Abstract
Purpose
We evaluated efficacy and tolerability of the combination of ofatumumab and lenalidomide in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), and explored whether immune system characteristics could influence the response to treatment.
Experimental Design
Thirty-four patients were enrolled in this phase II study. Ofatumumab was administered at a dose of 300 mg on day 1, 1,000 mg on days 8, 15, and 22 during course 1, 1,000 mg on day 1 during courses 3–6, and once every other course during courses 7–24 (28-day courses). Oral lenalidomide (10 mg daily) was started on day 9 and continued for as long as a clinical benefit was observed.
Results
The overall response rate was 71%. Eight patients (24%) achieved a complete remission (CR) or CR with incomplete recovery of blood counts, including 9% with minimal residual disease-negative CR. The median progression-free survival was 16 months, and the estimated 5-year survival was 53%. The most common treatment-related toxicity was neutropenia (grade >2 in 18% of the 574 patient courses). The most frequent infectious complications were pneumonia and neutropenic fever (24% and 9% of patients, respectively). We observed that patients who achieved a CR had at baseline higher numbers and a better preserved function of T cells and natural killer cells compared with non-responders.
Conclusions
The combination of ofatumumab and lenalidomide is a well-tolerated regimen that induces durable responses in the majority of patients with relapsed/refractory CLL. Our correlative data suggest a role of competent immune system in supporting the efficacy of this treatment.
Introduction
Chronic lymphocytic leukemia (CLL) is accompanied by a complex immune dysregulation, characterized by phenotypical and functional alterations of different host immune components. An increased number of circulating T cells in patients with CLL was observed for the first time more than 40 years ago (1), but the phenotypical abnormalities and functional defects are still under investigation (2–4). Regulatory T cells (Treg) are increased in patients with CLL (5). Natural killer (NK) cells are also increased and display a defective cytotoxic activity (6, 7).
Lenalidomide is an immunomodulatory agent that exerts both direct antitumor effect and a pleiotropic activity on the immune system, normalizing CD3+ T cell and Treg numbers in vivo (8, 9), promoting antigen-specific T cell activation, enhancing NK cell activity, and monocyte antibody-dependent cell cytotoxicity (ADCC; ref. 10), and restoring immunologic synapse formation (2).
Single-agent lenalidomide is active in patients with relapsed or refractory CLL (11, 12). In vitro, lenalidomide shows synergism with the anti-CD20 monoclonal antibody (mAb) rituximab by enhancing its CLL cell killing via NK cell- and monocyte-mediated ADCC (10). Our group evaluated combined rituximab and lenalidomide therapy in a phase II trial for patients with recurrent CLL, showing improvements in the quality and duration of responses when compared with lenalidomide monotherapy. Moreover, the addition of rituximab was accompanied by a decrease in the incidence and severity of lenalidomide-associated tumor flare reaction (TFR) and tumor lysis syndrome (TLS; ref. 13).
Ofatumumab is a type I fully human mAb that binds to a distinct epitope on CD20 (14), and has clinical activity in patients with refractory CLL (15, 16).
In the current study, we determined the activity and tolerability of combined ofatumumab and lenalidomide for the treatment of relapsed or refractory CLL. We also conducted correlative biologic studies to investigate whether immune system and tumor micro-environment characteristics, such as circulating T- and NK cell number and function, and plasma levels of chemokines, cytokines, and angiogenic factors, could be correlated with the response to treatment or could be modulated during the course of the therapy.
Materials and Methods
Patients
Patients with relapsed or refractory CLL were enrolled in a phase II study of combined ofatumumab and lenalidomide. The eligibility criteria included prior exposure to purine analogue-based therapy. Refractory disease was defined as no response to or progression of disease within 6 months from the most recent antileukemia therapy (17, 18). A complete list of inclusion and exclusion criteria is presented in Supplementary Table S1. The study protocol was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board and registered at clinicaltrials.gov (NCT01002755). Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki.
Treatment
Ofatumumab was administered intravenously at a dose of 300 mg on day 1, 1,000 mg on days 8, 15, and 22 during course 1, 1,000 mg on day 1 during courses 3–6, and on day 1 once every other course during courses 7–24. The patients self-administered lenalidomide 10 mg daily, orally, starting on day 9 of course 1. Each treatment course lasted 28 days. The planned treatment duration was 24 courses. However, patients who experienced a sustained partial response (PR) or complete response (CR) were allowed to continue treatment with lenalidomide monotherapy indefinitely. Patients were discontinued from the study if progression of disease or unacceptable toxicity were documented at any time. Treatment schema is depicted in Supplementary Fig. S1. TLS prophylaxis with allopurinol 300 mg daily was prescribed during the first 14 days of therapy. The use of granulocyte colony-stimulating factor (G-CSF) was allowed according to the American Society of Clinical Oncology guidelines (19). No anti-infectious, venous thromboembolism (VTE), or TFR prophylaxis was mandated on the basis of our previous experience with lenalidomide in the treatment of CLL.
Response and toxicity evaluation
Response was evaluated after courses 3 and 6, then every 6 courses thereafter. The clinical response to therapy was defined as the best response sustained for ≥2 courses at any time during treatment, and assessed according to the 2008 International Workshop on CLL (IWCLL) criteria (17). Four-color flow cytometry was also performed on bone marrow aspirates to assess minimal residual disease (MRD). CT scans were not mandated for response assessment and were used when clinically indicated according to the treating physician. Patients deemed candidate for stem cell transplant were transitioned to this procedure according to the principle of patient's best interest. Toxicity related to the study drugs was assessed using Common Terminology Criteria for Adverse Events (version 3.0). Treatment dose reduction plans for lenalidomide are summarized in Supplementary Table S2.
T and NK cell subset distribution
The absolute numbers of total (CD3+) T cells, helper (CD4+) T cells, cytotoxic (CD8+) T cells, Treg, CD16+CD56+ NK cells, and CD57+CD56+ NK cells were measured at baseline (n = 30) and at the beginning of courses 3 (n = 27), 6 (n = 21), 9 (n = 15), and 12 (n = 13), as described previously (8). Peripheral blood was incubated with fluorescein-conjugated mouse anti-human mAb to detect T-cell subsets (anti-CD3, anti-CD4, and anti-CD8), and NK cell subsets (anti-CD16, anti-CD56, and anti-CD57). Tregs were enumerated in peripheral blood mononuclear cells (PBMC) by using the Forkhead box protein P3 (FoxP3) staining kit (BD Pharmingen), according to the manufacturer's instructions. All reagents were purchased from BD Biosciences. Thirty-four age-matched healthy donors (HD) were used to establish normal ranges.
T cell functional assay
T cell cytotoxicity and cytokine production was assessed on 15 patients at baseline and at the beginning of courses 3 and 6, and on 3 HD. We preincubated PBMCs alone (negative control) or with CD3/CD28 magnetic beads (Invitrogen) for 6 hours at 37°C, in the presence of anti-CD107a-PECF594 mAb (clone H4A3, BD Biosciences) and Brefeldin A (Sigma-Aldrich). Cells were then stained with Live/Dead Aqua Viability Marker (Life Technologies), and anti-CD3-BV650, anti-CD8-FITC, and anti-CD4-APC-Cy7 mAbs (BD Biosciences). After surface staining, cells were lysed, fixed, and permeabilized (BD FACS solutions, BD Biosciences). Cytokine production was detected by intracellular staining with anti-IFNg-v450, anti-TNFα–Alexa 700, and anti-IL2-PE mAbs (all from BD Biosciences). Flow cytometry data were acquired on BD LSRFortessa (BD Biosciences) and analyzed with FlowJo software (Treestar).
NK cell functional assay
NK cell cytotoxicity and cytokine production was assessed on 15 patients at baseline and at the beginning of courses 3 and 6, and on 3 HDs. PBMCs were preincubated alone (negative control) or with K562 target (E:T ratio of 10:1) for 5 hours at 37°C, in the presence of anti-CD107a-PECF594 mAb, GolgiStop/monensin (BD Biosciences) and Brefeldin A. Cells were then stained with Live/Dead Aqua Viability Marker, anti-CD3-APC-Cy7, and anti-CD56-BV605 mAbs (both from Biolegend). After surface staining cells were lysed, fixed, and permeabilized. Cytokine production was detected by intracellular staining with anti-IFNγ-v450 and anti-TNFα- Alexa 700 mAb. Flow cytometry data were acquired on BD LSRFortessa and analyzed on FlowJo software.
Angiogenic factors and plasma cytokines quantification
Plasma levels of basic fibroblast growth factor (bFGF), VEGF, Thrombospondin-1, and IFNγ were measured at baseline (n = 32), and at the beginning of course 3 (n = 30), 6 (n = 23), 9 (n = 17), and 12 (n = 13). Chemokine (C-C motif) ligand 2, 3, and 4 (CCL2, CCL3, and CCL4), TNFα, IFNα2, and IL-12p70 plasma levels were evaluated in 22 patients at baseline and at the beginning of course 3. Samples were analyzed with multiplexed bead suspension arrays (MBAs; Millipore) according to the manufacturer's instructions and as described previously (12). MBAs were analyzed using a Luminex 200 machine and data were organized and analyzed using 3.1 xPONENT software (Luminex).
Study end points and statistical analysis
The primary endpoint of this study was the overall response rate (ORR). Differences in the response rates according to pretreatment characteristics were evaluated using a two-tailed Fisher exact test. The study's secondary objective was tolerance. Stopping rule for toxicity defined excessive toxicity as grade >2 nonhematologic adverse events observed in ≥50% of patients. Additional study objectives included the progression-free survival (PFS) duration, which was defined as the time from the start of therapy to death, disease progression, or initiation of the next therapy, and the overall survival (OS) duration, defined as time from the start of therapy to death from any cause. The PFS and OS durations were calculated using Kaplan–Meier estimates, and different groups of patients were compared using the log-rank test.
The modulation of T and NK cell subset distribution and function, angiogenic factors, and circulating cytokine/chemokine concentration between different time points was evaluated with paired t test, for values following Gaussian distribution, or with Wilcoxon matched-pairs signed rank test, for values not following Gaussian distribution. The differences in these same parameters between CR, PR, and non-responder (NR) patients and HD were compared for each specific time point with t test, for values following Gaussian distribution, whereas Mann–Whitney test was used for values not following Gaussian distribution. Results were considered statistically significant when P < 0.05. Statistical analyses were performed using SPSS software (version 19.0; SPSS) and GraphPad Prism (version 6.05 for Windows, GraphPad Software).
Results
Patients
Between January 2010 and January 2011, 36 patients were enrolled in this study. Two patients were not evaluable: one had concomitant myelodysplastic syndrome and one withdrew consent before the start of treatment. The remaining 34 patients were included in the analyses. Patients' pretreatment characteristics are summarized in Table 1.
Table 1. Baseline patients' characteristics.
| Characteristic | n = 34 |
|---|---|
| Age, years, median (range) | 64 (34–82) |
| Age ≥65 years, n (%) | 17 (50%) |
| Male/female | 26/8 |
| Hemoglobin, g/dL, median (range) | 12.5 (9.1–15.7) |
| Platelets, × 109/L, median (range) | 97 (41–273) |
| White blood cells, × 109/L, median (range) | 23.7 (2.5–125.8) |
| Lymphocytes, × 109/L, median (range) | 19.7 (0.7–119.5) |
| Neutrophils, × 109/L, median (range) | 2.5 (0.3–21.6) |
| β2-microglobulin, mg/L, median (range) | 4.1 (1.7–16.5) |
| β2-microglobulin ≥4 mg/dL, n. (%) | 20 (59%) |
| Rai stage, n (%) | |
| 0–II | 14 (41%) |
| III–IV | 20 (59%) |
| CD38+, n (%) | 19 (56%) |
| CD38% expression, median (range) | 32.8 (0.8–99.8) |
| ZAP70+, n (%)a | 21 (78%) |
| IGHV unmutated, n (%)b | 21 (70) |
| FISH abnormalities, n (%)c | |
| Deletion 13q | 7 (25%) |
| Negative | 5 (18%) |
| Trisomy 12 | 3 (11%) |
| Deletion 11q | 4 (14%) |
| Deletion 17p | 9 (32%) |
| ECOG Performance status | |
| 0 | 17 (50%) |
| 1 | 17 (50%) |
| Number of prior treatments, median (range) | 2 (1–8) |
| >3 prior treatments, n (%) | 8 (24%) |
| Prior stem cell transplant, n (%) | 3 (9%) |
| Prior FCR, n (%) | 33 (97%) |
| Fludarabine-refractory, n (%) | 13 (38%) |
| Refractory to previous therapy, n (%) | 10 (29%) |
| Bulky disease, n (%) | 3 (9%) |
Abbreviation: FCR, fludarabine-cyclophosphamide-rituximab.
Data available in 27 patients.
Data available in 30 patients.
Grouped according to Dohner hierarchical classification, data available in 28 patients.
A median of ten courses per patient (range 2–65) was administered. All patients have discontinued treatment at this time. Ten patients (29%) completed the planned 24 courses of combination therapy,the longest treatment duration being 59 months. Reasons for treatment discontinuation included disease progression (18 patients, 53%), adverse events [diarrhea, three patients (9%); infections, four patients (12%); other adverse events, four patients (12%)], stem cell transplant (three patients, 9%), and patient request (two patients, 3%).
Efficacy
Responses
Eight patients (24%) achieved CR (including 2 patients with incomplete recovery of blood counts, CRi) and 16 achieved PR (47%), for an ORR of 71%. Eight patients (24%) had stable disease (SD) and 2 (6%) experienced progression on treatment. The trend of response rates over time is depicted in Supplementary Fig. S2. Among the 24 responders, median time to PR was 3 months (range 3–12 months), and median time to CR was 4.5 months (range 3–18 months). Three patients achieved MRD-negative CR. Among these patients, a clinical CR was obtained after 3, 3 and 18 months, and the time to MRD negativity was 12, 18, and 24 months, respectively. None of these patients were fludarabine-refractory or had deletion of chromosome 17p (del17p).
Supplementary Table S3 shows different rates of response according to pretreatment characteristics. A β2-microglobulin level ≥4 mg/dL, more than three lines of prior treatment and fludarabine-refractoriness were associated with a lower CR rate (CRR). Fludarabine-refractoriness was also associated with a lower ORR. Patients with del17p showed a trend toward a lower CRR, but this difference was only of borderline significance, possibly because of the small number of patients. Finally, patients receiving an average daily lenalidomide dose of ≥5 mg showed a trend toward superior response rates, but this was not statistically significant (ORR 80% vs. 61%).
PFS
The estimated median PFS duration was 16 months [95% confidence interval (CI), 6–26 months] for the entire study population, and 25 months (95% CI, 16–34 months) for the 24 responders (Fig. 1A). Patients with del17p had significantly shorter PFS durations than those with other cytogenetic abnormalities (5 vs. 27 months, P = 0.001; Fig. 1B). Similarly, the estimated median PFS duration was shorter for patients with fludarabine-refractory disease (5 vs. 28 months, P < 0.0001) and patients who had not responded to their last treatment before entering the study (5 vs. 22 months, P = 0.003; Fig. 1C and D). Interestingly, responses to ofatumumab and lenalidomide were more durable than those to prior therapies in 15 patients (44%). Moreover, 4 of the 10 patients who were refractory to their last treatment experienced long-lasting responses (15, 16, 19, and 27 months) to this combination (Fig. 1E). Of the three patients who achieved MRD-negative CR, two discontinued therapy while in remission because of toxicity after 16 and 22 months, and further maintained the remission for additional 13 and 14 months, respectively. The third patient discontinued lenalidomide after 45 months of treatment due to the wish of conceiving a child, and was concomitantly found to have slowly progressive disease.
Figure 1.
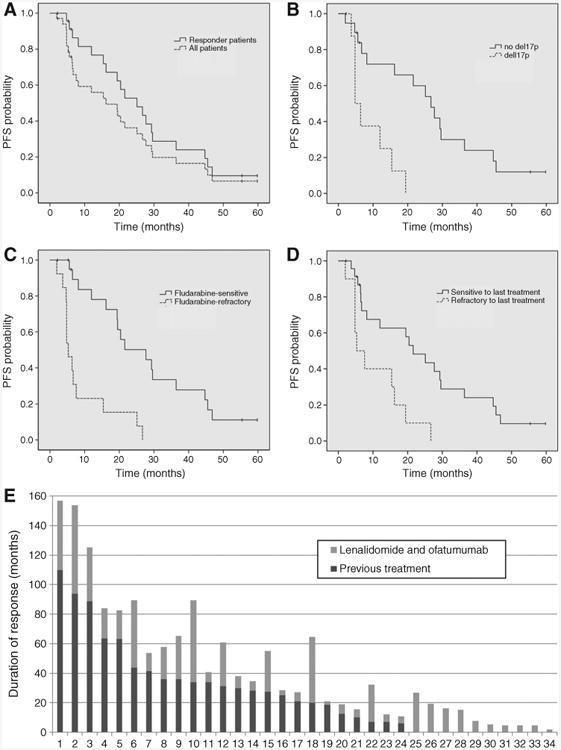
Progression-free survival (PFS) and duration of response. Kaplan–Meier estimates illustrate PFS for all patients and for responders (A). PFS is also shown for patients categorized on the basis of FISH aberrations (B), fludarabine sensitivity (C), and response to the previous treatment (D). E, the comparison between the duration of response to the previous treatment and the duration of response to ofatumumab and lenalidomide therapy is shown. Each bar represents a patient, ordered according to the duration of response to the previous line of therapy.
OS
The median follow-up duration is 50 months (range 4–61 months) for the entire population. No patient died while receiving the study treatment. Seven patients died within 6 months of treatment discontinuation, and 9 patients died at a later time. Causes of death are presented in Supplementary Table S4. The median OS has not been reached. The 1-year OS rate was 88% (95% CI: 78%–100%) and the 3-year OS rate was 65% (95% CI: 51%–83%). The estimated 5-year survival is 53% for all patients, and 62% for responding patients (Fig. 2). Patients with del17p, those with fludarabine-refractoriness, and those who had undergone more than three lines of treatment had significantly shorter survival duration (data not shown).
Figure 2.
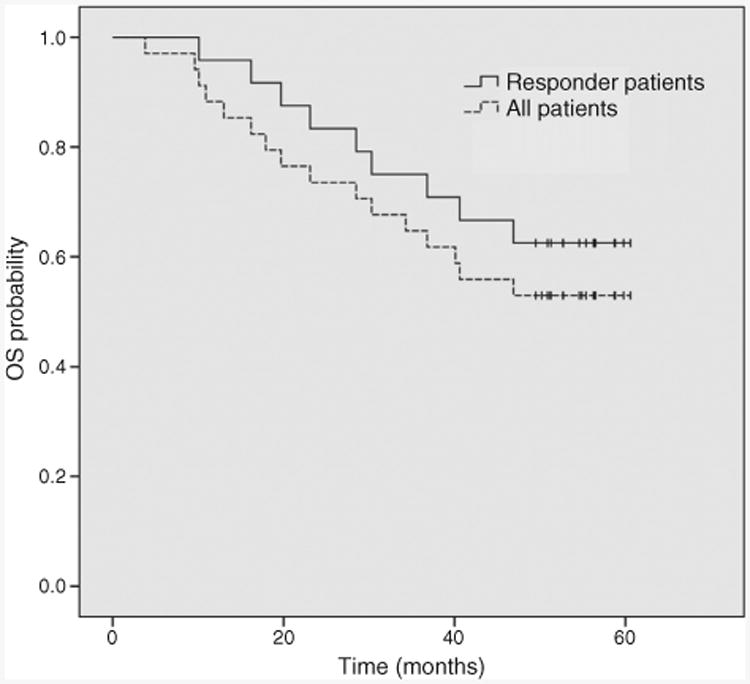
Overall survival (OS). Kaplan–Meier estimates illustrate OS for all patients and for responders.
Safety
Overall, 574 courses of therapy were administered during the study period, with a median of 10 courses per patient (range 2–65). The average daily dose of lenalidomide was 5.3 mg for the entire study cohort. It was higher in patients younger than 65 years than in those 65 years of age or older (6.6 mg vs. 4.1 mg, P < 0.0001). There was no correlation between the average daily dose of lenalidomide and serum creatinine level. Median daily dose was ≤2.5 mg in 179 (31%), 5 mg in 226 (40%), 7.5 mg in 12 (2%), and 10 mg in 157 (27%) patient-courses.
One patient developed a G3 infusion reaction at the time of the first dose of ofatumumab. Other grade 3 and 4 hematologic and nonhematologic toxicities observed during treatment are summarized in Table 2. We observed no grade 3 or 4 TFR or TLS. As expected, the most common hematologic toxicity was neutropenia, which was documented in 28 (82%) patients and 104 (18%) patient-courses. G-CSF was administered 37 times in 19 patients (56%). Treatment was temporarily interrupted due to neutropenia in 35 instances in 19 patients (56%).
Table 2. Hematologic and non-hematologic toxicities (n = 574 patient-courses).
| Grade 3 | Grade 4 | |||
|---|---|---|---|---|
|
|
|
|||
| Toxicity | Patients (%) | Patient-courses (%) | Patients (%) | Patient-courses (%) |
| Hematologic | ||||
| Neutropenia | 10 (29%) | 75 (13%) | 18 (53%) | 29 (5%) |
| Anemia | 2 (6%) | 3 (0.5%) | 0 | 0 |
| Thrombocytopenia | 4 (12%) | 9 (1.6%) | 2 (6%) | 2 (0.3%) |
| Non-hematologic | Patients (%) | Episodes | Patients (%) | Episodes |
|
| ||||
| Infections | ||||
| Pneumonia | 8 (24%) | 11 | 0 | 0 |
| Fever | 3 (9%) | 3 | 0 | 0 |
| Neutropenic fever | 3 (9%) | 3 | 0 | 0 |
| Bacteremia | 1 (3%) | 1 | 1 (3%) | 1 |
| Cellulitis | 2 (6%) | 2 | 0 | 0 |
| Toxoplasmosis | 1 (3%) | 1 | 0 | 0 |
| Parotitis | 1 (3%) | 1 | 0 | 0 |
| Appendicitis | 1 (3%) | 1 | 0 | 0 |
|
| ||||
| Other toxicities | ||||
| VTE | 2 (6%) | 2 | 1 (3%) | 1 |
| Dehydration | 1 (3%) | 1 | 0 | 0 |
| Fatigue | 1 (3%) | 1 | 0 | 0 |
| CHF exacerbation | 1 (3%) | 1 | 0 | 0 |
| Atrial fibrillation | 1 (3%) | 0 | 0 | 0 |
Abbreviation: CHF, congestive heart failure.
The most frequent infectious complication was pneumonia, with 11 episodes observed in 8 (24%) patients. Neutropenic fever occurred in 3 (9%) patients during the study period. Of the 24 infectious episodes observed, 16 (67%) occurred during the first 6 months of treatment. VTE were observed in 3 patients (9%), all with other concomitant risk factors (darbepoetin-α treatment and venostasis due to bulky adenopathy, raloxifene and epoetin-α treatment, prothrombin gene G20210A heterozygosis mutation with recurrent VTE despite warfarin prophylaxis).
Correlative studies
T and NK cell subset analysis
T and NK cell subset distribution was evaluated at baseline and during treatment with ofatumumab and lenalidomide to detect numerical changes and possible correlations with the response to therapy (Fig. 3). A significant decrease in all cell subsets during treatment was detected, and the greatest difference occurred at course 3 compared with baseline. When patients were categorized on the basis of their response to treatment (CR, n = 6; PR, n = 12; NR, n = 12), we found that patients who achieved a CR had higher baseline absolute numbers of CD4+ T cells and CD57+ CD56+ NK cells, compared with NR (median absolute number 1595/μL vs. 715/μL, P = 0.04, and 358/μL vs. 70/μL, P = 0.02, respectively). Moreover, patients who obtained a CR had significantly higher baseline numbers of CD16+CD56+ and CD57+CD56+ NK cell subsets compared with HD (median absolute number 356/μL vs. 80/μL, P = 0.03, and 358/μL vs. 29/μL, P = 0.0008, respectively). To evaluate the impact of the number of prior therapies on T and NK cell subset distribution, we compared patients who received ≤2 and >2 treatment regimens before lenalidomide and ofatumumab. We found a significantly lower number of CD56+CD57+ NK cells in patients who had received >2 treatment regimens (median absolute number 56/μL vs. 117/μL, P = 0.03), whereas the other T and NK cell subsets were not different between the two groups of patients.
Figure 3.
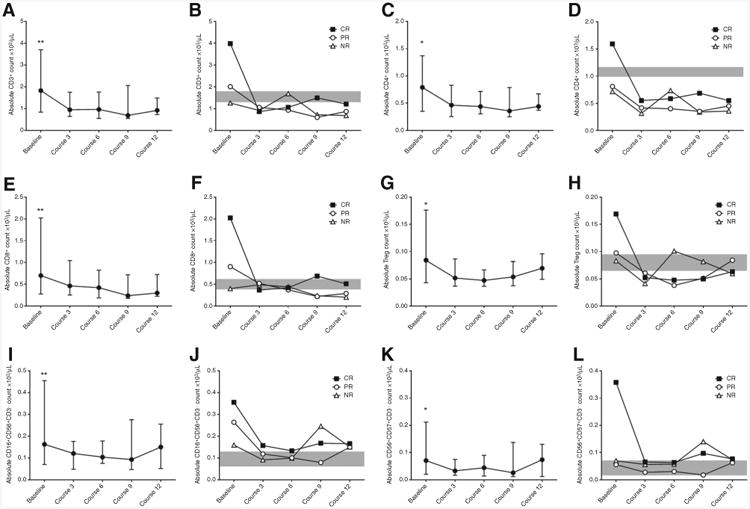
Number of T and NK cell subset at baseline are higher in patients achieving CR. Treatment with lenalidomide and ofatumumab induced a significant reduction in absolute number of CD3+ T cells compared with baseline levels (A, P always < 0.024), CD4+ T cells (C, P always < 0.021), CD8+ T cells (E, P always < 0.0046), Treg (G, P always < 0.027), CD16+CD56+ NK cells (I, P always < 0.0013), CD57+CD56+ NK cells (K, P always < 0.047). Graphs represent median values and interquartile ranges for each subpopulation at each time point. In panels B, D, F, H, J, and L, patients are divided on the basis of response to treatment, and median values for each subpopulation at every time point are plotted. Gray shade represents the interquartile ranges for HD.
T and NK cell function
Lenalidomide, and immunomodulatory drugs in general, have been reported to improve T and NK cell function in vitro, including proliferation, cytokine production, and cytotoxicity (20–23). Here, we performed functional studies on T and NK cells derived from peripheral blood of 15 patients before (baseline) and at course 3 and 6 of ofatumumab and lenalidomide therapy. We selected 5 patients who achieved CR, 5 patients who achieved PR, and 5 who failed to respond to treatment. Interestingly, patients who achieved CR had significantly better T and NK cell function at baseline, whereas T and NK cell function was significantly impaired in patients who achieved only PR and NR (Fig. 4 and Supplementary Fig. S3). During therapy, there was a gradual improvement in T cell function, significant only in patients who achieved CR, while NK cell function did not show any evidence of improvement. The number of prior treatment lines and the dose of lenalidomide had no significant impact on T and NK cell function.
Figure 4.
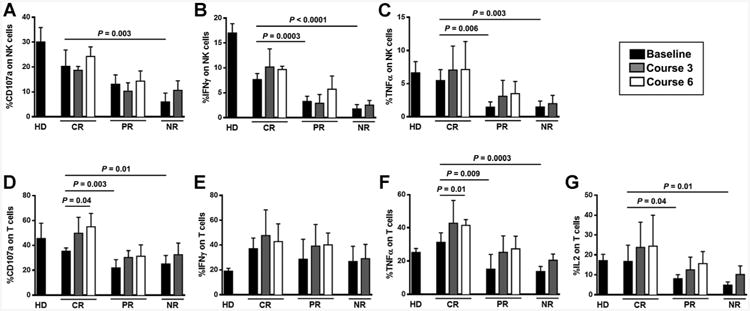
Better NK cell and T cell function before treatment is associated with response to ofatumumab and lenalidomide treatment. The function of NK cells derived from CLL patients against K562 cells was evaluated on the basis of the expression of CD107a (A), IFNγ (B), and TNFα (C), at baseline and at courses 3 and 6 of treatment. NK cell effector function before treatment was significantly higher in patients with CR compared to patients with PR or NR, and was predictive of outcome. No improvement of NK cell function over time was seen. The function of T cells derived from CLL patients in response to CD3/CD28 beads was evaluated on the basis of the expression of CD107a (D), IFNγ (E), TNFα (F), and IL2 (G) at baseline and at courses 3 and 6 of treatment. IL2 and TNFα production before treatment was significantly higher in CR patients compared to patients with PR or NR. A significant improvement in T cell function over time was seen in patients who achieved CR. Bars represent mean value of five patients per group ± SD.
Chemokines and cytokines
We have previously shown that during lenalidomide treatment patients who respond to therapy have decreased circulating levels of CCL3 and CCL4, compared with NR patients (24). In this series of patients, we evaluated a broader number of circulating chemokines/cytokines before and during treatment.
Baseline levels of CCL2, CCL3, CCL4, TNFα, IFNγ, IFNα2, and IL-12p70 were not significantly different in CR (n = 8), PR (n = 8), and NR (n = 6) patients (data not shown). A significant decrease in plasma level of IFNγ compared with baseline was observed during treatment, regardless of response (data not shown). During treatment, patients who achieved PR and CR showed significantly lower circulating levels of CCL2, CCL3, CCL4, IFNα2, and IL-12p70, compared with NR patients (Supplementary Fig. S4).
Angiogenic factors
No significant differences in baseline levels of bFGF, VEGF, and Thrombospondin-1 were found in patients grouped according to their response to treatment (CR: n = 8; PR: n = 12; NR: n = 12; data not shown). When compared with baseline, a significant decrease in patients' plasma level of bFGF and VEGF was observed during treatment, regardless of the response to therapy (Supplementary Fig. S5). No modulation of Thrombospondin-1 levels was detected (data not shown).
Discussion
Treatment regimens that are devoid of conventional cytotoxic agents are increasingly being developed for patients with CLL, both as initial therapy and as salvage treatment. The immunomodulatory properties of lenalidomide, combined with the reported activity of ofatumumab in patients with relapsed or refractory CLL, prompted us to evaluate the clinical activity of these two agents in combination and to perform correlative studies to measure the associated changes in T and NK cell number and function, and in circulating chemokines, cytokines, and angiogenic factors.
Initial studies exploring the activity of single-agent lenalidomide in relapsed or refractory CLL showed an ORR of 32%–47% (CR 7%–9%, PR 25%–38%; refs. 11, 12). In the current study, responses to treatment with ofatumumab and lenalidomide were observed in 71% of patients, and CR in 24%. Although comparisons between phase II studies have limitations, the activity of this combination compared favorably with that of chemoimmunotherapy (CIT) regimens that are considered standard salvage treatments for patients with CLL. The fludarabine-cyclophosphamide-rituximab (FCR) regimen was given to a comparable population at our institution and resulted in an ORR of 73%, a CRR of 29%, and a median PFS duration of 19 months (25). Outcomes reported by Fischer and colleagues for relapsed patients treated with combined bendamustine and rituximab yielded an ORR of 59%, CRR of 9%, and PFS duration of 14.7 months (26). Novel treatment strategies targeting the B-cell receptor pathway are showing promising results, with ORR of 63% to 91% and durable responses based on a limited duration of follow-up (27, 28). However, low CRR and the observation of progression at treatment discontinuation suggest the need to investigate new therapeutic associations. Interestingly, the combination of lenalidomide and ibrutinib is being explored in the treatment of relapsed or refractory CLL (29), as well as both indolent and aggressive non-Hodgkin lymphoma.
In our trial, lenalidomide was given continuously until progression or development of unacceptable toxicity. The majority of patients (24, 70%) could not complete the planned 24 months of treatment; however, the duration of response was heterogeneous and several long-lasting responses (11 patients, 32%, >2 years; 6 patients, 18%, >3 years) were achieved.
We observed that the quality of responses (SD vs.PR vs. CR) can improve with time during therapy, and interestingly, the three patients who achieved MRD negativity did so after one year of therapy, confirming a different kinetic of response as compared with conventional CIT.
With the limitation of a relatively small patient number, we compared the efficacy of the combination of ofatumumab and lenalidomide with the results of our previously reported experience with combined rituximab and lenalidomide in patients with relapsed or refractory CLL (13). Baseline patient characteristics were similar between the two studies, with the exception of a lower median β2-microglobulin level (3.5 vs. 4.1 mg/dL) and a lower proportion of fludarabine-refractory cases (20% vs. 38%) among patients treated with rituximab and lenalidomide. The two studies had similar ORR (66% for rituximab and lenalidomide, and 71% for ofatumumab and lenalidomide) and response durations (17 and 16 months, respectively). The CRR was 12% in patients treated with rituximab and lenalidomide and 24% in those treated with ofatumumab and lenalidomide. This difference could be related to a higher efficacy of ofatumumab compared with rituximab, or to other factors.
Achievement of an MRD-negative remission was independently correlated with longer PFS and OS durations in patients with CLL treated with CIT (30). Three patients treated with ofatumumab and lenalidomide achieved MRD-negative remission lasting 29, 36, and 45 months, supporting a relationship between quality and duration of remission in patients treated with this combination.
This regimen was relatively well tolerated. We did not observe any episode of severe TLS or TFR, which confirms our prior observation that administering anti-CD20 mAb before introducing lenalidomide is an effective strategy to limit these complications. About half of the patients experienced grade 4 neutropenia, similarly to our previous experience with lenalidomide in association with rituximab. Although episodes of pneumonia and fever appeared to be more frequent in this study (24% and 18%, respectively) compared with our previous study with lenalidomide and rituximab (15% and 13%, respectively), the small patient number limits this comparison. As the lenalidomide dose was adjusted during treatment according to the toxicities observed, reporting the MTD may not reflect the dose actually delivered for the entire treatment duration. We therefore reported the average dose of lenalidomide (5.3 mg). It was significantly higher in patients younger than 65 years than in those 65 years or older. Nevertheless, the difference in ORR between patients receiving an average daily lenalidomide dose of <5 mg and those receiving ≥5 mg did not reach the statistical significance. Our finding confirms previously published data that identified lenalidomide dosing for patients with CLL to be lower than that tolerated by patients with multiple myeloma and non-Hodgkin lymphomas.
The reported incidence of VTE in patients with CLL treated with lenalidomide-based regimens in the absence of thromboembolic prophylaxis is 2% to 6% (11–13, 24). In this study, we observed VTE in 3 patients (9%). However, these patients had concomitant risk factors, suggesting that, even if thromboembolic prophylaxis during lenalidomide treatment may not be necessary, the concomitant use of prothrombotic agents should be avoided.
We performed a series of correlative biologic studies to better understand the effects of the ofatumumab and lenalidomide combination on number and functional properties of T cell and NK cell populations, angiogenic mediators, and selected cytokines and chemokines known to have a role in tumor-microenvironment interactions. Moreover, we aimed to investigate whether some of these immune features could be correlated with response to treatment. Patients who achieved a CR had higher baseline numbers of T and NK cells, the difference being significant for CD4+T cells and CD57+CD56+ NK cells. Besides the number of immune cells, a better NK and T cell function at baseline appears to characterize patients responding to ofatumumab and lenalidomide treatment, while patients with dysfunctional NK and T cells had an inferior response to therapy. Although the number of patients evaluated is limited, our data suggest a fundamental role of the cellular immune system in the mechanism of action of lenalidomide and further suggest that the baseline T and NK cell number and function in patients with CLL may help predict their response to this treatment. Furthermore, our data support an important role for NK cell function in determining the efficiency of ofatumumab-mediated ADCC, further suggesting that better immune function at start of therapy is associated with a more favorable response to lenalidomide plus ofatumumab in CLL. Larger prospective studies are needed to determine if this association also applies to other therapies for CLL, and may also have future implications in the setting of T-cell–directed therapies.
The contribution of the tumor microenvironment in supporting CLL tumor cell survival and growth has been extensively studied (31). In our series, patients who responded to lenalidomide and ofatumumab combination had decreased circulating levels of CCL2, CCL3, CCL4, TNFα, IFNα2, and IL-12p70 when compared with NR. This is consistent with a potential effect of lenalidomide in modulating the interactions between the microenvironment and the CLL clone in responding patients. The observed changes are similar to our previous findings in patients treated with lenalidomide monotherapy (24), and ofatumumab does not seem to interfere with the immunomodulatory activity of lenalidomide.
In our patients, treatment with ofatumumab and lenalidomide induced a significant decrease in plasma levels of bFGF and VEGF, confirming an activity of this treatment combination in the disruption of proangiogenic signals (12, 32).
In conclusion, the combination of ofatumumab and lenalidomide is a feasible regimen that induces durable responses in the majority of patients with relapsed or refractory CLL and sustained MRD-negative CR in some. A higher number and preserved functional activity of NK and T cells were found in responding patients at baseline, as compared with NR, underscoring the importance of a competent immune system in supporting the effectiveness of this treatment and suggesting a need to further investigate these immune features as markers for prediction of response to therapy.
Supplementary Material
Translational Relevance.
Chronic lymphocytic leukemia (CLL) is characterized by a complex dysregulation of both humoral and cellular immunity. Lenalidomide, an immunomodulatory agent, is an active treatment for CLL, exerting a direct antitumor effect, but also a pleiotropic activity on the immune system. Here we show that the combination of lenalidomide with the anti-CD20 mAb ofatumumab is relatively well tolerated and can induce durable responses, including minimal residual disease-free complete responses (CR), in patients with relapsed or refractory CLL. We observed that patients who achieved a CR to treatment, when compared with non-responders, had higher numbers and better function of T cells and natural killer cells prior to treatment. These findings underscore the pivotal role, in terms of efficacy, played by a competent immune system at the time of treatment initiation, and pave the road for further studies, in an era of rapidly evolving immunotherapy and cell therapy approaches.
Acknowledgments
The authors thank Susan Lerner, Ana Ayala, Susan C. Smith, Kimberly Yerrow, Dawn Urbanovski, and Cristina Hinojosa for providing patient care, data collection, and data management during this trial.
Grant Support: The University of Texas MD Anderson Cancer Center is supported in part by the NIH through a Cancer Center Support Grant (P30 CA16672).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: S. O'Brien is a consultant/advisory board member for Celgene. J.M. Reuben reports receiving a commercial research grant from Hitachi Chemical Co. and is a consultant/advisory board member for Angle, PLC, and Hitachi Chemical Co. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions: Conception and design: C. Vitale, L. Falchi, K. Rezvani, M.J. Keating, A. Ferrajoli
Development of methodology: C. Vitale, L. Falchi, H. Gao, H. Shaim, K. Rezvani, J.M. Reuben, M.J. Keating, A. Ferrajoli
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C. Vitale, L. Falchi, H. Gao, H. Shaim, K. Van Roosbroeck, G. Calin, S. O'Brien, S. Faderl, K. Rezvani, J.M. Reuben, J.A. Burger, A. Ferrajoli
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C. Vitale, L. Falchi, E. ten Hacken, H. Gao, H. Shaim, K. Van Roosbroeck, G. Calin, X. Wang, K. Rezvani, J.M. Reuben, J.A. Burger, M.J. Keating, A. Ferrajoli
Writing, review, and/or revision of the manuscript: C. Vitale, L. Falchi, E. ten Hacken, H. Shaim, K.V. Roosbroeck, G. Calin, S. O'Brien, S. Faderl, X. Wang, W.G. Wierda, K. Rezvani, J.M. Reuben, J.A. Burger, M.J. Keating, A. Ferrajoli
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.V. Roosbroeck, A. Ferrajoli
Study supervision: L. Falchi, A. Ferrajoli
References
- 1.Catovsky D, Miliani E, Okos A, Galton DA. Clinical significance of T-cells in chronic lymphocytic leukaemia. Lancet. 1974;2:751–2. doi: 10.1016/s0140-6736(74)90944-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin invest. 2008;118:2427–37. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res. 2012;18:678–87. doi: 10.1158/1078-0432.CCR-11-2630. [DOI] [PubMed] [Google Scholar]
- 4.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–21. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Arena G, Laurenti L, Minervini MM, Deaglio S, Bonello L, De Martino L, et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leukemia Res. 2011;35:363–8. doi: 10.1016/j.leukres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Huergo-Zapico L, Acebes-Huerta A, Gonzalez-Rodriguez AP, Contesti J, Gonzalez-Garcia E, Payer AR, et al. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease. PLoS One. 2014;9:e108326. doi: 10.1371/journal.pone.0108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121:3658–65. doi: 10.1182/blood-2013-01-476606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BN, Gao H, Cohen EN, Badoux X, Wierda WG, Estrov Z, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strati P, Keating MJ, Wierda WG, Badoux XC, Calin S, Reuben JM, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood. 2013;122:734–7. doi: 10.1182/blood-2013-04-495341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 11.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–9. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 12.Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–7. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badoux XC, Keating MJ, Wen S, Wierda WG, O'Brien SM, Faderl S, et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31:584–91. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teeling J, Mackus W, Wiegman L, van den Brakel J, Beers S, French R, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 15.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–55. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118:5126–9. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-working group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating MJ. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 19.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 20.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–92. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489–98. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–24. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the german chronic lymphocytic leukemia study group. J Clin Oncol. 2011;29:3559–66. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 27.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollyea DA, Coutre S, Gore L, Adler N, Harris P, Phelps MA, et al. A dose escalation study of ibrutinib with lenalidomide for relapsed and refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2014;124:1987. [Google Scholar]
- 30.Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–8. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 31.Ten Hacken E, Burger JA. Microenvironment dependency in chronic lymphocytic leukemia: the basis for new targeted therapies. Pharmacol Ther. 2014;144:338–48. doi: 10.1016/j.pharmthera.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Maffei R, Fiorcari S, Bulgarelli J, Rizzotto L, Martinelli S, Rigolin GM, et al. Endothelium-mediated survival of leukemic cells and angiogenesis-related factors are affected by lenalidomide treatment in chronic lymphocytic leukemia. Exp Hematol. 2014;42:126–36.e1. doi: 10.1016/j.exphem.2013.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


