Abstract
Persistent activation of signal transducers and activators of transcription 3 (STAT3) is commonly detected in many types of cancer including pancreatic cancer. Whether STAT3 is activated in stem cell-like pancreatic cancer cells and the effect of STAT3 inhibition, is still unknown. Flow cytometry was used to isolate pancreatic cancer stem-like cells which are identified by both aldehyde dehydrogenase (ALDH)-positive (ALDH+) as well as cluster of differentiation (CD) 44-positive/CD24-positive subpopulations (CD44+/CD24+). STAT3 activation and the effects of STAT3 inhibition by STAT3 inhibitors, LLL12, FLLL32, and Stattic in ALDH+ and CD44+/CD24+ cells were examined. Our results showed that ALDH+ and CD44+/CD24+ pancreatic cancer stem-like cells expressed higher levels of phosphorylated STAT3, an active form of STAT3, compared to ALDH-negative (ALDH−) and CD44-negative/CD24-negative (CD44−/CD24−) pancreatic cancer cells, suggesting that STAT3 is activated in pancreatic cancer stem-like cells. Small molecular STAT3 inhibitors inhibited STAT3 phosphorylation, STAT3 downstream target gene expression, cell viability, and tumorsphere formation in ALDH+ and CD44+/CD24+ cells. Our results indicate that STAT3 is a novel therapeutic target in pancreatic cancer stem-like cells and inhibition of activated STAT3 in these cells by STAT3 inhibitors may offer an effective treatment for pancreatic cancer.
Keywords: aldehyde dehydrogenase, cancer stem cells, CD24, CD44, pancreatic cancer, STAT3
Introduction
Pancreatic cancer develops from cancerous cells in the tissues of the pancreas, a gland organ that lies inferior to the stomach. It is one of the most lethal cancers. According to the American Cancer Society, pancreatic cancer is expected to cause 48,960 new cases and 40,560 deaths in the United States in 2015 alone (1). Only 20% of patients live for more than a year after diagnosis and fewer than 6% survive past five years (2). Recent evidence suggests the existence of a small population of tumorigenic stem cells responsible for tumor initiation, resistance to chemotherapy and radiation, and metastasis. These cancer stem cells have the ability to self-renew, driving tumorigenicity, recurrence, and metastasis. They also have the capacity to differentiate aberrantly which gives rise to a heterogeneous subpopulation of cancer cells that constitute the bulk of the tumor. Surface markers cluster of differentiation 24 (CD24) and cluster of differentiation 44 (CD44), along with aldehyde dehydrogenase (ALDH1), a detoxifying enzyme responsible for the oxidation of intracellular aldehydes, have been identified as markers of stem cells of pancreatic adenocarcinomas (3,4). The role of signal transducers and activators of transcription 3 (STAT3) in pancreatic cancer stem cells, however, is still unknown. Hence, it is important to identify the regulatory mechanisms and signaling pathways involved in pancreatic cancer stem cells and develop novel agents to target pancreatic cancer stem cell populations.
The signal transducers and activators of transcription (STAT) protein family represents a group of transcription factors that play a role in relaying extracellular signals initiated by cytokines and growth factors from the cytoplasm to the nucleus (5–7). In response to extracellular signals, phosphorylated STAT proteins dimerize and translocate to the nucleus where they regulate the expression of numerous critical genes involved in cell cycle progression, proliferation, invasion, and survival. STAT3 activation is dependent upon the phosphorylation of a conserved tyrosine residue (Y705) which promotes the dimerization of STAT3 monomers via their Src-homology 2 (SH2) domains (8,9). The constitutive activation of STAT3 is frequently detected in primary human cancer cells including pancreatic cancer cells (10–12). Blockade of STAT3 signaling has been shown to effectively inhibit cell growth and induce apoptosis of pancreatic cancer cells in both in vitro (13) and in vivo studies (14,15). Although the role of STAT3 signaling in stem cell-like pancreatic cancer cells is unknown, this pathway may represent an attractive therapeutic target. Thus, it is important to determine the role of STAT3 activation in pancreatic stem cell-like cancer cells. We demonstrate for the first time that the ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells express higher levels of phosphorylated STAT3 (tyrosine 705) (P-STAT3, Y705) than subpopulations that do not express these markers. In addition, novel STAT3 inhibitors, LLL12, FLLL32, and Sttatic, inhibited STAT3 phosphorylation, cell viability, tumorsphere formation, and reduced STAT3 downstream target gene expression in ALDH+ and CD44+/CD24+ subpopulations. This report indicates that constitutively activated STAT3 has an important role in pancreatic stem cell-like cancer cell function and thus may serve as an attractive therapeutic target for pancreatic cancer.
Materials and methods
Pancreatic cancer cell lines
Human pancreatic cancer cell lines (Panc-1, BxPC3, and HPAC) were purchased from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 4.5 g/l L-glutamine, sodium pyruvate, and 1% penicillin/streptomycin. All cell lines were stored in a humidified 37°C incubator with 5% CO2. Cancer stem-like cells were grown in a serum-free mammary epithelial basal medium (MEBM) (Clonetics Division of Cambrex BioScience) supplemented with B27 (Invitrogen), 20 ng/ml EGF (BD Biosciences), 4 μg/ml gentamycin, 1 ng/ml hydrocortisone, 5 μg/ml insulin and 100 μM β-mercaptoethanol (Sigma-Aldrich).
STAT3 inhibitors, LLL12, FLLL32 and Stattic
Small molecules, LLL12 (16) and FLLL32 (17) that selectively target STAT3, were synthesized by Dr Pui-Kai Li's laboratory at the Ohio State University College of Pharmacy. Stattic, a previously reported STAT3 inhibitor (18), was purchased from Calbiochem (San Diego, CA, USA).
MTT cell viability assay
Pancreatic cancer stem-like cells (3,000/well in 96-well plates) were incubated with desired concentrations of compounds in triplicate at 37°C for 72 h. 3-(4,5-Dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) viability assays were done and the absorbance was read at 595 nm.
Isolation of cancer stem cells
The AldeFluor kit (StemCell Technologies, Durham, NC, USA) was used to isolate the population of cells with high ALDH enzymatic activity as previously described (19–21). Briefly, cells were trypsinized to single cells using 0.05% trypsin and subsequently suspended in AldeFluor assay buffer containing ALDH substrate (BAAA, 1 μmol/l per 1×106 cells) and then incubated for 40 min at 37°C. For each sample, an aliquot of cells was stained under identical conditions with 15 mmol/l diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor, as a negative control. In all experiments, the AldeFluor-stained cells treated with DEAB served as ALDH-negative controls. Anti-human PE-CD24 and PE/Cy5-CD44 antibodies (BioLegend) were used for CD44/CD24 identification. ALDH+ and CD44+/CD24+ subpopulations were separated from Panc-1, BxPC3, and HPAC pancreatic cancer cells by a FACS Wantage SE (Becton-Dickinson, Palo Alto, CA, USA) flow cytometer. After sorting, ALDH+ and CD44+/CD24+ cells were cultured in serum-free stem cell medium (MEBM) to maintain cancer stem cell characteristics. ALDH− and CD44−/CD24− cells were cultured in regular medium and replaced with stem cell medium for three days before harvesting.
Western blot analysis
After treatment with LLL12 (5 μM), FLLL32 (5 μM), Stattic (20 μM) or DMSO for 24 h, ALDH+ and CD44+/CD24+ Panc-1 and HPAC pancreatic cancer cells were lysed in cold RIPA lysis buffer containing protease inhibitors and subjected to SDS-PAGE. Proteins were transferred on to PVDF membrane and probed with antibodies (Cell Signaling Technology). Membranes were probed with a 1:1,000 dilution of antibodies (Cell Signaling Technology) against phospho-specific STAT3 (tyrosine 705), phospho-independent STAT3, phospho-specific ERK1/2 (threonine 202/tyrosine 204), and GAPDH. Membranes were analyzed using enhanced chemiluminescence Plus reagents and scanned with the Storm Scanner (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
ALDH+ and CD44+/CD24+ subpopulations of Panc-1 and HPAC pancreatic cancer cells were treated with LLL12 (5 μM), FLLL32 (5 μM), or DMSO for 24 h. RNA was then collected using RNeasy kits (Qiagen, Valencia, CA, USA). PCR amplification was done under the following conditions: 5 min at 94°C followed by 25 cycles of 30 sec at 94°C, 30 sec at 55°C, and 30 sec at 72°C with a final extension of 5 min at 72°C. Primer sequences and source information of STAT3 downstream target genes can be found in Table I.
Table I.
Primer sequences and source information of STAT3 downstream target genes.
| Gene | Primers | Size | Source |
|---|---|---|---|
| Cyclin D1 | Forward: 5′-GCTGGAGCCCGTGAAAAAGA-3′ Reverse: 5′-CTCCGCCTCTGGCATTTTG-3′ |
247 | (25) |
| Survivin | Forward: 5′-ACCAGGTGAGAAGTGAGGGA-3′ Reverse: 5′-AACAGTAGAGGAGCCAGGGA-3′ |
309 | (26) |
| Bcl-Xl | Forward: 5′-TTGGACAATGGACTGGTTGA-3′ Reverse: 5′-GTAGAGTGGATGGTCAGTG-3′ |
765 | (27) |
| Notch1 | Forward: 5′-CAACATCCAGGACAACATGG-3′ Reverse: 5′-GGACTTGCCCAGGTCATCTA-3′ |
229 | a |
| Notch3 | Forward: 5′-TGTCTTGCTGCTGGTCATTC-3′ Reverse: 5′-CATCTGGGCCACGCACATT-3′ |
413 | a |
Source unknown or designed with Primer 3 web application.
Tumorsphere culture
The ALDH+ and CD44+/CD24+ subpopulations of Panc-1, BxPC3, and HPAC pancreatic cancer cells were plated as single cells in ultra-low attachment 6-well plates (Corning, Lowell, MA, USA) at a density of 25,000 viable cells/well. Cells were grown in a serum-free mammary epithelial basal medium (MEBM) in a humidified incubator (5% CO2) at 37°C. On the second day after seeding, the ALDH+ cells were treated with 2.5–5 μM of LLL12, 2.5–5 μM of FLLL32, or 5–10 μM of Stattic. Tumorspheres were observed under microscope 21 to 28 days later. In order to compare tumorsphere-forming ability, ALDH+, CD44+/CD24+, ALDH− and CD44−/CD24− cells were plated as single cells in ultra-low attachment six-well plates at a density of 1,000 or 2,500 viable cells/well in triplicate in MEBM. Three to four weeks later, the content of all wells was collected, pooled, and transferred onto a collagen-coated 6-well dish in differentiating medium (DMEM+10% FBS). Tumorspheres adhered in these conditions in ~24 h, after which they were stained with crystal violet and counted under low magnification.
Results
ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells expressed higher levels of phosphorylated STAT3 and greater tumorsphere-forming ability than ALDH− and CD44−/CD24− subpopulations
To determine whether STAT3 is activated in pancreatic stem-like cancer cells, we separated ALDH+, ALDH−, CD44+/CD24+ and CD44−/CD24− subpopulations from Panc-1, BxPC3, and HPAC pancreatic cancer cell lines by flow cytometry as previously described (Fig. 1A and B) (19). ALDH+, CD44+, and CD24+ expressing subpopulations of pancreatic cancer cells have been reported to exhibit cancer stem-like cell properties (3,4). To confirm the cancer stem-like cell properties of ALDH+ and CD44+/CD24+ subpopulations, we first compared the tumorsphere-forming ability of ALDH+ and CD44+/CD24+ subpopulations with ALDH− and CD44−/CD24− subpopulations. As shown in Fig. 1C and D, ALDH+ and CD44+/CD24+ cells of Panc-1, BxPC3, and HPAC all generated more tumorspheres than ALDH− and CD44−/CD24− cells. We thus demonstrated that ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells have an increased ability to form tumorspheres than ALDH− and CD44−/CD24− cells which suggests that these markers are exhibited by pancreatic stem-like cancer cells.
Figure 1.
Identification of ALDH+, ALDH−, CD44+/CD24+, and CD44−/CD24− subpopulations of Panc-1, BxPC3, and HPAC pancreatic cancer cells by the AldeFluor kit or PE-CD24 and PE/Cy5-CD44 antibody, and separation by flow cytometry (A and B). Tumorsphere generation in ALDH+ (C) and CD44+/CD24+ (D) subpopulations of pancreatic cancer cells. ALDH+ and CD44+/CD24+ subpopulations generated more tumorspheres than ALDH− and CD44−/CD24− cells (*P<0.05 vs. ALDH+ or CD44+/CD24+ cells, tumorspheres were counted 3–4 weeks after the plating).
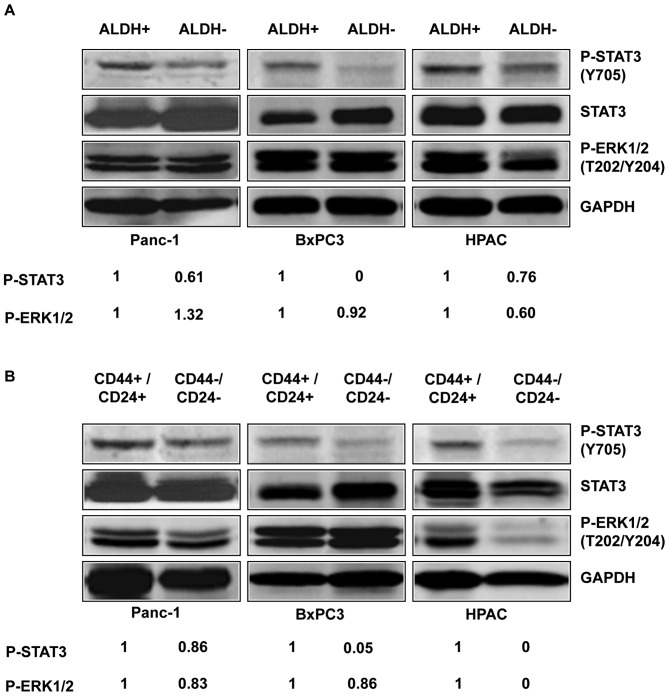
To determine the expression of activated P-STAT3 in pancreatic stem-like cancer cells, we separated the ALDH+ and CD44+/CD24+ subpopulations from the ALDH− and CD44−/CD24− subpopulations of three pancreatic cancer cell lines, Panc-1, BxPC3, and HPAC, and detected the level of P-STAT3 by western blot analysis. Our results showed that ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells expressed higher levels of P-STAT3 (Y705) compared to ALDH− (Fig. 2A) and CD44−/CD24− subpopulations (Fig. 2B). Phosphorylation of tyrosine residue 705 (Y705) is important for the activation of STAT3 (22). In contrast to the differences in STAT3 phosphorylation, the phosphorylation of P-ERK1/2 (T202/Y204) in the ALDH+ and ALDH− subpopulation were relatively similar in the three cell lines (Fig. 2A). The phosphorylation of P-ERK1/2 (T202/Y204) in the CD44+/CD24+ and CD44−/CD24− subpopulations were also similar in all three cell lines except HPAC (Fig. 2B). These results suggest that the STAT3 pathway plays an important role in pancreatic cancer stem-like cells and thus may serve as attractive pathway for targeting stem cell-like pancreatic cancer populations.
Figure 2.
P-STAT3 expression within ALDH+/− and CD44+/−/CD24+/− subpopulations of pancreatic cancer cells. ALDH+, CD44+/CD24+, ALDH−, and CD44−/CD24− subpopulations were separated from Panc-1, BxPC3, and HPAC pancreatic cancer cells by flow cytometry. STAT3 phosphorylation was determined by western blot analysis. (A) ALDH+ human pancreatic cancer stem-like cells express higher levels of P-STAT3 than ALDH− subpopulations. (B) CD44+/CD24+ human pancreatic cancer stem-like cells express higher levels of P-STAT3 than CD44−/CD24− subpopulations.
STAT3 inhibitors LLL12, FLLL32, and Stattic selectively inhibited STAT3 phosphorylation in ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells
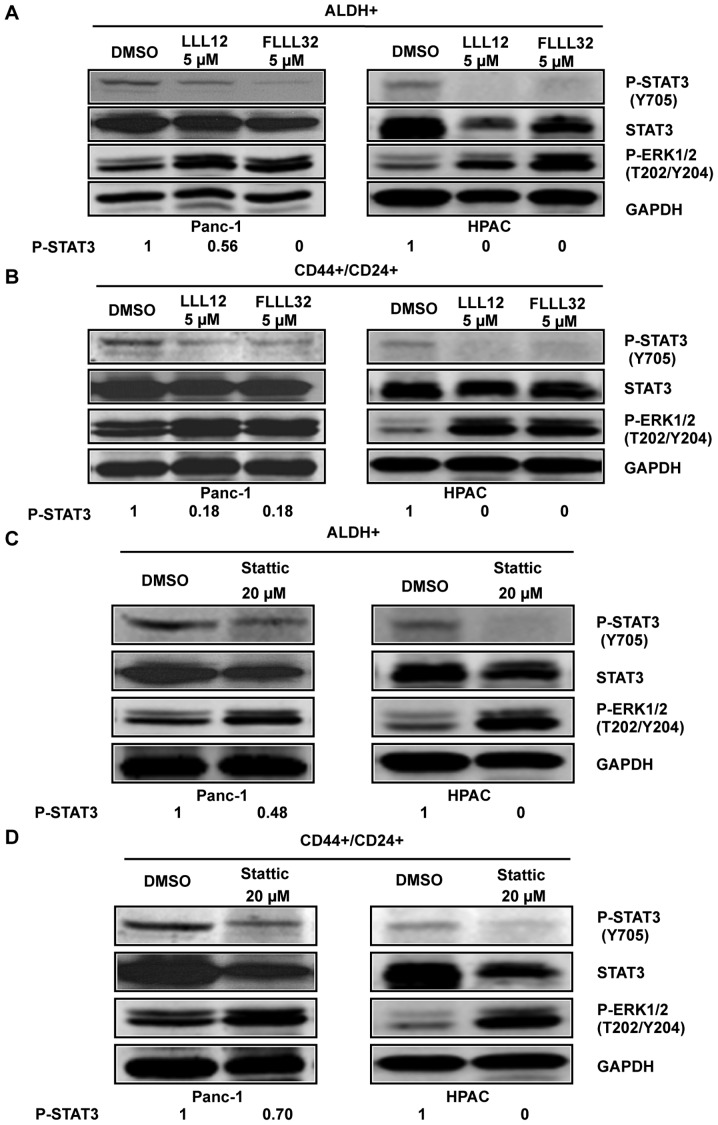
To confirm the importance of STAT3 in pancreatic cancer stem-like cells, STAT3 inhibitors, Stattic, LLL12, and FLLL32 were used to target STAT3 in these cells. To confirm the inhibition of phosphorylated STAT3 by Stattic, LLL12, and FLLL32 in pancreatic cancer stem-like cells, we examined STAT3 phosphorylation at tyrosine residue 705 (Y705) in ALDH+ and CD44+/CD24+ pancreatic cancer cells using phospho-STAT3 (tyrosine 705) antibody. Our results demonstrated that LLL12 and FLLL32 significantly inhibited STAT3 phosphorylation at tyrosine residue 705 (Y705) in Panc-1 and HPAC human pancreatic cancer cell lines in both ALDH+ (Fig. 3A) and CD44+/CD24+ subpopulations (Fig. 3B). Stattic also inhibited STAT3 phosphorylation (Y705) in ALDH+ (Fig. 3C) and CD44+/CD24+ (Fig. 3D) subpopulations of Panc-1 and HPAC pancreatic cancer cell lines at a higher concentration (20 μM). LLL12, FLLL32, and Stattic selectively inhibited P-STAT3 as demonstrated by the lack of inhibition of P-ERK1/2 in both ALDH+ and CD44+/CD24+ subpopulations of Panc-1 and HPAC (Fig. 3).
Figure 3.
STAT3 inhibitor-mediated inhibition of STAT3 phosphorylation in ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells. ALDH+ and CD44+/CD24+ subpopulations were separated from Panc-1 and HPAC pancreatic cancer cells by flow cytometry. ALDH+ and CD44+/CD24+ subpopulations of Panc-1 and HPAC were treated with LLL12, FLLL32, or Stattic for 24 h and STAT3 phosphorylation was determined by western blot analysis. STAT3 inhibitors, LLL12 and FLLL32, inhibited STAT3 phosphorylation in ALDH+ (A) and CD44+/CD24+ (B) pancreatic stem-like cancer cells. STAT3 inhibitor, Stattic, inhibited STAT3 phosphorylation in ALDH+ (C) and CD44+/CD24+ (D) pancreatic cancer stem-like cells.
STAT3 inhibitors LLL12, FLLL32, and Stattic inhibited STAT3 downstream targets in ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells
The inhibition of STAT3 by LLL12 and FLLL32 also downregulated the expression of many known STAT3-regulated genes in ALDH+ (Fig. 4A) and CD44+/CD24+ (Fig. 4B) pancreatic cancer stem-like cells related to cancer cell proliferation, survival, and angiogenesis, such as Cyclin D, Survivin, and Bcl-XL. Furthermore, LLL12 and FLLL32 inhibited Notch1 and Notch3 expression in ALDH+ (Fig. 4A) and CD44+/CD24+ cells (Fig. 4B), which have recently been reported as putative STAT3 downstream target genes (23,24). The Notch signaling pathway is known to be essential for normal stem cell self-renewal and differentiation in a variety of tissues, and is involved in the human cancer stem cell self-renewal capacity and tumorigenicity.
Figure 4.
Downregulation of STAT3-regulated genes via inhibition of STAT3 by LLL12 and FLLL32. ALDH+ and CD44+/CD24+ subpopulations were separated from Panc-1 and HPAC pancreatic cancer cells by flow cytometry. ALDH+ and CD44+/CD24+ subpopulations of Panc-1 and HPAC pancreatic cancer cells were treated with LLL12 (5 μM), FLLL32 (5 μM), or DMSO for 24 h. RNA was collected and gene expression was detected by RT-PCR.
STAT3 inhibitors, LLL12, FLLL32, and Stattic, inhibited cell viability of ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells
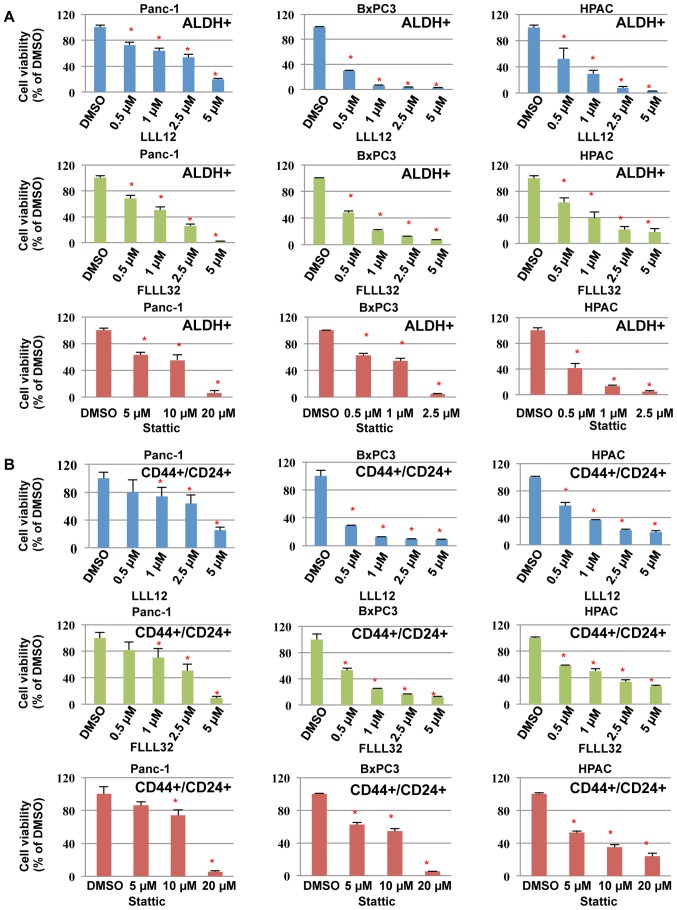
We next examined the inhibitory effects of LLL12, FLLL32, and Stattic on cell viability in pancreatic cancer stem-like cells. Our results demonstrated that LLL12, FLLL32, and Stattic could inhibit cell viability of the ALDH+ (Fig. 5A) and CD44+/CD24+ (Fig. 5B) subpopulations from Panc-1, BxPC3, and HPAC pancreatic cancer stem-like cells, further supporting the idea that these subpopulations of pancreatic cancer cells are sensitive to STAT3 inhibitors, LLL12, FLLL32, and Stattic. LLL12 and FLLL32 were more potent than Stattic in inhibiting cell viability of the ALDH+ and CD44+/CD24+ subpopulations from Panc-1, BxPC3, and HPAC (Fig. 5).
Figure 5.
STAT3 inhibitor-mediated inhibition of cell viability in ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells. Panc-1, BxPC3, and HPAC ALDH+ (A) and CD44+/CD24+ (B) subpopulations were seeded at 3,000 cells/well and exposed to the indicated concentrations of LLL12, FLLL32, or Stattic for 72 h. MTT dye was added for 4 h and the absorbance was read at 595 nm. The viability of DMSO treated cells was set at 100%.
STAT3 inhibitors, LLL12, FLLL32, and Stattic, inhibited tumorsphere forming capacity of ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells
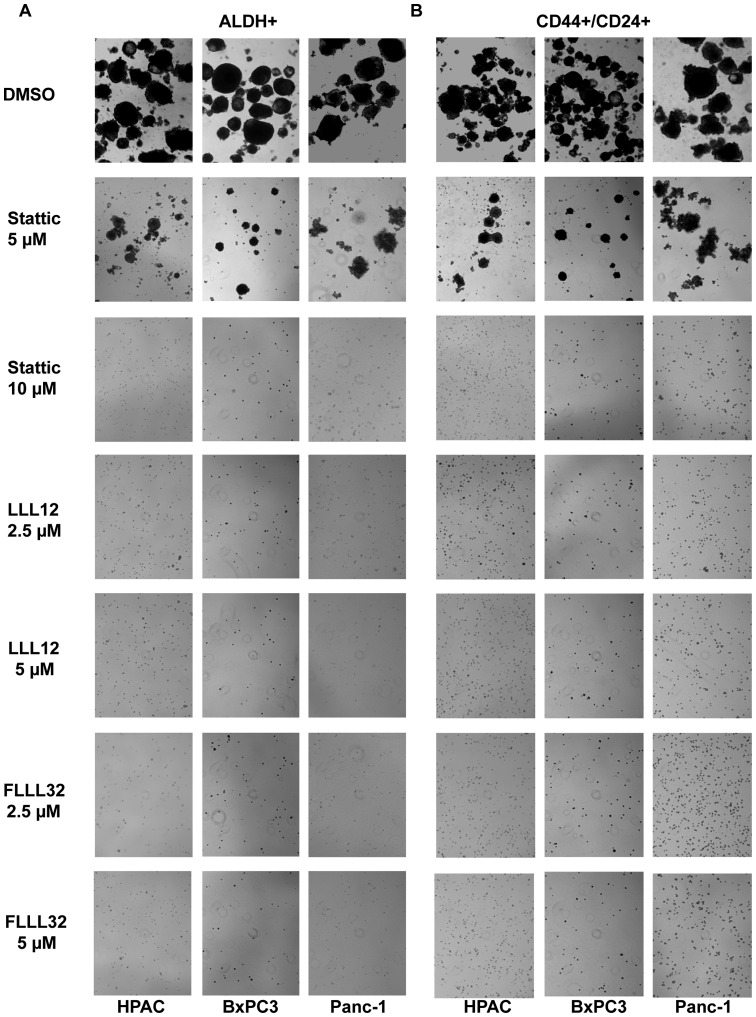
We also examined the efficacy of LLL12, FLLL32 and Stattic in inhibiting the survival and proliferation of pancreatic cancer stem-like cells in anchorage-independent conditions and ability to form tumor-spheres. Our results demonstrated that LLL12 and FLLL32 can significantly inhibit tumorsphere forming capacity in the ALDH+ and CD44+/CD24+ subpopulations of Panc-1, BxPC3, and HPAC pancreatic cell lines (Fig. 6A and B). As previously demonstrated, Stattic was not as potent in inhibiting tumorsphere forming capacity as LLL12 and FLLL32 in the ALDH+ and CD44+/CD24+ subpopulations (Fig. 6). Thus, we demonstrated that pancreatic cancer stem-like cells in the ALDH+ and CD44+/CD24+ cells expressed an activated form of STAT3 and are sensitive to inhibition by STAT3 inhibitors LLL12, FLLL32 and Stattic. Our results also show that STAT3 inhibition by LLL12, FLLL32, and Stattic decreases cell viability and tumorsphere forming ability, which indicates that STAT3 is an important pathway in pancreatic stem-like cancer cells and thus may serve as an attractive target for therapeutic drugs.
Figure 6.
LLL12, FLLL32 and Stattic-mediated inhibition of tumorsphere formation of CD44+/CD24+ (A) and ALDH+ (B) subpopulations of pancreatic cancer cells. ALDH+ and CD44+/CD24+ cells were plated as single cells in ultra-low attachment six-well plates at 25,000 cells/well). On the second day, the ALDH+ and CD44+/CD24+ cancer cells were treated with 2.5–10 μmol/l of LLL12, FLLL32, or Stattic. Tumorspheres were observed under a microscope 3–4 weeks later.
Discussion
Presently, the STAT3 pathway has been characterized in many cancers and the main effort to target constitutive STAT3 signaling is primarily on the bulk of cancer cells. No report has been published on the role of STAT3 in pancreatic stem-like cancer cells or on targeting the STAT3 pathway in these cells. Both CD44 and CD24, along with ALDH, have been used to isolate pancreatic cancer stem cells (3,4). We utilized pancreatic stem cell markers, ALDH, CD44, and CD24, to demonstrate that both ALDH+ and CD44+/CD24+ subpopulations express higher levels of P-STAT3 (Y705) than ALDH− and CD44−/CD24− subpopulations suggesting that the phosphorylation of STAT3 plays a role in their survival and proliferation. ALDH+ and CD44+/CD24+ subpopulations also generated more tumorspheres than ALDH− and CD44−/CD24− subpopulations of pancreatic cancer cells. Taken together, these data suggest that the STAT3 pathway may provide an attractive target for therapeutic treatment in pancreatic stem-like cancer cells.
To explore the inhibition of STAT3 in pancreatic cancer stem-like cells, we examined the inhibitory effects of three STAT3 inhibitors, LLL12, FLLL32, and Stattic, on two subpopulations of pancreatic stem-like cancer cells. All three molecules inhibited the expression of P-STAT3 in ALDH+ and CD44+/CD24+ subpopulations of pancreatic cancer cells. In addition, LLL12, FLLL32, and Stattic were also effective in inhibiting cell viability and tumorsphere formation in both ALDH+ and CD44+/CD24+ subpopulations of cells. Although Stattic did inhibit cell viability and tumorsphere formation in pancreatic stem-like cancer cells, it was not as potent as either LLL12 or FLLL32. This observation is consistent with the weaker predictive binding affinity of Stattic to STAT3 than either LLL12 or FLLL32.
In conclusion, this study demonstrates that STAT3 is activated in pancreatic cancer stem-like cells and that constitutively activated STAT3 in these cells enhances proliferation and survival. Our results show that STAT3 inhibition by small molecules could inhibit tumor stem-like cell growth, cell viability, and tumorsphere formation. Targeting STAT3 may provide an effective method for depleting stem cell-like pancreatic cancer cells and thus an effective treatment for pancreatic cancer. Our study also demonstrated that both LLL12 and FLLL32 significantly inhibited STAT3 in pancreatic cancer stem-like cells and thus are promising drug candidates for targeting constitutive STAT3 expression in these cells.
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (81001005, 81372402, 81570416), the Outstanding Young Investigator Foundation of Tongji Hospital (YXQN009) and the Fundamental Research Fund for the Central Universities, HUST: 0118540019 to Li Lin, and supported by funding from the AACR-Pancreatic Cancer Action Network to Jiayuh Lin.
Abbreviations
- STAT3
signal transducers and activators of transcription 3
- ALDH
aldehyde dehydrogenase
- CD44
cluster of differentiation 44
- MEBM
mammary epithelial basal medium
- DEAB
diethylaminobenzaldehyde
References
- 1.Society AC. Cancer Factor & Figures. American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 4.Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y, Wang CY. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat Dis Int. 2011;10:428–434. doi: 10.1016/S1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 5.Calò V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: From normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 6.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 7.Turkson J, Jove R. STAT proteins: Novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Z, Wen Z, Darnell JE., Jr Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 9.Sasse J, Hemmann U, Schwartz C, Schniertshauer U, Heesel B, Landgraf C, Schneider-Mergener J, Heinrich PC, Horn F. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol. 1997;17:4677–4686. doi: 10.1128/MCB.17.8.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Nagayasu T, Sekine I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833–838. doi: 10.1136/jcp.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;10:1569–1573. doi: 10.3748/wjg.v10.i11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, Freeman JW. Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor beta-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li H, Jiang T, Huang K, Cao J. RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci. 2007;98:1099–1106. doi: 10.1111/j.1349-7006.2007.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:176–193. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L, Hutzen B, Li PK, Ball S, Zuo M, DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, et al. A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells. Neoplasia. 2010;12:39–50. doi: 10.1593/neo.91196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Hutzen B, Zuo M, Ball S, Deangelis S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010;70:2445–2454. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, Lin J. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res. 2011;71:7226–7237. doi: 10.1158/0008-5472.CAN-10-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y, Noguchi S. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 22.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem. 1996;271:5961–5964. doi: 10.1074/jbc.271.11.5961. [DOI] [PubMed] [Google Scholar]
- 23.Grivennikov S, Karin M. Autocrine IL-6 signaling: A key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian X, Li J, Ma ZM, Zhao C, Wan DF, Wen YM. Role of hepatitis B surface antigen in the development of hepatocellular carcinoma: Regulation of lymphoid enhancer-binding factor 1. J Exp Clin Cancer Res. 2009;28:58. doi: 10.1186/1756-9966-28-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paydas S, Tanriverdi K, Yavuz S, Disel U, Sahin B, Burgut R. Survivin and aven: Two distinct antiapoptotic signals in acute leukemias. Ann Oncol. 2003;14:1045–1050. doi: 10.1093/annonc/mdg277. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Inokuchi K, Tarusawa M, Dan K. Mutation of bcl-x gene in non-Hodgkin's lymphoma. Am J Hematol. 2002;69:74–76. doi: 10.1002/ajh.10030. [DOI] [PubMed] [Google Scholar]