Fluid therapy is an integral and life-saving part of perioperative care. Based on strong experimental evidence, we know that hypovolemia results in insufficient oxygen delivery and flow-dependent-organ dysfunction whereas hypervolemia leads to interstitial edema with impaired oxygen diffusion and poor collagen regeneration.
Clinical studies including various types of surgical procedures have clearly shown an association between high perioperative fluids infusion and poor clinical outcome as expressed by composite mortality-morbidity endpoints (1–3). Overzealous fluid infusion promotes wound infections/dehiscence, anastomotic leakage and cardiac overload. Besides these quantitative aspects, fluid composition also deserves consideration since saline infusion exceeding 1.5–2L may cause hyperchloremic acidosis and colloids (e.g., hydroxyethyl starch) have been incriminated in renal damage when given in septic, trauma or other critically-ill patients (4–6).
We will focus in the first round more on “Fluid management in thoracic surgery”, because it can be considered as “patognomonic” for general fluid management.
In thoracic surgery, intraoperative fluid infusion exceeding 6–8 mL/kg/hour has been identified as a risk factor for lung injuries (acute lung injury [ALI], acute respiratory distress syndrome [ARDS]) but also other pulmonary complications (atelectasis, pneumonia, empyema) (7–11). From these cohort studies analyses, the “chicken and egg” question remains open: sicker patients and more complex operation may require more fluids whereas volume depletion may be masked by the administration of vasopressors.
With the recent advances in hemodynamic monitoring devices, the use of cardiovascular measurements such as stroke volume, stroke volume variations or pulse pressure variations have been encouraged along with the application of algorithms aiming to maximize cardiac output and oxygen delivery. Although a positive fluid balance with undesirable weight gain may result from this goal-directed therapy (GDT), recent meta-analysis of randomized controlled trials (RCTs) involving nonthoracic surgery have demonstrated a modest reduction in postoperative morbidity in the GDT groups compared with standard care (12–15). However, a Hawthorne effect could contribute to generate favorable clinical outcome as the clinicians acted as “positive (unblinded) interventionists” in the GDT group whereas the “standard care” management was most often poorly described, highly variable and likely suboptimal. More importantly, studies comparing GDT to a restrictive fluid approach have failed to demonstrate significant differences in terms of clinical outcome between the two treatment arms (16). Moreover, the restrictive regime that often results in relative perioperative oliguria was not associated with an increased risk of postoperative acute renal failure (17, 18).
More than in any other operation, reducing the amount of fluids and minimizing the hydrostatic pressure in the pulmonary capillaries is of paramount importance in thoracic surgery. The lungs of these surgical patients are prone to develop interstitial and alveolar edema given preexisting chronic illnesses, recent pneumonia or atelectasis in addition to the deleterious effects of one-lung ventilation and direct manipulations by the surgeon. Acute lung injuries following thoracic surgery may result from sequential multiple hits. Indeed, surgical trauma, ischemia-reperfusion phenomena, exposure to blood products and microbes, hyperglycemia, rapid fluid infusion as well as baro-and volotrauma associated with mechanical ventilation may all damage the glycocalix and the underlying endothelial cells as well as the epithelial alveolar cells and the surfactant (19, 20). Moreover, disruption of the lymphatic vessels by preoperative chemo-radiotherapy or surgical dissection may prevent proper fluid drainage and therefore worsen lung edema with its dramatic consequences on gas exchange.
Despite the clinical importance of fluids in thoracic surgery, no RCT has been designed so far to compare the effectiveness and safety of the restrictive and the GDT approaches. The current large variations in perioperative fluid practices among providers may contribute to significant waste and suboptimal health care outcomes. At the John Hopkins Hospitals, over a 4 year period, the median crystalloid volume that was infused during lung surgery was around 11.3 mL/kg/h and large variations were reported between anesthesiologists within the same department as expressed by a 55% coefficient of variation (ratio of standard deviation-to-mean volume infused) (21). Although the vast majority of anesthesiologists in this high ranking academic institution were well aware of the risks related to fluid overload, they did not apply a restrictive fluid strategy, or failed to do so.
In mechanically ventilated patients presenting with ALI/ARDS, the conservative or restrictive fluids management has clearly demonstrated benefits in terms of earlier weaning from the ventilator and better oxygenation compared with the liberal fluid regime (22). Although scientific evidence based on RCTs is currently lacking in thoracic surgery, physiological arguments strongly supports the conservative fluid approach that has been coined the “zero-balance” approach (23). This restrictive fluid strategy is intended to minimize the postoperative weight gain that results from the dual effects of the exogenous fluid administration and the retention of salt and water in response to surgical stress-induced release of anti- diuretic hormone and the activation of both the hypothalamo-sympatho-adrenal axis and the renin-angiotensin-aldosterone system. Successful application of the “zero balance” regime often requires the concomitant administration of vasopressors and a limited amount of fluids to counteract the vasodilatory effects of anesthetic agents and/thoracic epidural blockade while maintaining intravascular normovolemia and stable hemodynamic variables.
Keeping the lung dry and the circulatory compartment close to normovolemia remains a wise statement that has been claimed for more than two decades (24, 25). In the toolbox of cardiorespiratory and metabolic monitors connected to our thoracic patient, which indicators might be helpful to cautiously guide the administration of fluids and cardiovascular drugs without dropping on the bad hypo- or hypervolemic sides? We suggest a simple pragmatic approach to achieve these goals throughout several perioperative processes of care (26, 27). Firstly, patient should be encouraged to take carbohydrate drinks up to two hours before surgery to promote a “fed” euvolaemic state (28). Secondly, as standard intraoperative fluids infusion, buffer/balanced crystalloids should be limited to 3 to 4 mL/kg/h in order to replace fluid losses by perspiration (airways) and evaporation (surgical field) as well as urine output and digestive secretions. Additional crystalloids (or colloids) can be a given to compensate blood losses and/or exudative liquids (29). Replacement of the “third space” is not anymore justified as it merely results from the excessive administration of “salty solutions”. Thirdly, the administration of vasopressors is helpful to counteract the anesthetic-induced vasorelaxation and to convert the relative hypovolemia into normovolemia. In the early postoperative period, attention should also be paid to fluid balance and the patient’body weight. Oral hydration and realimentation can be resumed within the first 12 hours after surgery and coupled with the removal of IV lines, urinary catheter and chest tubes to facilitate mobilization.
In conclusion, the thoracic surgical patient should receive an individualised fluid management plan that takes into account his co-morbidities and the operative complexity. A zero-balance fluid approach can be applied with balanced salt crystalloid and be part of the enhanced recovery program (30). Since heart rate, arterial pressure and central venous pressure are unreliable indicators of volume status, the clinicians should best rely on close observation of ongoing fluid losses (in/out fluid balance) and maintenance of vital signs. Monitoring of cardiac output (by pulse contour analysis or Doppler ultrasound), extravascular lung water (transpulmonary thermodilution), cerebral oxygenation (by near-infra-red spectroscopy) and/or central venous oximetry are valuable adjuncts in high-risk patients and complex procedures (31, 32).
To make a long story short, clinicians caring for thoracic surgical patients should remember: “keep the lungs clean, sealed, fully expanded and dry to make the patient fit for rapid and happy discharge!”
“Fluid management” (FM) is a complicated, unsolved riddle. I think we should first find the “good questions”; or rather, we have to formulate the questions in an appropriate way. Otherwise, it may the case that you compare “liberal” (?) vs ”restrictive” (?) strategies; and find at the end that you have given even more fluid in the restrictive group because of the “rescue”. Not to mention is that what you call “liberal” in some studies is defined in some other studies as “restrictive”.
Now we have a third way: “optimised FM”. It sounds and seems to be rational, but are there also some limitations or drawbacks of this approach? Or rather: “Can optimal FM be really optimal?”.
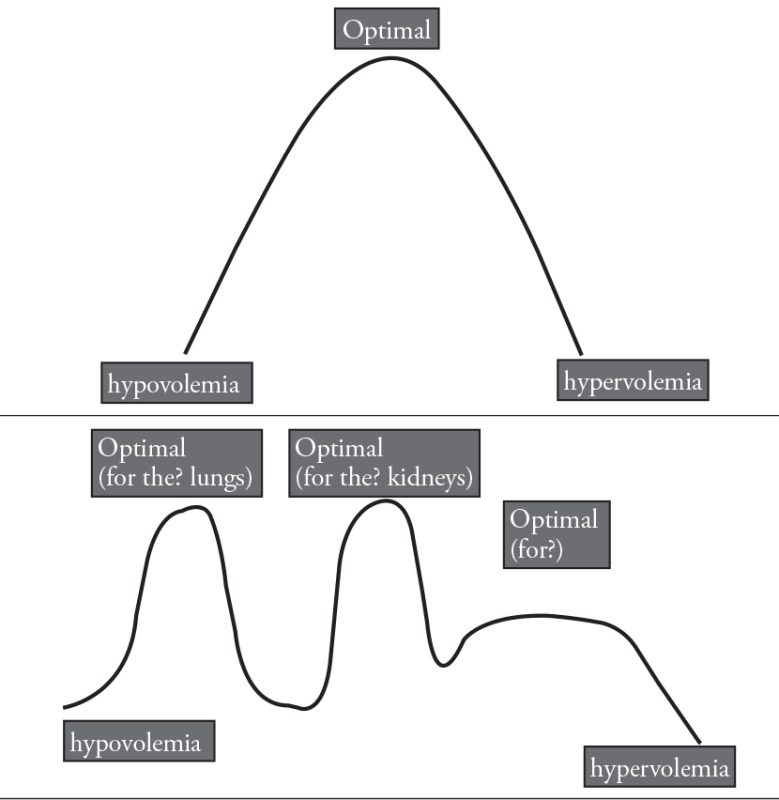
Let me expand my question with further questions: Some authors advocate that being restrictive is optimal to avoid edema, whereas others defend that FM should not cost AKI (acute kidney injury). Is “optimal FM” indeed a peak between “hypovolemia” and “hypervolemia” (curve A); or is the FM curve maybe an irregular, fluctuant curve with several peaks, maybe “u”-shaped instead of “v”? (curve B).
In those terms, I want you to remind the keyword “glycocalyx”. With increasing information about glycocalyx, we recognize that we have to revise all the knowledge we have, such as Frank-Starling Curve.
My last question is a stupid one: what is actually the “3rd space”? Does it really exist somewhere in the body?
Mert Şentürk
Editor
References
- 1.Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, et al. Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care. 2014;29:936–41. doi: 10.1016/j.jcrc.2014.06.023. http://dx.doi.org/10.1016/j.jcrc.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth. 2012;109:69–79. doi: 10.1093/bja/aes171. http://dx.doi.org/10.1093/bja/aes171. [DOI] [PubMed] [Google Scholar]
- 3.Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting-a clinical review. J Intensive Care. 2016;4:27. doi: 10.1186/s40560-016-0154-3. http://dx.doi.org/10.1186/s40560-016-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–21. doi: 10.1213/ANE.0b013e318293d81e. http://dx.doi.org/10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 5.Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;15:346. doi: 10.1136/bmj.f839. http://dx.doi.org/10.1136/bmj.f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–88. doi: 10.1001/jama.2013.430. http://dx.doi.org/10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 7.Zeldin RA, Normandin D, Landtwing D, Peters RM. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg. 1984;87:359–65. [PubMed] [Google Scholar]
- 8.Alam N, Park BJ, Wilton A, Seshan VE, Bains MS, Downey RJ, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg. 2007;84:1085–91. doi: 10.1016/j.athoracsur.2007.05.053. http://dx.doi.org/10.1016/j.athoracsur.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8. doi: 10.1097/01.sla.0000094387.50865.23. http://dx.doi.org/10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–65. doi: 10.1213/01.ANE.0000087799.85495.8A. http://dx.doi.org/10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 11.Arslantas MK, Kara HV, Tuncer BB, Yildizeli B, Yuksel M, Bostanci K, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg. 2015;149:314–20. doi: 10.1016/j.jtcvs.2014.08.071. http://dx.doi.org/10.1016/j.jtcvs.2014.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Ripollés J, Espinosa A, Martínez-Hurtado E, Abad-Gurumeta A, Casans-Francés R, Fernández-Pérez C, et al. Intraoperative goal directed hemodynamic therapy in noncardiac surgery: a systematic review and meta-analysis. Braz J Anesthesiol. 2016;66:513–28. doi: 10.1016/j.bjane.2015.02.001. http://dx.doi.org/10.1016/j.bjan.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90. doi: 10.1001/jama.2014.5305. http://dx.doi.org/10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 14.Rollins KE, Lobo DN. Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg. 2016;263:465–76. doi: 10.1097/SLA.0000000000001366. http://dx.doi.org/10.1097/SLA.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care. 2014;18:584. doi: 10.1186/s13054-014-0584-z. http://dx.doi.org/10.1186/s13054-014-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69:488–98. doi: 10.1017/S0029665110001734. http://dx.doi.org/10.1017/S0029665110001734. [DOI] [PubMed] [Google Scholar]
- 17.Egal M, de Geus HR, van Bommel J, Groeneveld AB. Targeting oliguria reversal in perioperative restrictive fluid management does not influence the occurrence of renal dysfunction: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;34:1–12. doi: 10.1097/EJA.0000000000000416. http://dx.doi.org/10.1097/eja.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 18.Matot I, Dery E, Bulgov Y, Cohen B, Paz J, Nesher N. Fluid management during video-assisted thoracoscopic surgery for lung resection: a randomized, controlled trial of effects on urinary output and postoperative renal function. J Thorac Cardiovasc Surg. 2013;146:461–6. doi: 10.1016/j.jtcvs.2013.02.015. http://dx.doi.org/10.1016/j.jtcvs.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35:331–7. doi: 10.1183/09031936.00098709. http://dx.doi.org/10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res. 2010;87:320–30. doi: 10.1093/cvr/cvq146. http://dx.doi.org/10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Gani F, Spolverato G, Ejaz A, Xu L, Buettner S, et al. Variation in crystalloid administration: an analysis of 6248 patients undergoing major elective surgery. J Surg Res. 2016;203:368–77. doi: 10.1016/j.jss.2016.02.045. http://dx.doi.org/10.1016/j.jss.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network1. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. http://dx.doi.org/10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 23.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40. doi: 10.1097/ALN.0b013e3181863117. http://dx.doi.org/10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 24.Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol. 2013;26:31–9. doi: 10.1097/ACO.0b013e32835c5cf5. http://dx.doi.org/10.1097/ACO.0b013e32835c5cf5. [DOI] [PubMed] [Google Scholar]
- 25.Evans RG, Naidu B. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury? Interact Cardiovasc Thorac Surg. 2012;15:498–504. doi: 10.1093/icvts/ivs175. http://dx.doi.org/10.1093/icvts/ivs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro LH, Bloomstone JA, Auler JO, Jr, Cannesson M, Rocca GD, Gan TJ, et al. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioper Med (Lond) 2015;4:3. doi: 10.1186/s13741-015-0014-z. http://dx.doi.org/10.1186/s13741-015-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iijima T, Brandstrup B, Rodhe P, Andrijauskas A, Svensen CH. The maintenance and monitoring of perioperative blood volume. Perioper Med (Lond) 2013;2:9. doi: 10.1186/2047-0525-2-9. http://dx.doi.org/10.1186/2047-0525-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32:34–44. doi: 10.1016/j.clnu.2012.10.011. http://dx.doi.org/10.1016/j.clnu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–9. doi: 10.1093/bja/aet307. http://dx.doi.org/10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Gan TJ. Peri-operative fluid management to enhance recovery. Anaesthesia. 2016;71(Suppl 1):40–5. doi: 10.1111/anae.13309. http://dx.doi.org/10.1111/anae.13309. [DOI] [PubMed] [Google Scholar]
- 31.Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014;112:617–20. doi: 10.1093/bja/aet590. http://dx.doi.org/10.1093/bja/aet590. [DOI] [PubMed] [Google Scholar]
- 32.Downs EA, Isbell JM. Impact of hemodynamic monitoring on clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28:463–76. doi: 10.1016/j.bpa.2014.09.009. http://dx.doi.org/10.1016/j.bpa.2014.09.009. [DOI] [PubMed] [Google Scholar]


