SUMMARY
Transporting epithelial cells interact with the luminal environment using a tightly packed array of microvilli known as the ‘brush border’. During intestinal epithelial differentiation, microvillar packing and organization are driven by cadherin-dependent adhesion complexes, which localize to the distal tips of microvilli where they drive physical interactions between neighboring protrusions. Although enrichment of the “intermicrovillar adhesion complex” (IMAC) at distal tips is required for proper function, the mechanism driving tip accumulation of these factors remains unclear. Here, we report that the actin-based motor, Myo7b, promotes the accumulation of IMAC components at microvillar tips. Myo7b is highly enriched at the tips of microvilli in both kidney and intestinal brush borders, and loss of Myo7b in differentiating intestinal epithelial cells disrupts intermicrovillar adhesion and thus, brush border assembly. Analysis of cells lacking Myo7b revealed that IMAC components and the resulting intermicrovillar adhesion links are mislocalized along the microvillar axis rather than enriched at the distal tips. We also found that Myo7b motor domains are capable of supporting tip-directed transport. However, motor activity is supplemented by other passive targeting mechanisms, which together drive highly efficient IMAC accumulation at the tips. These findings illuminate the molecular basis of IMAC enrichment at microvillar tips and hold important implications for understanding apical morphogenesis in transporting and sensory epithelial tissues.
Keywords: actin, brush border, protrusion, cytoskeleton, motor protein, epithelia, intermicrovillar adhesion, Myo7b
Graphical Abstract
INTRODUCTION
Transporting epithelia, like those that line the luminal surface of the intestine and kidney, build cylindrical actin-based protrusions called microvilli on their apical surface to increase capacity for solute uptake. A single microvillus is supported by a core actin bundle of about 20–30 parallel filaments 0.5 – 5 μm in length, with the barbed ends oriented toward the distal tips and the pointed ends anchored in a mesh of intermediate filaments and spectrin referred to as the terminal web [1]. Enterocytes, the transporting epithelial cells that line the intestinal tract, represent one of the most elaborate cases where thousands of microvilli extend from the surface in a tightly packed array referred to as the brush border (BB). In this context, the BB also serves as a barrier against harmful compounds and microbes in the lumen [2]. The physiological role of the BB in normal gut function is underscored by the fact that numerous intestinal diseases are characterized by perturbation to or complete loss of apical microvilli, including microvillus inclusion disease [3, 4], Type 1C Usher Syndrome [5], and infections with attaching/effacing microbes such as enterohemorrhagic and enteropathogenic E. coli [6].
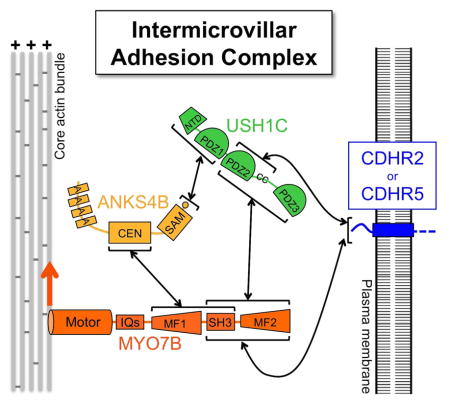
Recent studies have begun to explore the molecular mechanisms that promote microvillar growth and organization [7, 8]. We previously reported that adhesion between the distal tips of microvilli plays an important role in driving and optimizing the packing of these protrusions during BB assembly [7]. Such intermicrovillar adhesion is mediated by a trans-heterophilic complex formed by two tip-targeted protocadherins, protocadherin-24 (PCDH24/CDHR2) and mucin-like protocadherin (MLPCDH/CDHR5) [7]. Both protocadherins interact with cytoplasmic factors including multi-PDZ domain protein, harmonin-a/USH1C, and ankyrin repeat protein, ANKS4B [7–9], which also localize to the distal tips of microvilli and together form the intermicrovillar adhesion complex (IMAC) [7, 8]. Proper assembly of the IMAC and its enrichment at microvillar tips are critical for normal microvillar clustering and BB assembly [7, 8]. Focusing adhesion activity at microvillar tips provides a mechanism for maximizing the packing density of these protrusions and may also contribute to microvillar length uniformity in this system. However, the mechanisms responsible for tip localization of the IMAC have not been elucidated.
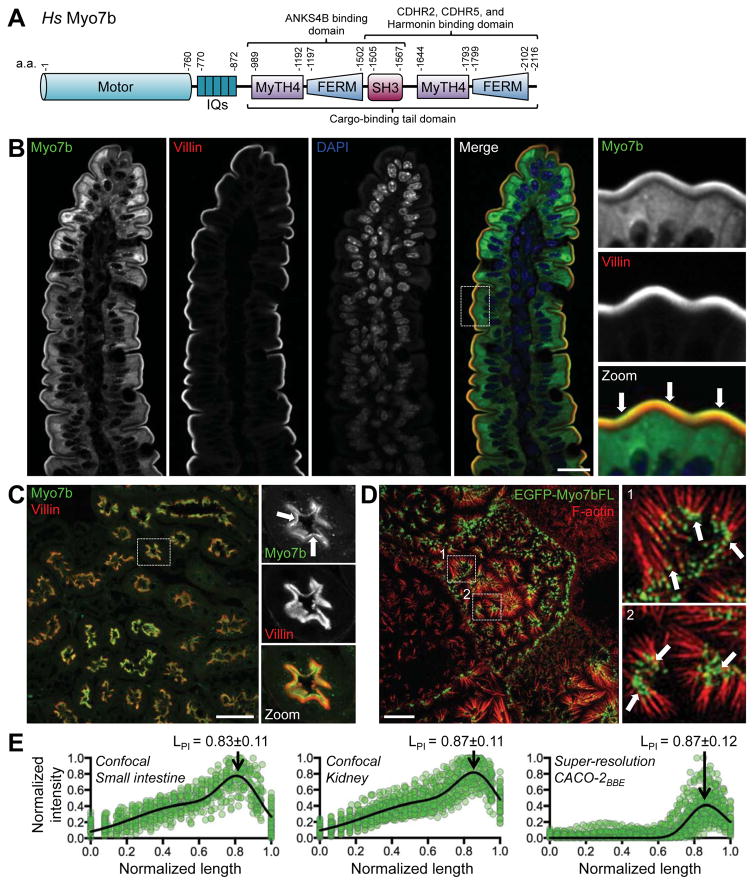
A fifth component of the IMAC is the actin-based motor, myosin-7b (Myo7b). Myo7b was originally identified in a PCR screen for myosin-like genes in epithelial cells as a product with high sequence homology to myosin-7a [10]. As a class VII myosin, Myo7b contains a highly conserved N-terminal motor domain with ATP and actin bindings sites, a central ‘neck’ region containing at least five recognizable IQ motifs, and a C-terminal tail that consists of tandem MyTH4-FERM domains separated by an intervening SH3 domain (Figure 1A)[11]. Using its tail domain, Myo7b binds directly to other components of the IMAC; the N-terminal MyTH4-FERM domain binds specifically to ANKS4B, whereas the C-terminal MyTH4-FERM domain interacts with CDHR2, CDHR5, and USH1C [7–9]. Binding partners for the SH3 domain have yet to be identified. Myo7b protein is highly expressed in epithelial tissues of the kidney and intestinal tract, where it localizes to the apical surface [11]. More recent work revealed localization at the distal tips of microvilli on the surface of CACO-2BBE intestinal epithelial cells [7]. Although enrichment of Myo7b at the distal tips of microvillar actin bundles could reflect barbed-end-directed transporter activity, this possibility has yet to be investigated.
Figure 1. Myo7b localization in native tissue and CACO-2BBE cells.
(A) Domain diagram of full-length Myo7b. Numbers indicate amino acids. (B) Confocal images of mouse small intestinal tissue stained for Myo7b (green), villin (red), and DAPI (blue). Boxed region indicates field shown in zoomed images. Arrows point to distal tip enrichment of Myo7b within the BB. Scale bar, 20 μm. (C) Confocal images of mouse kidney tissue stained for Myo7b (green) and villin (red). Boxed area indicates region in zoomed images. Arrows point to distal tip enrichment of Myo7b within the BB. Scale bar, 50 μm. (D) Structure illumination microscopy of CACO-2BBE cells overexpressing full-length Myo7b N-terminally tagged with EGFP (green) and stained for F-actin (red). Boxed areas indicate regions in zoomed images. Arrows highlight distal tip enrichment. Scale bar, 5 μm. (E) Line scans of Myo7b intensity parallel to the microvillar axis in tissue sections or CACO-2BBE cells overexpressing EGFP-Myo7b; 0=base and 1=tip. N = 55 scans for SI, 53 scans for kidney, and 62 scans for CACO-2BBE. Gaussian curve fits are marked with length at peak intensity (LPI) ± distribution width (SD). See also Figure S1.
To date, no barbed-end directed transporters have been identified in microvilli. However, myosin-6 has been implicated in cargo retention and/or movement towards the pointed-ends of microvillar actin bundles, which are embedded in the terminal web at the base of the BB [12, 13]. Unconventional myosin motors have also been implicated in tipward transport in other actin-supported protrusions, playing roles in the formation and maintenance of these structures. Myosins-3 [14, 15], -7a [16–19], and -15 [20–22], have all been implicated in cargo transport within stereocilia, the mechanosensory protrusions that extend from the apical surface of inner ear hair cells. Additionally, myosin-10 has been shown to processively transport a variety of cargoes to the tips of filopodia in motile cells [23–26].
Whether microvilli employ a barbed-end directed transport motor or use other mechanisms to position specific cargoes at their distal tips remains unknown, but Myo7b is an intriguing candidate in either case. Kinetic studies of mouse Myo7b showed ADP release is rate limiting, which leads to a long-lived actin bound state and correspondingly high duty ratio (~0.8) [27]. Myo7b from Drosophila also exhibits a very high duty ratio [28]. Although these properties are consistent with a role in processive transport, Myo7b does not possess a coiled-coil domain that would allow for robust dimerization. Previously characterized transporters deal with the same structural limitation using a mechanism in which oligomerization/dimerization is induced by cargo binding [18, 29, 30].
Because Myo7b is the only component of the IMAC with a recognizable actin-binding domain, we hypothesized that this motor could function as a physical link to the actin cytoskeleton and play a role in localizing the IMAC at the distal ends of microvillar actin bundles. Here, we provide evidence in support of this hypothesis and show that Myo7b serves to enrich the IMAC at the distal tips of microvilli. By properly localizing the IMAC, Myo7b plays a crucial role in microvillar organization and BB formation, a role that requires functional motor and cargo-binding tail domains. Taken together, these findings provide a molecular mechanism underpinning the localization and thus function of the IMAC; this work also holds implications for understanding apical morphogenesis in a variety of epithelial contexts.
RESULTS
Myo7b localizes to the distal tips of microvilli
Components of the IMAC are enriched at the distal tips of microvilli in intestinal and kidney epithelia [7, 8]. Additionally, all endogenous IMAC proteins and exogenous fluorescent protein-tagged constructs of ANKS4B, CDHR2, and CDHR5 also localize to microvillar tips in the CACO-2BBE intestinal epithelial cell culture model [7, 8]. Previous studies showed that Myo7b is expressed in kidney and intestinal tissue where it localizes to the BB in kidney, and the distal portion of microvilli in intestinal epithelial cells [11]. To extend these results and determine the precise localization of Myo7b, we stained paraffin-embedded kidney and intestinal tissue sections using a newer Myo7b-specific antibody (Sigma HPA039131). In the intestine, tissue staining revealed distal tip enrichment of the motor within BB microvilli, along the length of the villus (Figure 1B and 1E). Expression of Myo7b is decreased in the crypt compartments, with little detection in crypt microvilli (Figure S1, open arrowheads). Increased expression and tip enrichment of Myo7b appeared in cells transitioning out of the crypt and onto the base of the villus (Figure S1, filled arrowheads and arrows). Myo7b also showed striking enrichment at the distal tips of microvilli on the surface of tubule epithelial cells in cortical kidney sections (Figure 1C and 1E). In both intestine and kidney, the position of the Myo7b intensity peak with respect to the microvillar axis was remarkably similar (LPI = 0.83 ± 0.11 and 0.87 ± 0.11 respectively, where 0 = base and 1 = tip).
We previously showed that endogenous Myo7b enriches towards the distal tips of clustering microvilli in CACO-2BBE cells after two weeks of differentiation [7]. To determine if tagged variants of Myo7b also target to microvillar tips in this model, we stably expressed an N-terminally EGFP-tagged full-length construct of human Myo7b (Figure S1B) in CACO-2BBE cells. After two weeks of differentiation, this construct localizes to the distal tips of microvilli in a manner similar to endogenous Myo7b (LPI = 0.87 ± 0.12, Figure 1D and 1E). Thus, Myo7b is well positioned to play a role in localizing IMAC components to microvillar tips.
Myo7b is required for normal BB assembly
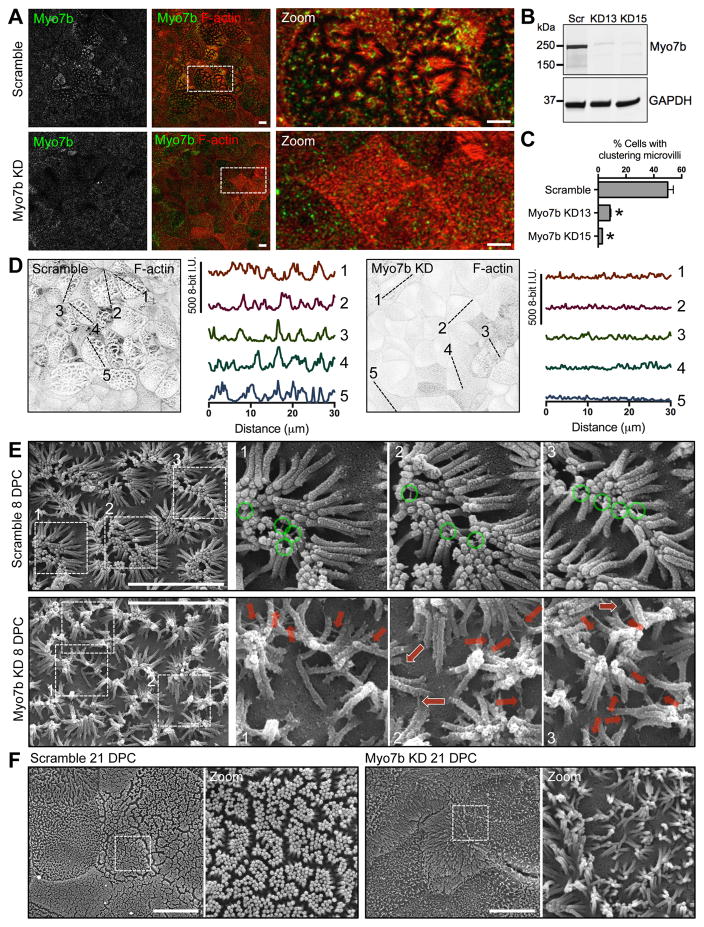
Previous studies showed that loss of IMAC components (CDHR2, CDHR5, USH1C, or ANKS4B) results in the disruption of microvillar clustering and BB formation [7, 8]. Based on our observations using both light and scanning electron microscopy (SEM) [7, 8], differentiating CACO-2BBE monolayers typically exhibit a heterogeneous surface morphology, although the majority of cells present prominent “tipi”-shaped clusters of microvilli interacting via their tips and surrounded by regions of free apical membrane space (Figure S2B). Tipi-clustered microvilli represent an intermediate packing state that is critical for the progression to a mature BB [7, 8]. Other cells exhibit immature microvillar growth, with a peripheral F-actin signal that is much higher than medial regions of the cell surface (Figure S2B). To determine if Myo7b also plays a role in organizing microvilli during BB assembly, we generated two independent, stable lentivirus-mediated knockdowns (KDs) of Myo7b in CACO-2BBE cells, confirmed by western blot and immunostaining (Figure 2A, 2B, and S2A). Myo7b KD cells were still able to assemble microvilli, although cell surface morphology was perturbed. The presence of tipi-shaped clusters was scored as described previously [7, 8] after two weeks of differentiation. Myo7b KD resulted in a significant decrease in clustering (Figure 2C), which was evident in confocal images of phalloidin-stained monolayers. Line scans across the long axis of control cells revealed large variations in phalloidin-labeling intensity caused by the juxtaposition of free apical space (low intensity) with well-formed tipi clusters of microvilli (high intensity) (Figure 2D). These large variations in phalloidin signal were absent in Myo7b KD cells. Moreover, in Myo7b KD cells that were able to build long microvilli, we noticed aberrant “fan”-like arrays (Figure S2B), which indicated failure of these protrusions to extend away from the cell surface. SEM of 8 days post confluency (DPC) scramble control cells revealed large microvillar clusters surrounded by free apical space with almost exclusively tip-to-tip interactions (Figure 2E, top row). Most observable linkages were localized to the tips of adjacent microvilli (Figure 2E, green circles). In contrast, KD of Myo7b caused disorganization of microvilli with little free apical space, resulting in a disheveled BB (Figure 2E, bottom row). Aberrant contacts between microvilli (e.g. tip-to-base and base-to-base interactions) were readily observed in KD cells. We also observed extracellular links along the full length of microvilli (Figure 2E, red arrows) as well as aberrant links between microvilli and the cell surface (Figure 2E, white outlined red arrows). Perturbations in Myo7b KD cell apical surface organization were also observed at 21 DPC, a time point by which microvillar packing is typically optimized in control cultures (Figure 2F). Together, these findings allow us to conclude that Myo7b is required for normal BB assembly.
Figure 2. Myo7b KD in CACO-2BBE cells results in defects in BB assembly.
(A) Confocal images of Myo7b (green) and F-actin (red) of scramble and Myo7b KD15 CACO-2BBE cells at 14 DPC. Boxed area indicates region in zoomed images. Scale bars, 10 μm; 5 μm in zooms. (B) Western blots show near complete KD of Myo7b in CACO-2BBE cells expressing two distinct shRNAs (KD13 or KD15). (C) Quantification of percentage of cells with clustering microvilli. Bars indicate mean ± SD. N = 1,313 cells for Scramble, 721 cells for KD13, and 1,451 cells for KD15. *p<0.0001, t test. (D) Line scan analysis of five cells (indicated with dashed line), derived from F-actin channel images from (A). (E) SEM images of scramble and Myo7b KD15 cells at 8 DPC. Boxed area indicates region in zoomed images. Green circles show IMAC links at the tips of clustering microvilli. Solid red arrows and outlined red arrows show IMAC links at the base of adjacent microvilli and between microvilli and the plasma membrane, respectively. Scale bars, 4 μm. (F) SEM images of scramble and Myo7b KD15 cells at 21 DPC. Boxed area indicates region in zoomed images. Scale bars, 10 μm. See also Figure S2.
Distal tip enrichment of the IMAC is dependent on Myo7b
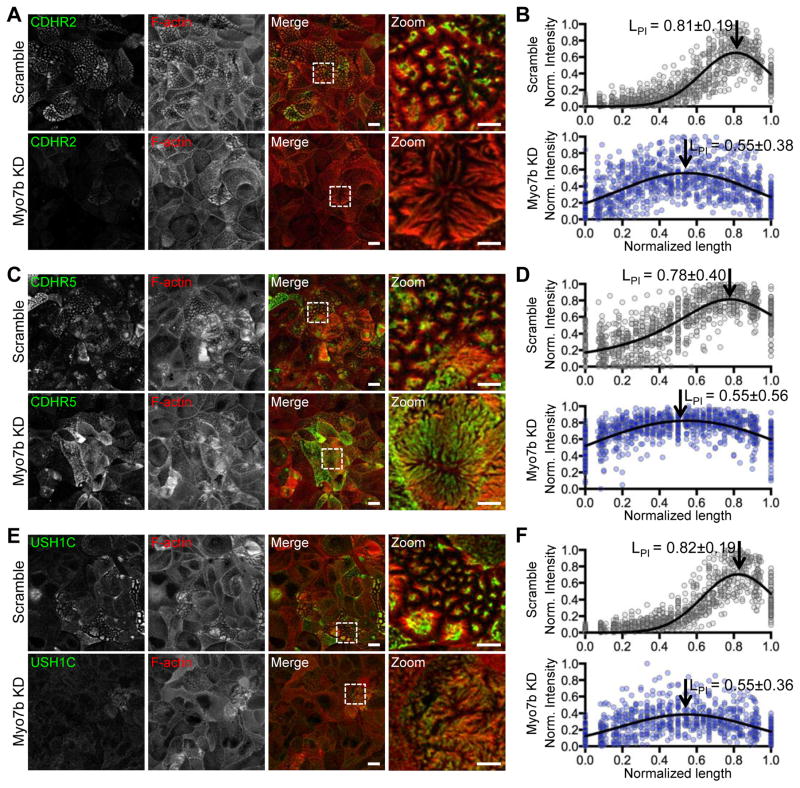
Myo7b directly interacts with all other IMAC components [7–9]. To examine the role of Myo7b in targeting the IMAC to microvillar tips, we stained Myo7b KD cells for individual components of the complex, including CDHR2, CDHR5, and USH1C. In each case, KD of Myo7b resulted in a striking loss of distal tip enrichment (Figure 3, and S3A). Using z-axis confocal sections, we quantified the localization of each IMAC component by generating line scans along the length of individual microvilli (Figure S3B). The resulting LPI values for all IMAC components were significantly reduced, indicating loss of distal tip enrichment in Myo7b KD cells (Figure 3B,D,F). Immunofluorescence imaging and western blots revealed decreased expression levels of CDHR2 and USH1C in KD cells (Figure 3A, 3E, and S3). Strikingly, in cells that were able to maintain higher levels of IMAC proteins, we observed diffuse localization along the microvillar axis (Figure 3C and S3A). Therefore, the defects in microvillar organization observed in Myo7b KD cells are most likely due to loss of distal tip enrichment of IMAC cargoes.
Figure 3. Myo7b KD results in loss of IMAC enrichment at microvillar tips.
(A, C, E) Confocal images of CDHR2, CDHR5, and USH1C staining (green) and F-actin labeling (red) in Myo7b KD15 CACO-2BBE cells at 14 DPC. Boxed area indicates region in zoomed images. Scale bars, 10 μm; 5 μm in zooms. (B, D, F) Line scan analysis of CDHR2, CDHR5, and USH1C staining intensity parallel to the microvillar axis. 0=base and 1=tip. N = 64 scans for each plot. Gaussian curve fits are marked with length at peak intensity (LPI) ± distribution width (SD). See also Figure S3.
Myo7b microvillar tip localization requires a functional motor domain
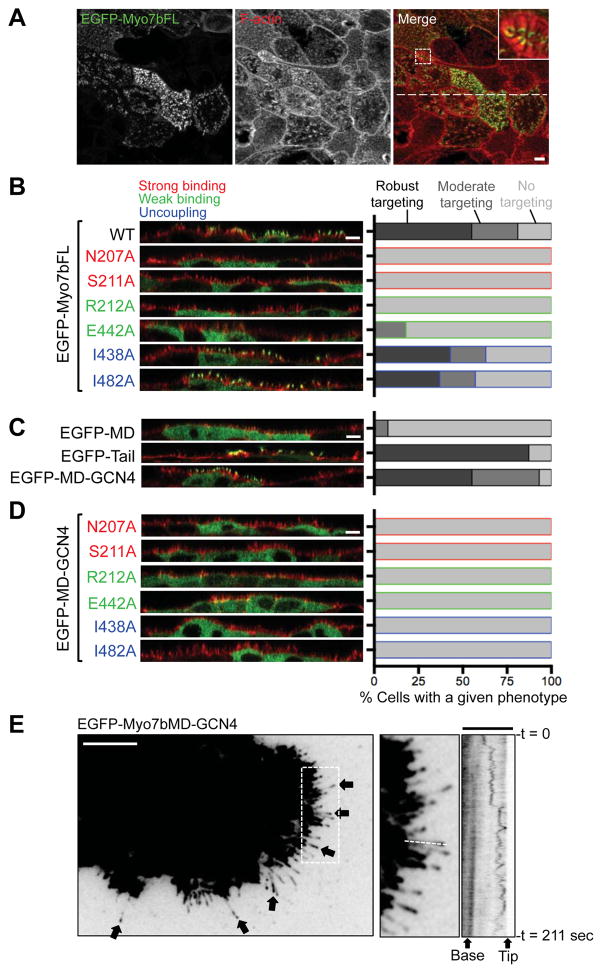
In studies of unconventional myosins that localize to actin-based protrusions in other systems, tip targeting is viewed as a telltale sign of processive barbed-end directed motility [21, 24]. To examine the role of the motor domain in Myo7b targeting, we used mutagenesis to create variants of Myo7b predicted to be deficient in motor activity, and then assessed their ability to target to microvillar tips. Many residues that are critical for myosin catalytic and mechanical function are perfectly conserved across eukaryotes. Thus, we used information from previous studies to generate variants of Myo7b deficient in specific aspects of motor function (Figure S4A). A similar strategy has been used previously by others to assess the significance of myosin motor function in cells [18, 21, 31]. We introduced three classes of mutations into the Myo7b motor domain: (1) mutations expected to prevent nucleotide binding and lock the motor in a strong actin bound state (N207A and S211A) [32], (2) mutations expected to block phosphate release and inhibit actin binding (R212A and E442A) [32, 33], and (3) mutations expected to ‘uncouple’ conformational changes in the nucleotide binding pocket from lever arm rotation, which would result in normal ATPase activity and actin binding, but no motor activity (I438A and I482A) [34, 35].
Mutant variants of Myo7b were tagged with EGFP and stably expressed in LLC-PK1-CL4 kidney epithelial cells to assay microvillar tip targeting. Construct expression was confirmed using western blot analysis (Figure S4B). LLC-PK1-CL4 cells express endogenous Myo7b (Figure S4B) and differentiate rapidly (~3–4 days), which allowed efficient production of the numerous stable lines required for these experiments. We used the ratio of microvillar tip intensity:cytoplasmic intensity to quantify the extent of tip targeting for each construct. Ratio values from single cells were binned into the three categories based on the efficiency of targeting: none (ratio of <1), moderate (ratio of 1–2.5), and robust (ratio of >2.5). Our positive control, EGFP-tagged WT full-length Myo7b (EGFP-Myo7bFL) localized as expected in this system (Figure 4A); ~50% of cells expressing this WT construct exhibited robust tip targeting (Figure 4B). Myo7b mutants expected to be locked in either strongly or weakly bound states were unable to target to microvillar tips (Figure 4B and S4C). Thus, active ATPase cycling and normal actin binding contribute to the tip enrichment of full-length Myo7b. Mutants expected to strongly bind actin also demonstrated aberrant stress fiber localization (Figure S4C). Interestingly, Myo7b mutants expected to exhibit uncoupling of catalytic and mechanical activity showed only a moderate decrease in targeting when compared to WT (Figure 4B and S4C), suggesting that motor activity per se may be at least partially dispensable for tip enrichment.
Figure 4. Structure-function analysis of Myo7b tip targeting.
(A) Confocal images of full-length EGFP-tagged Myo7b (green) stable overexpression in LLC-PK1-CL4 cells stained for F-actin (red) at 4 DPC. Boxed area indicates region in zoomed inset. Dashed line indicates WT X-Z vertical section shown in (B). Scale bar, 5 μm. (B) Vertical sections from confocal images of cells expressing EGFP-tagged full-length Myo7b constructs (EGFP-Myo7bFL) with point mutations indicated. Quantification of tip targeting in each case was performed by binning tip:cytoplasmic intensity ratios into three categories as indicated. Scale bar, 5 μm. (C) Vertical sections from confocal images of cells expressing EGFP-tagged Myo7b motor domain (EGFP-MD), Myo7b tail domain (EGFP-Tail), or a forced dimer of Myo7b motor domains (EGFP-MD-GCN4), quantified as in (B). Scale bar, 5 μm. (D) Vertical sections from confocal images of cells expressing a forced dimer of Myo7b motor domains (EGFP-MD-GCN4) with point mutations indicated, quantified as in (B). Scale bar, 5 μm. (E) TIRF images of a Cos-7 cell co-transfected with mCherry-fascin (not shown) and the EGFP-tagged Myo7b forced dimer construct (EGFP-MD-GCN4). Black arrows point to filopodial tip enrichment. Boxed area indicates region in zoomed image and Movie S1. Dashed line indicates line used to generate accompanying kymograph. Scale bars, 10 μm; 3.5 μm in kymograph. See also Figure S4 and Movie S1.
Myo7b cargo-binding tail domain also contributes to tip targeting
The fact that uncoupled Myo7b mutants exhibit near normal localization suggests that actin binding (predicted to be still active in these mutants) and binding partner interactions with the tail domain make significant contributions to tip targeting. To further dissect the relative contributions of motor and tail domains, we examined the targeting potential of truncated constructs consisting of only these regions (Figure S1B). A construct containing only the motor and neck domains (EGFP-MD) was unable to enrich at microvillar tips (Figure 4C and Figure S4D), suggesting that actin binding alone is not sufficient for normal targeting. However, when the cargo-binding tail domain (EGFP-Tail) was expressed by itself, we observed robust tip localization, most likely due to interactions with endogenous IMAC components or possibly other unidentified binding partners at microvillar tips (Figure 4C and Figure S4D). Thus, the Myo7b cargo-binding tail domain holds significant tip targeting potential.
A forced dimer of Myo7b motor domains uses motor activity to tip target
We next sought to further examine the role of Myo7b motor activity in the absence of potentially confounding effects due to tail domain interactions with endogenous factors. To this end, we fused Myo7b motor and neck domains in frame with a GCN4 dimerization motif (MD-GCN4, Figure S1B) [36]. This ‘forced dimer’ of Myo7b motor domains exhibited robust tip targeting to a level that was comparable to full-length Myo7b (Figure 4C). Because the Myo7b motor domain exhibits a high duty ratio [27, 28], the tip targeting of MD-GCN4 likely reflects barbed-end directed movement along microvillar core actin bundles. Consistent with this conclusion, when we overexpressed EGFP-MD-GCN4 in Cos-7 cells, we observed accumulation at the tips of filopodia; in some cases, we also observed streaming of fluorescent puncta to and from the tips of these structures with tip-ward velocities in the range of ~200 nm/sec (Figure 4E and Movie S1). We also introduced the mutations described above (Figure S4A) into the EGFP-MD-GCN4 construct and generated stable cell lines. Similar to full-length Myo7b, mutations expected to prevent ATPase cycling impaired tip targeting of MD-GCN4 (Figure 4D and Figure S4E). In contrast to experiments with full-length Myo7b, mutations expected to uncouple catalytic and mechanical activity completely inhibited distal tip targeting of the forced dimer (Figure 4D and Figure S4E). Based on these results, we conclude that the Myo7b motor domain is mechanically active in cells and the minimal unit of a motile complex is two Myo7b motor domains. In combination with the studies presented above, these results tell us that Myo7b motor activity can drive tip targeting, but enrichment at distal tips is likely reinforced by actin binding and binding partner (IMAC or other) interactions with the tail domain.
Complete rescue of Myo7b KD requires functional motor and cargo-binding domains
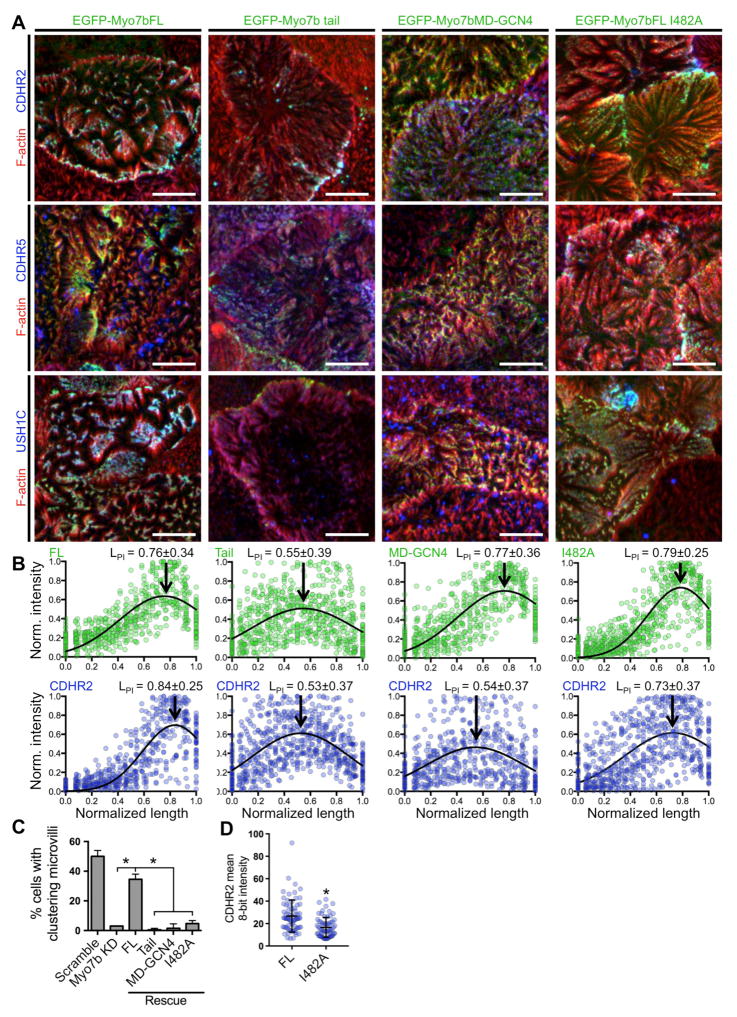
To further probe the interactions and functions of Myo7b required for IMAC component localization and microvillar clustering, we performed rescue experiments in Myo7b KD CACO-2BBE cells. We were able to rescue expression and distal tip enrichment of IMAC components by stably expressing a full-length Myo7b construct designed to be refractory to KD (Figure 5A–5B, EGFP-Myo7bFL). Expression of this construct also rescued microvillar clustering (Figure 5C). The cargo-binding tail construct failed to target to microvillar tips in the absence of endogenous Myo7b and instead accumulated in the cytoplasm (Figure 5A–5B, EGFP-Myo7b tail). The forced dimer construct allowed us to disconnect the tip targeting of Myo7b from its interactions with endogenous IMAC cargoes. This construct exhibited robust tip targeting in Myo7b KD cells, but was unable to rescue tip localization of IMAC components (Figure 5A–5B, EGFP-Myo7bMD-GCN4), and the level of microvillar clustering was comparable to the Myo7b KD alone (Figure 5C). Finally, we used a variant of full-length Myo7b harboring the I482A mutation (expected to uncouple catalytic and mechanical activity) to analyze the role of motor activity in tip targeting of the IMAC and microvillar clustering. Expression of this construct only partially rescued the distal tip enrichment of IMAC proteins (Figure 5A–5B, EGFP-Myo7bFL I482A). However, this mutant was unable to rescue microvillar clustering (Figure 5C). To better understand this disconnect, we examined levels of CDHR2 recruitment to the BB in cells expressing EGFP-Myo7b FL vs. EGFP-Myo7bFL I482A constructs. In gain-matched images, cells expressing the uncoupling mutant exhibited significantly lower CDHR2 levels at the apical surface relative to cells expressing the WT full-length variant (Figure 5D), suggesting that this mutant was unable to rescue the localization of enough CDHR2 to support microvillar clustering. These results indicate that normal Myo7b motor activity is needed for robust, efficient accumulation of CDHR2 to the tips of microvilli, to a level that supports intermicrovillar adhesion.
Figure 5. Motor and tail domains are required for complete rescue of Myo7b KD phenotypes.
(A) Confocal images of 14 DPC Myo7b KD15 CACO-2BBE cells expressing the EGFP-tagged Myo7b rescue constructs indicated (green), and stained for F-actin (red) and either CDHR2, CDHR5, or USH1C (blue). Scale bars, 5 μm. (B) Line scan analysis of Myo7b rescue construct signal (green) and CDHR2 intensity (blue) parallel to the microvillar axis. 0=base and 1=tip. N = 51 scans for each plot. Gaussian curve fits are marked with length at peak intensity (LPI) ± distribution width (SD). (C) Quantification of cells with clustering microvilli expressed as a total percentage of cells. Only EGFP-positive (i.e. rescue construct-expressing) cells were scored. Bars indicate mean ± SD. N = 145 cells for FL, 194 cells for Tail, 168 cells for MD-GCN, and 164 cells for I482A. *p<0.0001, t test. (D) Mean 8-bit intensity measurements of CDHR2 in gain-matched images of FL rescue (24.7 ± 14.3; n = 72 cells) and I482A rescue (16.6 ± 8.8; n = 67 cells). *p<0.0001, t test. See also Figure S5.
DISCUSSION
Myo7b plays a role in intermicrovillar adhesion
Tip localization of the IMAC is essential for its function in organizing microvillar protrusions during BB assembly [7]. Initial studies showed that deletion of the cytoplasmic domain of CDHR2 resulted in a loss of distal tip enrichment, and thus intermicrovillar adhesion [7]. These data were the first to suggest that links to cytoplasmic binding partners were needed to generate and maintain the tip enrichment of IMAC components. Additionally, mice lacking the scaffolding molecule USH1C demonstrated a striking loss of Myo7b from the BB; in these cells, CDHR5 localized along the length of microvilli rather than exhibiting tip enrichment. SEM of these animals also revealed perturbations in BB morphology in regions of both small intestine and colon, further underscoring the importance of IMAC tip enrichment.
The data we present here indicate that Myo7b plays a role in promoting the distal tip enrichment of IMAC components. KD of Myo7b in CACO-2BBE cells resulted in decreased microvillar clustering and disruption of BB organization (Figure 2 and S2), as well as a significant loss of distal tip enrichment of several IMAC components (Figure 3 and S3A). We also observed decreased expression of certain IMAC components, which is most likely explained by mislocalization-induced turnover (Figure 3 and S3). By concentrating IMAC components at the distal tips, Myo7b focuses the resulting adhesion capacity to a singular point along the microvillar axis; this in turn increases the probability that collisions between adjacent microvilli will lead to productive, organized tipi-like clustering. Tip localized adhesion may also play a role in unifying the length of adjacent microvilli [7]. Although the CACO-2BBE cell culture model requires days to achieve enterocyte-like differentiation, the differentiation that occurs as native enterocytes exit stem cell-containing crypts is likely complete in hours rather than days. Thus, in an in vivo context, Myo7b is probably critical for targeting the IMAC to microvillar tips in a timely manner.
Significance of Myo7b motor activity in IMAC localization
The most obvious role for Myo7b motor activity in IMAC localization and function would be in powering transport along the microvillar axis, towards the distal tips. Although the mechanical properties of Myo7b have yet to be characterized in vitro, several lines of evidence suggest that Myo7b is an active motor, and that motor activity contributes to cargo enrichment at microvillar tips. First, a forced dimer of Myo7b motors domains, which lacks any cargo-interacting motifs, exhibits robust targeting to microvillar tips (Figure 4C). Second, mutations expected to prevent ATPase cycling or ‘uncouple’ (i.e. prevent) lever arm rotation abolish targeting of the forced dimer to microvillar tips (Figure 4D). Third, the plus-end directed movement of Myo7b forced dimer puncta can be directly visualized in Cos-7 cell filopodia (Figure 4E and Movie S1). Together, these data suggest that Myo7b mechanical activity is capable of driving transport out to the tips of microvilli.
Although Myo7b motor activity is sufficient for targeting this molecule to microvillar tips, the mechanisms employed by full-length, cargo-binding Myo7b molecules may be more complex. Indeed, our rescue experiments provide information on the requirements for distal tip enrichment of IMAC components and microvillar organization. Whereas full-length WT Myo7b was capable of fully rescuing both tip localization of IMAC cargoes and microvillar clustering, a Myo7b tail fragment or forced dimer of Myo7b motor domains (lacking the tail) were both unable to rescue either readout (Figure 5A–5D). Interestingly, a full-length Myo7b construct expressing the I482A mutation (expected to uncouple catalytic and mechanical activity) was able to target to microvillar tips and partially rescue the tip enrichment of CDHR2 (Figure 5A–5B). These results strongly suggest that one aspect of Myo7b function could be retention of cargoes at microvillar tips, which may not require force generation. However, this mutant failed to rescue microvillar clustering (Figure 5C), most likely because the amount of CDHR2 rescued in this case was significantly reduced relative to WT (Figure 5D). Thus, Myo7b force generation is needed to optimize the efficiency of IMAC accumulation at the distal tips of microvilli, either by enhancing retention or by actively transporting IMAC cargoes to these sites.
How might a Myo7b mutant deficient in motor activity target to microvillar tips and partially rescue CDHR2 localization? One mechanism might involve cooperative interactions between actin binding (still active in uncoupled mutants) and tail-mediated binding partner interactions. Future studies will need to dissect how specific regions of the tail, and interactions with specific IMAC cargoes, contribute to the targeting and function of Myo7b.
Regulation of Myo7b activity
Motor proteins are subject to tight regulation to prevent unnecessary ATP hydrolysis and control both temporal and spatial activation for proper function. One common form of regulation is ‘auto-inhibition’, where a cargo-binding tail folds back to directly interact with the motor domain and inhibit catalytic and mechanical activity. Release of auto-inhibition typically involves cargo binding to the tail, but can also be caused by calcium-dependent light chain binding or phosphorylation. There is no direct evidence indicating that full-length Myo7b adopts an auto-inhibitory conformation. However, structural studies of closely related Myo7a have shown that the tail domain binds to and inhibits the motor domain [17, 37, 38]. If Myo7b is regulated in a similar manner, our data suggest that complete release from any auto-inhibited state may also require ATPase activity and normal actin binding. Indeed, the Myo7b tail domain exhibited the most robust distal tip localization in our targeting assay (Figure 4C), whereas the non-cycling full-length Myo7b mutants were unable to tip target even though they contain an intact tail domain (Figure 4B). Previous studies have demonstrated that actin-binding is sufficient to relieve auto-inhibition of myosin-6, allowing for dimerization, motor clustering, and processive movement [39]. Myo7b may have similar requirements for exiting an auto-inhibited state and achieving full activation in cells.
MyTH4-FERM myosins as transporters in finger-like protrusions
Myo7b shares structural and functional similarities with other MyTH4-FERM myosins including Myo7a, Myo10, and Myo15, each of which have been implicated in anchoring or transporting cargoes at/to the tips of other actin bundle-supported protrusions [16–26]. Myo10 is an inducer of filopodial formation and has been implicated in the trafficking of a number of factors that play roles in the growth and stabilization of these protrusions, including VASP and integrins [25, 26, 40]. Previous experiments with Myo10 provide compelling evidence in support of barbed-end directed transport, with direct observation of motility at the single molecule level using TIRF microscopy [41]. Myo15a also plays a role in the enrichment of specific cargoes at the ends of protrusions, in this case Whirlin [21] and EPS8 [22] at the tips of stereocilia. Shaker-2 mice lacking Myo15a are unable to enrich these cargoes at the tips of stereocilia, which are shorter as a result [21, 22]. Myo7a, which has been investigated extensively in connection to its role in Type 1 Usher syndrome [42], is most closely related to Myo7b in domain structure and organization [11]. Myo7a is highly expressed in hair cells of the vestibular and cochlear systems, where it contributes to the localization of Sans and other components of the Usher complex (CDH23 and USH1C) at upper tip-link densities [16]. Together, these factors are essential for tip-link assembly, positioning, and function in mechanotransduction [43]. Because a number of mutations that give rise to hearing loss are located in the motor domain [44], Myo7a likely uses motor activity to position or exert tension on the ends of tip-links. Mice lacking Myo7a exhibit striking parallels in phenotype relative to the Myo7b KD CACO-2BBE cell lines reported here. Shaker-1 mice, which lack functional Myo7a, exhibit disorganized bundles of stereocilia and defects in mechanotransduction [45]. Loss of functional Myo7a also disrupts the localization of USH1C at upper tip-link densities [46]. Interestingly, Myo7a contains a stable α-helix and short coiled-coil, which are noticeably absent in Myo7b. These domains likely mediate the robust filopodial tip targeting and cargo-induced dimerization seen in cultured cells [18].
With our current findings, we now know that all three major classes of actin bundle-supported protrusions take advantage of MyTH4-FERM myosins for their assembly and organization. This role is conserved as Myo7 in Dictyostelium targets to the tips of filopodia where it plays a role in their adhesion with extracellular substrates [47]. The use of MyTH4-FERM myosins to mediate similar functions in diverse systems suggests structural diversification from a common ancestral gene. Indeed, phylogenetic studies indicate that a MyTH4-FERM myosin was one of three actin-based motors present in the cenancestral eukaryote [48].
Conclusions
Our studies reveal Myo7b as a MyTH4-FERM myosin that targets to the tips of microvilli and promotes the distal tip enrichment of IMAC cargoes, which are essential for normal BB assembly. Beyond the implications for understanding mechanisms of enterocyte differentiation, our results also imply that microvillar core actin bundles may support tip-directed transport by other myosins normally found in these protrusions. Indeed, myosin-1d and myosin-5 have been show to enrich at both the distal tips and terminal web in the intestinal BB [49, 50]. Additional studies will be needed to determine how these motors contribute to the assembly and maintenance of microvilli. Other future experiments will need to explore the regulation of Myo7b motor and cargo binding activities. While the minimal unit sufficient for tip targeting is a dimer of Myo7b motor domains, the stoichiometry of the complex in vivo is unknown. The vast network of protein interactions between Myo7b and other IMAC components could support the formation of a large macromolecular complex, associated with an ensemble of Myo7b motors. Investigating molecular mechanisms of IMAC formation will further develop our understanding of the requirements for proper function and provide additional insight into conserved functions of MyTH4-FERM myosins.
EXPERIMENTAL PROCEDURES
Cell culture and stable cell line generation
CACO-2BBE, LLC-PK1-CL4, Cos-7, and HEK293FT cells were cultured at 37°C and 5% CO2 in DMEM with high glucose and 2 mM L-glutamine supplemented with 20% FBS for CACO-2BBE cells and 10% FBS for LLC-PK1-CL4, Cos-7, and HEK293FT cells. For generation of stable cell lines, CACO-2BBE and LLC-PK1-CL4 cells were grown to 90% confluency in T25 flasks. For lentivirus transduction of CACO-2BBE cells, the media was supplemented with 8 μg/ml polybrene and incubated with lentivirus overnight (ON). For stable transfections of LLC-PK1-CL4 cells, transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions and the cells were allowed to recover ON. The following day, cells were reseeded into T75 flasks and grown for 3 days. Cells were then reseeded into T182 flasks with media containing 50 μg/ml of puromycin or 1 mg/ml of G418 and grown to select for stable integration. Additional details are found in the Supplemental Experimental Procedures.
Microscopy
Tissue sections and cells were imaged using a Leica TCS SP5 or Nikon A1R laser-scanning confocal microscope. Live cell imaging was performed on a Nikon TiE inverted light microscope equipped with 488 and 561 excitation LASERS, a 100x/1.49 NA TIRF objective, and a Roper Evolve EM-CCD detector (Photometrics). Structure illumination microscopy was performed using an Applied Precision DeltaVision OMX (GE Healthcare) equipped with a 60x Plan-Apochromat N/1.42 NA oil immersion objective (Olympus) and processed using softWorx software (GE Healthcare). SEM was performed using a Quanta 250 Environmental scanning electron microscope operated in high vacuum mode with an accelerating voltage of 5–10 kV. Images were contrast enhanced and cropped using ImageJ software (NIH). For details on sample preparation and data analysis, see the Supplemental Experimental Procedures.
Statistical analysis
For all figures, error bars indicate SD and n values are reported in the figure legends. All graphs were generated and statistical analyses performed in Prism v.6 or 7 (GraphPad). Unpaired t tests were used to determine statistical significance between reported values.
Supplementary Material
Document S1. Figures S1–S5 and Supplemental Experimental Procedures.
Movie S1. Related to Figure 4, forced dimerization of Myo7b motor domains results in enrichment at filopodial tips.
Acknowledgments
We thank all members of the Tyska laboratory, Vanderbilt Microtubule and Motors Club, Vanderbilt Epithelial Biology Center, and Vanderbilt Program in Developmental Biology for advice and support. Super-resolution and electron microcopy was performed through the use of the VUMC Cell Imaging Shared Resource. This work was supported by the Vanderbilt Training Program in Stem Cell and Regenerative Developmental Biology (M.L.W.), American Heart Association Predoctoral Fellowship (M.L.W.), NRSA Predoctoral Fellowship F31DK108528 (M.L.W.), American Heart Association Postdoctoral Fellowship (S.W.C.), and National Institutes of Health Grants R01-DK075555 and R01-DK095811 (M.J.T.).
Footnotes
AUTHOR CONTRIBUTIONS
M.L.W. and M.J.T. designed experiments, analyzed data, and wrote the manuscript. M.L.W. performed experiments and M.J.T. supervised the study. S.W.C and C.R.S. helped with data collection. All authors contributed to editing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. The Journal of cell biology. 1975;67:725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shifrin DA, Jr, McConnell RE, Nambiar R, Higginbotham JN, Coffey RJ, Tyska MJ. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial-microbial interactions. Current biology : CB. 2012;22:627–631. doi: 10.1016/j.cub.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khubchandani SR, Vohra P, Chitale AR, Sidana P. Microvillous inclusion disease--an ultrastructural diagnosis: with a review of the literature. Ultrastructural pathology. 2011;35:87–91. doi: 10.3109/01913123.2010.537438. [DOI] [PubMed] [Google Scholar]
- 4.Wilson W, Scott RB, Pinto A, Robertson MA. Intractable diarrhea in a newborn infant: microvillous inclusion disease. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2001;15:61–64. doi: 10.1155/2001/743925. [DOI] [PubMed] [Google Scholar]
- 5.Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, Hussain K, Furth-Lavi J, Cosgrove KE, Shepherd RM, et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nature genetics. 2000;26:56–60. doi: 10.1038/79178. [DOI] [PubMed] [Google Scholar]
- 6.Vallance BA, Chan C, Robertson ML, Finlay BB. Enteropathogenic and enterohemorrhagic Escherichia coli infections: emerging themes in pathogenesis and prevention. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2002;16:771–778. doi: 10.1155/2002/410980. [DOI] [PubMed] [Google Scholar]
- 7.Crawley SW, Shifrin DA, Jr, Grega-Larson NE, McConnell RE, Benesh AE, Mao S, Zheng Y, Zheng QY, Nam KT, Millis BA, et al. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell. 2014;157:433–446. doi: 10.1016/j.cell.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawley SW, Weck ML, Grega-Larson NE, Shifrin DA, Jr, Tyska MJ. ANKS4B Is Essential for Intermicrovillar Adhesion Complex Formation. Developmental cell. 2016;36:190–200. doi: 10.1016/j.devcel.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, He Y, Lu Q, Zhang M. Mechanistic Basis of Organization of the Harmonin/USH1C-Mediated Brush Border Microvilli Tip-Link Complex. Developmental cell. 2016;36:179–189. doi: 10.1016/j.devcel.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZY, Hasson T, Zhang DS, Schwender BJ, Derfler BH, Mooseker MS, Corey DP. Myosin-VIIb, a novel unconventional myosin, is a constituent of microvilli in transporting epithelia. Genomics. 2001;72:285–296. doi: 10.1006/geno.2000.6456. [DOI] [PubMed] [Google Scholar]
- 12.Chen T, Hubbard A, Murtazina R, Price J, Yang J, Cha B, Sarker R, Donowitz M. Myosin VI mediates the movement of NHE3 down the microvillus in intestinal epithelial cells. Journal of cell science. 2014;127:3535–3545. doi: 10.1242/jcs.149930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. Journal of the American Society of Nephrology : JASN. 2005;16:2890–2896. doi: 10.1681/ASN.2005040366. [DOI] [PubMed] [Google Scholar]
- 14.Merritt RC, Manor U, Salles FT, Grati M, Dose AC, Unrath WC, Quintero OA, Yengo CM, Kachar B. Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions. Current biology : CB. 2012;22:320–325. doi: 10.1016/j.cub.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salles FT, Merritt RC, Jr, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dose AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nature cell biology. 2009;11:443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai T, Jung HS, Sato O, Yamada MD, You DJ, Ikebe R, Ikebe M. Structure and Regulation of the Movement of Human Myosin VIIA. The Journal of biological chemistry. 2015;290:17587–17598. doi: 10.1074/jbc.M114.599365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai T, Umeki N, Ikebe R, Ikebe M. Cargo binding activates myosin VIIA motor function in cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7028–7033. doi: 10.1073/pnas.1009188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Kovacs M, Sakamoto T, Zhang F, Kiehart DP, Sellers JR. Dimerized Drosophila myosin VIIa: a processive motor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5746–5751. doi: 10.1073/pnas.0509935103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belyantseva IA, Boger ET, Friedman TB. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13958–13963. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nature cell biology. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 22.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Current biology : CB. 2011;21:167–172. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almagro S, Durmort C, Chervin-Petinot A, Heyraud S, Dubois M, Lambert O, Maillefaud C, Hewat E, Schaal JP, Huber P, et al. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Molecular and cellular biology. 2010;30:1703–1717. doi: 10.1128/MCB.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nature cell biology. 2002;4:246–250. doi: 10.1038/ncb762. [DOI] [PubMed] [Google Scholar]
- 25.Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochemical and biophysical research communications. 2004;319:214–220. doi: 10.1016/j.bbrc.2004.04.167. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Berg JS, Li Z, Wang Y, Lang P, Sousa AD, Bhaskar A, Cheney RE, Stromblad S. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nature cell biology. 2004;6:523–531. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 27.Henn A, De La Cruz EM. Vertebrate myosin VIIb is a high duty ratio motor adapted for generating and maintaining tension. The Journal of biological chemistry. 2005;280:39665–39676. doi: 10.1074/jbc.M507667200. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Kovacs M, Xu Q, Anderson JB, Sellers JR. Myosin VIIB from Drosophila is a high duty ratio motor. The Journal of biological chemistry. 2005;280:32061–32068. doi: 10.1074/jbc.M506765200. [DOI] [PubMed] [Google Scholar]
- 29.Phichith D, Travaglia M, Yang Z, Liu X, Zong AB, Safer D, Sweeney HL. Cargo binding induces dimerization of myosin VI. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17320–17324. doi: 10.1073/pnas.0909748106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C, Feng W, Wei Z, Miyanoiri Y, Wen W, Zhao Y, Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. doi: 10.1016/j.cell.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 31.Aschenbrenner L, Naccache SN, Hasson T. Uncoated endocytic vesicles require the unconventional myosin, Myo6, for rapid transport through actin barriers. Molecular biology of the cell. 2004;15:2253–2263. doi: 10.1091/mbc.E04-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada T, Sasaki N, Ohkura R, Sutoh K. Alanine scanning mutagenesis of the switch I region in the ATPase site of Dictyostelium discoideum myosin II. Biochemistry. 1997;36:14037–14043. doi: 10.1021/bi971837i. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki N, Shimada T, Sutoh K. Mutational analysis of the switch II loop of Dictyostelium myosin II. The Journal of biological chemistry. 1998;273:20334–20340. doi: 10.1074/jbc.273.32.20334. [DOI] [PubMed] [Google Scholar]
- 34.Kambara T, Rhodes TE, Ikebe R, Yamada M, White HD, Ikebe M. Functional significance of the conserved residues in the flexible hinge region of the myosin motor domain. The Journal of biological chemistry. 1999;274:16400–16406. doi: 10.1074/jbc.274.23.16400. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki N, Ohkura R, Sutoh K. Dictyostelium myosin II mutations that uncouple the converter swing and ATP hydrolysis cycle. Biochemistry. 2003;42:90–95. doi: 10.1021/bi026051l. [DOI] [PubMed] [Google Scholar]
- 36.O’Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 37.Umeki N, Jung HS, Watanabe S, Sakai T, Li XD, Ikebe R, Craig R, Ikebe M. The tail binds to the head-neck domain, inhibiting ATPase activity of myosin VIIA. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8483–8488. doi: 10.1073/pnas.0812930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Baboolal TG, Siththanandan V, Chen M, Walker ML, Knight PJ, Peckham M, Sellers JR. A FERM domain autoregulates Drosophila myosin 7a activity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4189–4194. doi: 10.1073/pnas.0808682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park H, Ramamurthy B, Travaglia M, Safer D, Chen LQ, Franzini-Armstrong C, Selvin PR, Sweeney HL. Full-length myosin VI dimerizes and moves processively along actin filaments upon monomer clustering. Molecular cell. 2006;21:331–336. doi: 10.1016/j.molcel.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12411–12416. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerber ML, Jacobs DT, Campagnola L, Dunn BD, Yin T, Sousa AD, Quintero OA, Cheney RE. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Current biology : CB. 2009;19:967–973. doi: 10.1016/j.cub.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weil D, Kussel P, Blanchard S, Levy G, Levi-Acobas F, Drira M, Ayadi H, Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nature genetics. 1997;16:191–193. doi: 10.1038/ng0697-191. [DOI] [PubMed] [Google Scholar]
- 43.Pan L, Zhang M. Structures of usher syndrome 1 proteins and their complexes. Physiology. 2012;27:25–42. doi: 10.1152/physiol.00037.2011. [DOI] [PubMed] [Google Scholar]
- 44.Weston MD, Kelley PM, Overbeck LD, Wagenaar M, Orten DJ, Hasson T, Chen ZY, Corey D, Mooseker M, Sumegi J, et al. Myosin VIIA mutation screening in 189 Usher syndrome type 1 patients. American journal of human genetics. 1996;59:1074–1083. [PMC free article] [PubMed] [Google Scholar]
- 45.Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 46.Lefevre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin JP, Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 47.Tuxworth RI, Weber I, Wessels D, Addicks GC, Soll DR, Gerisch G, Titus MA. A role for myosin VII in dynamic cell adhesion. Current biology : CB. 2001;11:318–329. doi: 10.1016/s0960-9822(01)00097-5. [DOI] [PubMed] [Google Scholar]
- 48.Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- 49.Benesh AE, Nambiar R, McConnell RE, Mao S, Tabb DL, Tyska MJ. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Molecular biology of the cell. 2010;21:970–978. doi: 10.1091/mbc.E09-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. Journal of cell science. 1994;107(Pt 12):3535–3543. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Document S1. Figures S1–S5 and Supplemental Experimental Procedures.
Movie S1. Related to Figure 4, forced dimerization of Myo7b motor domains results in enrichment at filopodial tips.