycle for repeat evaluation. Subjects with at least one follicle with a mean diameter >10 mm were excluded to avoid spuriously high ovarian volume and hormonal effects from the emergent dominant follicle. The AFC was the number of antral follicles visible on ultrasound in both ovaries.
Anthral follicle counts (AFC)
All visible follicles in both ovaries were counted, and diameters of follicles were determined as the mean of two diameters (longitudinal and antero-posterior). Women with ovarian follicles or cysts >10 mm were asked to return in the next cycle for repeat evaluation. Women with at least one follicle with a mean diameter >10mm were excluded to avoid spuriously high ovarian volume and hormonal effects from the emergent dominant follicle.
Ovarian volume
After determining the longest medial axis of the ovary, the length and height of the ovaries were measured, and the probe was then turned to measure the width. Ovarian volume was calculated using the formula for the volume of a prolate ellipsoid: V = 0.523 x length x height x width. The measure used for analysis was the mean of the left and right ovarian volumes.
Anti-mullerian hormone
Anti-mullerian hormone (AMH), a surrogate measure of ovarian function, was measured with the Gen II ELISA assay, following the recommended premix procedure, and using the semi-automated programmed Evolis immunoassay system (Bio-Rad, Hercules, CA, USA)
Metabolic outcomes
Body mass index (BMI), defined as the ratio of body weight to height squared, and waist to hip ratio (WHR) were determined. Levels of glucose, insulin and lipids were measured using fasted serum. Glucose, insulin and lipids (triglycerides, total cholesterol, HDL, and LDL) were measured using assays and instruments from Beckman Coulter Inc. The homeostasis model assessment-estimated insulin resistance (HOMA-IR), an indicator of insulin resistance, was calculated as (glucose x insulin)/22.5 [17]. We defined the women in the highest quartile of the HOMA-IR distribution as insulin resistant. This method has been shown to be a reliable alternative to the euglycemic hyperinsulinemic clamp in defining insulin resistance [18]. The upper quartile cut-off value for HOMA-IR was 1.96 in our study sample. Metabolic syndrome was defined using the International Diabetes Federation criteria for South Asian women.
All assay performance characteristics were determined contemporaneously and shown in S1 Table.
Statistical analysis
Associations between shift work, exercise or sleep duration with participants’ reproductive and metabolic outcomes were examined using Student’s t-test for continuous variables with a normal distribution (average ovarian volume, AMH, testosterone, SHBG, BMI, WHR, average diastolic blood pressure, cholesterol, LDL and HDL), the Mann-Whitney U test for continuous variables with a non-normal distribution (anthral follicular count, mFG score, FAI, average systolic blood pressure, fasting triglycerides, fasting glucose, fasting insulin and HOMA-IR) and the chi-square test for the categorical variable of menstrual cycle length. Multivariable linear regression and logistic regression were used, where appropriate, to adjust for confounding factors (age, race (Chinese or non-Chinese), education (university or below university), stress levels (scale of 1 to 10), having given birth in the last 5 years and BMI) that could affect the associations of sleep length or exercise with the reproductive and metabolic outcomes. Only 12 women were smokers, 2 women consumed alcohol once a week or more, and 10 women had a previous medical history of depression and/or anxiety, so these variables were not considered further. Coffee consumption was not included as a potential confounder, as the temporal sequence between coffee consumption and sleep is unclear. All statistical analyses were performed using SPSS software 20.0 (SPSS, Chicago, Illinois). The area-proportional Venn diagram was generated using EulerAPE v3.0 [19].
Results
Characteristics of the study women (n = 231) are shown in Table 1. Average age was 31.6 years, 73% were of Chinese ethnic origin, 64% had at least a university education, and 62% had executive or professional occupations and 21% gave birth in the last 5 years (i.e., had a young child to care for). All study women had menstrual cycle lengths of <60 days and there were no cases of amenorrhea or grossly irregular menses.
Table 1. Characteristics of study women (N = 231).
| Characteristic | Number (%) |
|---|---|
| Age* | 31.6 (5.6) |
| Race | |
| Chinese | 170 (73.6) |
| Non-Chinese | 61 (26.4) |
| Education | |
| Below university | 81 (35.1) |
| University | 150 (64.9) |
| Occupation | |
| Executive | 35 (15.2) |
| Professional | 110 (47.6) |
| Technical support | 11 (4.8) |
| Sales | 6 (2.6) |
| Clerical/admin support | 18 (7.8) |
| Service | 17 (7.4) |
| Self-Employed | 7 (3.0) |
| Unemployed | 16 (6.9) |
| Others | 11 (4.8) |
| Medical history of depression and/or anxiety | |
| Yes | 10 (4) |
| No | 221 (96) |
| No of hours of sleep | |
| <6 hours | 55 (23.8) |
| > = 6 hours | 176 (76.2) |
| Shift work | |
| Yes | 57 (24.7) |
| No | 174 (75.3) |
| Coffee consumption | |
| > = 1 cup/day | 69 (29.9) |
| <1 cup/day | 162 (70.1) |
| Stress levels* | 5.3 (1.8) |
| Gave birth in last 5 years* | |
| Yes | 48 (20.8) |
| No | 183 (79.2) |
| Regular Exercise | |
| Yes | 65 (24.2) |
| No | 175 (75.8) |
| Smoking | |
| Yes | 12 (5.2) |
| No | 219 (94.8) |
| Alcohol consumption | |
| Never/Occasionally | 229 (99.1) |
| Once a week or more | 2 (0.9) |
* Mean (SD)
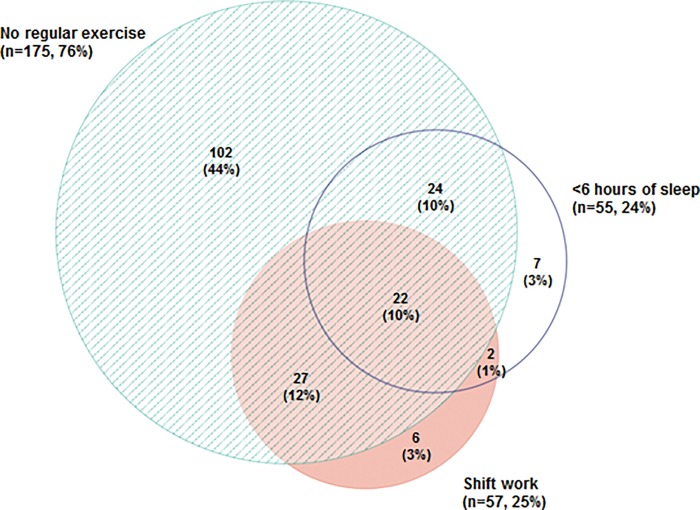
Fig 2 shows the prevalence and overlap of these risk factors in the study women. The majority (n = 190, 82%) of study women reported at least one of the three lifestyle risk factors under study: shift work, no regular exercise or short sleep duration.
Fig 2. Venn diagram showing the prevalence and overlap of lifestyle risk factors.
Numbers (and percentages of total study sample) refer to study women with one or more of the three lifestyle risk factors alone and in combination.
Twenty-four percent of the study women reported doing shift work. Shift work was not associated with any of the reproductive outcomes. Shift work was associated with increased WHR, but not with any of the other metabolic outcomes analyzed (Table 2).
Table 2. Lifestyle factors and reproductive and metabolic outcomes.
| Shiftwork | No Regular Exercise | Sleep hours | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Significance | No | Yes | Significance | > = 6 hours | <6 hours | Significance | |
| Reproductive | |||||||||
| Frequency (%) | |||||||||
| Menstrual cycle length (days) | |||||||||
| <25 | 9 (5.2) | 7 (12.3) | 1 (1.8) | 15 (8.6) | 8 (4.5) | 8 (14.5) | ** | ||
| 25–34 | 119 (68.4) | 36 (63.2) | 40 (71.4) | 115 (65.7) | 127 (72.2) | 28 (50.9) | |||
| > = 35 days | 46 (26.4) | 14 (24.6) | 15 (26.8) | 45 (25.7) | 41 (23.3) | 19 (34.5) | |||
| Mean (SD) | |||||||||
| Avg ovarian volume | 5.09 (2.28) | 5.48 (2.50) | 5.10 (2.16) | 5.22 (2.40) | 5.19 (2.30) | 5.19 (2.49) | |||
| Anthral follicle count | 15.8 (11.7) | 16.9 (11.1) | 15.1 (10.1) | 16.4 (11.9) | 15.7 (10.4) | 17.2 (14.7) | |||
| AMH (pmol/L) | 41.9 (34.1) | 47.6 (41.8) | 40.1 (30.5) | 44.3 (37.8) | 43.0 (33.8) | 44.3 (43.2) | |||
| mFG score | 1.09 (1.39) | 1.09 (1.78) | 1.21 (1.39) | 1.05 (1.52) | 1.09 (1.48) | 1.07 (1.52) | |||
| SHBG (nmol/L) | 60.5 (31.2) | 54.8 (23.8) | 62.5 (32.6) | 57.9 (28.6) | 61.1 (31.2) | 52.6 (22.8) | * | ||
| Testosterone (nmol/L) | 1.32 (0.71) | 1.25 (0.65) | 1.45 (0.74) | 1.25 (0.67) | 1.31 (0.70) | 1.28 (0.67) | |||
| FAI | 3.19 (3.49) | 3.06 (2.58) | 3.24 (3.71) | 3.13 (3.15) | 3.14 (3.46) | 3.22 (2.69) | |||
| Mean (SD) | |||||||||
| Metabolic | |||||||||
| BMI | 22.5 (4.0) | 23.1 (4.2) | 22.1 (3.3) | 22.8 (4.3) | 22.1 (3.6) | 24.2 (5.1) | ** | ||
| WHR | 0.77 (0.06) | 0.80 (0.06) | ** | 0.76 (0.06) | 0.78 (0.06) | * | 0.78 (0.06) | 0.79 (0.06) | |
| Average systolic blood pressure (mm Hg) | 111 (14) | 112 (12) | 113 (15) | 112 (13) | 111 (12) | 115 (17) | |||
| Average diastolic blood pressure (mm Hg) | 67.5 (9.8) | 68.0 (9.3) | 66.8 (8.4) | 67.9 (10.0) | 67.0 (9.4) | 69.5 (10.3) | |||
| Cholesterol (mmol/L) | 4.65 (0.76) | 4.72 (0.84) | 4.71 (0.80) | 4.65 (0.77) | 4.62 (0.76) | 4.79 (0.82) | |||
| Triglycerides (mmol/L) | 0.91 (0.46) | 0.96 (0.73) | 0.93 (0.54) | 0.92 (0.54) | 0.89 (0.46) | 1.01 (0.72) | |||
| HDL (mmol/L) | 1.50 (0.34) | 1.50 (0.44) | 1.49 (0.30) | 1.50 (0.39) | 1.51 (0.36) | 1.48 (0.37) | |||
| LDL (mmol/L) | 2.72 (0.66) | 2.76 (0.74) | 2.75 (0.71) | 2.72 (0.67) | 2.70 (0.66) | 2.82 (0.74) | |||
| Glucose (mmol/L) | 4.65 (0.45) | 4.57 (0.40) | 4.65 (0.43) | 4.62 (0.45) | 4.62 (0.44) | 4.65 (0.44) | |||
| Insulin (mU/L) | 7.96 (6.74) | 7.90 (4.37) | 6.14 (3.85) | 8.52 (0.63) | ** | 7.19 (5.23) | 10.4 (8.3) | *** | |
| HOMA-IR | 1.68 (1.53) | 1.62 (0.97) | 1.29 (0.88) | 1.78 (1.52) | ** | 1.51 (1.30) | 2.13 (1.63) | *** | |
| Frequency (%) | |||||||||
| Insulin resistance | |||||||||
| No | 132 (75.9) | 42 (73.7) | 47 (83.9) | 127 (72.6) | 143 (81.2) | 31 (56.4) | *** | ||
| Yes | 42 (24.1) | 15 (26.3) | 9 (16.1) | 48 (27.4) | 33 (18.8) | 24 (43.6) | |||
| Metabolic Syndrome | |||||||||
| No | 167 (96.0) | 54 (94.7) | 51 (91.1) | 170 (97.1) | 171 (97.2) | 50 (90.9) | |||
| Yes | 7 (4.0) | 3 (5.3) | 5 (8.9) | 5 (2.9) | 5 (2.8) | 5 (9.1) | |||
* P<0.05
** P<0.01
*** P<0.001
Seventy-six percent of study women did not exercise regularly. No differences were observed in menstrual cycle length or in ovarian or androgen outcomes in women who exercised regularly compared to those who did not (Table 2). Women who did not exercise regularly had significantly higher mean WHR (0.78 ± 0.06 vs 0.76 ± 0.06, p = 0.02), but did not have a significantly higher mean BMI. Lack of regular exercise was associated with significantly higher mean fasting insulin (difference in means = 2.3 mU/L; 95% CI, 0.5 to 4.1) and HOMA-IR (difference in means = 0.49; 95% CI, 0.09 to 0.90) levels but not with the risk of insulin resistance, after adjusting for potential confounders and the other lifestyle factors (shift work and sleep length).
Twenty-three percent of the study women reported fewer than 6 hours of sleep. Menstrual cycle length was significantly associated with sleep length (χ2 = 10.9, p = 0.004, see Table 2). After adjustment for confounders and the other lifestyle factors (exercise and shift work) women who reported less than 6 hours of sleep had significantly higher odds of an abnormal cycle length (short or long) than women with 6 or more hours of sleep (OR = 2.1; 95% CI, 1.1 to 4.2). In multinomial logistic regression analyses adjusting for potential confounders and exercise other lifestyle factors (exercise and shift work), short sleep duration was significantly associated with having short cycles (vs normal cycles) (OR = 3.7; 95% CI,1.1 to 12.7) and non-significantly associated with having longer cycles (vs normal cycles) (OR = 1.7, 95% CI, 0.8 to 3.7) compared to those who had 6 or more hours of sleep. No significant interactions (synergistic effects) were observed between sleep and shift work or exercise and menstrual cycle length. SHBG was significantly lower in women who reported less than 6 hours of sleep (52.6 ± 22.8 vs 61.1 ± 31.2 nmol/L, p = 0.03), but no changes were observed in the other ovarian and androgenic measures.
With respect to metabolic outcomes, women who reported fewer than 6 hours of sleep had a significantly higher mean BMI (24.2 ± 5.1 vs 22.1 ± 3.6, p = 0.008), fasting insulin levels (10.4 ± 8.3 vs 7.2 ± 5.2) mU/L, P<0.001) and HOMA-IR (2.1 ± 1.6 vs 1.5 ± 1.3, p<0.001). After adjustment for confounders and other lifestyle factors, short sleep duration remained significantly associated with increased fasting insulin levels (difference in means = 2.1; 95% CI, 0.3 to 4.0 mU/L) and higher odds of insulin resistance (OR = 2.6; 95% CI, 1.2 to 5.8) and non-significantly associated with HOMA-IR (difference in means = 0.40; 95% CI, -0.02 to 0.82) compared to women who reported 6 or more hours of sleep. No significant interactions were observed with shift work or insufficient exercise for fasting insulin levels or HOMA-IR.
Discussion
The lifestyle factors we examined (shift work, short sleep duration and regular exercise) were not significantly associated with any ovarian (ovarian volume, anthral follicle count, serum AMH) or androgenic measures (mFG score, serum testosterone) that define PCOS. Analyses of women who reported fewer than 6 hours of sleep, those who reported no regular exercise, and those who performed shift work revealed no significant associations with the ovarian and androgenic parameters studied. Nor did shift work or insufficient exercise have an impact on menstrual cycle length. Thus, our findings do not support the hypothesis that shift work or lack of exercise results in the clinical or biochemical characteristics typical of PCOS. These findings differ from those reported in previous studies reporting that night shift workers have lower mean serum testosterone levels than day shift workers [13], and that women with more than 20 months of rotating shift work were at risk of short (<21 days) or long (>40 days) menstrual cycles [8].
We observed that short sleep duration, rather than shiftwork, was significantly associated with changes in menstrual cycle length. In women who reported fewer than 6 hours of sleep, 15% had short cycle lengths and 35% had long cycle lengths. In contrast, among women who reported 6 or more hours of sleep, only 5% had short cycle lengths and 23% had long cycle lengths. After adjustment for confounders, women reporting fewer than 6 hours of sleep had a 3.9-fold increased risk of having short menstrual cycles (less than 25 days).
Recent findings suggest that there are circadian rhythm and sleep disturbances in women with diagnosed PCOS [20, 21]. We also observed that women with less than 6 hours of sleep had a 1.7-fold increased risk of having long menstrual cycles (more than 35 days) typical of PCOS, although this association did not reach statistical significance. The small number of women with long cycles in our cohort limited our statistical power to detect such an association.
Interestingly, we found that a lack of sleep is significantly associated with shorter menstrual cycles. It is tempting to speculate that disruption of pulsatile FSH and LH secretion associated with disordered sleep [12, 21, 22] may adversely affect luteinization of granulosa cells, resulting in luteal phase defect [23], which can lead to short menstrual cycles. However, short sleep duration was not significantly associated with any changes in reproductive hormone levels (FSH, LSH, estrogens, androgens) or ovarian variables (volume, anthral follicle counts or AMH).
Strong experimental evidence [24] indicates that recurrent nights of insufficient sleep can cause insulin resistance and diabetes in healthy adults. This has also been confirmed in epidemiological studies [25]; nurses working night shifts have been noted to have a five-fold increased risk of metabolic syndrome [7]. Short sleep duration had also been associated with a higher risk of the pre-diabetic state when compared with individuals with 7 or more hours of sleep per night [26]. Such an association may be attributable to increased concentrations of insulin and HbA1c in individuals with short sleep duration, which may be a consequence of their higher BMI [27]. Insufficient or disordered sleep has been associated with an increased risk of type 2 diabetes [26, 28].
Consistent with the findings of previous studies, we observed that short sleep duration is significantly associated with metabolic abnormalities, including higher BMI, raised fasting insulin levels, and increased risk of insulin resistance. The associations between sleep and fasting insulin levels and insulin resistance remained significant even after adjustment for BMI and other confounders. In particular, women who reported fewer than 6 hours of sleep had a 2.6 fold higher adjusted risk of insulin resistance compared to women who reported 6 or more hours of sleep. Women with short sleep duration were also at increased risk of short menstrual cycles. Given our findings, the link between menstrual cycle length, insulin metabolism and ovarian function should be pursued in further studies.
An important strength of our study is that it is not based on a referred sample of symptomatic women, but on healthy hospital workers and volunteers from a university community who agreed to undergo detailed clinical assessment for hyperandrogenism, ultrasound examination of ovarian morphology and measurement of reproductive and metabolic hormone levels. A limitation of our study includes its recruitment from a single center, which might compromise the generalizability of our results. In addition, information on sleep duration was based on recall, and we were unable to perform polysomnography to determine other aspects of sleep, such as the quality of sleep and duration of rapid eye movement sleep. Although we have adjusted for as many factors that could affect the associations of sleep length or exercise with the reproductive and metabolic outcomes, there is the possibility of residual confounding due to factors which we lack information on, such as diet and current depression status. Also, there may be a possible over-reporting of exercise due to a lack of accurate actigraphy data, but any such over-reporting should not differ according to sleep duration or quality and therefore would bias any observed association towards the null.
In conclusion, our results suggest that women with insufficient sleep are at increased risk of insulin resistance and menstrual disturbances. Sufficient sleep may be important to protect women against ovulatory dysfunction, possible fertility problems and insulin resistance. Larger epidemiologic studies are required to confirm our results, and mechanistic investigations should help delineate the pathways underlying the associations we observed.
Supporting Information
(DOCX)
Acknowledgments
We acknowledge our research staff, the National University Hospital Department of Laboratory Medicine and all our participants for their contributions to this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Bedside & Bench Grant from the Singapore National Medical Research Council (NMRC/BnB/0007c/2013) (http://www.nmrc.gov.sg/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. 10.1016/S0140-6736(07)61345-2 . [DOI] [PubMed] [Google Scholar]
- 2.Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83(6):1717–23. 10.1016/j.fertnstert.2005.01.096 . [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–32. 10.1016/j.molmed.2006.05.006 . [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38(9):1165–74. . [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv. 2004;59(2):141–54. 10.1097/01.OGX.0000109523.25076.E2 . [DOI] [PubMed] [Google Scholar]
- 6.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001;24(3):608 . [DOI] [PubMed] [Google Scholar]
- 7.Pietroiusti A, Neri A, Somma G, Coppeta L, Iavicoli I, Bergamaschi A, et al. Incidence of metabolic syndrome among night-shift healthcare workers. Occup Environ Med. 2010;67(1):54–7. 10.1136/oem.2009.046797 . [DOI] [PubMed] [Google Scholar]
- 8.Lawson CC, Whelan EA, Lividoti Hibert EN, Spiegelman D, Schernhammer ES, Rich-Edwards JW. Rotating shift work and menstrual cycle characteristics. Epidemiology. 2011;22(3):305–12. 10.1097/EDE.0b013e3182130016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messing K, Saurel-Cubizolles MJ, Bourgine M, Kaminski M. Menstrual-cycle characteristics and work conditions of workers in poultry slaughterhouses and canneries. Scand J Work Environ Health. 1992;18(5):302–9. . [DOI] [PubMed] [Google Scholar]
- 10.Su SB, Lu CW, Kao YY, Guo HR. Effects of 12-hour rotating shifts on menstrual cycles of photoelectronic workers in Taiwan. Chronobiol Int. 2008;25(2):237–48. . [DOI] [PubMed] [Google Scholar]
- 11.Chung FF, Yao CC, Wan GH. The associations between menstrual function and life style/working conditions among nurses in Taiwan. J Occup Health. 2005;47(2):149–56. . [DOI] [PubMed] [Google Scholar]
- 12.Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77(4):738–44. . [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Acebo I, Dierssen-Sotos T, Papantoniou K, Garcia-Unzueta MT, Santos-Benito MF, Llorca J. Association between exposure to rotating night shift versus day shift using levels of 6-sulfatoxymelatonin and cortisol and other sex hormones in women. Chronobiol Int. 2015;32(1):128–35. 10.3109/07420528.2014.958494 . [DOI] [PubMed] [Google Scholar]
- 14.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–47. 10.1016/j.smrv.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu R, Lee BH, Huang Z, Raja Indran I, Li J, Shen L, et al. Anti-mullerian hormone, antral follicle count and ovarian volume predict menstrual cycle length in healthy women. Clin Endocrinol (Oxf). 2015. 10.1111/cen.12984 . [DOI] [PubMed] [Google Scholar]
- 16.DeUgarte CM, Woods KS, Bartolucci AA, Azziz R. Degree of facial and body terminal hair growth in unselected black and white women: toward a populational definition of hirsutism. J Clin Endocrinol Metab. 2006;91(4):1345–50. 10.1210/jc.2004-2301 . [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. . [DOI] [PubMed] [Google Scholar]
- 18.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond). 2006;30(8):1308–14. 10.1038/sj.ijo.0803189 . [DOI] [PubMed] [Google Scholar]
- 19.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9(7):e101717 10.1371/journal.pone.0101717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod. 2015;30(2):466–72. 10.1093/humrep/deu318 . [DOI] [PubMed] [Google Scholar]
- 21.Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill CM, et al. Poor sleep in PCOS; is melatonin the culprit? Hum Reprod. 2013;28(5):1348–53. 10.1093/humrep/det013 . [DOI] [PubMed] [Google Scholar]
- 22.Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90(4):2050–5. 10.1210/jc.2004-2033 . [DOI] [PubMed] [Google Scholar]
- 23.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60(1):1–13. . [DOI] [PubMed] [Google Scholar]
- 24.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985). 2005;99(5):2008–19. 10.1152/japplphysiol.00660.2005 . [DOI] [PubMed] [Google Scholar]
- 25.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20. 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabet Med. 2013;30(6):676–80. 10.1111/dme.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ES, Wheaton AG, Chapman DP, Li C, Perry GS, Croft JB. Associations between self-reported sleep duration and sleeping disorder with concentrations of fasting and 2-h glucose, insulin, and glycosylated hemoglobin among adults without diagnosed diabetes. J Diabetes. 2014;6(4):338–50. 10.1111/1753-0407.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–4. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.