Abstract
Spondylocarpotarsal synostosis syndrome (SCT) is a rare Mendelian disorder (OMIM #272460) characterized by prenatal vertebral fusion, scoliosis, short stature and carpal and tarsal synostosis. SCT is typically known as an autosomal recessive disease caused by variants in the FLNB gene. The genetic basis of the rarer cases of vertical transmissions remains unknown. In two independent families with symptoms related to autosomal dominant SCT, we identified – by exome sequencing – two protein-altering variants in the embryonic myosin heavy chain 3 (MYH3) gene. As MYH3 variants are also associated with distal arthrogryposis (DA1, DA2A, DA2B) and autosomal dominant multiple pterygium syndromes (MPS), the present study expands the phenotypic spectrum of MYH3 variants to autosomal dominant SCT. Vertebral, carpal and tarsal fusions observed in both families further confirm that MYH3 plays a key role in skeletal development.
Introduction
Spondylocarpotarsal synostosis syndrome (SCT; OMIM #272460) is a rare Mendelian disorder characterized by vertebral fusion, scoliosis, short stature and carpal and tarsal synostosis. Several additional manifestations have been described including cleft palate, sensorineural or mixed hearing loss, joint limitations, clinodactyly and enamel hypoplasia. The disease was first recognized more than 40 years ago.1 To date, however, only 30 cases have been reported (reviewed by one of us in 2008).2, 3, 4, 5 The vast majority of patients show autosomal recessive inheritance with variants in the gene, FLNB, encoding the cytoskeleton protein filamin B.6 We identified two families with dominant disease transmission: one with typical SCT previously reported by one of us2 and the second with multiple pterygium syndrome (MPS; DA8) associated with skeletal manifestations. The present genetic study reveals that embryonic myosin heavy chain 3 (MYH3) mutations are associated with a spectrum of phenotypes including distal arthrogryposis (DA), MPS and SCT.
MYH3 encodes the heavy chain of embryonic myosin. The gene is known to be involved in DA) syndromes that constitute a group of disorders characterized by multiple congenital contractures of the upper and lower limbs.7, 8, 9, 10 Variants in MYH3 cause DA1 (OMIM #108120),11, 12 DA2A (Freeman–Sheldon syndrome; OMIM #193700)13, 14, 15 and DA2B (Sheldon–Hall syndrome; OMIM #601680).16, 17 Interestingly, recent findings from Chong et al18 indicate that MYH3 is also involved in autosomal dominant MPS that, in addition to congenital contractures of the limbs, is characterized by multiple pterygia and skeletal anomalies including fusion of vertebra and scoliosis. The latter report further suggests for embryonic myosin to play a key role in skeletal development.
The present study expands the spectrum of MYH3 variant to SCT that is characterized by a predominant bone phenotype and therefore confirms the role of MYH3 in skeletal development.
Subjects and methods
Subjects and exome sequencing
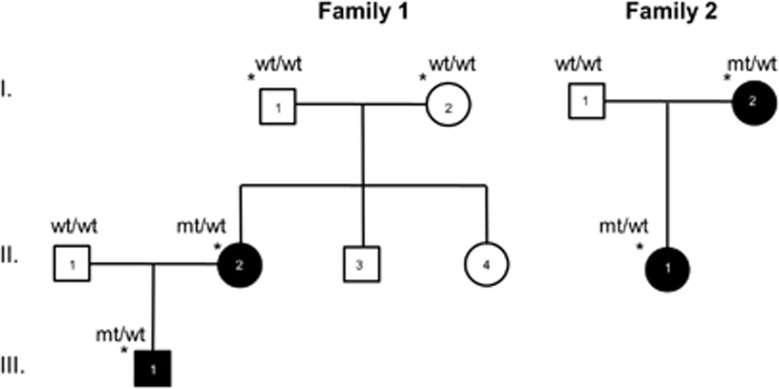
Subjects reported in this study are members of two unrelated families, both presenting vertical transmission of SCT. The following individuals were exome sequenced: in the first family, the proband (a boy) 1-III1, his affected mother (1-II2) and his healthy grandparents (1-I1 and 1-I2); in the second family, the proband (girl) (2-II1) and her affected mother (2-I2) (Figure 1). All subjects gave written informed consent for genetic analyses.
Figure 1.
Pedigrees of families 1 and 2. Black icons denote affected individuals. Asterisks indicate exome-sequenced subjects.
Genomic DNA was extracted and purified from peripheral blood using standard protocols. Exome capture was performed with the SureSelect 50MB Target Enrichment System v3 (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer's protocol. Prepared libraries were subjected to paired-end sequencing on a SOLiD 5500 platform (Life Technologies, Carlsbad, CA, USA) with 75 bases forward reads and 35 bases reverse reads. An average of 7.9 Gb of mappable sequence data per individual was obtained and mapped to the hg19 reference genome using Lifescope software v2.5.1 (Life Technologies). The mean coverage was 142-fold (median of 142-fold) and 84.1% of the target sequence was covered at a minimum of 10 ×. Variants were called using the GATK software (Broad Institute, Boston, MA, USA) and LifeScope (Life Technologies) algorithms with medium-stringency calling settings. Only variants called by both methods and covered by more than five variant reads were considered. Annotation was performed with the KGGSeq software package (Hong Kong University, Hong Kong, China). As SCT is extremely rare (30 cases reported to date), we focused only on protein-altering variants (missense, nonsense, splice-site variants and coding indels) with alternative allele frequency <0.0001 in dbSNP (build 135), 1000 Genomes Project data and an in-house database including 200 exomes. To identify potential causal variants in family 1, we focused on de novo variants of patient 1-II.2 (ie, absent in 1-I1 and 1-I2) that were also present in 1-III.1. In the second family, all variants common to 2-I2 and 2-II1 were analyzed.
Sanger sequencing
Candidate variants were checked using conventional capillary Sanger sequencing. Briefly, 344 and 318 bp DNA stretches of MYH3 exons 11 and 30 were amplified using the Expand Long Template PCR System (Roche, Meylan, France), following the manufacturer's recommendations. The PCR primer pairs were 5′-GGTGAAGCCCAGGATGTC-3′ (forward) and 5′-GGTAAAAGTTCCAGCCCAGG-3′ (reverse) for exon 11 and 5′-AGTATTTTGTTAGAAACCTAGAGGAGAGCTC-3′ (forward) and 5′-CTGAGTGATGAAGCCACACCCA-3′ (reverse) for exon 30. After purification with the Exostar kit (GE Healthcare, Little Chalfont, UK), PCR products were sequenced bidirectionally with the same amplification primers using Big Dye Terminator Kit v3.1 (Life Technologies). Sequence reactions were run on an ABI PRISM 3730xl sequencer (Life Technologies). The identified variants have been submitted to the MYH3-specific Leiden Open Variation Database (www.lovd.nl/MYH3) with individual IDs 00060260 (1-II.2), 00060275 (1-III.1), 00060276 (2-I.2) and 00060277 (2-II.1).
Results
Clinical examination
Table 1 summarizes the clinical findings in the two cases of SCT (family 1) and the two cases of MPS (family 2). In each family two cases concordant with an autosomal dominant inheritance of the disease were studied.
Table 1. Comparison of clinical features in the four patients.
|
Family 1 |
Family 2 |
|||
|---|---|---|---|---|
| II.2 | III.1 | I.2 | II.1 | |
| Sex (M/F) | F | M | F | F |
| Short stature | + | + | − | + |
| Spine anomalies | ||||
| Short trunk | + | + | − | + |
| Thoracolumbar fusions | + | + | − | + |
| Scoliosis | + | + | − | + |
| Cervical fusion | + | − | + | + |
| Short neck | + | + | + | + |
| Sacral anomaly | + | + | − | + |
| Limb anomalies | ||||
| Carpal fusion | + | a | + | + |
| Tarsal fusion | + | a | + | + |
| Joint mobility limitation | + | + | + | + |
| Clinodactyly 5th | + | + | + | − |
| Elbow, knee and shoulder pterygium | − | − | − | + |
| Single palmar crease | − | − | + | − |
| ORL | ||||
| Hearing loss | + | + | − | − |
| High arched/cleft palate | + | + | + | + |
| Inguinal hernia | − | + | − | + |
| Other clinical features | ||||
| Facial dysmorphy | + | + | + | + |
| Pterygium colli | − | − | + | + |
| Respiratory failure | − | − | − | + |
Evaluation of this feature was impossible because of severe bone age delay.
Family 1: patient 1-II.2
This presently 37-year-old woman was first described by some of us in Isidor et al.2 As described in that report, at her last medical examination (age 26 years) she presented the following symptoms: a short neck with limited mobility, low hairline, limited extension of both elbows, moderate hearing deficit, a cleft palate, bilateral single palmar crease and an absence of the flexion crease of the third phalange of all fingers except the thumb. Hands further showed bilateral fusion of trapezium and scaphoid, lunate–triquetrum and the right-hand fusion of capitate–hamate. Standard X-rays (see Isidor et al2 – cited above – for radiographs) showed progressive thoracic scoliosis, lumbar lordosis, lack of normal segmentation of T7–11 vertebral bodies with progressive vertebral fusion extending to T2, fusions of C1–C4 vertebral bodies and partial tarsal fusion between the scaphoid and the cuneiform. For further clinical phenotyping see Isidor et al.2
Family 1: patient 1-III.1 (proband)
This 16-year-old boy is the unique child of patient II.2 described above. He was also part of the original clinical report by some of us.2 At his last visit (9 years old) he showed a posterior cleft palate, a short neck, low set and posteriorly rotated ears, positional plagiocephaly without torticollis, short hands, bilateral single palmar crease and clinodactyly of both fifth fingers. He had a mild hearing deficit. X-rays revealed narrowing of the intervertebral space T8–T11, progressive scoliosis and fusion of T5–8 vertrebral bodies. For further clinical phenotyping see Isidor et al.2
Family 2: patient 2-I.2
Patient 2-I.2 was seen at 27 years of age for genetic counseling. She presented a Klippel–Feil syndrome (C1–C2 and C6–C7 fusions) along with feet malformations, both diagnosed in childhood. Bilateral pes cavus was also recorded at birth. Her left foot became painful at 11 years of age and required surgery. A bilateral tarsal coalition between navicular and first cuneiform was also documented. Psychomotor development was normal.
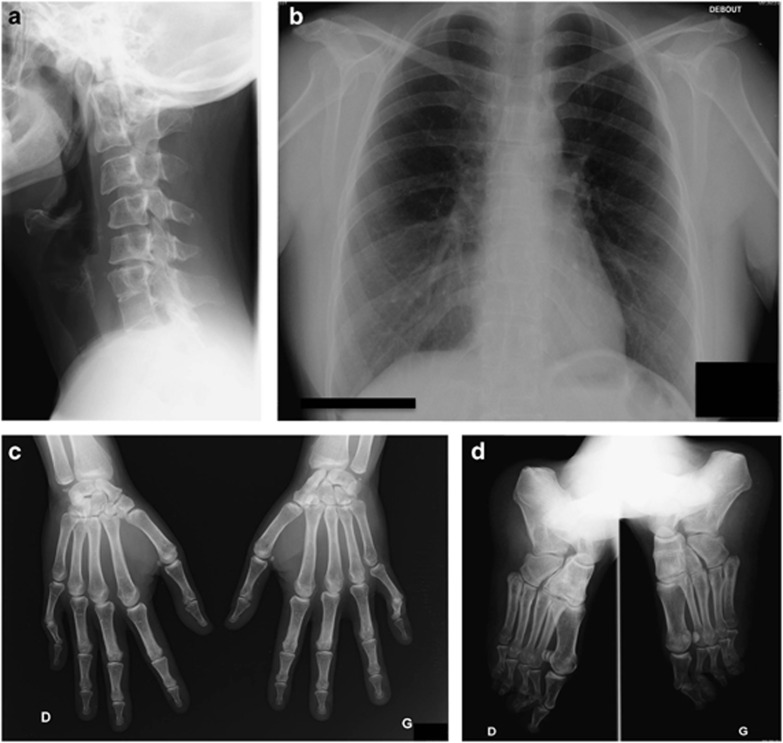
Clinical examination at 36 years of age showed normal stature (159 cm), pterygium colli, low posterior hair line, high arched palate and dysmorphic facial features with down-turned palpebral fissures and low set ears. Feet examination showed pes cavus but normal toes. Neurological examination and muscular tone were normal, as were the hands, except for bilateral fifth-digit camptodactyly and single palmar crease. X-rays showed fusions of C1–C2 and C6–C7 vertebral bodies (Figure 2a) and decreased height of the intervertebral discs T9–T10 and T11–T12 (Figure 2b). Hand and feet X-rays confirmed carpal and tarsal synostosis (Figures 2c and d). Renal and cardiac ultrasounds were normal as was audition and standard karyotyping.
Figure 2.
X-ray imaging of patient 2-I.2. (a) Lateral X-ray of cervical spine showing C1–C2 and C6–C7 anterior fusion associated with C1–C3 and C5–C7 posterior fusion. (b) AP pulmonary X-ray showing narrowing of intervertebral space T9–T10 and T11–T12, suggesting anterior fusions. Note the absence of spine or rib cage deformation. (c) AP X-rays of hands showing capitate–hamate and lunate–triquetrum coalitions of both hands associated with scaphoid–trapezium coalition of the left hand. (d) Oblique X-rays of feet showing a coalition between the navicular and the three cuneiforms of both feet.
Family 2: patient 2-II.1
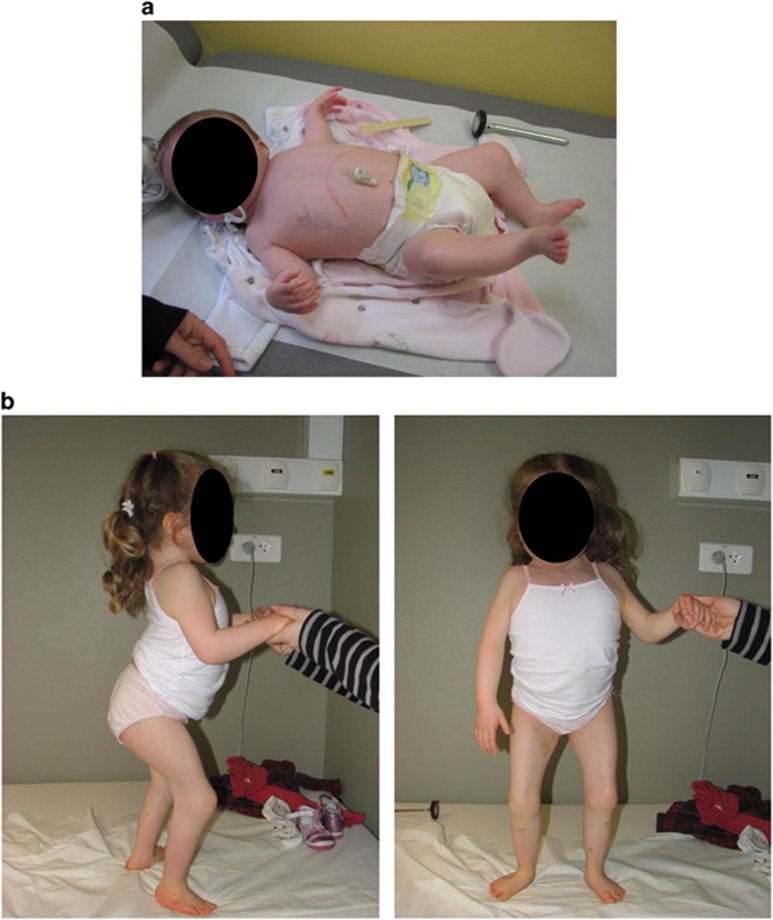
This 6-year-old girl is the unique child of patient 2-I.2, described above. Increased nuchal translucency and abnormal rachis were uncovered during pregnancy by ultrasound at 10 WG. Standard prenatal karyotyping was normal. Prenatal sonographic follow-up showed diffuse severe vertebral fusions. Termination of pregnancy was considered but the mother chose not to do so. The girl was born at 41 weeks, and she presented a short stature (43.6 cm; below 3rd percentile) but normal weight (3.08 kg 10th–50th percentile). Neonatal examination revealed low spontaneous mobility, torticollis, a large and short neck and severe joint limitations with pterygia of knees, ankles, shoulders and open hands with unmarked palmar creases. She also carried a posterior cleft palate and neonatal failure to thrive that led to gastrostomy. Facial features included ptosis, low set ears and short neck. A simple sacral dimple was noted, and spinal cord was low set (L2–L3; Figure 3).
Figure 3.
Phenotypic characteristics of patient 2-II.1. (a) Photograph of patient 2-II.1 at 6 months of age. (b) Photographs of the patient 2-II.1 at 4 years of age. Note the short stature, short trunk and neck, scoliosis, knee pterygium and neck webbing. A detailed description of the phenotype of the patient is presented in Table 1.
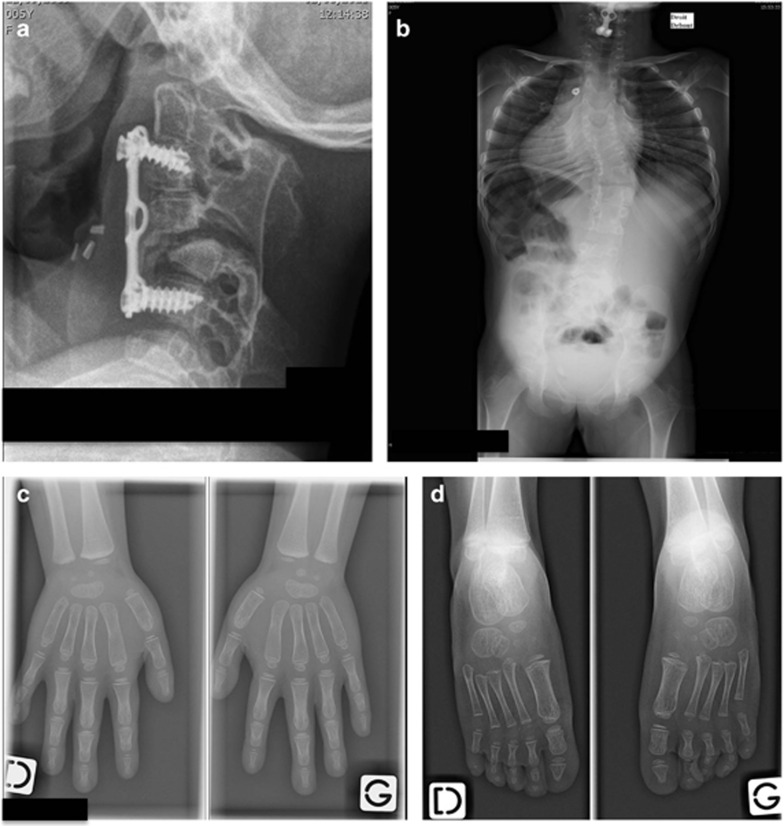
Diffuse vertebral segmentation anomalies were confirmed, involving thoracic, cervical and lumbar segments with fusions of vertebral bodies, posterior arches and spinous process, leading to several block vertebra and severe scoliosis (Figure 4).
Figure 4.
Radiographic examination of patient 2-II.1 at 5 years and 6 months of age (a) and at 3 years and 8 months of age (b, c, d). (a) Lateral X-ray of cervical spine 3 months after a C3–C6 anterior epiphysiodesis with tibial bone graft and plate fixation showing an incomplete reduction of a C4–C5 dislocation and posterior spinal fusions. (b) PA X-ray of thoracolumbar spine showing thoracic vertebral fusions without rib fusions responsible for growth disturbance with a short trunk and a right thoracolumbar curve. (c) AP X-rays of hands showing capitate–hamate coalition in both hands. (d) Dorsal plantar X-rays of feet where no coalition is observed.
Soon after birth, she showed motor development delays because of these orthopedic defects; walking was acquired at 2 and a half years. She kept a very short stature (height 4 SD, weight 2 SD and OFC 1 SD) with severe disproportion because of the impaired growth of her thoracic cage. A bicuspid aortic valve with grade 1 aortic insufficiency, slight aortic supravalvular stenosis and a patent ductus arteriosus (which required surgery) were also diagnosed. Furthermore, she presented a left inguinal hernia that required surgery as well. A few episodes of hypercalcemia and hypercalciuria were observed but remained unexplained. She had a respiratory failure episode following severe bronchiolitis with an aspergillus superinfection and her restrictive lung disease. Muscular examination was normal. Renal and spinal cord sonography as well as brain MRI were normal as was cognitive development. Surgery was necessary on the popliteal pterygium. At 4 years of age, C4–C5 dislocation with spinal cord involvement was diagnosed and an anterior epiphysiodesis was performed. Carpotarsal fusions were revealed by radiographic examinations.
Exome sequencing and identification of the candidate gene
Before exome sequencing, CGH array analysis and direct sequencing of the plausible causal genes – FLNB, NOG and GDF6 – failed to identify any chromosomal rearrangements or variants. The exome of a total of six subjects (family 1: index patient 1-III1, his healthy maternal grandparents and his affected mother; family 2: index patient 2-II1 and her affected mother (2-I2)) were sequenced. On average, 49 529 SNPs (median of 49 481) and 1861 indels (median of 1926) were identified for each individual. For SNPs, we focused only on the protein-altering events, represented by an average of 8251 variants (median of 8252) and corresponding to missense, frameshift, non-frameshift, splicing, stoploss and stopgain SNPs. In the case of indels, only frameshift, non-frameshift, stoploss, stopgain and splicing events were taken into account, corresponding to an average number of 168 (median of 187).
After filtering out variants with an alternative allele frequency >0.0001 in dbSNP (build 135), 1000 Genome Project data and an in-house database from each proband (1-III1 and 2-II1), a dominant heredity model was applied to further reduce the number of candidates to 2 in family 1 (MYH3 and SYT14, each with one de novo variant) and 30 in family 2 (Supplementary Table 1 and Supplementary Figure 1). The unique mutated gene in both families was MYH3 (NM_002470.3, NG_011537.1). In family 1, a de novo variant located in exon 11, which belongs to the coding region of the head domain (c.998C>G; p.(Thr333Arg)), was identified. In family 2, we uncovered a variant located in exon 30 that is part of the coding region of the protein tail (c.4031T>C; p.(Leu1344Pro)). Both missense variants were validated by Sanger sequencing and were shown to segregate with the disease in each family (Supplementary Figure 2). Both variants have deleterious predictive values using various tools such as Sift, Polyphen or GERP (Supplementary Table 1). In addition, neither variant was found in ESP6500, 1000 Genomes, the ExAC Browser (60 706 unrelated individuals) and an internal exome database (200 exomes).
Discussion
The two families reported here show both vertical disease transmission with MYH3 mutations and present phenotypic spectra going from pure SCT (family 1) to MPS (family 2). In family 2, the phenotype is characterized by a strong intrafamilial variability in terms of severity of vertebral fusions and orthopedic deformities. The child is severely disabled with short stature, major orthopedic deformations, persistent feeding difficulties and cardiac disease, although the mother is not disabled and shows normal stature and normal mobility except for that of the cervical spine. Carpotarsal and vertebral fusions are rare and appear in different clinical entities in which SCT is well characterized. Other rare entities include carpotarsal and vertebral coalition in different clinical and radiologic context (spondylocostal dysplasia, Robinow syndrome, multiple synostosis, proximal symphalangism). Two cases with a form of syndromic carpotarsal and vertebral fusion with cardiac disease and severe feeding difficulties may overlap with our cases,19 but cardiac findings seem different.
To date, MYH3 variants have been associated only with autosomal dominant DA) syndromes, including DA1, DA2A, DA2B and autosomal dominant MPS (DA8). Autosomal dominant MPS is a rare form of distal arthrogryposis quite different from the others because of the absence of myotonic facies and severe pterygia possibly associated with skeletal abnormalities reported as scoliosis and vertebral fusions.18, 20, 21, 22, 23 In the description of Chong et al,18 vertebral fusions and scoliosis seem very similar to those observed in the present report and the patients share many clinical features including camptodactyly, hypoplastic flexion creases, short stature, ptosis and neck webbing. Although family 2 presents with a carpotarsal coalition that has not been described by Chong et al,18 the important overlapping symptomatology and the fact that both carpal and tarsal fusions have been reported previously in families affected by dominantly inherited MPS indicate that this family has MPS rather than SCT.20, 21, 22 Although hearing loss has been reported in several studies related to DA2A14, 24 and MPS,25 to our knowledge this feature was never associated with underlying MYH3 mutations. Altogether, these observations indicate that MYH3 mutations cause a spectrum of diseases including DA, MPS and SCT. The extensive phenotypic variability of MYH3-related diseases may be due to modifier genes or additional mutations. Identification of additional cases would be helpful to clarify the extent of the associated phenotypes.
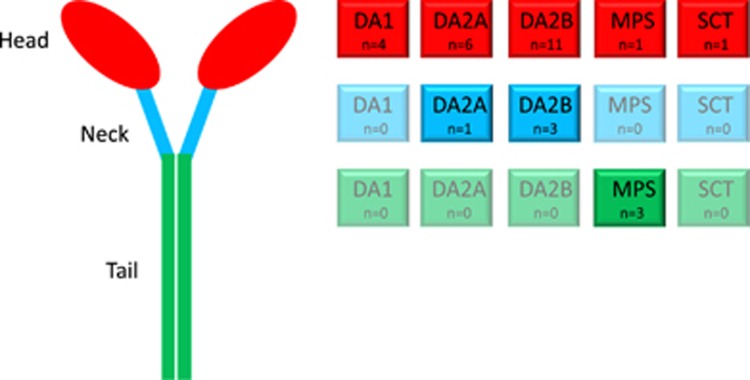
The MYH3 gene encodes the skeletal muscle myosin MYH3, composed of a myosin head-like domain (amino acids 1–779), a neck domain (amino acids 779–840) and a long coiled-coil rod domain (amino acids 840–1940), for a majority corresponding to the protein tail (amino acids 1070–1940). In DA1, DA2A and DA2B, all variants described so far are located within the head and neck domains (Figure 5). Variants in the tail domain have so far exclusively been observed in association with bone abnormalities. Such domain-specific phenotypes have previously been shown for MYH9, where variants in the motor domain coding region were associated with more severe clinical phenotypes than those in the tail domain coding part.26 Localization of variants in MYH7 have also various phenotypic impacts: those associated with myosin storage myopathy modify the distal rod region of the protein, whereas those associated with distal myopathy are located either in the rod or in the globular myosin head coding regions.27
Figure 5.
Protein localization of variants that cause MYH3-related diseases. The numbers of known variants in each of the three main protein domains (head, neck and tail) are indicated in the boxes of the right panel. DA1, distal arthrogryposis type 1; DA2A, distal arthrogryposis type 2A; DA2B, distal arthrogryposis type 2B; MPS, multiple pterygium syndrome; SCT, spondylocarpotarsal synostosis; n, number of variants observed.
Our present data confirm the conclusions of the recent report by Chong et al,18 suggesting that MYH3 plays a role in skeletal development and variants in this protein may perturb this development and in fine cause MPS or in our case SCT. Several further observations support this hypothesis: (1) MYH3 transcripts have been detected in bones of 17-week-old fetuses;18 (2) another skeletal muscle protein – troponin I type 2 (TNNI2) – has a proven role in skeletal development;28 (3) expression of myosins 2, 4 and 7 is associated with osteoblast activity and differentiation;29 and (4) severe deformation and failure of maturation of cranial skeletogenesis have been observed in zebrafish mutated in the myod gene, a key player in cranial myogenesis.30
In conclusion, the present report broadens the phenotype associated with MYH3 variants to autosomal dominant SCT and confirms the role of MYH3 in bone development.
Acknowledgments
This work was supported by funds from the Strasbourg High Throughput Next Generation Sequencing facility (GENOMAX) and INSERM UMR_S 1109. We are grateful to Christine Bole-Feyssot (Institut Imagine, Necker Hospital, Paris, France) for screening Necker's internal exome database for presence of MYH3 variants, Jocelyn Laporte and Catherine Koch (IGBMC, Illkirch, France) for helpful interactions and Rose-Marie Javier (Rheumatology Department, Hôpitaux Universitaires de Strasbourg, Strasbourg, France) for critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Jones KL, Guthrie RD, Smith DW: Case report 8. Syndr Identification 1973; I (2): 10–11. [Google Scholar]
- Isidor B, Cormier-Daire V, Le Merrer M et al: Autosomal dominant spondylocarpotarsal synostosis syndrome: phenotypic homogeneity and genetic heterogeneity. Am J Med Genet A 2008; 146A: 1593–1597. [DOI] [PubMed] [Google Scholar]
- Patil SJ, Bhat M, Rao S, Krishnan RS: Spondylocarpotarsal synostosis: a rare case of vertebral segmentation defect. Indian J Pediatr 2009; 76: 417–419. [DOI] [PubMed] [Google Scholar]
- Assir MZ, Waseem T: Spondylocarpotarsal synostosis with hydromyelia, mega cisterna magna, and pachydermoperiostosis. Clin Dysmorphol 2012; 21: 144–147. [DOI] [PubMed] [Google Scholar]
- Singh A, Kapoor S, Pradhan G: Urolithiasis in a child with spondylocarpotarsal synostosis syndrome: a co-incidence. J Clin Diagn Res 2013; 7: 2031–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow D, Robertson SP, King LM et al: Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat Genet 2004; 36: 405–410. [DOI] [PubMed] [Google Scholar]
- Hall JG, Reed SD, Greene G: The distal arthrogryposes: delineation of new entities—-review and nosologic discussion. Am J Med Genet 1982; 11: 185–239. [DOI] [PubMed] [Google Scholar]
- Hall JG: Arthrogryposis (multiple congenital contractures): diagnostic approach to etiology, classification, genetics, and general principles. Eur J Med Genet 2014; 57: 464–472. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Jorde LB, Carey JC: A revised and extended classification of the distal arthrogryposes. Am J Med Genet 1996; 65: 277–281. [DOI] [PubMed] [Google Scholar]
- Beals RK: The distal arthrogryposes: a new classification of peripheral contractures. Clin Orthop Relat Res 2005; 203–210. [PubMed]
- Alvarado DM, Buchan JG, Gurnett CA, Dobbs MB: Exome sequencing identifies an MYH3 mutation in a family with distal arthrogryposis type 1. J Bone Joint Surg Am 2011; 93: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AE, McMillin MJ, Gildersleeve HI et al: Spectrum of mutations that cause distal arthrogryposis types 1 and 2B. Am J Med Genet A 2013; 161A: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EA, Sheldon JH: Cranio-carpo-tarsal dystrophy. Arch Dis Child 1938; 13: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson DA, Carey JC, Palumbos J, Rutherford A, Dolcourt J, Bamshad MJ: Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics 2006; 117: 754–762. [DOI] [PubMed] [Google Scholar]
- Toydemir RM, Rutherford A, Whitby FG, Jorde LB, Carey JC, Bamshad MJ: Mutations in embryonic myosin heavy chain (MYH3) cause Freeman-Sheldon syndrome and Sheldon-Hall syndrome. Nat Genet 2006; 38: 561–565. [DOI] [PubMed] [Google Scholar]
- Krakowiak PA, Bohnsack JF, Carey JC, Bamshad M: Clinical analysis of a variant of Freeman-Sheldon syndrome (DA2B). Am J Med Genet 1998; 76: 93–98. [DOI] [PubMed] [Google Scholar]
- Toydemir RM, Bamshad MJ: Sheldon-Hall syndrome. Orphanet J Rare Dis 2009; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JX, Burrage LC, Beck AE et al: Autosomal-dominant multiple pterygium syndrome is caused by mutations in MYH3. Am J Hum Genet 2015; 96: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SB, Baujat G, Abadie V et al: Postnatal growth retardation, facial dysmorphism, spondylocarpal synostosis, cardiac defect, and inner ear malformation (cardiospondylocarpofacial syndrome?)—-a distinct syndrome? Am J Med Genet A 2010; 152A: 539–546. [DOI] [PubMed] [Google Scholar]
- Kawira EL, Bender HA: An unusual distal arthrogryposis. Am J Med Genet 1985; 20: 425–429. [DOI] [PubMed] [Google Scholar]
- McKeown CM, Harris R: An autosomal dominant multiple pterygium syndrome. J Med Genet 1988; 25: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prontera P, Sensi A, Merlo L, Garani G, Cocchi G, Calzolari E: Familial occurrence of multiple pterygium syndrome: expression in a heterozygote of the recessive form or variability of the dominant form? Am J Med Genet A 2006; 140: 2227–2230. [DOI] [PubMed] [Google Scholar]
- Frias JL, Holahan JR, Rosenbloom AL, Felman AH: An autosomal dominant syndrome of multiple pterygium, ptosis, and skeletal abnormalities. Proceedings of the Fourth International Conference on Birth Defects. Excerpta Medica 1973;. 19.
- Zampino G, Conti G, Balducci F, Moschini M, Macchiaiolo M, Mastroiacovo P: Severe form of Freeman-Sheldon syndrome associated with brain anomalies and hearing loss. Am J Med Genet 1996; 62: 293–296. [DOI] [PubMed] [Google Scholar]
- Thompson EM, Donnai D, Baraitser M, Hall CM, Pembrey ME, Fixsen J: Multiple pterygium syndrome: evolution of the phenotype. J Med Genet 1987; 24: 733–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rocco D, Zieger B, Platokouki H et al: MYH9-related disease: five novel mutations expanding the spectrum of causative mutations and confirming genotype/phenotype correlations. Eur J Med Genet 2013; 56: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsharghi H, Oldfors A: Myosinopathies: pathology and mechanisms. Acta Neuropathol 2013; 125: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang F, Zhao Y et al: A gain-of-function mutation in Tnni2 impeded bone development through increasing Hif3a expression in DA2B mice. PLoS Genet 2014; 10: e1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lazarenko OP, Blackburn ML et al: Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One 2011; 6: e24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinits Y, Williams VC, Sweetman D et al: Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev Biol 2011; 358: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.