Abstract
Background
Evidence is mixed regarding whether diabetes confers equivalent risk of coronary heart disease (CHD) as prevalent CHD. We investigated whether diabetes and severe diabetes are coronary heart disease (CHD) risk equivalents.
Methods
At baseline, participants in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study (black and white US adults ≥45 years old recruited 2003–2007) were categorized as having prevalent CHD only (self-reported or electrocardiogram evidence) (n=3,043), diabetes only (self-reported or elevated glucose) (n=4,012), diabetes and prevalent CHD (n=1,529) and neither diabetes nor prevalent CHD (n=17,155). Participants with diabetes using insulin and/or with albuminuria (urinary albumin-to-creatinine ratio ≥30 mg/g) were categorized as having severe diabetes. Participants were followed through 2011 for CHD events (myocardial infarction or fatal CHD).
Results
During a mean follow-up of 5 years, 1385 CHD events occurred. The hazard ratios (HRs) of CHD events comparing participants with diabetes only, diabetes and prevalent CHD and neither diabetes nor prevalent CHD to those with prevalent CHD were 0.65 (95% CI: 0.54, 0.77), 1.54 (95% CI: 1.30, 1.83) and 0.41 (95% CI: 0.35, 0.47), respectively, after adjustment for demographics and risk factors. Compared to participants with prevalent CHD, the HR of CHD events for participants with severe diabetes was 0.88 (95% CI: 0.72, 1.09).
Conclusions
Participants with diabetes had lower risk of CHD events than those with prevalent CHD. However, participants with severe diabetes had similar risk as those with prevalent CHD. Diabetes severity may need consideration when deciding whether diabetes is a CHD risk equivalent.
Keywords: albuminuria, coronary heart disease, diabetes mellitus, insulin, myocardial infarction, risk
Although some studies have found that diabetes confers a risk of coronary heart disease (CHD) events similar to a history of CHD or cardiovascular disease (CVD), others have reported that this risk is considerably lower.1–8 Prior studies have varied widely in terms of age and sex of participants, time periods, racial composition of study populations, and definitions of prior CHD or CVD. The optimal intensity of CHD prevention therapy in people with diabetes may depend on whether diabetes is truly a CHD risk equivalent. Rates of CHD have declined dramatically over time;9, 10 information about the risk of CHD events associated with diabetes in a contemporary population could help patients and physicians make informed decisions about therapy for the primary prevention of CHD.
The severity of diabetes may be important when assessing the risk of CHD among people with diabetes and making decisions regarding the intensity of CHD prevention therapy.11 Diabetes severity may be measured by treatment intensity, biomarkers of diabetes complications or glycemic control,12–14 disease duration and age,15–17 or comorbid CVD risk factors.18 One of the early signs of diabetic nephropathy is albuminuria which increases the risk for myocardial infarction (MI) and other CVD.19, 20 Insulin use among people with diabetes may be a marker of both more severe disease and an increased risk of CVD.21, 22 Using data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, we compared the risk of CHD events between participants with diabetes but no prevalent CHD and those with prevalent CHD but no diabetes and investigated whether the relative risk of CHD events associated with diabetes versus history of CHD varied by age. Additionally, we investigated the risk of CHD events associated with more severe diabetes, defined as diabetes with insulin use and/or albuminuria.
Methods
Population description
The REGARDS study is a prospective cohort of 30,239 English-speaking, community-dwelling black and white US adults ≥45 years of age at baseline in 2003–2007.23 The study was designed to investigate differences in stroke mortality by geographic region and race.23 REGARDS oversampled black individuals and people living in the US stroke buckle (coastal regions of North Carolina, South Carolina and Georgia) and the rest of the stroke belt (remaining areas of North Carolina, South Carolina and Georgia and Alabama, Arkansas, Louisiana, Mississippi, and Tennessee).23 The study protocol was approved by institutional review boards at participating centers, and all participants provided written informed consent.23 For this analysis, participants were excluded if they were missing data on diabetes or history of CHD (n=1,660), insulin use or albuminuria (n=2,417) or follow-up for CHD (n=423). After exclusions, 25,739 participants remained in the sample.
Data collection
Computer assisted telephone interviews were conducted to obtain information about socio-demographic factors, CVD risk factors, cigarette smoking, physical activity, and medication use.24 An in-home study visit was conducted by health professionals to obtain ECGs, medication inventories, systolic and diastolic blood pressure, weight and height measurements and blood and spot urine samples.24 Participants were asked to fast for 10–12 hours the night before the visit.23 After collection, blood and urine samples were shipped overnight with ice packs to a central laboratory at the University of Vermont where a BNII ProSpec nephelometer (Siemens AG) was used to measure urine albumin and a Modular-P chemistry analyzer (Roche/Hitachi) was used to measure urine creatinine by the rate Jaffe method.23, 24 Laboratory assays were performed on the blood samples to obtain lipid profiles, glucose, creatinine and C-reactive protein levels.23 ECGs were analyzed at Wake Forest University.23
Exposures
The primary exposure groups were: 1) prevalent CHD but no diabetes, 2) diabetes but no prevalent CHD, 3) both diabetes and prevalent CHD, and 4) neither prevalent CHD nor diabetes. Prevalent CHD was defined as self-reported history or ECG evidence of a prior MI or self-reported CABG, coronary angioplasty, or coronary stenting. Baseline diabetes was defined as fasting blood glucose levels ≥126 mg/dL, non-fasting glucose levels ≥200 mg/dL for 13% of participants who did not fast for at least 8 hours,25 or self-reported use of oral diabetes medication or insulin. We further categorized participants with diabetes at baseline based on evidence of severe diabetes [self-reported insulin use and/or presence of albuminuria (urinary albumin-to-creatinine ≥30 mg/g)] and by insulin use and albuminuria, separately.
Covariates
Age, race, sex, region of residence, income, education, cigarette smoking and physical activity were self-reported. BMI (kg/m2) was calculated from height and weight as measured during the study visit. Information on the use of medications (aspirin, statins, ARBs, ACE inhibitors) was collected in the medication inventory. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or self-reported use of antihypertensive medication. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, C-reactive protein and serum creatinine (used to estimate glomerular filtration rate with the CKD-Epi equation26) were measured in blood samples.
Outcome
The primary outcome was CHD events (definite/probable MI or CHD death). Secondary outcomes were MI and fatal CHD (CHD death or death within 28 days of a definite or probable MI). When examining MI, only the first event was included and for fatal CHD, participants were censored if they experienced a non-fatal MI. Participants or their proxies were called every 6 months to gather information about hospitalizations and deaths. Deaths were also detected through linkage to the Social Security Administration’s Master Death File and the National Death Index. Records from deaths and heart-related hospitalizations were retrieved for adjudication. Adjudication was conducted by pairs of clinician-adjudicators based on published guidelines, with committee review to resolve disagreements.27–29 Adjudication of MIs was based on signs and symptoms of ischemia; a rising and/or falling pattern in cardiac troponin or creatinine phosphokinase-MB level with the peak level more than two times the normal upper limit; and ECG changes which indicated ischemia.29, 30 Adjudication of CHD death was based on review of medical history, hospital records, interviews with next of kin or proxies, autopsy reports, death certificates, and National Death Index data.29 Kappa for agreement between adjudicators was >0.80 for definite or probable MI and definite or probable acute CHD death.29
Statistical Analysis
We calculated means and standard deviations or percentages of participant characteristics by exposure status (prevalent CHD but no diabetes, diabetes but no prevalent CHD, both diabetes and prevalent CHD and neither diabetes nor prevalent CHD) and severity of diabetes (insulin and/or albuminuria). We calculated cumulative incidence of CHD, MI and fatal CHD events by exposure status using the Kaplan-Meier method and tested for differences in cumulative incidence curves using log-rank tests. We estimated crude incidence rates and 95% confidence intervals (CIs) by exposure status. Multivariable-adjusted Cox proportional hazards models were used to estimate hazard ratios (HRs) for CHD events comparing participants with diabetes but no prevalent CHD, both diabetes and prevalent CHD and neither diabetes nor prevalent CHD to participants with prevalent CHD but no diabetes. Model 1 was adjusted for age (continuous), race (black vs. white), sex (male vs. female), and region of residence (Stroke Buckle vs. Stroke Belt vs. Non-Belt). Model 2 was further adjusted for income (<$20,000 vs. ≥$20,000), education (high school or less vs. some college or college graduate), systolic and diastolic blood pressures (continuous), hypertension (yes vs. no), cigarette smoking (current vs. past vs. never), total cholesterol (continuous), HDL cholesterol (continuous), triglycerides (continuous), and use of aspirin, statins, ACE inhibitors or ARB (yes vs. no), BMI (continuous), physical activity (none vs. 1–3 times per week vs. ≥4 times per week), C-reactive protein (<1 vs. 1–3 vs. >3 mg/L), estimated glomerular filtration rate (<60 vs. ≥60 mL/min/1.73 m2), and urinary albumin-to-creatinine ratio (≤30 vs. >30 mg/g). There were no variance inflation factors greater than 5 suggesting that multi-collinearity among the covariates was not a concern. Analyses were repeated for the outcomes of MI and, separately, fatal CHD. We also conducted the analyses further stratifying participants with diabetes but no prevalent CHD based on diabetes severity; insulin use and/or albuminuria and, separately, by insulin use and albuminuria. We used Cox proportional hazards models adjusted as above, excluding urinary albumin-to-creatinine ratio in models comparing people with diabetes stratified based on insulin use and/or albuminuria and, separately, by albuminuria alone to those with prevalent CHD but no diabetes. The proportional hazards assumption was evaluated by including interaction terms between the exposure categories and the natural logarithm of time in the models. There was no evidence of violation of the proportional hazards assumption. We further tested whether age was an effect modifier of the association between exposure status (prevalent CHD but no diabetes, diabetes but no prevalent CHD, both diabetes and prevalent CHD and neither diabetes nor prevalent CHD) and CHD risk using a cross-product (interaction) term (P-value for interaction = 0.04). Therefore, we calculated HRs for CHD events stratified by age categories (<65 and ≥65 years). To handle missing data on covariates in the Cox proportional hazards models, we performed multiple imputation by chained equations, with ten datasets.31 Analyses were conducted using SAS, version 9.3, SAS Institute, Cary, NC and Stata Statistical Software, version 12.1, College Station, TX.
Sources of Funding
The REGARDS study is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Additional support for this project was provided by grants from the National Heart, Lung, and Blood Institute (K24 HL111154 and R01 HL080477) and National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK095928). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
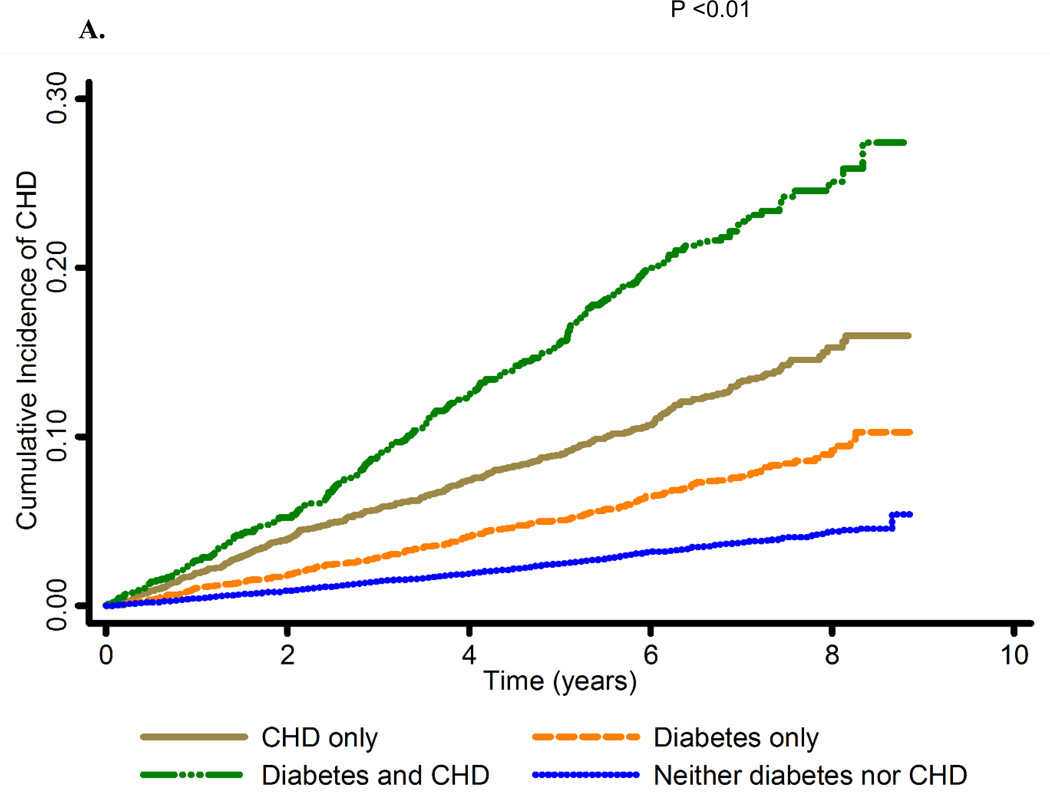
Baseline characteristics of the population by prevalent CHD and diabetes status are presented in Table 1 and by diabetes severity in Supplemental Table 1. Participants with diabetes but no prevalent CHD were more likely to have hypertension, higher mean triglycerides and BMI and were more likely to have C-reactive protein >3 mg/L compared to participants with prevalent CHD but no diabetes (Table 1). Over a mean follow up of 5 years, 1,385 CHD events occurred, (1,019 MIs and 506 fatal CHD) among 25,739 participants. The cumulative incidence of CHD events was highest for those with both prevalent CHD and diabetes, followed by those with prevalent CHD but no diabetes, those with diabetes but no prevalent CHD, and finally those with neither prevalent CHD nor diabetes (Figure 1A). The same pattern was observed for MI and fatal CHD events (Supplemental Figures 1A and 1B). Crude incidence rates of CHD were 19.9 (95% CI: 17.8, 22.0); 11.3 (95% CI: 9.9, 12.7); 35.3 (95% CI: 31.1, 39.5) and 5.3 (95% CI: 4.9, 5.8) events per 1,000 person-years of follow up among those with prevalent CHD but no diabetes, diabetes but no prevalent CHD, both diabetes and prevalent CHD and neither diabetes nor prevalent CHD, respectively (Table 2). After adjustment for covariates, HRs for CHD, MI and fatal CHD events comparing those with diabetes but no prevalent CHD to those with prevalent CHD but no diabetes were 0.65 (95% CI: 0.54, 0.77), 0.70 (95% CI: 0.57, 0.87) and 0.53 (95% CI: 0.40, 0.71), respectively.
Table 1.
Characteristics of REGARDS participants by diabetes and prevalent coronary heart disease status at baseline
| Characteristics | Prevalent CHD only‡ |
Diabet es only§ |
Diabetes and prevalent CHD |
Neither diabetes nor prevalent CHD |
|---|---|---|---|---|
| n = 3,043 | n = 4,012 |
n = 1,529 | n = 17,155 | |
| Age, years, mean ± SD | 67.0 ± 9.2 | 64.9 ± 8.7 |
67.4 ± 8.2 | 63.8 ± 9.4 |
| Black, n (%) | 880 (28.9) | 2,473 (61.6) |
695 (45.5) | 6,267 (36.5) |
| Female, n (%) | 1,147 (37.7) |
2,277 (56.8) |
595 (38.9) | 9,932 (57.9) |
| Region, n (%) | ||||
| Stroke belt* | 1,059 (34.8) |
1,478 (36.8) |
505 (33.0) | 5,856 (34.1) |
| Stroke buckle† | 616 (20.2) | 888 (22.1) |
338 (22.1) | 3,539 (20.6) |
| Non-stroke belt or buckle | 1,368 (45.0) |
1,646 (41.0) |
686 (44.9) | 7,760 (45.2) |
|
Annual household income <$20,000, n (%) |
574 (21.3) | 1,008 (28.6) |
401 (30.0) | 2,452 (16.3) |
| Education ≤ High school, n (%) | 1,248 (41.0) |
1,883 (47.0) |
775 (50.8) | 5,799 (33.8) |
|
Fasting blood glucose, mg/dL, mean ± SD |
93.8 ± 11.3 | 132.9 ± 50.5 |
135.1 ± 52.8 | 92.4 ± 10.6 |
| Diabetes treatment, n (%) | ||||
| No pharmacologic treatment | - | 496 (12.4) |
119 (7.8) | - |
| Oral medications | - | 2,543 (63.4) |
877 (57.4) | - |
| Insulin | - | 514 (12.8) |
288 (18.8) | - |
| Both oral medications and insulin |
- | 459 (11.4) |
245 (16.0) | - |
|
Systolic blood pressure, mm Hg, mean ± SD |
128.5 ± 17.1 |
131.7 ± 17.0 |
132.3 ± 17.7 | 125.9 ± 16.2 |
|
Diastolic blood pressure, mm Hg, mean ± SD |
75.6 ± 9.7 | 77.0 ± 10.1 |
75.4 ± 10.7 | 76.6 ± 9.5 |
| Hypertension prevalence, n (%) | 2,044 (67.3) |
3,111 (77.6) |
1,254 (82.3) | 8,635 (50.4) |
| Smoker, n (%) | ||||
| Current | 476 (15.7) | 548 (13.7) |
223 (14.6) | 2,427 (14.2) |
| Never | 1,105 (36.5) |
1,834 (45.9) |
503 (33.0) | 8,205 (48.0) |
| Past | 1,449 (47.8) |
1,616 (40.4) |
799 (52.4) | 6,456 (37.8) |
|
Total cholesterol, mg/dL, mean ± SD |
180.3 ± 40.1 |
182.6 ± 41.0 |
171.5 ± 40.9 | 198.3 ± 38.1 |
| HDL-C, mg/dL, mean ± SD | 49.1 ± 15.5 | 47.9 ± 14.2 |
43.3 ± 13.4 | 54.0 ± 16.5 |
| LDL-C, mg/dL, mean ± SD | 104.4 ± 33.6 |
105.6 ± 34.7 |
95.1 ± 32.5 | 119.2 ± 33.6 |
|
Triglycerides, mg/dL, mean ± SD |
134.6 ± 91.5 |
146.1 ± 98.0 |
167.4 ± 119.9 | 125.6 ± 79.0 |
| Other medication use, n (%) | ||||
| Aspirin | 2,055 (67.6) |
1,917 (47.8) |
1,137 (74.4) | 6,078 (35.5) |
| Statins | 1,681 (55.2) |
1,719 (42.9) |
1,003 (65.6) | 3,734 (21.8) |
| ACE inhibitors or ARBs | 1,385 (45.5) |
2,459 (61.3) |
1,059 (69.3) | 4,287 (25.0) |
| BMI, kg/m2, mean ± SD | 28.1 ± 5.5 | 32.5 ± 6.7 |
31.9 ± 6.1 | 28.5 ± 5.8 |
| Physical activity, n (%) | ||||
| None | 1,013 (33.8) |
1,550 (39.2) |
687 (45.6) | 5,272 (31.2) |
| 1–3 times per week | 1,009 (33.7) |
1,421 (35.9) |
449 (29.8) | 6,380 (37.8) |
| 4+ times per week | 972 (32.5) | 984 (24.9) |
371 (24.6) | 5,250 (31.1) |
| C-reactive protein, mg/L, n (%) | ||||
| <1 | 781 (26.2) | 763 (20.2) |
308 (21.5) | 4765 (28.3) |
| 1 to 3 | 1,037 (34.8) |
1,131 (30.0) |
463 (32.3) | 5,760 (34.2) |
| >3 | 1,165 (39.1) |
1,875 (49.8) |
661 (46.2) | 6,321 (37.5) |
|
Estimated glomerular filtration rate, <60 mL/min/1.73 m2, n (%) |
515 (16.9) | 581 (15.1) |
397 (27.0) | 1,308 (7.6) |
|
Ratio of albumin to creatinine, ≥ 30 mg/g, n |
486 (16.0) | 1,131 (28.2) |
585 (38.3) | 1,692 (9.9) |
Abbreviations: REGARDS, Reasons for Geographic and Racial Differences in Stroke; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein cholesterol; CHD, prevalent coronary heart disease
Defined as the states of Alabama, Arkansas, Louisiana, Mississippi, Tennessee and the noncoastal regions of North Carolina, South Carolina and Georgia.
Defined as the coastal regions of North Carolina, South Carolina and Georgia
Coronary heart disease was assessed using ECG evidence of MI or self-report of MI or revascularization.
Diabetes was defined as blood glucose (fasting ≥ 126 mg/dL or non-fasting ≥ 200 mg/dL) or self-reported use of diabetes medication.
The frequencies and percentages may not add up to the total sample size due to missing data.
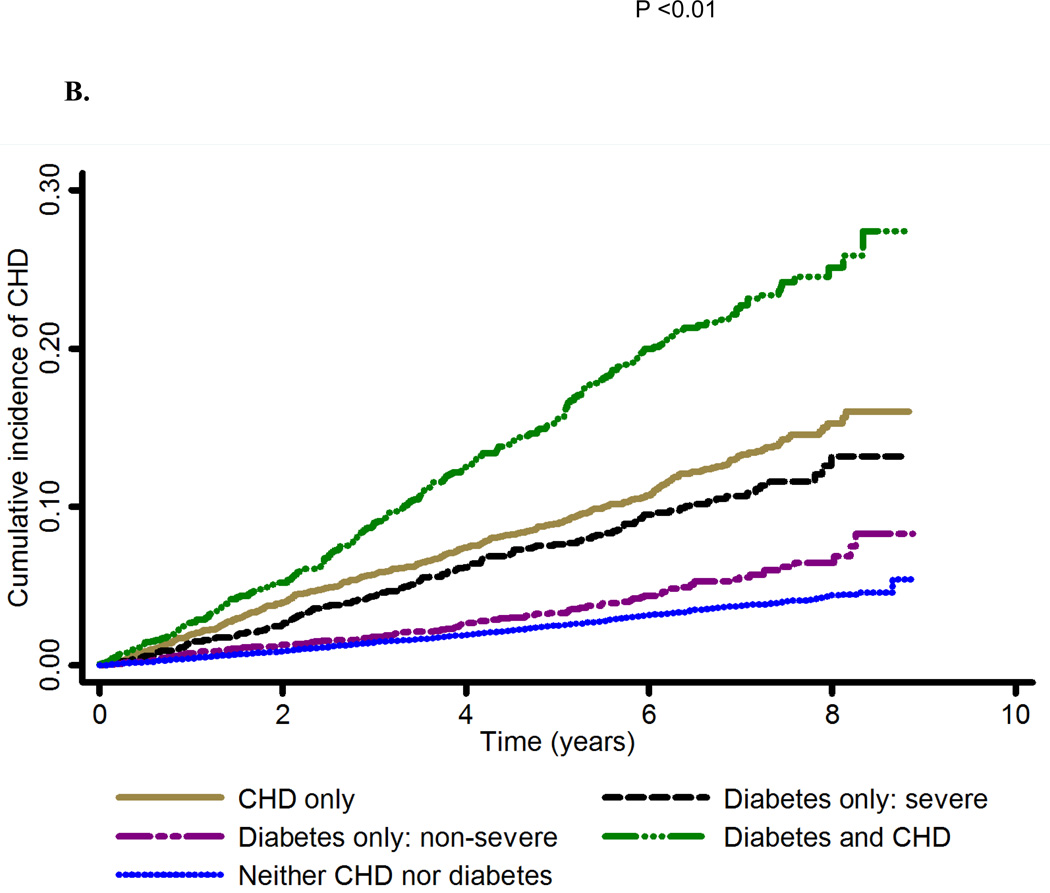
Figure 1. Cumulative incidence of coronary heart disease by A. prevalent coronary heart disease and diabetes status and B. prevalent coronary heart disease and diabetes (insulin use and/or albuminuria) status.
Abbreviations: CHD, coronary heart disease; CHD only, prevalent coronary heart disease; diabetes and CHD, diabetes and prevalent coronary heart disease; neither diabetes nor CHD, neither diabetes nor prevalent coronary heart disease
Exposure groups: CHD only, Diabetes only, Diabetes and CHD, Neither diabetes nor CHD. CHD was assessed using ECG evidence of MI or self-report of MI or revascularization. Diabetes was defined as blood glucose (fasting ≥ 126 mg/dL or non-fasting ≥ 200 mg/dL) or self-reported use of diabetes medication.
Outcome: Y-axis
Description of illustration: The cumulative incidence of CHD events was highest for those with both prevalent CHD and diabetes, followed by those with prevalent CHD but no diabetes, those with diabetes but no prevalent CHD, and finally those with neither prevalent CHD nor diabetes.
Table 2.
Incidence rates and adjusted hazard ratios and 95% confidence intervals for coronary heart disease events, myocardial infarction and fatal coronary heart disease events according to diabetes and prevalent coronary heart disease status at baseline
| Prevalent CHD only§ |
Diabetes only‖ |
Diabetes and prevalent CHD |
Neither diabetes nor prevalent CHD |
|
|---|---|---|---|---|
| CHD | ||||
|
Number of events |
332 | 248 | 272 | 533 |
|
Person-years of follow up |
16,654 | 21,993 | 7,703 | 99,848 |
|
Incidence rate (95% CI)* |
19.9 (17.8, 22.0) |
11.3 (9.9, 12.7) |
35.3 (31.1, 39.5) | 5.3 (4.9, 5.8) |
|
Hazard Ratio (95% CI)† |
1.0 (Reference) |
0.74 (0.62, 0.87) |
1.95 (1.66, 2.29) | 0.36 (0.31, 0.42) |
|
Hazard Ratio (95% CI)‡ |
1.0 (Reference) |
0.65 (0.54, 0.77) |
1.54 (1.30, 1.83) | 0.41 (0.35, 0.47) |
| MI | ||||
|
Number of events |
238 | 184 | 192 | 405 |
|
Person-years of follow up |
16,654 | 21,993 | 7,703 | 99,848 |
|
Incidence rate (95% CI)* |
14.3 (12.5, 16.1) |
8.4 (7.2, 9.6) |
24.9 (21.4, 28.4) | 4.1 (3.7, 4.5) |
|
Hazard Ratio (95% CI)† |
1.0 (Reference) |
0.79 (0.65, 0.97) |
1.95 (1.61, 2.37) | 0.38 (0.32, 0.45) |
|
Hazard Ratio (95% CI)‡ |
1.0 (Reference) |
0.70 (0.57, 0.87) |
1.54 (1.26, 1.89) | 0.43 (0.36, 0.51) |
| Fatal CHD | ||||
|
Number of events |
132 | 86 | 112 | 176 |
|
Person-years of follow up |
16,654 | 21,993 | 7,703 | 99,848 |
|
Incidence rate (95% CI)* |
7.9 (6.6, 9.3) | 3.9 (3.1, 4.7) |
14.5 (11.8, 17.2) | 1.8 (1.5, 2.0) |
|
Hazard Ratio (95% CI)† |
1.0 (Reference) |
0.62 (0.47, 0.83) |
2.02 (1.56, 2.60) | 0.31 (0.25, 0.40) |
|
Hazard Ratio (95% CI)‡ |
1.0 (Reference) |
0.53 (0.40, 0.71) |
1.57 (1.20, 2.06) | 0.34 (0.27, 0.44) |
Abbreviations: CHD, coronary heart disease events; MI, myocardial infarction; CHD only; prevalent coronary heart disease; diabetes and CHD, diabetes and prevalent coronary heart disease; neither diabetes nor CHD, neither diabetes nor prevalent coronary heart disease; CI, confidence interval
Per 1,000 person years
Adjusted for age (continuous), race (categorical), sex (categorical) and region of residence (categorical).
Adjusted for model 1 covariates, income (categorical) and education (categorical), systolic and diastolic blood pressure (continuous), hypertension (hypertensive based on SBP, DBP and self-reported use of antihypertensive medications) (categorical),cigarette smoking (categorical), total cholesterol (continuous), HDL cholesterol (continuous), triglycerides (continuous) and use of other medications (aspirin; statins; ACE inhibitors or ARBs) (categorical), BMI (continuous), physical activity (categorical), C-reactive protein (categorical), estimated glomerular filtration rate (categorical), urinary albumin to creatinine ratio (categorical).
CHD was assessed using ECG evidence of MI or self-report of MI or revascularization.
Diabetes was defined as blood glucose (fasting ≥ 126 mg/dL or non-fasting ≥ 200 mg/dL) or self-reported use of diabetes medication.
Among 4,012 participants with diabetes but no prevalent CHD, 973 (24.3%) used insulin, 1,131 (28.2%) had albuminuria, 416 (10.4%) used insulin and had albuminuria, and 2,323 (57.9%) had neither of the diabetes severity markers. The crude incidence rate was 16.2 (95% CI: 13.6, 18.9) and 7.9 (95% CI: 6.4, 9.4) CHD events per 1,000 person-years of follow up among participants with diabetes who used insulin and/or had albuminuria and those who neither used insulin nor had albuminuria, respectively (Table 3). The risk of CHD events was similar between participants with prevalent CHD but no diabetes and participants with severe diabetes defined as insulin use and/or albuminuria (Figure 1B). In separate analyses, the risk of CHD events was similar both between participants with prevalent CHD but no diabetes and those who used insulin (Supplemental Figure 2A) as well as between participants with prevalent CHD but no diabetes and those with albuminuria (Supplemental Figure 2B). These patterns were also present for the outcomes of MI and fatal CHD (Supplemental Figures 3A and 3B, Supplemental Figures 4A and 4B, Supplemental Figures 5A and 5B). In multivariable-adjusted models, HRs for CHD, MI and fatal CHD events comparing participants with diabetes who used insulin and/or had albuminuria to participants with prevalent CHD but no diabetes at baseline were 0.88 (95% CI: 0.72, 1.09), 0.93 (95% CI: 0.73, 1.19) and 0.75 (95% CI: 0.54, 1.06), respectively (Table 3). Similar HRs for CHD, MI and fatal CHD events were present when comparing participants with diabetes who used insulin and separately, participants with diabetes who had albuminuria, each compared to participants with prevalent CHD but no diabetes (Supplemental Table 2). Although there was a statistically significant interaction between age and exposure status (prevalent CHD but no diabetes, diabetes but no prevalent CHD, both diabetes and prevalent CHD and neither diabetes nor prevalent CHD), the associations were largely similar for participants <65 and ≥65 years of age (Supplemental Table 3). The hazard ratio for CHD events comparing participants with diabetes but no prevalent CHD to participants with CHD at baseline was 0.61 (95% CI: 0.45, 0.82) among participants <65 years of age and 0.65 (95% CI; 0.52, 0.80) among participants ≥65 years of age (p = 0.74).
Table 3.
Incidence rates and adjusted hazard ratios and 95% confidence intervals for coronary heart disease events, myocardial infarction and fatal coronary heart disease according to severity of diabetes (insulin use and/or albuminuria) and prevalent coronary heart disease status at baseline
| Prevalent CHD only§ | Diabetes only‖ | ||
|---|---|---|---|
|
Insulin use or albuminuria or both |
Neither insulin use nor albuminuria |
||
| CHD | |||
| Number of events | 332 | 144 | 104 |
| Person-years of follow up | 16,654 | 8,879 | 13,114 |
| Incidence rate (95% CI)* | 19.9 (17.8, 22.0) | 16.2 (13.6, 18.9) | 7.9 (6.4, 9.4) |
| Hazard Ratio (95% CI)† | 1.0 (Reference) | 1.05 (0.85, 1.28) | 0.52 (0.42, 0.66) |
| Hazard Ratio (95% CI)‡ | 1.0 (Reference) | 0.88 (0.72, 1.09) | 0.53 (0.42, 0.67) |
| MI | |||
| Number of events | 238 | 103 | 81 |
| Person-years of follow up | 16,654 | 8,879 | 13,114 |
| Incidence rate (95% CI)* | 14.3 (12.5, 16.1) | 11.6 (9.4, 13.8) | 6.2 (4.8, 7.5) |
| Hazard Ratio (95% CI)† | 1.0 (Reference) | 1.09 (0.86, 1.39) | 0.59 (0.45, 0.76) |
| Hazard Ratio (95% CI)‡ | 1.0 (Reference) | 0.93 (0.73, 1.19) | 0.60 (0.46, 0.78) |
| Fatal CHD | |||
| Number of events | 132 | 53 | 33 |
| Person-years of follow up | 16,654 | 8,879 | 13,114 |
| Incidence rate (95% CI)* | 7.9 (6.6, 9.3) | 6.0 (4.4, 7.6) | 2.5 (1.7, 3.4) |
| Hazard Ratio (95% CI)† | 1.0 (Reference) | 0.92 (0.66, 1.27) | 0.41 (0.28, 0.61) |
| Hazard Ratio (95% CI)‡ | 1.0 (Reference) | 0.75 (0.54, 1.06) | 0.42 (0.28, 0.62) |
Abbreviations: CHD, coronary heart disease; MI, myocardial infarction; CHD only; prevalent coronary heart disease; CI, confidence interval
Per 1,000 person years
Adjusted for age (continuous), race (categorical), sex (categorical) and region of residence (categorical) for the overall models.
Adjusted for model 1 covariates, income (categorical) and education (categorical), systolic and diastolic blood pressure (continuous), hypertension (hypertensive based on SBP, DBP and self-reported use of antihypertensive medications) (categorical), cigarette smoking (categorical), total cholesterol (continuous), HDL cholesterol (continuous), triglycerides (continuous) and use of other medications (aspirin; statins; ACE inhibitors or ARBs) (categorical), BMI (continuous), physical activity (categorical), C-reactive protein (categorical), estimated glomerular filtration rate (categorical)
CHD was assessed using ECG evidence of MI or self-report of MI or revascularization.
Diabetes was defined as blood glucose (fasting ≥ 126 mg/dL or non-fasting ≥ 200 mg/dL) or self-reported use of diabetes medication
Discussion
In the REGARDS study, the risks of CHD, MI and fatal CHD events were lower in participants with diabetes but no prevalent CHD compared to their counterparts with prevalent CHD but no diabetes. However, more severe diabetes requiring insulin and/or accompanied by albuminuria conferred a risk for total CHD and MI events similar to prevalent CHD but a slightly lower risk for fatal CHD events. In this population, 42% of the participants with diabetes had one or both of the severity measures. The REGARDS study enrolled a large and racially diverse contemporary population including black and white men and women across the continental US and rigorously adjudicated CHD events.
Some prior studies have found that diabetes as a broad category and prior MI confer equivalent risks of CHD events while others found the risk of CHD among those with diabetes is lower compared to those with prior MI.1–8 The differences among these studies were not explained by study country, gender, follow-up time or age of the participants. However, changes in the definition, diagnosis and aggressiveness of treatment of diabetes may have contributed to differences in diabetes severity across studies. In a landmark study, Haffner and colleagues found that diabetes was associated with equivalent risk of MI events as prior MI in a Finnish population.1 Boyko and Meigs reported that participants with diabetes but no history of MI in this study had a mean fasting blood glucose of 210 mg/dL (11.7 mmol/L), compared to 132.9 mg/dL (7.4 mmol/L) in the current study.1, 32 Therefore, the results obtained by Haffner and colleagues may be explained by the potentially more severe diabetes present in the Finnish participants. It is unclear to what extent severity of diabetes could explain the results in the other studies suggesting that diabetes was a CHD risk equivalent.2–4, 7 However, these studies were conducted prior to the reduction in the fasting glucose threshold for diagnosing and treating diabetes which was implemented in 1997 by the American Diabetes Association (ADA)33 and 1998 by the World Health Organization.34 In the REGARDS study, participants were recruited from 2003–2007 and followed up until 2011, after the 1997 revision to the definition of diabetes.
Other studies have found that kidney disease and insulin use among individuals with diabetes were associated with risk of CVD (MI, stroke and CVD deaths). Among participants with diabetes in the Cardiovascular Health Study, HRs for CVD events among those with creatinine >1.25 mg/dL (>110.5 μmol/L) compared to creatinine ≤1.25 mg/dL (≤110.5 μmol/L) and for participants treated with oral hypoglycemic agents or insulin use compared to no pharmacologic treatment were 1.31 (95% CI: 0.96, 1.78) and 1.57 (95% CI: 1.21, 2.03), respectively.35 Among participants with diabetes in the Heart Outcomes Prevention Evaluation (HOPE) trial randomized to 10 mg of ramipiril or placebo, the relative risk of CVD events was 1.89 (95% CI: 1.52, 2.36) for those with urinary albumin-to-creatinine ratio >14.3 mg/g (>1.62 mg/mmol) vs. <1.9 mg/g (<0.22 mg/mmol).36 In the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study, for every 10-fold increase in urinary albumin-to-creatinine ratio, the HR for CVD was 2.48 (95% CI: 1.74, 3.52).37
We considered insulin use an indicator of diabetes severity. While clinical trials of insulin use have not shown an increased risk of CVD in people with diabetes and pre-diabetes, some observational studies have shown an increased risk.21 One proposed risk of insulin use in the treatment of type 2 diabetes is an average weight gain of 10–12 pounds over the first year of treatment.38, 39 The increase in fat mass may worsen metabolic syndrome and increase inflammation and thrombosis leading to higher risk of CVD, but this pathway has not been confirmed.39, 40 Additionally, there is some evidence that people with more severe diabetes indicated by presence of chronic kidney disease, have higher platelet reactivity, and so, they may be a target for more intensive treatment.41, 42 In the current study, only 10% of participants both used insulin and had albuminuria. Similar results were obtained for both diabetes severity measures, suggesting that these are distinct proxies for diabetes severity.
The results of the current study provide confirmation of data from prior studies which found that an increased risk of MI or CHD has been observed among people with severe diabetes, defined by longer diabetes duration but no prevalent CHD, compared to those with prevalent MI or CHD.15–17 In another study, Howard and colleagues, there was a higher risk of fatal CHD among people with multiple CHD risk factors in addition to diabetes compared to people with diabetes without multiple additional CHD risk factors.18 In the current study, participants with diabetes had CHD risk factor profiles that were worse than risk factor profiles of participants with prevalent CHD at baseline. This may be the result of treatment recommendations which have improved risk factor management for individuals with CHD. However, contemporary treatment guidelines also emphasize CHD risk factor reduction among those with diabetes in this time frame.
The previous Adult Treatment Panel (ATP) III guidelines on management of cholesterol suggested that diabetes should be considered a CHD risk equivalent for the purpose of risk stratification and CHD prevention therapy.43 People with a history of CHD and people with diabetes were recommended equivalently intensive cholesterol lowering therapy with drugs such as statins.43 The 2013 American Heart Association (AHA)/American College of Cardiology guidelines, the 2015 AHA/ADA statement on CVD prevention in adults with diabetes and the 2015 ADA’s Standard of Medical Care in Diabetes also recommend statins for patients with diabetes, though not necessarily high intensity statins.44–47 Although diabetes was associated with a higher cumulative incidence of CHD events compared to those with neither diabetes nor CHD in this study, only those with severe diabetes had a similar risk of CHD events as those with prevalent CHD. However, the decision to consider diabetes as a CHD risk equivalent was based on considerations in addition to risk of CHD events.48 For example, the MI case-fatality rate in patients with diabetes is twice that of those without diabetes, and there is strong evidence that statins are effective in people with diabetes.48 Because of the elevated risk of CHD and the effectiveness of preventive therapies, lifestyle and pharmaceutical interventions for prevention of CHD are indicated for many people with diabetes.46 Nevertheless, our findings indicate that among people living with diabetes, there is a subgroup at particularly elevated CHD risk, thus these findings may be helpful to clinicians to guide the intensity of risk reduction therapies among patients taking insulin or with albuminuria or both.
Study limitations
The results should be interpreted in light of the limitations. Prevalent CHD and diabetes exposures were measured at a single time point, increasing the potential for misclassification. Hemoglobin A1c, a measure of glycemic control and disease severity, and duration of diabetes, an additional marker of diabetes severity, were not assessed in REGARDS. However, duration of diabetes can be difficult to interpret since length of time between diabetes onset and diagnosis is highly variable. Further, we were unable to differentiate type 1 from type 2 diabetes; it is likely that the observations here apply mostly to type 2 diabetes given the age of the population and the fact that type 2 diabetes represents 90–95% of diabetes in the US.49 In addition, some of the exposures, such as prevalent CHD, and covariates relied on self-report. As a result, there was potential for misclassification. While a rigorous procedure was used to adjudicate CHD events, it is possible that some events were missed. Despite available information on a host of important CHD risk factors, there was also potential for residual confounding.
Conclusions
The current study suggests that diabetes as a broad category may not be a CHD risk equivalent. However, diabetes requiring insulin and/or with albuminuria was associated with similar risk of CHD events as prevalent CHD. Therefore, severity of diabetes may warrant consideration when deciding whether diabetes should be treated as a CHD risk equivalent. While the high risk of CHD events and proven benefits of statins and treatment of hypertension in people with diabetes mean that CHD risk factor control is an important goal in this population, our findings may assist clinicians in targeting their efforts at aggressive risk factor control to those at highest risk.
Supplementary Material
Acknowledgments
T.M.B. has received research grants from Amgen. P.M. has received research grants from Amgen. R.W.D. has received research grants from Amgen. A.P.C. has received research funding from Amgen. M.M.S. has received salary support for investigator-initiated research from Amgen. E.B.L. has received research grants from Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions. F.L.M. contributed to the design of the study, analyzed and interpreted the data, wrote the manuscript, had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. T.M.B. adjudicated the CHD events, provided expertise on diabetes and coronary heart disease and revised the manuscript for intellectually important content. P.M. contributed to the design of the study and critically revised the manuscript for intellectually important content. R.W.D. adjudicated the CHD events, provided expertise on diabetes and coronary heart disease and revised the manuscript for intellectually important content. A.P.C. provided expertise on diabetes and revised the manuscript for intellectually important content. M.M.S. contributed to the design of the study, adjudicated the CHD events, provided expertise on diabetes and revised the manuscript for intellectually important content. E.B.L. conceived and designed the study, provided statistical expertise and revised the manuscript for intellectually important content.
Disclosures: F.L.M has no conflicts to report.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Mukamal KJ, Nesto RW, Cohen MC, et al. Impact of Diabetes on long-term survival after acute Myocardial Infarction comparability of risk with prior myocardial infarction. Diabetes Care. 2001;24:1422–1427. doi: 10.2337/diacare.24.8.1422. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley L, Padmanabhan S, Hole D, et al. Should diabetes be considered a coronary heart disease risk equivalent? Results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care. 2005;28:1588–1593. doi: 10.2337/diacare.28.7.1588. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Archives of Internal Medicine. 2001;161:1717. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 5.Do Lee C, Folsom AR, Pankow JS, et al. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation. 2004;109:855–860. doi: 10.1161/01.CIR.0000116389.61864.DE. [DOI] [PubMed] [Google Scholar]
- 6.Pajunen P, Koukkunen H, Ketonen M, et al. Myocardial infarction in diabetic and non-diabetic persons with and without prior myocardial infarction: the FINAMI Study. Diabetologia. 2005;48:2519–2524. doi: 10.1007/s00125-005-0019-0. [DOI] [PubMed] [Google Scholar]
- 7.Juutilainen A, Lehto S, Rönnemaa T, et al. Type 2 Diabetes as a “Coronary Heart Disease Equivalent” An 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–2907. doi: 10.2337/diacare.28.12.2901. [DOI] [PubMed] [Google Scholar]
- 8.Bulugahapitiya U, Siyambalapitiya S, Sithole J, et al. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–148. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–1857. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 12.Aronow WS, Ahn C, Weiss MB, et al. Relation of Increased Hemoglobin A1c Levels to Severity of Peripheral Arterial Disease in Patients With Diabetes Mellitus. The American Journal of Cardiology. 2007;99:1468–1469. doi: 10.1016/j.amjcard.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Coresh J, Golden SH, et al. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Wattanakit K, Steffes MW, et al. HbA1c and peripheral arterial disease in diabetes the Atherosclerosis Risk in Communities study. Diabetes Care. 2006;29:877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- 15.Wannamethee SG, Shaper AG, Whincup PH, et al. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med. 2011;171:404–410. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- 16.Yeap BB, McCaul KA, Flicker L, et al. Diabetes, myocardial infarction and stroke are distinct and duration-dependent predictors of subsequent cardiovascular events and all-cause mortality in older men. The Journal of Clinical Endocrinology and Metabolism. 2015;100:1038–1047. doi: 10.1210/jc.2014-3339. [DOI] [PubMed] [Google Scholar]
- 17.Rana JS, Liu JY, Moffet HH, et al. Diabetes and Prior Coronary Heart Disease are Not Necessarily Risk Equivalent for Future Coronary Heart Disease Events. Journal of General Internal Medicine. 2016;31:387–393. doi: 10.1007/s11606-015-3556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard BV, Best LG, Galloway JM, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29:391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 19.Yuyun MF, Khaw KT, Luben R, et al. A prospective study of microalbuminuria and incident coronary heart disease and its prognostic significance in a British population: the EPIC-Norfolk study. American Journal of Epidemiology. 2004;159:284–293. doi: 10.1093/aje/kwh037. [DOI] [PubMed] [Google Scholar]
- 20.Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 21.Roumie CL, Greevy RA, Grijalva CG, et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA. 2014;311:2288–2296. doi: 10.1001/jama.2014.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safford MM. Comparative effectiveness research and outcomes of diabetes treatment. JAMA. 2014;311:2275–2276. doi: 10.1001/jama.2014.4313. [DOI] [PubMed] [Google Scholar]
- 23.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez OM, Khodneva YA, Muntner P, et al. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706–714. doi: 10.1001/jama.2013.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carson AP, Muntner P, Kissela BM, et al. Association of prediabetes and diabetes with stroke symptoms: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2012;35:1845–1852. doi: 10.2337/dc11-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; and the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 29.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prineas RJ, Crow RS, Zhang Z. The Minnesota Code Manual of Electrocardiographic Findings: Including Measurement and Comparison with the Novacode ; Standards and Procedures for ECG Measurement in Epidemiologic and Clinical Trials. Springer. 2009 [Google Scholar]
- 31.Royston P and White IR. Multiple imputation by chained equations (MICE): implementation in Stata. Journal of Statistical Software. 2011;45:1–20. [Google Scholar]
- 32.Boyko EJ, Meigs JB. Does diabetes always confer coronary heart disease risk equivalent to a prior myocardial infarction? Implications for prevention. Diabetes Care. 2011;34:782–784. doi: 10.2337/dc10-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Mukamal KJ, Kizer JR, Djousse L, et al. Prediction and classification of cardiovascular disease risk in older adults with diabetes. Diabetologia. 2013;56:275–283. doi: 10.1007/s00125-012-2772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 37.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. Journal of the American Society of Nephrology. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 39.Aas AM, Ohrvik J, Malmberg K, et al. Insulin-induced weight gain and cardiovascular events in patients with type 2 diabetes. A report from the DIGAMI 2 study. Diabetes, Obesity & Metabolism. 2009;11:323–329. doi: 10.1111/j.1463-1326.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Aronne LJ. Weight management for type 2 diabetes mellitus: global cardiovascular risk reduction. Am J Cardiol. 2007;99:68B–79B. doi: 10.1016/j.amjcard.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Angiolillo DJ, Bernardo E, Capodanno D, et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Baber U, Bander J, Karajgikar R, et al. Combined and independent impact of diabetes mellitus and chronic kidney disease on residual platelet reactivity. Thrombosis and Haemostasis. 2013;110:118–123. doi: 10.1160/TH13-01-0004. [DOI] [PubMed] [Google Scholar]
- 43.Blumenthal RS. Overview of the adult treatment panel (ATP) III guidelines. Advanced Studies Med. 2002;2:148–157. [Google Scholar]
- 44.Goff DCL-JD, Jr, Bennett G, Coady S, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;00 [Google Scholar]
- 45.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 46.(8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl):S49–S57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 47.Fox CS, Golden SH, Anderson C, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundy SM. Diabetes and Coronary Risk Equivalency What does it mean? Diabetes care. 2006;29:457–460. doi: 10.2337/diacare.29.02.06.dc05-1904. [DOI] [PubMed] [Google Scholar]
- 49.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.