Summary
When the Multi-Ethnic Study of Atherosclerosis (MESA) began, the Framingham Risk Score (FRS) was the preferred tool for 10-year global coronary heart disease (CHD) risk assessment. The FRS had limitations including derivation in a homogenous population lacking racial and ethnic diversity and exclusive reliance on traditional risk factors without consideration of subclinical disease measures. MESA was designed to study the prognostic value of subclinical atherosclerosis and other risk markers in a multi-ethnic population. In a series of landmark publications, MESA demonstrated that measures of subclinical cardiovascular disease add significant prognostic value to the traditional Framingham risk variables. In head-to-head studies comparing these markers, MESA established that the coronary artery calcium (CAC) score may be the single best predictor of CHD risk. Results from MESA have directly influenced recent prevention guidelines including the recommendations on risk assessment and cholesterol-lowering therapy. The MESA study has published its own risk score, which allows for the calculation of 10-year risk of CHD before and after knowledge of a CAC score.
Introduction
The Framingham Heart Study, the first major longitudinal cohort study of cardiovascular disease in the United States, identified and described the major traditional risk factors for coronary heart disease (CHD): high cholesterol, high blood pressure, smoking, and diabetes.1,2 Recognizing that these risk factors acted synergistically, Framingham investigators developed risk equations for the calculation of 10-year risk that became the basis for global risk assessment for over 25 years.3 In 2001, the third Adult Treatment Panel (ATPIII) of the National Cholesterol Education Program adopted a version of the 10-year Framingham Risk Score for CHD (FRS) in their guidelines, which solidified the role of global risk assessment in the decision to treat asymptomatic individuals free of known CHD with lipid-lowering therapy.4
The Multi-Ethnic Study of Atherosclerosis (MESA), following the original Framingham cohort by approximately 50 years, began enrollment in an era when the traditional CHD risk factors were well known.5 MESA was distinct in its aim to study the prevalence, burden, progression, and clinical significance of subclinical cardiovascular disease (Figure 1). At the time MESA was conceived, it was not at all clear if routine measurement of subclinical cardiac or vascular disease would add clinical value and predict risk beyond the FRS. Therefore, the initial objectives in MESA sought to investigate whether new risk markers, especially those representing subclinical atherosclerosis, added prognostic value when combined with the FRS or with the individual traditional risk factors.6
Figure 1.
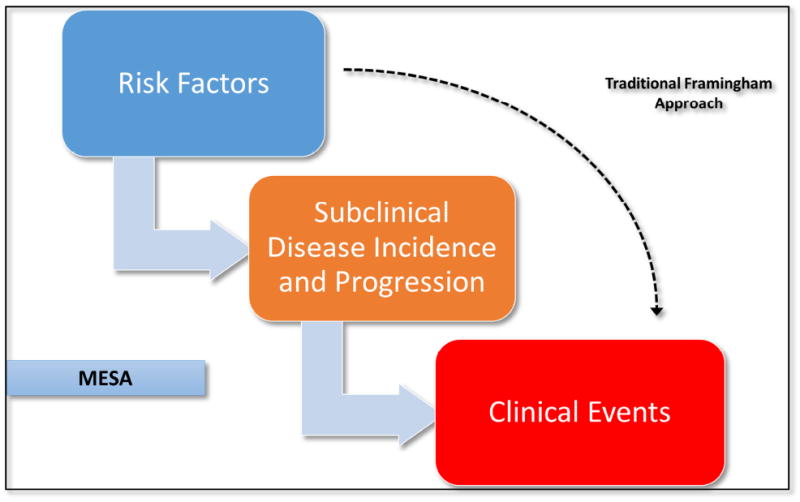
The design of MESA allows the study the associations between risk factors, subclinical disease burden and progression, and clinical events.
It was reassuring that the traditional risk factors were not only associated with subclinical disease in MESA, but that they predicted the progression of subclinical disease. In a paper by Kronmal et al in 2007, MESA authors demonstrated that age, male sex, white race/ethnicity, hypertension, body mass index, diabetes, and family history not only predicted incident coronary artery calcium (CAC) over 2.4 years of follow-up, but also progression of existing CAC.7 These data were recently replicated over 10-year follow-up.8 Coupled with data demonstrating that subclinical disease predicts CHD events,9 MESA helped solidify subclinical disease as a true precursor lesion on the causal pathway between risk factors and hard events. Other MESA studies have established a wide range of more novel risk factors, ranging from air pollution to lifestyle variables to insulin resistance, as predictors of both subclinical disease progression and CHD events.10-14
The FRS itself in fact predicts CAC progression. In 2011, DeFilippis et al demonstrated a 40% higher risk of incident CAC per 5% higher absolute FRS risk, and a mean 7 Agatston score increase per 5% higher FRS among those with existing CAC.15 However given concerns about possible limitations of the FRS,16 including lack of race and ethnic diversity in the derivation sample and the absence of certain newly identified risk factors, competing risk scores including the Reynolds Risk Score (RRS) were also studied. The 2008 RRS added family history and high sensitivity C-reactive protein (hsCRP) to the risk algorithm along with the traditional Framingham risk factors.17 The DeFilippis et al. paper showed that when the FRS and RRS were discordant, the RRS better predicted CAC incidence and progression.15
Adding to the Framingham Risk Factors
Many novel risk markers have been proposed to improve CHD risk prediction when added to the traditional Framingham risk factors. In MESA, these most prominently have included measures of subclinical cardiovascular disease (CAC, carotid intima media thickness, carotid plaque, and ankle brachial index), vascular function (flow mediated dilation), inflammation (especially high-sensitivity C-reactive protein), and family history of CHD.
Coronary Artery Calcium
In the first landmark MESA paper, Detrano et al reported on the relationship between CAC and CHD events in the 4 race/ethnicity groups in MESA.18 Over a median follow up of 4 years, CAC was associated with a graded increase in risk of both hard and all CHD events (Figure 2). In multivariable models controlling for the traditional risk factors, a CAC score of 1-100 was associated with a nearly 4-fold higher risk of hard events (95% confidence interval (CI) 1.72, 8.79), while a CAC score >300 was associated with a nearly 7-fold higher hard event risk (95% CI 2.93, 15.99) compared to those who had a CAC score of 0. Each doubling of CAC was associated with a 20% increased risk of events (95% CI 1.12, 1.29). Similar trends were noted for each of the 4 race/ethnic groups, and there was no interaction between CAC and race/ethnicity. In the overall population as well as for each racial/ethnic group, CAC improved discrimination for incident CHD. Overall, there was a significant increase in the C-statistic from 0.79 to 0.83 after addition of CAC to a model with only traditional risk factors.18
Figure 2.
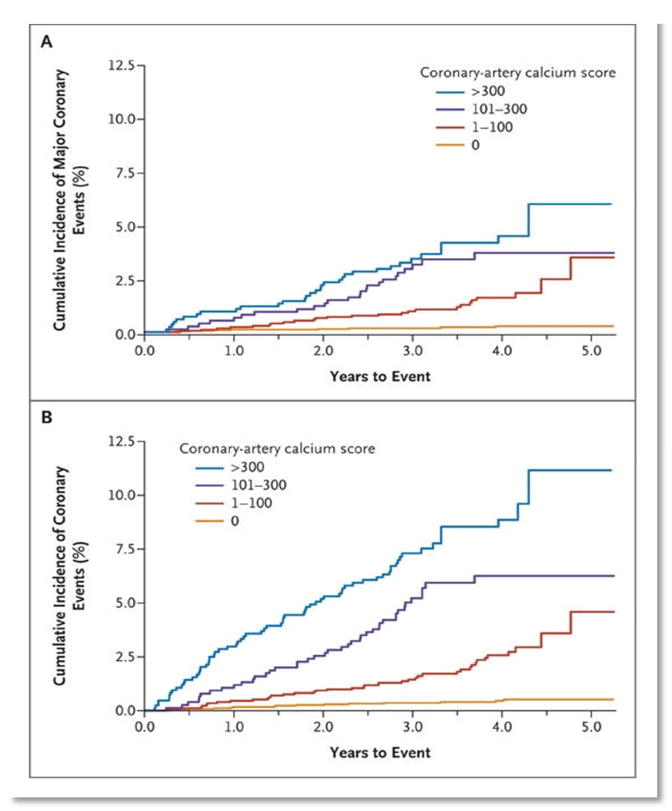
Unadjusted Kaplan-Meier cumulative-event curves for coronary events among participants with coronary artery calcium Scores of 0, 1 to 100, 101 to 300, and >300.
To better understand the impact of CAC on CHD risk classification, Polonsky et al calculated the net reclassification improvement (NRI) using models with traditional risk factors before and after addition of the CAC score. In an analytic sample of 5878 individuals, the addition of CAC resulted in an overall NRI of 0.25 (95% CI 0.16, 0.34) whereby 728 individuals were reclassified to a higher risk category and 814 to a lower risk category. This translated to a higher proportion of individuals classified as either high or low risk (77% vs. 69%). Importantly, the addition of CAC to the model resulted in an additional 23% of participants who had events and an additional 13% of those who did not experience events to be classified as high- or low-risk respectively.19 Several other MESA papers have supported these general findings.20-25
The legacy of MESA in the field of risk prediction has perhaps been most solidified by these results for CAC, driving the current shift in the risk assessment paradigm from a purely risk factor-based enterprise to a multifaceted approach including measurement of subclinical disease.
Carotid Intima Media Thickness and Carotid Plaque
While the strongest results have been observed for CAC, MESA has provided additional insight about other markers. Polak et al used baseline ultrasound measurements of the carotid arteries to study the association of different plaque indices (carotid intima media thickness (cIMT) and carotid plaque stenosis) and incident cardiovascular disease over a follow up of 7.8 years. Each metric was significantly associated with CHD and CVD risk and modestly improved the AUC when added to a baseline model with risk factors only. Only carotid plaque causing >25% narrowing at the carotid bulb was associated with a higher risk of stroke (hazard ratio (HR) (95% CI) 1.60 (1.08, 2.35)), but this did not improve discrimination compared to risk factors only. Importantly, different plaque metrics resulted in improved reclassification depending on the outcome of interest. For CHD, the NRI was small but significant for all metrics except for the maximum internal carotid artery IMT >1.5 mm, and the largest NRI was observed for mean of the maximum IMT. For CVD events, the NRI was significant only for the mean of the maximum internal carotid artery IMT, while NRI values were not significant for any metric for stroke.26
Flow mediated dilation
Yeboah et al assessed the predictive value of brachial flow mediated dilation (FMD) for incident cardiovascular events over 5 years of follow up. An increase in 1 standard deviation of FMD was significantly associated with decreased CVD risk (HR (95% CI) 0.80 (0.63, 0.97)) independent of the FRS. Similarly, FMD was inversely associated with incident CHD and CVD death in fully adjusted models. In race-stratified analyses, FMD was no longer significantly associated with incident CVD after full adjustment. Addition of FMD to the FRS did not improve overall global discrimination of incident CVD as measured by the C-statistic. However, FMD correctly reclassified 52% of participants with no incident CVD event but also incorrectly reclassified 23% of subjects who developed CVD; the overall NRI was 29% (p<0.001).27
Ankle Brachial Index
Criqui et al evaluated the association of high and low ankle brachial index (ABI) with incident cardiovascular events over a mean follow up of 5.3 years. Both high (ABI ≥1.4) and low (ABI <1) were associated with higher risk of CVD (HR (95% CI) 1.82 (0.98, 3.34) and 1.78 (1.32, 2.39) respectively) after adjusting for traditional risk factors. After additional adjustment for markers of inflammation, thrombosis, subclinical CVD and kidney function, only low ABI remained a significant predictor of CVD (HR (95% CI 1.46 (1.06, 2.00)). In analyses of ABI as a continuous variable, excluding ABI ≥1.4, a 0.1 unit increment in ABI was associated with an 11% lower risk of CVD (95% CI 0.81, 0.97) after adjusting for traditional risk factors. Interaction testing between ABI and each of sex and race/ethnicity was not significant. Similarly, both low and high ABIs were associated with CHD events independent of traditional risk factors (HR= 1.87 (p =0.001) and 2.15 (p =0.029) respectively). Results for stroke were not significant. Addition of ABI to traditional risk factors increased the C-statistic from 0.78 to 0.79 (p=0.022) and the integrated discrimination improvement (IDI) demonstrated a significant role for ABI for reclassification of events and non-events (p=0.003).28
Inflammation
Jenny et al examined the cross-sectional association between inflammatory markers and coronary atherosclerosis measured by presence of CAC. Compared to the lowest quartile of hsCRP, there was a 13% higher risk of CAC >0 in the highest quartile (95% CI 1.06, 1.19) in age, sex and ethnicity adjusted models. For interleukin 6 (IL-6), the corresponding relative risk was 22% (95% CI 1.15, 1.30) and 18% (95% CI 1.11, 1.24) for fibrinogen. After adjustment for FRS variables, the relative risk estimates were attenuated and were as follows: 1.05 (95% CI 0.99, 1.12) for hsCRP, 1.12 (95% CI 1.06, 1.20) for IL-6, and 1.09 (95% CI 1.02, 1.16) for fibrinogen. Similar trends were noted in sex- and ethnicity-stratified analyses.29
HsCRP is the most clinically accepted inflammatory biomarker and has been the subject of many important papers from MESA. For example, Yeboah et al demonstrated that hsCRP was mildly associated with incident CHD but not CVD over a median follow-up 7.6 years in multivariable models that controlled for traditional risk factors (HR (95% CI) 1.28 (1.00, 1.64) and 1.15 (0.92, 1.45) respectively).30 Other studies suggested that the association of hsCRP with subclinical atherosclerosis was at least moderately attenuated by adjustment for obesity, and after stratification, there was a stronger association between obesity and cIMT as compared to hsCRP and cIMT.31
Family History of Coronary Heart Disease
Nasir et al examined the cross-sectional association of a family history (FH) of premature CHD with prevalence of CAC. After adjustment for the FRS, a FH of premature CHD was associated with a 78% higher odds ratio (OR) of CAC >0 (95% CI 1.48, 2.13). The corresponding odds ratio (OR) for CAC ≥75th percentile was 2.00 (95% CI, 1.66, 2.41). The association of FH of late-onset CHD and CAC was weaker compared to FH of premature CHD. In race/ethnicity-stratified analyses, a FH of premature CHD was associated with a higher prevalence of CAC ≥75th percentile in both low and intermediate FRS categories. When considering the relationship to the affected family member, a FH of premature CHD in a sibling had a stronger association with CAC >0 compared to a parent only, while a FH in both parents and siblings had the strongest association (OR (95% CI) 1.90 (1.49 to 2.40), 1.48 (1.15 to 1.91), and 3.23 (1.85 to 5.63) respectively). The association of CHD risk factors and CAC did not differ according to FH of premature CHD status.32 Among individuals with a CAC score of 0, a positive FH of CHD portended a greater 10-year risk of CVD and CHD events compared to those without a FH of CHD.33
Head-to-Head Comparisons of Novel Risk Markers – A Primary Contribution of MESA
MESA uniquely allowed head-to-head comparison of the strength of novel markers for a variety of outcomes.
Folsom et al compared CAC and cIMT for the prediction of cardiovascular events over approximately 5 years of follow-up. In multivariable models adjusted for traditional risk scores and both CAC and cIMT (modeled continuously), CAC was a stronger predictor of CVD and CHD than cIMT. The HRs (95% CI) of CVD and CHD per standard deviation increase of CAC versus cIMT were (2.1 (1.8, 2.5) vs. 1.3 (1.1, 1.4) and 2.3 (1.9, 2.8) vs. 1.1 (1.0, 1.3). Only cIMT was significantly associated with incident stroke (HR (95% CI) 1.3 (1.1, 1.7)) while the HR (95% CI) for CAC was 1.1 (0.8, 1.4). Similar results were obtained in analyses using categorical CAC and cIMT. In analyses of discrimination, CAC was better able to discriminate CVD events compared to cIMT. Addition of CAC to traditional risk factors improved the C-statistic from 0.772 (95% CI 0.74, 0.80) to 0.808 (95% CI 0.78, 0.83) while addition of cIMT led to an increase to 0.782 (95% CI 0.75, 0.81). The C-statistic after including both cIMT and CAC was 0.811 (0.78, 0.84). Similar trends were obtained for CHD such that addition of CAC to risk factors alone increased the C-statistic from 0.771 (95% CI 0.74, 0.80) to 0.823 (95% CI 0.79, 0.85) while addition of cIMT increased it to 0.782 (95% CI 0.75, 0.82). Similarly, addition of both cIMT and CAC had a similar effect on the C-statistic as did addition of CAC only (AUC 0.824 (95% CI 0.79, 0.85)).34
Gepner et al compared the predictive use of CAC, carotid plaque, and cIMT for incident CVD, CHD and stroke/TIA. CAC presence was the strongest predictor of CVD events after adjustment for traditional risk factors (HR (95% CI) 3.12 (2.44, 3.99)). Presence of carotid plaque was also significantly associated with incident CVD (HR (95% CI) 1.61 (1.17, 2.21)). Carotid plaque/cIMT ≥75th percentile was a better predictor of CVD compared to carotid plaque only (HR (95% CI) 2.06 (1.46, 2.91)). CAC presence was a stronger predictor of CHD events (HR (95% CI) 4.48 (3.24, 6.17)) than CVD. CAC presence, carotid plaque presence, and carotid plaque/cIMT ≥75th percentile independently predicted stroke/TIA (HR (95% CI) 1.54 (1.09, 2.18), 1.40 (1.35, 1.45), and 1.86 (1.10, 3.13) respectively). In analyses of discrimination of incident CVD, addition of CAC presence to traditional risk factors increased the C-statistic from 0.756 to 0.776 (p<0.001). Addition of carotid plaque presence increased the C-statistic to 0.760 (p=0.033), while cIMT ≥75th percentile did not have an effect on the C-statistic compared to traditional risk factors alone (p=0.110). The improvement in discrimination for carotid plaque/cIMT ≥75th percentile was similar to carotid plaque alone. The results were similar for incident CHD. For combined stroke/TIA, only addition of carotid plaque led to a statistically significant improvement in the AUC (C-statistic=0.787, p=0.045). In reclassification analyses, only CAC presence resulted in a statistically significant improvement in NRI for CVD and CHD events.35
Criqui et al studied the joint association of ABI and CAC with incident CVD by analyzing the relationship of ABI and CVD risk within strata of CAC. Among those with CAC=0, incidence rates were low regardless of ABI group. In those with CAC >0, ABI was found to have a U-shaped association within CAC groups (1-100 and >100). In analyses using ABI as a continuous variable, ABI was inversely related to the CVD event rate among those with presence of CAC.28
Blaha et al studied the prognostic significance of CAC in MESA participants who met the JUPITER trial entry criteria (LDL-C >130 mg/dL and hsCRP ≥2 mg/L). Among those with CAC=0, CHD and CVD event rates were low (0.8 and 3.7 events per 1000 person-years, respectively), while event rates were high for CAC >100 (20.2 and 26.4 events per 1000 person years). Importantly, over a median follow up of 5.8 years, hsCRP did not predict CHD (HR (95% CI) 0.98 (0.62, 1.57)) or CVD events (HR (95% CI) 1.15 (0.78 1.68)) after adjusting for basic demographics. Presence of CAC however was significantly associated with both CHD (HR (95% CI) 6.65 (2.99, 14.78)) and CVD (HR (95% CI) 3.06 (1.82, 5.13)) in similarly adjusted models. CAC prevalence, and increasing CAC burden, remained significant predictors of events after full adjustment. This comparative effectiveness study helped conclude that CAC is a stronger predictor of CHD and CVD risk than hsCRP.36
Yeboah et al compared novel risk markers in MESA participants who were at intermediate risk of CHD (FRS >5% - <20%) to determine which marker most improved risk prediction. All risk markers were associated with incident CHD; however, after adjusting for traditional risk factors, cIMT and FMD were no longer significant. Among the risk markers, CAC had the strongest association (HR (95% CI) 2.60 (1.94, 3.50)). Similar results were obtained for CVD except that hsCRP was not significant in univariable analyses. Addition of each of the 6 risk markers to FRS improved the AUC; the C-statistic for risk factors alone was 0.623. CAC showed the highest increment while FMD showed the least increment for incident CHD (C-statistic: 0.784 and 0.639 respectively). CAC also showed the highest increment while hsCRP showed the least increment for incident CVD (Figure 3). For incident CHD, CAC resulted in the highest NRI of 0.659. The respective NRI was 0.024 for FMD, 0.036 for ABI, 0.102 for cIMT, 0.160 for FH of CHD, and 0.079 for hsCRP. Similar results were obtained for incident CVD.30
Figure 3.
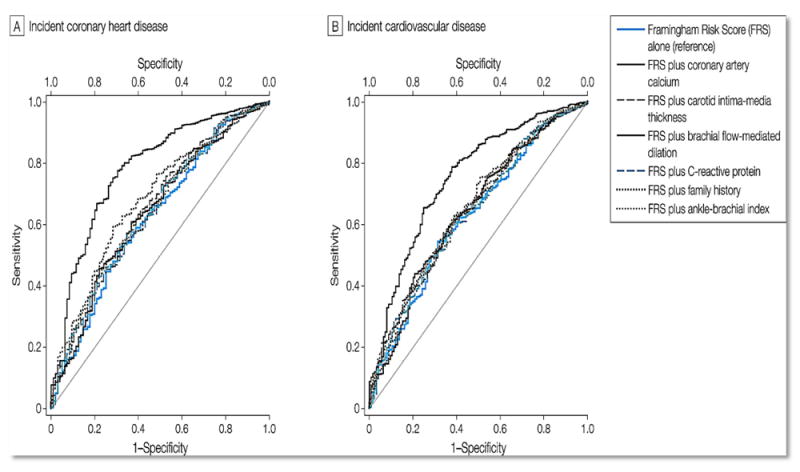
Receiver operator characteristic curves showing area under the curve for FRS alone and FRS in addition to novel risk markers.
Assessing Risk Score Performance: The Pooled Cohort Equations and 2013 ACC/AHA Prevention Guidelines
In 2013 the American College of Cardiology (ACC) and the American Heart Association (AHA) published a new set of prevention guidelines, and for the first time since 2001, a new risk score was introduced. As opposed to the FRS, which was derived solely from the original Framingham Heart Study cohort, the new Pooled Cohort Equations (PCE) was derived from 4 cohorts representing a mix of white and African-American participants. Instead of CHD as the outcome, the PCE modeled the 10-year risk of both CHD and stroke (so-called atherosclerotic cardiovascular disease [ASCVD]). However, the risk factors included in the PCE (except for race) are exactly the same as the FRS.37
MESA, although not a part of the derivation dataset, played a major role in risk score evaluation. In a limited validation exercise, the guideline writers themselves noted moderate discrimination (C-statistic for men and women ranging from 0.70-0.71 in Whites, and 0.67-0.77 in African Americans) and just fair calibration of the PCE in MESA, with a trend toward overestimation of risk.37
In a subsequent paper by DeFilippis et al, MESA authors conducted a comprehensive analysis of the discrimination and calibration of not just the PCE, but also the original FRS, the more popular ATPIII version of FRS, the RRS, and another Framingham risk formula for total cardiovascular disease (Table 1). In MESA, the PCE displayed moderate discrimination, similar to that seen for the ATPIII FRS (C-statistic 0.71 vs. 0.71). The PCE showed slightly better discrimination that the ATPIII FRS in women (C-statistic 0.71 vs. 0.67). Calibration for both scores was poor, with both the PCE and the ATPIII FRS overestimating 10-year CHD risk (discordance 78% and 115%, respectively). Overestimation was more notable in men (85% and 154%) than in women (67% and 46%).38 The DeFilippis paper clearly demonstrated that a traditional risk factor model alone had limited performance in MESA. Similar poor discrimination with the FRS was also noted by other MESA papers.30,39
Table 1.
Calibration and discrimination of various risk scores undergoing validation in the MESA study
| Risk Score | Predicted Events, n(%) | Observed Events, n(%) | Signed Absolute Difference | Discordance, %* | c-Statistic | Discrimination Slope |
|---|---|---|---|---|---|---|
| Total (n = 4227) | ||||||
| FRS-CHD† | 397.6 (9.41) | 263 (6.22) | 3.18 | 51 | 0.68 | 0.05 |
| FRS-CVD‡ | 561.3 (13.28) | 448 (10.60) | 2.68 | 25 | 0.71 | 0.09 |
| ATPIII-FRS-CHD¶ | 288.7 (6.83) | 134 (3.17) | 3.66 | 115 | 0.71 | 0.06 |
| RRS□ | 314.0 (7.43) | 323 (7.64) | -0.21 | -3 | 0.72 | 0.07 |
| AHA-ACC-ASCVD§ | 387.2 (9.16) | 218 (5.16) | 4.00 | 78 | 0.71 | 0.06 |
| Men (n = 1961) | ||||||
| FRS-CHD† | 251.1 (12.80) | 164 (8.36) | 4.44 | 53 | 0.69 | 0.05 |
| FRS-CVD‡ | 358.7 (18.29) | 261 (13.31) | 4.98 | 37 | 0.71 | 0.09 |
| ATPIII-FRS-CHD¶ | 218.6 (11.15) | 86 (4.39) | 6.76 | 154 | 0.71 | 0.05 |
| RRS□ | 213.5 (10.89) | 196 (9.99) | 0.89 | 9 | 0.70 | 0.06 |
| AHA-ACC-ASCVD§ | 232.1 (11.84) | 125 (6.37) | 5.46 | 86 | 0.71 | 0.06 |
| Women (n = 2266) | ||||||
| FRS-CHD† | 146.5 (6.47) | 99 (4.37) | 2.10 | 48 | 0.60 | 0.01 |
| FRS-CVD‡ | 202.6 (8.94) | 187 (8.25) | 0.69 | 8 | 0.70 | 0.05 |
| ATPIII-FRS-CHD¶ | 70.2 (3.10) | 48 (2.12) | 0.98 | 46 | 0.67 | 0.02 |
| RRS□ | 100.5 (4.44) | 127 (5.60) | -1.17 | -21 | 0.72 | 0.05 |
| AHA-ACC-ASCVD§ | 155.1 (6.84) | 93 (4.10) | 2.74 | 67 | 0.70 | 0.05 |
ACC = American College of Cardiology; AHA = American Heart Association; ASCVD = atherosclerotic cardiovascular disease; ATP = Adult Treatment panel III; CHD = coronary heart disease; CVD = cardiovascular disease; FRS = Framingham Risk Score; RRS = Reynolds Risk Score
Percentage discordance calculation: ([expected percentage – observed percentage) / observed percentage] × 100).
End points are myocardial infarction, death from CHD, and angina.
End points are myocardial infarction, death from CHD, angina, stroke, transient ischemic attach, peripheral vascular disease, and heart failure.
End points are myocardial infarction and death from CHD.
End points are myocardial infarction, death from CHD, stroke, and coronary revascularization.
End points are myocardial infarction, death from CHD, and stroke.
MESA and the Clinician-Patient Risk Discussion
A prominent feature of the new cholesterol treatment guidelines was the so-called Clinician-Patient Risk Discussion (CPRD), a two-way conversation between clinicians and patients about risk, potential benefits and harms of cholesterol therapy, and patient preferences.40 Several studies from MESA directly influenced the details of the CRPD, including recommended strategies for advanced risk stratification.
Under the guidelines, clinicians may consider a number of additional risk markers in patients “for whom after quantitative risk assessment a risk-based treatment decision is uncertain”. These include an abnormal CAC score (≥300 or ≥75th percentile for age/gender/race), hsCRP ≥2 mg/L, abnormal ABI, family history of premature CHD, and LDL-C >160 mg/dL. Carotid intima-media thickness was not included on the list (Class III recommendation), while the CAC score was described as single strongest predictor of risk.37
Yeboah et al examined the utility of these risk markers to reclassify risk among individuals who are below the threshold for statin therapy. Using a calibrated version of the pooled cohort equation (cPCE), MESA participants with an initial cPCE <7.5% and elevated levels of additional risk markers whose new calculated risk was ≥7.5% were considered statin eligible. More than half of ASCVD events occurred among participants whose cPCE was <7.5% at baseline. Within this subgroup, 264 (6.8%) participants had a CAC score that exceeded the threshold recommended in the new guidelines and became statin eligible. Accordingly, the needed to screen to identify 1 potential statin-eligible participant (NNSI) for CAC was 14.7. The corresponding NNSI for the other markers was higher with 21.8 for a FH of ASCVD, 39.2 for hsCRP, 176 for ABI, and 193.3 for LDL-C. Using at least one of the additional risk marker criteria, 431 of 3882 of participants with an initial cPCE <7.5% (11.1%) became statin eligible (reclassified to ≥7.5% cPCE).41
In another pivotal study, Yeboah et al assessed whether the risk markers improved discrimination and reclassification of incident ASCVD beyond the cPCE. The markers that were studied only included CAC, hsCRP, ABI (all modeled continuously) and FH of ASCVD as these remained significant predictors of ASCVD over 10 years of follow up independent of traditional risk factors. While each of the risk markers improved the AUC when added to the cPCE, only CAC was significant. Furthermore, adding CAC to the cPCE resulted in a larger improvement in NRI compared the other risk markers but this was limited to an improvement in classification for events (event NRI: 0.178; 95% CI: 0.080, 0.256; nonevent NRI: −0.059; 95% CI: −0.075, −0.030). ABI yielded a very modest improvement but the highest nonevent NRI (event NRI: 0.013; 95% CI: −0.034, 0.051; nonevent NRI: 0.004; 95% CI: −0.004, 0.011). Similar analyses were conducted for incident CHD using the calibrated FRS (cFRS). CAC was the only risk marker to significantly improve discrimination of CHD when added to the cFRS. Similar to ASCVD, addition of CAC resulted in a larger NRI compared to the other additional risk markers.42
One of the most prominent criticisms of the PCE and the new cholesterol guidelines was the potential for overestimation and overtreatment.43 The MESA study played a prominent role in describing how so-called “negative risk factors” can be used to down-classify risk in certain situations.36,44-46 In a study of 13 negative risk factors, Blaha et al. used risk factor-adjusted diagnostic likelihood ratios (DLRs) to demonstrate that a CAC score of zero was the strongest negative risk factor (0.41), followed by a normal carotid ultrasound (0.65) and a negative family history of CHD (0.76) (Figure 4).47
Figure 4.
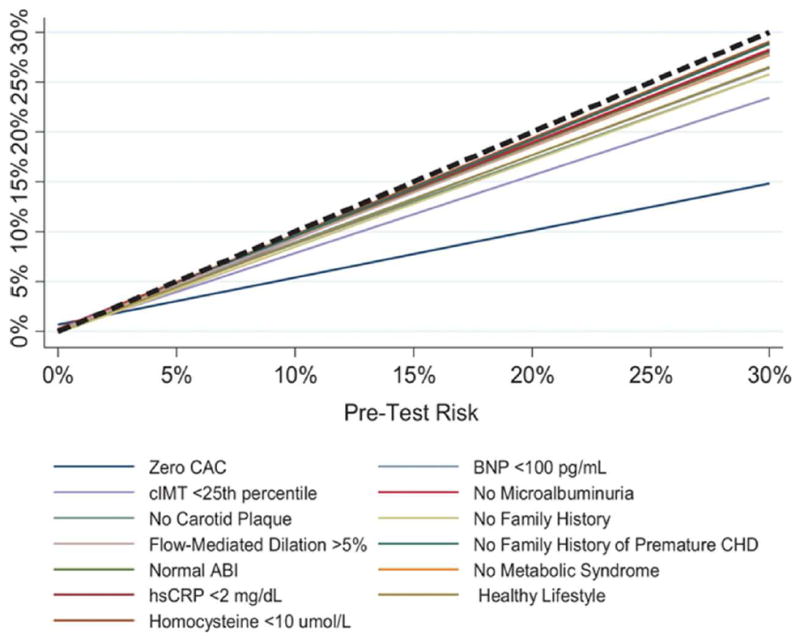
Relationship between pretest and posttest cardiovascular disease (CVD) risk after the knowledge of the negative result of each risk marker.
A paper by Nasir et al looked specifically at clinical situations where a finding of CAC=0 might change clinician decision making for initiating lipid-lowering therapy. In MESA participants with 10-year ASCVD risk of between 5-20% using the PCE, a finding of CAC=0 was associated with observed ASCVD event rates below the guideline-treatment threshold of 7.5% (Figure 5).48
Figure 5.
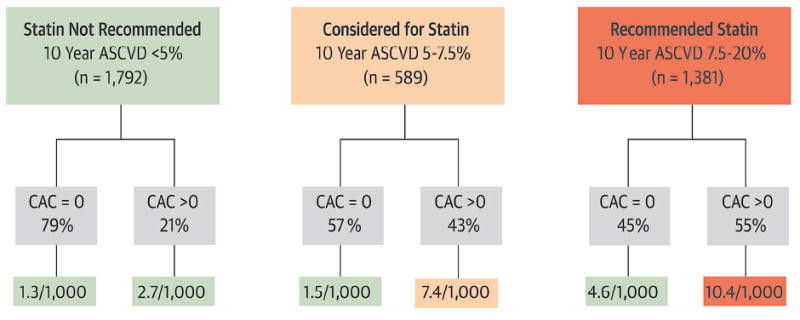
Impact of the absence of CAC in reclassifying risk below the threshold for statin consideration suggested by ACC/AHA cholesterol management guidelines, by estimated 10-Year ASCVD risk.
The MESA CHD Risk Score
Despite the wealth of data supporting the superior predictive value of CAC and its potential value in clinical practice, until recently there was no tool for formally incorporating CAC into 10-year risk estimates. In 2016, the MESA CHD Risk Score was published in Journal of the American College of Cardiology. 49 In this paper, McClelland et al. used the traditional risk factors as well as family history of CHD to fit two models for predicting the 10-year risk or hard CHD: one without CAC, and one adding CAC to the model. A striking feature of the results was the degree to which the predictive value of the traditional risk factors was reduced when CAC was added to the model (Table 2). Using just the traditional risk factors plus family history, the C-statistic was 0.75. After adding CAC, the C-statistic increased to 0.80. The MESA CHD Risk Score was validated in both the Dallas Heart Study and the Heinz-Nixdorf Recall study with similar discrimination (C-statistic 0.82 and 0.78, respectively) and excellent calibration (Table 3).
Table 2.
MESA 10-Year CHD risk prediction models without and with CAC
| Risk Factors Only | Risk Factors and CAC | |||||
|---|---|---|---|---|---|---|
| Hazards Ratio | Beta Coefficient | p Value | Hazards Ratio | Beta Coefficient | p Value | |
| Age, yrs | 1.05 | 0.0455 | <0.0001 | 1.02 | 0.0172 | 0.007 |
| Male | 2.12 | 0.7496 | <0.0001 | 1.5 | 0.4079 | <0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | Ref | 0 | — | Ref | 0 | — |
| Chinese American | 0.6 | -0.5055 | <0.01 | 0.71 | -0.3475 | 0.07 |
| African American | 0.81 | -0.2111 | 0.066 | 1.04 | 0.0353 | 0.7 |
| Hispanic | 0.83 | -0.1900 | 0.11 | 0.98 | -0.0222 | 0.88 |
| Diabetes | 1.68 | 0.5168 | <0.0001 | 1.48 | 0.3892 | 0.002 |
| Current smoker | 1.61 | 0.4732 | <0.001 | 1.45 | 0.3717 | 0.005 |
| Total cholesterol, mg/dl | 1.01 | 0.0053 | <0.0001 | 1 | 0.0043 | <0.001 |
| HDL cholesterol, mg/dl | 0.99 | -0.0140 | <0.001 | 0.99 | -0.0114 | 0.003 |
| Lipid-lowering meds | 1.28 | 0.2473 | 0.003 | 1.13 | 0.1206 | 0.32 |
| Systolic blood pressure, mm Hg | 1.01 | 0.0085 | 0.0002 | 1.01 | 0.0066 | 0.004 |
| Antihypertensive meds | 1.4 | 0.3381 | 0.0013 | 1.26 | 0.2278 | 0.033 |
| Family history of heart attack | 1.57 | 0.4522 | <0.0001 | 1.38 | 0.3239 | <0.001 |
| ln (CAC + 1) | NA | NA | NA | 1.32 | 0.2743 | <0.0001 |
| Baseline survival at 10 yrs, S(10) | 0.99963 | 0.99833 | ||||
Table 3.
Validation of the MESA CHD risk score in the Heinz-Nixdorf Recall (HNR) and Dallas Heart Study (DHS) cohorts.
| MESA | HNR | DHS | |
|---|---|---|---|
| Sample size | 6,726 | 3,692 | 1,080 |
| CHD events, n | 422 | 274 | 58 |
| Model with risk factors only | |||
| Harrell’s C-statistic | 0.75 | 0.72 | 0.782 |
| Discrimination slope | 0.052 | 0.053 | 0.046 |
| Calibration slope | 0.834 | 0.74 | 1.55 |
| Model with risk factors and CAC | |||
| Harrell’s C-statistic | 0.8 | 0.779 | 0.816 |
| Discrimination slope | 0.086 | 0.095 | 0.078 |
| Calibration slope | 0.857 | 0.899 | 1.19 |
Using the online MESA CHD Risk Score calculator (https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspxone) the clinician can now determine the estimated 10-year risk of patient before and after knowledge of the CAC score (Figure 6). Such information can be used to guide preventive pharmacotherapy and for enriching CAC score reporting from CT labs. A MESA CVD Risk Score is currently under development, which will allow separate modeling of CHD and stroke, rather than the composite outcomes chosen by the PCE.
Figure 6.
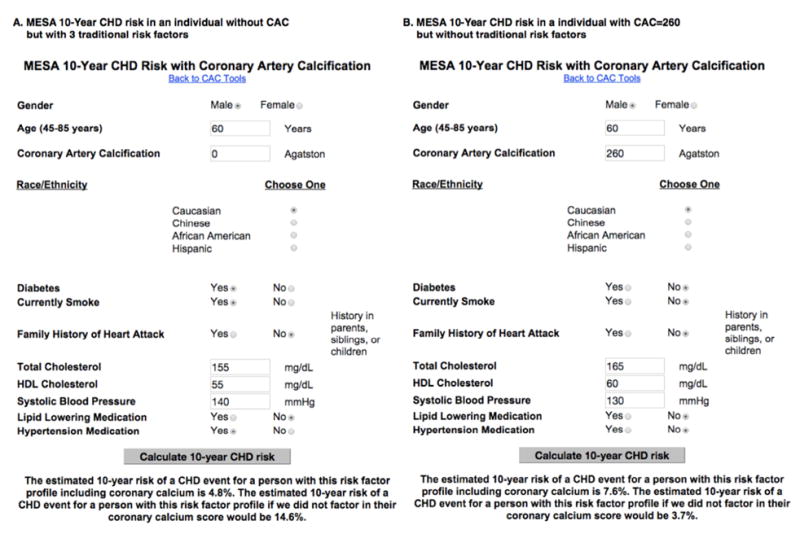
The MESA CHD Risk Score online calculator using two case examples.
Future Directions
MESA has moved the field of risk prediction forward, raising subclinical disease detection up to a standing alongside traditional risk factors as the preeminent tools for optimal risk prediction. The most important finding from MESA for risk prediction is the superior risk prediction provided by CAC. While MESA has also made critical discoveries in advanced serum biomarkers (for example LpPLA2, homocysteine, IL-6, and others),50 magnetic resonance imaging,51 and genetics,52 these have not reached clinical practice guidelines to date.
The future will bring an enhanced understanding of CAC.53 For example, recent MESA studies have suggested that the rate of CAC progression adds additional prognostic value on top of traditional risk factors and the baseline CAC score.54 A new study by Criqui et al. has challenged the long-standing assumption that the density of CAC is a predictor of events; in fact, adjusted for the volume of CAC, increasing CAC density is a protective marker.55 The regional distribution of CAC also appears to have prognostic value. In a study by Blaha et al, a more diffuse distribution of CAC is association with more risk compared to a more concentrated patter for a given absolute CAC score.56 Extra-coronary calcification, which can also be detected on a CAC scan, appears to add prognostic value for cardiovascular disease outcomes including stroke as well as all-cause mortality.57
While MESA had a significant impact on the 2013 ACC/AHA Prevention Guidelines, we expect there to be an even greater influence on the next guideline iteration. Coinciding the call for precision medicine is the recognition that all preventive therapies should be matched to absolute risk to best maximize net benefit, including non-statin lipid-lowering therapy, aspirin therapy, blood pressure therapy and intensification, and possible anti-inflammatory therapy. Studies from MESA have helped inform the balance between number needed to treat (NNT) and number needed to harm (NNH) of new and existing therapies.58
It is truly an exciting time for MESA, and arguably the greatest legacy of MESA is the paradigm shift towards routine consideration of subclinical disease measurement in clinical risk assessment.
MESA is the first NHLBI cohort dedicated to the study of subclinical cardiovascular disease, including its predictors, progression, and influence on outcomes
MESA has demonstrated that coronary artery calcium (CAC) significantly adds risk predictive value beyond traditional risk factors
MESA has directly influenced recommendations in the new 2013 ACC/AHA prevention guidelines, including risk prediction and the treatment of blood cholesterol
MESA recently published the first 10-year risk score incorporating both CAC and risk factors
Footnotes
There are conflicts of interest for this paper for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DAWBER TR, MEADORS GF, MOORE FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawber TR, Kannel WB, Lyell LP. AN APPROACH TO LONGITUDINAL STUDIES IN A COMMUNITY: THE FRAMINGHAM STUDY. Ann N Y Acad Sci. 2006;107:539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 6.Pletcher MJ, Sibley CT, Pignone M, Vittinghoff E, Greenland P. Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2013;128:1076–84. doi: 10.1161/CIRCULATIONAHA.113.002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 8.Gassett AJ, Sheppard L, McClelland RL, et al. Risk Factors for Long-Term oronary Artery Calcium Progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e001726. doi: 10.1161/JAHA.114.001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain A, McClelland RL, Polak JF, et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA) Circ Cardiovasc Imaging. 2011;4:8–15. doi: 10.1161/CIRCIMAGING.110.959403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diez Roux AV, Auchincloss AH, Franklin TG, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–75. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- 11.Blaha MJ, DeFilippis AP, Rivera JJ, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–51. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman JD, Adar SD, Allen RW, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–37. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed HM, Blaha MJ, Nasir K, et al. Low-risk lifestyle, coronary calcium, cardiovascular events, and mortality: results from MESA. Am J Epidemiol. 2013;178:12–21. doi: 10.1093/aje/kws453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFilippis AP, Blaha MJ, Ndumele CE, et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:2076–83. doi: 10.1016/j.jacc.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin NP, Martin SS, Blaha MJ, Nasir K, Blumenthal RS, Michos ED. Headed in the right direction but at risk for miscalculation: a critical appraisal of the 2013 ACC/AHA risk assessment guidelines. J Am Coll Cardiol. 2014;63:2789–94. doi: 10.1016/j.jacc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman MG, Blaha MJ, Krumholz HM, et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35:2232–41. doi: 10.1093/eurheartj/eht508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care. 2011;34:2285–90. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as ‘low risk’ based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–42. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 24.Blankstein R, Budoff MJ, Shaw LI, et al. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:364–74. doi: 10.1016/j.jacc.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 25.Tota-Maharaj R, Blaha MJ, Blankstein R, et al. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin Proc. 2014;89:1350–9. doi: 10.1016/j.mayocp.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1506–12. doi: 10.1016/j.jacc.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenny NS, Brown ER, Detrano R, et al. Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–9. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaha MJ, Rivera JJ, Budoff MJ, et al. Association between obesity, high-sensitivity C-reactive protein ≥2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1430–8. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasir K, Budoff MJ, Wong ND, et al. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;116:619–26. doi: 10.1161/CIRCULATIONAHA.107.688739. [DOI] [PubMed] [Google Scholar]
- 33.Cohen R, Budoff M, McClelland RL, et al. Significance of a positive family history for coronary heart disease in patients with a zero coronary artery calcium score (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;114:1210–4. doi: 10.1016/j.amjcard.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–92. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFilippis AP, Young R, Carrubba CJ, et al. An Analysis of Calibration and Discrimination Among Multiple Cardiovascular Risk Scores in a Modern Multiethnic Cohort. Ann Intern Med. 2015;162:266–75. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villines TC, Taylor AJ. Multi-ethnic study of atherosclerosis arterial age versus framingham 10-year or lifetime cardiovascular risk. Am J Cardiol. 2012;110:1627–30. doi: 10.1016/j.amjcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. J Am Coll Cardiol. 2015;65:1361–8. doi: 10.1016/j.jacc.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeboah J, Polonsky TS, Young R, et al. Utility of Non-Traditional Risk Markers in Individuals Ineligible for Statin Therapy According to the 2013 ACC/AHA Cholesterol Guidelines. Circulation. 2015;132:916–22. doi: 10.1161/CIRCULATIONAHA.115.016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeboah J, Young R, McClelland RL, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139–47. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cainzos-Achirica M, Desai CS, Wang L, et al. Pathways Forward in Cardiovascular Disease Prevention One and a Half Years After Publication of the 2013 ACC/AHA Cardiovascular Disease Prevention Guidelines. Mayo Clin Proc. 2015;90:1262–71. doi: 10.1016/j.mayocp.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bittencourt MS, Blaha MJ, Blankstein R, et al. Polypill therapy, subclinical atherosclerosis, and cardiovascular events-implications for the use of preventive pharmacotherapy: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:434–43. doi: 10.1016/j.jacc.2013.08.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miedema MD, Duprez DA, Misialek JR, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–60. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2016;133:849–58. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015;66:1657–68. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 49.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Stu. J Am Coll Cardiol. 2015;66:1643–53. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnett DK, McClelland RL, Bank A, et al. Biomarkers of inflammation and hemostasis associated with left ventricular mass: The Multiethnic Study of Atherosclerosis (MESA) Int J Mol Epidemiol Genet. 2011;2:391–400. [PMC free article] [PubMed] [Google Scholar]
- 51.Bluemke DA, Kronmal RA, Lima JAC, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alluri K, Joshi PH, Henry TS, Blumenthal RS, Nasir K, Blaha MJ. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239:109–17. doi: 10.1016/j.atherosclerosis.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–9. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–8. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silverman MG, Harkness JR, Blankstein R, et al. Baseline subclinical atherosclerosis burden and distribution are associated with frequency and mode of future coronary revascularization: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2014;7:476–86. doi: 10.1016/j.jcmg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tison GH, Guo M, Blaha MJ, et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 9:406–14. doi: 10.1016/j.jcct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeboah J, Sillau S, Delaney JC, et al. Implications of the new American College of Cardiology/American Heart Association cholesterol guidelines for primary atherosclerotic cardiovascular disease event prevention in a multi ethnic cohort: Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2015;169:387–95.e3. doi: 10.1016/j.ahj.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


