Abstract
Objective
Increased liver fat and type 2 diabetes are prevalent in women with polycystic ovarian syndrome (PCOS) and cause excess mortality, yet little is known about their development during adolescence. Our goal was to measure hepatic steatosis and related metabolic contributors in girls with obesity, with and without PCOS.
Methods
Nondiabetic adolescents with obesity, 41 with PCOS (PCOS; age 15.0(13.0,16.0) years, BMI 35.2±0.61 kg/m2) and 30 without PCOS (OB; age 14.5(13.0,17.0), BMI 33.2±1.8) were studied. Visceral and liver fat were assessed with MRI. Serum measures included androgens and 16 and 18 n7 fatty acids specific to de novo lipogenesis. Adipose, hepatic and peripheral insulin sensitivity (IS) were assessed with a 4-phase hyperinsulinemic-euglycemic clamp with isotope tracers.
Results
49% PCOS had hepatic steatosis vs. 14% OB (p=0.02), and PCOS had higher n7 (43±4 nmol/g vs. 29±5; p=0.02). Peripheral IS was lower in PCOS (9.4(7.2,12.3) mg/lean kg/min vs. 14.5(13.1,18.05); p<0.001) as was hepatic (p=0.006) and adipose IS (p=0.005). Percent liver fat correlated with n7 (R=0.46, p=0.02) and visceral fat (R=0.42, p<0.001), not androgens or peripheral IS.
Conclusions
Nearly 50% of nondiabetic girls with PCOS and obesity have hepatic steatosis, which related to visceral fat and lipogenesis, but not to IS or androgens.
Keywords: PCOS, Hepatic Steatosis, Insulin Resistance, Adolescence
Introduction
Polycystic Ovarian Syndrome (PCOS) affects 6–10% of all women and is increasing in prevalence worldwide in parallel with the obesity epidemic (1, 2). The PCOS phenotype in adults includes hyperandrogenism, insulin resistance and hepatic steatosis (HS), leading to nonalcoholic fatty liver disease (NAFLD), type 2 diabetes (T2D), and cardiovascular disease (3, 4). Obesity-related PCOS contributes strongly to the earlier onset and rising incidence of T2D and NAFLD (3–6), and both lead to early mortality. Of note, NAFLD- is anticipated to become the leading cause of hepatic transplant. Current PCOS therapies are limited, including lifestyle modification and metformin, which are only marginally effective, especially in youth (7, 8). Further understanding of the pathophysiology underlying PCOS is needed to develop improved therapeutics.
HS was recently implicated as being central to insulin resistance and development of the metabolic syndrome. Approximately 50–70% of adult women with obesity and PCOS have HS, vs. 20–30% of adult women with obesity but without PCOS (5, 9). Decreased insulin sensitivity (IS) in muscle, liver and adipose tissue is reported in PCOS adults (10–13). Furthermore, adult women with PCOS and HS are less IS than those without HS, indicating a link between HS and worsening IS (6). Only one study assessed tissue-specific IS with tracers in PCOS adolescents, reporting low hepatic and muscle IS in the subset with obesity (10). Studies directly comparing youth with obesity and PCOS with BMI-similar cohorts without PCOS are limited, and have yet to examine underlying mechanisms (14). Among a cohort of PCOS youth, HS correlated with increased age, adiposity and estimated IS (15).
PCOS youth are at high risk for cardiometabolic disease due to long disease duration, yet HS rates in PCOS youth with obesity have not been well studied, especially regarding tissue-specific IS. Importantly, the initial mechanism(s) impacting HS development is unclear, and we therefore hypothesized that it may relate to increased free fatty acid delivery (FFA), de novo lipogenesis, or decreased triglyceride secretion (16). Thus, the aim of this study was to measure hepatic fat in nondiabetic youth with PCOS vs. equally obese nondiabetic girls with regular menses, as well as examining mechanisms potentially contributing to HS.
Methods
Study population
71 participants were enrolled; 41 with PCOS and 30 without. Inclusion criteria: female sex, obesity (BMI percentile >95%) and sedentary status (<3 hours of exercise/week; validated with both a 3 day activity recall and 7 day accelerometer use). Exclusion criteria: diabetes, alanine transferase (ALT) >80 IU/mL, BP >140/90 mmHg, hemoglobin <9mg/dl, serum creatinine >1.5 mg/dl, smoking, medications affecting IS (oral steroids, metformin, thiazolidinediones, atypical antipsychotics, hormonal contraceptives), antihypertensive medications, statins, pregnancy, and breastfeeding. PCOS was diagnosed per NIH criteria with adolescent adaptation: oligomenorrhea (<8 menses a year), clinical or biochemical signs of hyperandrogenism, and at least 2 years post-menarche (8). The study was approved by the University of Colorado AMC Institutional Review Board. Informed consent was obtained from all participants 18–20 years old, and parental consent and participant assent from all participants <18 years old.
Insulin Sensitivity
The study day was preceded by 3 days of restricted physical activity and a fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, 15% protein). Participants were admitted to the Colorado Clinical Translational Research Center for an isocaloric meal, followed by a 12 hour overnight monitored fast. The following morning, a four-phase hyperinsulinemic euglycemic clamp was performed as previously described (17, 18) to determine adipose, hepatic and muscle IS. Following a 2 hour basal equilibration phase, insulin doses for each 1.5 hour phase were 10, 16, and 80 mU/m2/min, based on our (17, 18) and others (19) previous experience with the higher insulin requirements in pubertal youth. 20% dextrose (with 6,6-2H2 glucose) was infused to maintain blood glucose at 95 mg/dl, with samples drawn every 5 minutes and analyzed with a bedside Yellow Springs Instrument glucose analyzer (20). Glucose infusion rate, (GIR) (mg/kg of fat free mass [FFM]•min) was measured based on steady-state measurements from the final 30 min of the final, high-dose phase of the clamp. Body composition by DEXA was performed to determine FFM (10).
Tracer infusion protocol
At 6AM, blood samples to measure background enrichment of glucose and glycerol, and concentrations of glucose, insulin, glycerol and FFA were obtained. Then, a bolus of 4.5 mg/kg [6,6-2H2] glucose (Isotec, Miamisville, IA), followed by a continuous infusion at 0.03 mg/kg/min [6,6-2H2] glucose was paired with a primed (1.6 µmol/kg) then constant (0.11 µmol/kg/min) infusion of 2H5glycerol (21). During the last 30 minutes of each of the 4 clamp phases, 4 samples, each 10 minutes apart, were drawn for glucose, glycerol, FFA and insulin concentrations, and glucose and glycerol tracer enrichments (22).
Indirect calorimetry was performed at the end of each phase using a Vmax Encore metabolic cart system (Carefusion Corp, San Diego, CA) with a hood attachment. Carbohydrate oxidation was calculated as 4.55 * volume CO2 – 3.21 volume O2 and fat oxidation was calculated as 1.67 * volume CO2 – 1.67 volume O2 with O2 and CO2 in liters/min (23).
Physical Activity
A 3 day pediatric activity recall (3DPAR) was completed (24). Participants wore an Actigraph GT3x accelerometer (Actigraph Corp, Pensacola, FL) for 7 days. All data collected were used, corrected for wear time and categorized into the following age appropriate activity levels: sedentary, light, lifestyle, moderate, vigorous and very vigorous (25).
Dietary intake
Customary macronutrient pattern was ascertained by diet interview using a SEARCH food frequency questionnaire, modified to incorporate common food choices among ethnically and regionally diverse youth aged 10–19 (48).
Sample analysis
Analysis of 2H5glycerol and 6,6-2H2 glucose were done using gas chromatography mass spectrometry as described (22, 26, 27). Serum insulin, leptin and adiponectin were analyzed with radioimmunoassay (Millipore, Billerica, MA); plasma glycerol (R-Biopharm, Marshall, MI) and FFA (Wako Chemicals, Inc., Richmond, VA) enzymatically. Hemoglobin A1c by DCCT-calibrated ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, Calif). Total cholesterol, high density lipoprotein cholesterol, and triglyceride assays were performed enzymatically (Hitachi 917 autoanalyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). Low density lipoprotein cholesterol was calculated by the Friedewald equation (11). hs-C-reactive protein via immunoturbidimetric assay (Beckman Coulter, Brea, CA), C-peptide via chemiluminescent immunoassay (DiaSorin, Stillwater, MN) and estradiol and progesterone via chemiluminescent immunoassay (Beckman Coulter, Brea, CA). AST and ALT were measured on a Vitros® 5600 (Ortho Clinical Diagnostics, Rochester, NY).
Testosterone was measured via LC/MS/MS; DHEAS, sex hormone binding globulin (SHBG) and anti-mullerien hormone by Electrochemiluminescence immunoassay, all by Esoterix (Calbassas Hills, CA). Free androgen index (FAI) was calculated from total testosterone and SHBG. Serum FFA 16:1n7 and 18:1n7 were measured by LC/MS (Lipomics division of Metabolomics, Sacramento, CA).
Tracer calculations
All isotopic enrichments were corrected for background enrichments. The glucose and glycerol rate of appearance (Ra), rate of disappearance (Rd), and metabolic clearance rate (MCR) over the last 30 minutes of each phase of the clamp were calculated using the Steele non-steady-state equation, accounting for “spiked” glucose in the 20% dextrose infusion (26). To describe the interaction between Ra across the different insulin concentrations of each phase, the 50% inhibitory concentration (IC 50) was calculated. The Ra and log insulin for each phase were plotted, and the slope of the curve of all four points was used to calculate the insulin concentration at the location on the curve equal to 50% suppression of the basal Ra, as described previously (26). The inverse of this relationship, or predicted Ra at the average insulin concentration for the group was also calculated, and called the “predicted Ra”.
Statistics
The distributions of all variables were examined and results presented as mean ± standard deviation (SD), median (minimum, maximum), or proportions, as appropriate. Group comparisons were made using chi-square or Fisher’s exact test for proportions and the t-test or Kruskal-Wallis test for continuous variables. Repeated measures mixed models with a group by time interaction were used to compare outcomes measured at multiple time points during the clamp. Differences in the trends of the repeated measures over time were assessed by testing the significance of the group by time interaction. The associations between visceral fat, IC 50 glucose, IC 50 glycerol, FAI, testosterone, hs-CRP, waist/hip ratio, 3 day mets, total n7, time in lifestyle/light, time in mod/vigorous, and the two primary outcomes, GIR and % hepatic fat, were examined using Pearson’s correlation coefficients. P-values < 0.05 were considered significant. All statistical analyses were performed with SAS Software, Version 9.4 (Cary, NC).
Results
41 nondiabetic girls with PCOS and 30 (OB) nondiabetic girls without PCOS were enrolled. Participant demographic and physical attributes are shown in Table 1. The groups had similar age of menarche, had equal obesity, and were similar in terms of pubertal stage. By design, the PCOS group had less frequent menses, and worse hirsutism and acne scores (p=0.001). Whereas the waist/hip ratio and percent visceral fat were the same between groups, girls with PCOS had a higher percent liver fat. Of note, 47% of PCOS and 14% of OB met criteria for HS (>5% hepatic fat). We also evaluated the role of ethnicity in the presence of HS. Among OB non-Hispanic white (NHW) girls, the % liver fat was 2.08 ± 0.84 vs 5.96 ± 4.04; P=0.003 in the NHW PCOS group. In the Hispanic group, OB was 2.79 (1.34,14.23) vs. 5.79 (2.44,10.09); P=0.9 in the OB PCOS.
Table 1. Participant Descriptors.
Demographics, physical characteristics and lifestyle measurements are shown above for girls with and without PCOS.
| OB | PCOS | ||
|---|---|---|---|
| Number | 30 | 41 | |
| Age (years) | 14.5 (13.00,17.00) | 15.0 (13.00,16.00) | |
| BMI (kg/m2) | 33.2±1.79 | 35.2±0.61 | |
| Ethnicity Caucasian Hispanic Black American Indian Asian |
12(40.00) 8(26.67) 7(23.33) 2(6.67) 1(3.33) |
15(36.59) 23(56.10)* 3(7.32) 0(0.00) 0(0.00) |
|
| Menarche Age (years) | 11 (10, 12) | 12 (11,12) | |
| Hirsutism (Ferriman-Gallwey Score) | 1 (0, 2) | 7 (4,11)** | |
| Waist Circumference (cm) | 95.0 (86.5,104.2) | 103 (96.0,111.0)* | |
| Waist/Hip Ratio | 0.89±0.02 | 0.89±0.01 | |
| % total body fat | 43.6 (39.55,45.35) | 42.7 (40.45,45.95) | |
| % liver fat | 2.23 (1.38, 3.04) | 5.64 (2.17, 8.89)* | |
| % Visceral Fat | 16.0 (11.70,36.01) | 16.2 (12.29,22.32) | |
| Daily mets from 3-day PAR | 58.9 (54.6,62.3) | 55.6 (46.7,60.0) | |
| Accelerometer Data: | |||
| % sedentary | 0.70 (0.63, 0.83) | 0.66 (0.61, 0.71) | |
| % Lifestyle+light | 0.29 (0.14, 0.31) | 0.31 (0.25, 0.35) | |
| % Moderate+vigerous+very vigorous | 0.04 (0.02, 0.04) | 0.04 (0.03, 0.05) | |
Data is shown as mean ± standard error of the mean or median (25,75).
Significance is noted as
<0.05–0.01,
= <0.01–0.001,
= <0.001
Both groups were equally sedentary and had a similar dietary intake of both micro and macronutrients including total calories, fat, carbohydrate, protein, saturated fat, fructose and intake of 16:1 and 18:1 fats (data not shown), per dietary recall questionnaire.
Fasting laboratory measurements are shown in Table 2. Girls with PCOS had a higher FAI, testosterone anti-mullerian hormone and lower SHBG. There were no differences in DHEAS, estradiol or progesterone between groups, and progesterone was low, confirming that studies were performed in the follicular phase of the menstrual cycle. Adiponectin was lower in PCOS, but not leptin. The serum lipid profile was similar between groups, as were fasting glucose, C-peptide and HbA1c. Fasting insulin was higher in PCOS (p=0.007). ALT and C-reactive protein were higher in the PCOS group, whereas other markers of inflammation were similar.
Table 2. Participant Fasting Morning Labs.
Laboratory measures drawn at 6 AM after a 12 hour monitored inpatient fast are shown above for girls with and without PCOS.
| OB | PCOS | |
|---|---|---|
| Free Androgen Index (FAI) | 3.5 (3.0, 4.0) | 7.9 (6.6,14.6)** |
| Total Testosterone (ng/dL) | 25.5 (23.00,37.00) | 47.0 (34.00,61.00)** |
| SHBG (nmol/L) | 26 (15,38) | 16 (12, 22.)+ |
| DHEAS (µg/dL) | 117 (90,150) | 150 (75,230) |
| Estradiol (pg/mL) | 43 (28,75) | 38 (33, 48) |
| Progesterone (ng/dL) | 1.0 (0.6, 4.8) | 0.9 (0.7, 1.1) |
| AMH (ng/mL) | 3.0 (2.0, 4.9) | 5.9 (4.7, 8.9)** |
| Leptin (ng/mL) | 33 (28, 49) | 33 (26, 41) |
| Adiponectin (ng/mL) | 7.6 (5.4,10.0) | 5.5 (4.1, 7.2)+ |
| Cholesterol (mg/d) | 163±7 | 162±5 |
| HDL (mg/dL) | 40±2 | 37±1 |
| TG (mg/dL) | 98 (88,184) | 122 (76,158) |
| LDL (mg/dL) | 96 (82,123) | 110 (84,131) |
| Total N7 (nmol/g) | 29.2±3.7 | 42.5±3.9* |
| Glucose (mg/dL) | 85±1 | 85±1 |
| Insulin (uIU/mL) | 20±2 | 27±1+ |
| C-peptide (ng/mL) | 2.70 (1.90, 3.50) | 3.30 (2.80, 3.95) |
| HbA1C (%) | 5.2±0.1 | 5.3±0.1 |
| AST (U/L) | 30±3 | 36±2 |
| ALT (U/L) | 31 (24, 37) | 37 (29, 43)* |
| WBC (109cells/L) | 7.17±0.42) | 8.07±0.29 |
| Platelets (109cells/L) | 261 (223, 316) | 282 (248, 307) |
| hs-CRP (mg/dL) | 1.3 (0.4, 2.1) | 2.6 (1.2, 5.1)+ |
Data is shown as mean ± standard error of the mean or median (25,75).
Significance is noted as
<0.05–0.01
= <0.01–0.001,
= <0.001
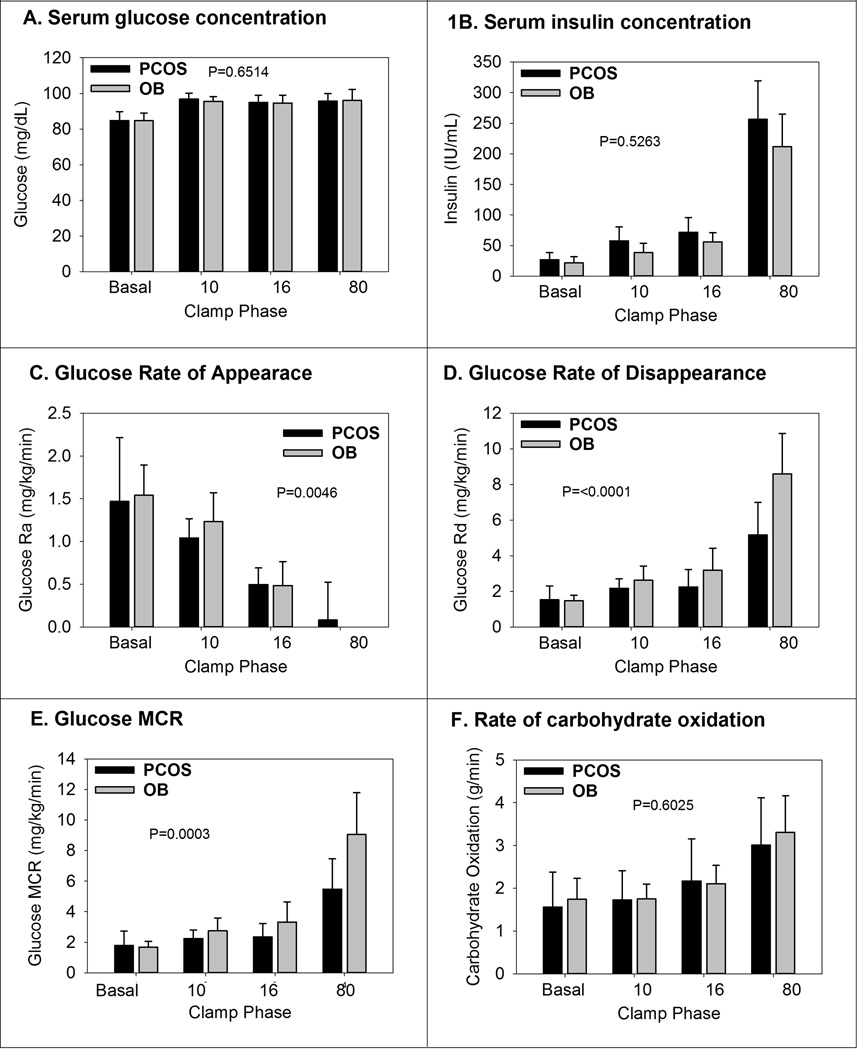
Glucose clamp measurements are shown in Figure 1. 1A shows the average serum glucose concentrations and there was no difference between groups in any phase, and the glucose was steady at 95 mg/dL throughout. Insulin concentrations (Figure 1B) increased in both groups with increasing doses of insulin. The fasting insulin was different between the groups, as noted in Table 2. However, there was no difference in the insulin concentrations during hyperinsulinemia (p=0.5263). The glucose Ra in mg/kg/min (Figure 1C) decreased with increases in the insulin dose, and the difference during hyperinsulinemia between groups was statistically significant (p=0.0046) with incomplete suppression of Ra in the PCOS group. The Rd of glucose (Figure 1D) increased with each increase in insulin dose, as expected, and was lower at each clamp stage in the PCOS group (p<0.0001). Glucose MCR (Figure 1E) increased with each increase in insulin dose, and was significantly lower in the PCOS group at each clamp stage (p=0.0003). Whole body carbohydrate oxidation (Figure 1F) increased with increasing insulin dose, but was not different between groups (p=0.6025). Non-oxidative glucose disposal increased with each phase of the clamp, but was not different between the groups (data not shown).
Figure 1.
Glucose related measurements from the hyper-insulinemic clamps are shown above. Data are shown by PCOS and non-PCOS status, and then by phase of the clamp, named per insulin dose. The P-values are from the test of the group by time interaction from the repeated measures models.
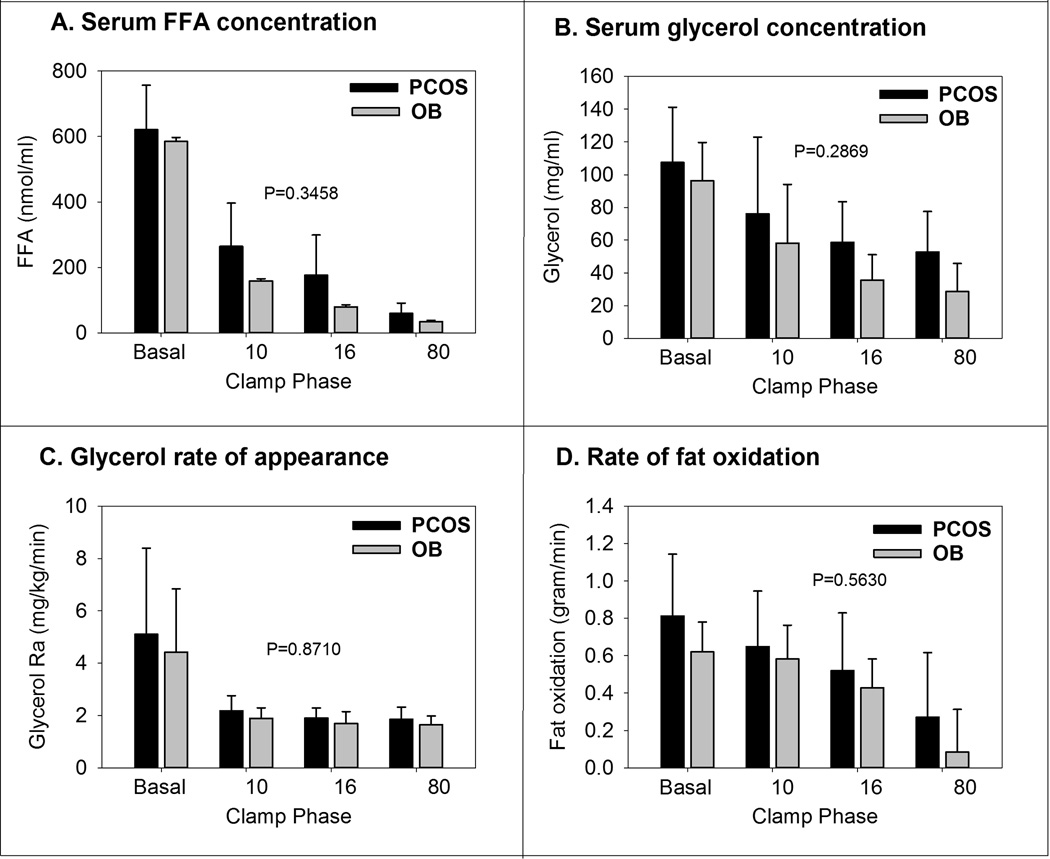
Results from FFA and glycerol measurements are shown in Figure 2. As expected, FFA (Figure 2A) decreased with increasing insulin doses. Overall, the trend was not different between the groups (p=0.34). Again, glycerol concentrations (Figure 2B) decreased per stage, but there was no difference between groups (p=0.28). Glycerol Ra (Figure 2C) was suppressed in the first stage of hyperinsulinemia, and remained so for the rest of the clamp, and there was no difference between groups (p=0.87). The rate of fat oxidation (Figure 2D) decreased progressively per stage, and there was no difference between groups (p=0.56).
Figure 2.
Fat related measurements from the hyper-insulinemic clamps are shown above. Data are shown by PCOS and non-PCOS status, and then by phase of the clamp, named per insulin dose. The P-values are from the test of the group by time interaction from the repeated measures models.
The final data from the hyperinsulinemic clamp are shown in Table 3. The GIR during the highest dose of insulin was significantly lower in PCOS, controlled for either total body weight, or FFM (p<0.001 for both). In terms of suppression of endogenous glucose Ra, there was no difference when expressed as percent suppression of basal, however when accounting for insulin concentrations with the IC50 method, the PCOS group had higher glucose Ra (p=0.006), suggestive of hepatic insulin resistance. As measured with glycerol, there was no difference in adipose response to insulin, either expressed as percent suppression of basal, or the insulin concentration required to suppress 50 (IC50) percent of release. However, when assessed with FFA, the percent suppression of FFA was significantly lower in PCOS (P=0.006) and the FFA IC50 was significantly higher in the PCOS groups (p=0.005).
Table 3. Summary measures of insulin sensitivity.
Summary measures of insulin sensitivity per tissue type are shown, with several different methods of calculation shown, including the percent suppression from the basal concentration, and the insulin concentration required to suppress 50% of the basal rate (IC 50).
| OB | PCOS | |
|---|---|---|
| Peripheral insulin sensitivity | ||
| Glucose infusion rate (mg/kg/min) | 7.9 (7.4,10.7) | 5.1 (4.2, 6.8)** |
| Glucose infusion rate (mg/lean kg/min) | 14.5 (13.1,18.1) | 9.4 (7.2,12.3)** |
| Hepatic insulin sensitivity | ||
| Glucose IC 50 (IU/ml) | 61±6 | 86±6+ |
| % Suppression glucose (basal to 16mU/m2/min phase) | 67 (57,82) | 57 (54,71) |
| Adipose insulin sensitivity | ||
| Glycerol IC 50 (IU/ml) | 76 (51, 94) | 75 (49,147) |
| % Suppression glycerol (basal to 10mU/m2/min phase) | 100 (92, 109) | 93 (83, 112) |
| FFA IC 50 (IU/ml) | 40 (36,45) | 62 (50,82)+ |
| % Suppression FFA (basal to 10mU/m2/min phase) | 57±18 | 73±12+ |
Data is shown as mean ± standard error of the mean or median (25,75).
Significance is noted as
= <0.01–0.001,
= <0.001
Contributors to liver fat
Univariate analysis of the entire cohort indicated that percent liver fat related to visceral fat (R=0.42, p=0.001), reported physical activity per the 3 day questionnaire (R=−0.34, p=0.01) and markers of de novo lipogenesis: the sum of 16:1 and 18:1 N7 concentrations (R=0.47, p=0.02). There was no relationship between % liver fat and waist/hip ratio, FAI or total testosterone, CRP, accelerometer measures of physical activity, glucose IC 50 or glycerol IC 50.
Contributors to insulin resistance
Visceral fat (R=−0.49, p=0.0003), FAI (−0.47, p=0.0002), physical activity from the questionnaire (R=0.42, p=0.0024), the sum of 16:1 and 18:1 N7 concentrations (R=−0.51, p=0.02) and FFA IC 50 (R=−0.65, p<0.001) were univariately associated with GIR in the entire cohort. Glycerol IC 50, physical activity per accelerometer, CRP, and waist:hip ratio were not related to GIR.
Discussion
We found that nondiabetic adolescents with PCOS and obesity had significantly more hepatic fat vs. controls with similar obesity, with approximately 50% of PCOS girls having clinically significant HS. PCOS girls also had lower peripheral, hepatic and adipose IS compared to controls. Hepatic fat correlated with N7 FFA’s as a marker of lipogenesis and with visceral fat and physical activity, but not androgens or IS. We also demonstrated that peripheral IS correlated with androgens, physical activity and the sum of 16:1 and 18:1 N7 FFA’s. These findings indicate that HS is common in PCOS girls with obesity, is multi-factorial, but mostly related to altered hepatic fat production, rather than androgens or IS as typically assumed.
This is the first time that HS and IS as assessed with a hyperinsulinemic euglycemic clamp are reported in PCOS youth vs. controls, and confirms findings in adult PCOS women with obesity. 55% of women with PCOS in their thirties had increased hepatic fat (5) and 64% of women with PCOS and of an average age of 25 years had increased hepatic fat (9). Both studies included women of varied BMI, thus HS would likely be even higher if only PCOS women with obesity were included. A recent study in PCOS youth found that rates of HS were 2 fold higher in PCOS girls, but IS was not assessed (14). Moreover, our results likely underestimate the prevalence of HS, as girls with hypertension, weight >300 pounds, ALT >80 IU/mL and T2D were excluded.
HS was related to N7 FFA’s, but could be secondary to alterations in of hepatic lipogenesis, triglyceride secretion or hepatic FFA acid uptake with re-esterification. Limited studies in adults with NAFLD indicate that upregulated lipogenesis and FFA delivery are the primary contributors to HS (16, 28). The source of FFA is of dietary, visceral adipose and peripheral origins. We did find that visceral fat correlated with hepatic fat, yet we found no relationship between whole body lipolysis and HS. It is likely that our measures are more reflective of peripheral lipolysis. The visceral adipose release of FFA’s to the liver unfortunately cannot be assessed noninvasively. However, when hepatic FFA delivery was experimentally decreased, hepatic and muscle IS improved despite no change in HS (29). Others found that altered hepatic lipogenesis may drive HS in non-PCOS adults (28, 30). Our finding of correlations with N7 FFA’s, but not glycerol IC 50 glycerol or IC 50 FFA with HS are thus inconsistent with peripheral adipose IS leading to excess hepatic FFA delivery and instead favors lipogenesis as the source. The synergy between obesity and hyperandrogenism in adult PCOS influences fat metabolism, inflammation, NAFLD and IS and likely is multifactorial in youth as well (31, 32).
In contrast to other reports, we did not find associations between HS and markers of IS. In adults with T2D, HS was related to hepatic basal glucose Ra (25) and adipocyte IS was associated with fasting glucose (26). It may be that in T2D, variations in fasting glucose are larger, allowing for detection of these relationships. Additionally, PCOS status seems to contribute greatly to HS prevalence in NHW, whereas Hispanic ethnicity may be more important in the development of HS vs. PCOS per se, although we are underpowered to make this conclusion with our data set.
There is a high prevalence of insulin resistance and T2D in women with PCOS, and our findings indicate that this process is well established by an average age of 15 years. We found that peripheral IS was at least a third lower in girls with PCOS compared to controls. 30–40% of women with PCOS and obesity in their third decade have IR but not yet T2D, compared to 5–10% in women without PCOS. Women with PCOS and obesity are 2.5 times more likely and women with PCOS but no obesity are 3.3 times more likely to have IR compared to weight matched controls (12, 33, 34). Reported IS in PCOS girls is much more varied, likely an artifact of differing methods used to assess IS (10, 35, 36). Thus, the prevalence of IR in PCOS teens may be similar to that of adult women, but that it is in pre-clinical stages, and only detectable with post-prandial or more sensitive assessment methods, such as a hyperinsulinemic euglycemic clamp that we utilized.
The mechanism(s) of IR in PCOS are not well understood but may be similar to T2D, though very few studies exist in adolescents. We found evidence of decreased peripheral, hepatic and adipose IS in girls with PCOS. Two previous studies involving hyperinsulinemic euglycemic clamps reported lower hepatic and muscle IS in adolescents with PCOS. These studies used a single insulin dose during the clamp, and hepatic IS was extrapolated, whereas our multiphase insulin clamp directly confirmed these findings. Studies in adults with PCOS show decreased IS in muscle, liver and adipose tissue, yet none have used glycerol and glucose tracers in the same study, nor performed a 4 phase clamp (10–12). Defects in the insulin signaling cascade in adipocytes, fibroblasts and muscle tissue from adults appear similar between T2D and PCOS (37, 38). We have previously demonstrated mitochondrial dysfunction in the skeletal muscle of girls with PCOS (39). In teens with obesity of both sexes with insulin resistance but not T2D, mitochondrial dysfunction begins in mid-puberty, and insulin resistance is associated with elevated androgens (13, 40). Finally, markers of inflammation have been shown to be increased in women with PCOS and thus were also assessed in our study (31, 32). The PCOS group had higher markers of inflammation and liver transaminases than the control group, but these did not correlation with measures of IS.
There are several strengths and weakness to the study. Strengths include the large cohort for a study of this intensity. We performed the gold standard measurement for IS with a 4 phase hyperinsulinemic euglycemic clamp, and used glucose and glycerol tracers to directly measure adipose and hepatic IR. We used the NIH definition of PCOS which is the most exclusive diagnostic criteria for PCOS. We also controlled for variations in physical activity, diet and menstrual stage, all known to influence IS. One of the weaknesses of the study was that the PCOS group trended to be more obese, although this was not clinically or statistically significant. We were unable to measure free testosterone in all volunteers, yet have the less accurate total testosterone (which can be low with a low SHBG) and FAI. We may have detected a relationship between liver fat and androgens if we had free testosterone in all subjects, although we did not in the subset with the measurement. As discussed previously, our findings likely underestimate metabolic disease in obese girls with PCOS, due to our exclusion criteria. For example, we have previously found that obese youth with T2D have a peripheral IS during a similar clamp that is 50% worse than what we measured in our PCOS group. The negative correlations could be due to the smaller sample size, yet these studies are costly to perform in large cohorts. Further, if large cohorts are necessary for correlations, measures may be of small physiologic significance.
In conclusion, we found that nondiabetic girls with PCOS and obesity have a 3-fold increased prevalence of HS and significant multi-tissue insulin resistance when compared to normally cycling girls of similar BMI. Increased lipogenesis may be a primary drivers of HS in these girls, although physical activity is important. Clinicians should have a low threshold for suspecting NAFLD and insulin resistance and youth with PCOS should be encouraged to decrease sedentary behavior.
What is known about this subject?
Women with PCOS have increased risk of all components of the metabolic syndrome including type 2 diabetes, fatty liver disease, hyperlipidemia and hypertension.
There is limited evidence that girls with PCOS have an increased risk of hepatic steatosis and whether this relates to multi-tissue insulin resistance.
In adult with nonalcoholic fatty liver disease, increased de novo lipogenesis seems to be the primary metabolic contribution to hepatic steatosis, but this has not been studied in PCOS (adult or youth).
What does this study add?
This is the first study in which multi-tissue insulin sensitivity with dual tracers has been assessed as well as liver fat and markers of de novo lipogenesis in girls with obesity, with and without PCOS – to determine the relationship between the two.
We found that the prevalence of hepatic steatosis in PCOS is tripled compared to BMI similar controls with regular menses. Further, girls with PCOS had multi-tissue insulin resistance. However, the hepatic steatosis and peripheral IR did not relate, and hepatic steatosis was related to de novo lipogenesis and visceral fat.
Metabolic changes in non-diabetic youth with moderately obesity and PCOS are severe and hepatic steatosis is common.
Acknowledgments
Participants and their families; CTRC nurses and staff.
Funding: K.J.N.: NCRR K23 RR020038-01, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, NIH/NIDDK 1R56DK088971-01, JDRF5—2008-291, ADA 7-11-CD-08.
M.C.G.: AHA 13CRP 14120015, Thrasher Pediatric Research Foundation Mentored Pilot Grant, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, Pediatric Endocrinology Society Fellowship, Endocrine Society Fellowship in Women’s health, NIDDK T32 DK063687, BIRCWH K12HD057022.
This research was also supported by Adult CTRC NIH Grant M01-RR00051, Pediatric CTRC NIH Grant 5MO1-RR00069 and NIH/NCRR Colorado CTSI Grant UL1 RR025780.
Footnotes
Disclosure: The authors declare no conflict of interest
Author Contributions: M.C.G designed the study, researched data and wrote the manuscript, B.C.B researched data and edited the manuscript, G.V.C. researched data and edited the manuscript, L.N. researched data and edited the manuscript, A.D.B. researched data and edited the manuscript, S.B researched data and edited the manuscript A.S. researched data and edited the manuscript, L.P. performed all statistical analysis and edited the manuscript, K.J.N designed the study, researched data, contributed to discussion and edited the manuscript.
References
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. PubMed PMID: 9745406. [DOI] [PubMed] [Google Scholar]
- 2.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical epidemiology. 2013;6:1–13. doi: 10.2147/CLEP.S37559. Epub 2014/01/01. PubMed PMID: 24379699; PubMed Central PMCID: PMC3872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blank SK, Helm KD, McCartney CR, Marshall JC. Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. PubMed PMID: 18574211. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):48–53. doi: 10.1210/jc.2005-1329. Epub 2005/10/27. PubMed PMID: 16249284. [DOI] [PubMed] [Google Scholar]
- 5.Gambarin-Gelwan M, Kinkhabwala SV, Schiano TD, Bodian C, Yeh HC, Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(4):496–501. doi: 10.1016/j.cgh.2006.10.010. Epub 2007/02/09. PubMed PMID: 17287148. [DOI] [PubMed] [Google Scholar]
- 6.Baranova A, Tran TP, Birerdinc A, Younossi ZM. Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Alimentary pharmacology & therapeutics. 2011;33(7):801–814. doi: 10.1111/j.1365-2036.2011.04579.x. Epub 2011/01/22. PubMed PMID: 21251033. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez L, Diaz M, Sebastiani G, Sanchez-Infantes D, Salvador C, Lopez-Bermejo A, et al. Treatment of androgen excess in adolescent girls: ethinylestradiol-cyproteroneacetate versus low-dose pioglitazone-flutamide-metformin. J Clin Endocrinol Metab. 2011;96(11):3361–3366. doi: 10.1210/jc.2011-1671. Epub 2011/08/26. PubMed PMID: 21865363. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350. Epub 2013/10/24. PubMed PMID: 24151290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerda C, Perez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Journal of hepatology. 2007;47(3):412–417. doi: 10.1016/j.jhep.2007.04.012. Epub 2007/06/15. PubMed PMID: 17560682. [DOI] [PubMed] [Google Scholar]
- 10.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86(1):66–71. doi: 10.1210/jcem.86.1.7123. PubMed PMID: 11231980. [DOI] [PubMed] [Google Scholar]
- 11.Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab. 2009;94(1):157–163. doi: 10.1210/jc.2008-1492. PubMed PMID: 18854391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65(3):499–507. doi: 10.1210/jcem-65-3-499. PubMed PMID: 3305551. [DOI] [PubMed] [Google Scholar]
- 13.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. doi: 10.1210/jc.2006-2002. PubMed PMID: 17118995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayonrinde OT, Adams LA, Doherty DA, Mori TA, Beilin LJ, Oddy WH, et al. Adolescent females with NAFLD and PCOS have an adverse metabolic phenotype compared with other females and males. Journal of gastroenterology and hepatology. 2015 doi: 10.1111/jgh.13241. Epub 2015/11/22. PubMed PMID: 26589977. [DOI] [PubMed] [Google Scholar]
- 15.Michaliszyn SF, Lee S, Tfayli H, Arslanian S. Polycystic ovary syndrome and nonalcoholic fatty liver in obese adolescents: association with metabolic risk profile. Fertil Steril. 2013;100(6):1745–1751. doi: 10.1016/j.fertnstert.2013.08.015. Epub 2013/09/17. PubMed PMID: 24034940; PubMed Central PMCID: PMC3844059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol. 2014;25(3):213–220. doi: 10.1097/MOL.0000000000000080. Epub 2014/05/03. PubMed PMID: 24785962. [DOI] [PubMed] [Google Scholar]
- 17.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 95(2):513–521. doi: 10.1210/jc.2009-1756. PubMed PMID: 19915016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–3695. doi: 10.1210/jc.2008-2844. PubMed PMID: 19584191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91(2):401–404. doi: 10.1210/jc.2005-1672. Epub 2005/11/18. doi: jc.2005-1672 [pii] 10.1210/jc.2005-1672. PubMed PMID: 16291705. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. PubMed PMID: 382871. [DOI] [PubMed] [Google Scholar]
- 21.Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 2006;14(12):2163–2172. doi: 10.1038/oby.2006.253. Epub 2006/12/26. PubMed PMID: 17189542; PubMed Central PMCID: PMC2832608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem. 1992;205:172–178. doi: 10.1016/0003-2697(92)90595-x. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR, Chinkes DL. Principles and Pracetice of Kinetic Analysis. Hoboken, NJ: Wiley-Liss; 2005. Isotope Tracers in Metabolic Research. [Google Scholar]
- 24.Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc. 1997;29(1):138–143. doi: 10.1097/00005768-199701000-00020. Epub 1997/01/01. PubMed PMID: 9000167. [DOI] [PubMed] [Google Scholar]
- 25.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. Epub 1998/05/20. PubMed PMID: 9588623. [DOI] [PubMed] [Google Scholar]
- 26.Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97(5):1663–1672. doi: 10.1210/jc.2011-3172. Epub 2012/03/01. PubMed PMID: 22362823; PubMed Central PMCID: PMC3339891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Clement TW, et al. The importance of palmitoleic acid to adipocyte insulin resistance and whole-body insulin sensitivity in type 1 diabetes. J Clin Endocrinol Metab. 2013;98(1):E40–E50. doi: 10.1210/jc.2012-2892. Epub 2012/11/15. PubMed PMID: 23150678; PubMed Central PMCID: PMC3537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. Epub 2013/12/10. PubMed PMID: 24316260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigazio S, Lehto HR, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, et al. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab. 2008;295(2):E413–E419. doi: 10.1152/ajpendo.00744.2007. Epub 2008/05/29. PubMed PMID: 18505832. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Lambert JE, Hovhannisyan Y, Ramos-Roman MA, Trombold JR, Wagner DA, et al. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101(1):34–43. doi: 10.3945/ajcn.114.092262. Epub 2014/12/21. PubMed PMID: 25527748; PubMed Central PMCID: PMC4266891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20(4):291–302. PubMed PMID: 8680455. [PubMed] [Google Scholar]
- 32.Glintborg D, Andersen M, Hagen C, Frystyk J, Hulstrom V, Flyvbjerg A, et al. Evaluation of metabolic risk markers in polycystic ovary syndrome (PCOS). Adiponectin, ghrelin, leptin and body composition in hirsute PCOS patients and controls. Eur J Endocrinol. 2006;155(2):337–345. doi: 10.1530/eje.1.02207. PubMed PMID: 16868149. [DOI] [PubMed] [Google Scholar]
- 33.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318. PubMed PMID: 9408743. [DOI] [PubMed] [Google Scholar]
- 34.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 16(4):347–363. doi: 10.1093/humupd/dmq001. PubMed PMID: 20159883. [DOI] [PubMed] [Google Scholar]
- 35.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1017–1023. doi: 10.1210/jcem.87.3.8305. Epub 2002/03/13. PubMed PMID: 11889155. [DOI] [PubMed] [Google Scholar]
- 36.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol. 2009;25(5):335–343. doi: 10.1080/09513590802630146. Epub 2009/11/12. PubMed PMID: 19903040. [DOI] [PubMed] [Google Scholar]
- 37.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281(2):E392–E399. doi: 10.1152/ajpendo.2001.281.2.E392. PubMed PMID: 11440917. [DOI] [PubMed] [Google Scholar]
- 38.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–332. doi: 10.1016/j.molmed.2006.05.006. PubMed PMID: 16769248. [DOI] [PubMed] [Google Scholar]
- 39.Cree-Green M, Newcomer BR, Coe G, Newnes L, Baumgartner A, Brown MS, et al. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308(9):E726–E733. doi: 10.1152/ajpendo.00619.2014. Epub 2015/02/26. PubMed PMID: 25714677; PubMed Central PMCID: PMC4420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleischman A, Kron M, Systrom DM, Hrovat M, Grinspoon SK. Mitochondrial function and insulin resistance in overweight and normal-weight children. J Clin Endocrinol Metab. 2009;94(12):4923–4930. doi: 10.1210/jc.2009-1590. PubMed PMID: 19846731. [DOI] [PMC free article] [PubMed] [Google Scholar]