Abstract
For the past 20 years, research on biodiversity and ecosystem functioning (B-EF) has only implicitly considered the underlying role of environmental change. We illustrate that explicitly re-introducing environmental change drivers in B-EF research is needed to predict the functioning of ecosystems facing changes in biodiversity. Next, we show how this reintroduction improves experimental control over community composition and structure, which helps to obtain mechanistic insight about how multiple aspects of biodiversity relate to function, and how biodiversity and function relate in food-webs. We also highlight challenges for the proposed re-introduction, and suggest analyses and experiments to better understand how random biodiversity changes, as studied by classic approaches in B-EF research, contribute to the shifts in function that follow environmental change.
Keywords: Biodiversity, Richness, Environmental change, Traits, Modelling, Food-webs
Predicting effects on ecosystem functions from changes in biodiversity: a brief history
Various types of environmental change, such as climate change, habitat fragmentation, or chemical pollution, can profoundly alter multiple facets of biodiversity [1–4]. The past 25 years have seen a rise in different empirical approaches to examine how such changes affect ecosystem functions and services [5, 6]. Many focus on altering biodiversity while observing corresponding changes in function [7]. These approaches can be first classified based on the nature of the manipulation, whether species densities are altered randomly or non-randomly (see ‘Glossary’). Random manipulations assume a random extinction or colonization order, while non-random manipulations are done based on the (presumed) response of species to environmental change [8], or based on the effects of species on function (e.g. species with a greater effect on function are removed first) [9]. A second distinction can be based on whether manipulations of biodiversity are direct or indirect (see ‘Glossary’). Direct biodiversity manipulations are performed by manually altering species densities [10], whereas with indirect manipulations, a relevant environmental change is introduced to alter biodiversity [11, 12].
Indirect and non-random manipulations of biodiversity make intuitive sense because they are rooted in a recognition that environmental change drivers (see ‘Glossary’) are often the cause of biodiversity alterations [3] and that these alterations are non-random [9, 13]. As a consequence, early research on biodiversity and ecosystem functioning (‘B-EF research’ [7]) often adopted indirect and non-random biodiversity manipulations [11, 12, 14]. However, such approaches were increasingly subject to controversy and disagreement. In his seminal paper, Huston [15] criticized indirect and non-random biodiversity manipulations for difficulties in separating ‘true’ biodiversity effects from the effects of ‘hidden treatments’. Huston argued that by indirectly altering biodiversity using an environmental variable, researchers precluded partitioning the biodiversity-mediated effects on ecosystem function from the many other effects environmental change can have on function (see ‘Glossary’). Non-random manipulations were also shown to suffer from inherent bias, because results were highly dependent on the chosen order of species removal or addition. Collectively, the critiques by Huston and others [15–18] pushed the field towards direct and random biodiversity manipulations [7, 10]. The advantage of this methodological shift was that the causal relationship between biodiversity and ecosystem functioning, a main research gap at that time, could be more rigorously established. Today, however, a main research gap in ecology is to understand how the data produced using random and/or direct manipulations of biodiversity can be used to meet two of ecology’s current challenges: (1) to support quantitative prediction of the ecological effects of anthropogenic activities [7]; and (2) to unravel the mechanisms linking community structure (relative abundances) and composition to ecosystem function [19, 20]. In the present contribution, we submit that re-introducing non-random and indirect manipulations of biodiversity using environmental change drivers [21–25] (1) is a prerequisite to predicting the functioning of ecosystems facing changes in biodiversity that are caused by environmental change (section 2); and (2) facilitates unravelling mechanistic insight into the connections between community structure and composition and ecosystem function (section 3).
The re-introduction of environmental change drivers is needed to predict ecosystem functioning following changes in biodiversity
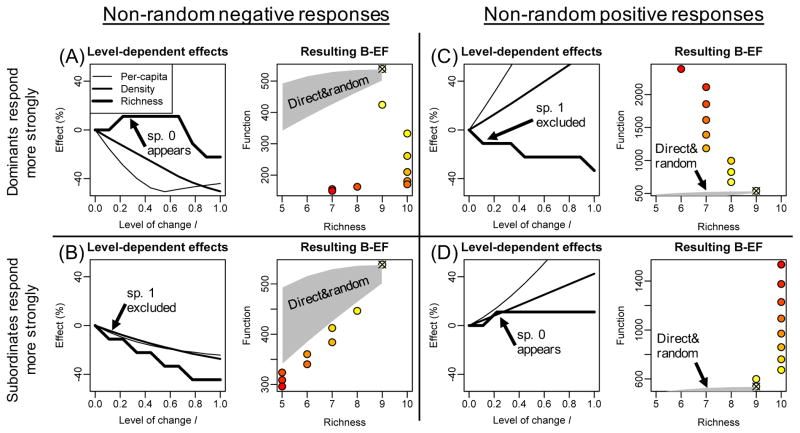
In many ecosystems, environmental change causes biodiversity declines or increases [26–29]. Experiments that directly and randomly manipulate biodiversity are unlikely to predict function in these ecosystems (Fig. 1, shaded area). This is because biodiversity changes that are non-random with respect to species’ contributions to function will affect ecosystem functioning more or less than do random biodiversity changes [9, 30]. In addition, environmental change can alter the effect species have on ecosystem functions by altering (1) per-capita contributions to function [31, 32], and (2) population density [33, 34]. Depending on the type of environmental change, these alterations can be mostly positive (e.g. nutrient enrichment [35]), mostly negative (e.g. drought [36] or pollution [37]), or negative for some species and positive for others (e.g. warming [38–40]).
Figure 1.
Indirect and non-random manipulations of biodiversity can result in a multitude of biodiversity-ecosystem function relationships (‘Resulting B-EF’, simulated from the model in Box 1; l is the level of environmental change and colours represent a scale from l=0 (yellow) to l=1 (red), the value for l=0 is indicated with a ‘x’ for clarity). These relationships emerge as a consequence of effects on richness, per-capita contributions to function (average effect across all species), and total density (sum of all species). The strength of these effects depends on l (‘level-dependent effects’) and the shape of the resulting B-EF critically depends on whether dominants (A and C) or subordinates (B and D) respond more strongly to environmental change, and on whether the elicited responses are negative (A and B) or positive (C and D). The shaded area indicates the expected B-EF under direct and random biodiversity manipulations.
Trait-based frameworks are available to predict how non-random effects of environmental change on per-capita contributions to function, population densities, and biodiversity translate to changes in ecosystem function [9, 30]. A simple extension of this framework with species interactions (Box 1) and using richness as a biodiversity indicator illustrates two important points. First, environmental change can cause a variety of B-EF relationships (Fig. 1). The shape of this relationship critically depends on (1) whether the responses elicited by the environmental change driver are positive or negative, and (2) the type of non-randomness exerted by the environmental change driver [28, 41] (Box 1). Second, changes in function are expected before any change in species richness is observed (Fig. 1A and D; levels 0–0.1), and – more generally – the variability of ecosystem function within one level of species richness is substantial (Box 1, Box 3, ‘Outstanding questions’). The ensemble of B-EF relationships constructed through direct and random biodiversity manipulation (Fig. 1, shaded area) does not capture the variation in B-EF shapes arising from indirect and non-random biodiversity manipulation, and can both over- (e.g. Fig. 1B) and underestimate variation of function within one biodiversity level (e.g. Fig. 1C).
Box 1. Non-random and indirect vs. random and direct biodiversity manipulations.
We simulate richness and ecosystem functioning in a community of 10 species responding to a level l of an environmental change driver and contributing to an ecosystem function F [9]:
| (based on [ 51]) |
The αi,j are per-capita effects of species jon species i (αi,j = αj,i = −0.2; intraspecific effects αi,i are set to −1). Ni is the density of species i (asterisks denote equilibrium densities); μi(l) and fi(l) are growth rates and per-capita contributions to F as a function of l:
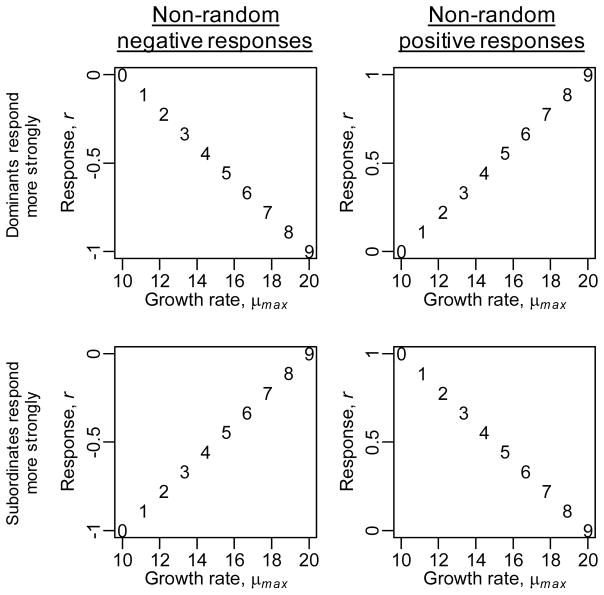
whereri represents the response of species i to environmental change and the division by two ensures per-capita contributions to function responds more strongly than density [77]. All species have fi,max = 10, respond differently to environmental change (Fig. I), have different growth rates (Fig. I) and therefore different competitive strengths (Fig. II).
We manipulated richness indirectly and non-randomly by exposing the community to levels l between 0 (no change) and 1 (100% increase or decrease of μ of the most responsive species), and measured the corresponding F (Fig. 1, colored symbols). When dominants respond most negatively (Fig. 1A), function decreases but richness is higher with than without environmental change because of competitive release of species 0. Thus, environmental change promotes co-existence and richness only decreases at high levels of change. The resulting B-EF relationship is therefore non-monotonic. When environmental change mostly elicits negative responses of subordinates (Fig. 1B), richness decreases already at low levels of change because subordinates (species 1) combine a low density, which makes them inherently prone to competitive exclusion, with a large negative response. In this case, a monotonic positive B-EF relationship emerges. When environmental change elicits positive responses, negative (Fig. 1C) or positive B-EF relationships (Fig. 1D) emerge from exactly the same mechanisms as in Fig. 1A and 1B.
We also manipulated richness directly and randomly by removing all possible combinations of 1 to 5 species from the community and measuring the corresponding F while setting l=0 (Fig. 1, shaded area, identical for all four scenarios).
Figure I.
Environmental change elicits negative (left panels) or positive responses (right panels) that are strongest for species with high (top row) or low (bottom row) growth rates, i.e. species that are dominant and subordinate in pre-change conditions, respectively (Fig. II). Numbers give species identity.
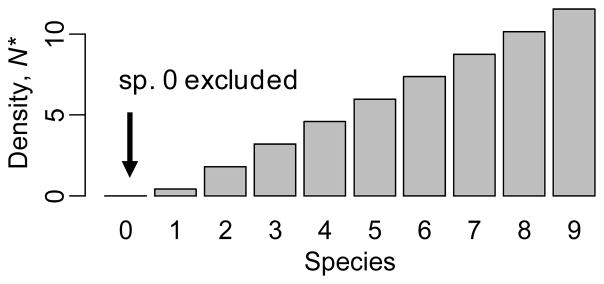
Figure II.
Equilibrium densities in absence of environmental change.
Box 3. Outstanding questions.
Theory indicates that environmental change can affect function without changing richness but how important are such effects in real ecosystems? How do effects on function at invariant richness vary among ecosystems?
Biodiversity-ecosystem functioning research has mostly focused on the effects of random species loss on functions. How do these effects compare to those occuring following environmental change?
How does environmental change alter per-capita species interactions and how does this affect our capacity to manipulate biodiversity using environmental change drivers?
How can knowledge about a selection of well-studied environmental change drivers be used to manage ecosystems exposed to other types of environmental change?
The re-introduction of environmental change drivers can augment mechanistic insight
Many descriptors of biodiversity (e.g. richness and evenness, and based on traits, taxonomy, or genes), but also community structure and composition, total density (community size) and per-capita contributions to function, can affect ecosystem functioning [33, 42–45]. A main research theme in ecology is to understand their relative importance to functioning [7, 46, 47]. Using environmental change drivers to indirectly manipulate biodiversity, community structure and composition, total density, and per-capita contributions to function facilitates such studies. This is because different environmental change levels trigger effects on different subsets of these variables (Fig. 1). For example, in Fig. 1A, environmental change levels between 0.25 and 0.7 will all lead to the same species richness, but will alter total density and per-capita contributions to function. In Fig. 1B, effects on richness are always more important than effects on total density or per-capita contributions to function. In Fig. 1A and D, low levels of change only affect per-capita contributions to function and total density. In general, the fact that different levels of environmental change cause different effects offers greater control over the different mechanisms underlying change of function than do direct manipulations of biodiversity. Controlling per-capita contributions to function is by definition impossible through direct manipulations of biodiversity, since per-capita contribution to function is no descriptor of biodiversity. However, even community composition, structure, and richness will often be uncontrollable through direct manipulations. For example, in the model presented in Fig. 1, persistence of species 0 or dominance by any other species than species 9 is only possible in the continuous presence of an appropriate environmental change driver, i.e. through indirect manipulations. Without this presence, community structure will always converge to the one shown in Box 1, and richness will be 9, even when all 10 species are added to the initial community. Many examples illustrate community compositions and structures that only emerge in the presence of specific environmental change drivers and do not occur in their absence. For example, drought in streams reduces the relative density of large-bodied consumers, predators, and encrusting green algae [36]. Nitrogen enrichment in grasslands increases the relative density of nitrogen demanding grasses [35], while increased precipitation in grasslands increases the relative density of nitrogen-fixing forbs [48]. Even though most of the available studies are based on taxonomic diversity, case studies showing how environmental change drivers can cause loss or gain of genetic diversity are rapidly accumulating [49, 50].
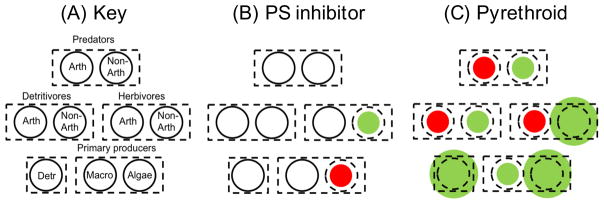
The relationship between biodiversity and functioning in multi-trophic communities (food-webs) has been an important research theme in ecology since the 1990s [7, 51–53]. For example, the biodiversity of one food-web compartment can drive functions performed by other parts of the food-web [54], or both can be unrelated [55]. Using environmental change drivers to indirectly and non-randomly manipulate food-webs facilitates studying such links. This is because environmental change drivers often target specific food-web compartments so that it becomes possible to experimentally alter biodiversity and related functions of specific food-web compartments and measure corresponding changes in other compartments. For example, resource enrichment can be used to increase functions performed by basal species groups (e.g. bacterial decomposition, water purification, primary production), while desiccation can be used to target functions performed by non-basal species [36]. In addition to the well-known cases of resource addition or manipulation of climate variables, chemical stressors comprise an exceptionally useful group of experimental agents that can be used for both non-random manipulations as well as for manipulations that are random with respect to the effects species have on function. This is illustrated by the many studies that have exposed relatively complex food-webs composed of field organisms (typically primary producers and invertebrate grazers and predators) to concentration series of chemical stressors during several weeks to months (Fig. 2). For example, many pyrethroid insecticides will target arthropod consumers and predators [56, 57], while photosystem-inhibiting herbicides will target specific algal taxa [58, 59]. Certain biocides such as triphenyltin [60] and narcotic chemicals [61] are examples of chemical stressors that exert effects that are random with respect to the effects species have on function. Directly manipulating food-webs to persistently exclude certain trophic levels or functional groups (e.g. small-bodied benthic grazers, specific bacterial communities or, algal taxa) will be nearly impossible. Indirect non-random manipulations might therefore be the only solution.
Figure 2.
Chemical stressors can be used to non-randomly and indirectly manipulate food-webs. This is illustrated by empirically observed effects of continuous exposure of freshwater ditch food-webs to chemical stressors in published micro- and mesocosm experiments. A: Predators, herbivores and detritivores are separated into arthropod (Arth) and non-arthropod (Non-arth) species; primary producers are separated into macrophytes (Macro) and algae; Det. represents detrital material and its associated microflora. B: Results for exposure to 50μg•L linuron, a photosystem (‘PS’) inhibitor [58, 59]. C: Results for exposure to 35 μg•L chlorpyrifos, a pyrethroid insecticide [56, 57]. Significant primary responses by the corresponding chemical stressor are shown in red, secondary effects mediated by species interactions are shown in green. White circles indicate that there was no effect. The relative sizes of the coloured and dotted circles indicate whether the effect was positive (increase in abundance - coloured circle larger than dotted circle) or negative (decrease of abundance -coloured circle smaller than dotted circle).
Back to the future: methods to connect indirect and non-random manipulations with classic B-EF research
Most classic B-EF designs focus on the effect of random biodiversity changes on ecosystem function through direct manipulations. To quantify the contribution of such effects to the functioning of ecosystems following environmental change (Box 3, ‘Outstanding questions’) [23] analysing available data is a useful starting point. The literature is replete with studies exposing communities to environmental gradients. When a sufficient number of change levels has been tested across a sufficiently broad gradient of change, the contributions of biodiversity-mediated effects can be separated from the other effects of environmental change on ecosystem function using available analytical techniques. One possible way to do so is by applying multivariate statistical techniques, such as structural equation modelling [62, 63] (Box 2). However, sophisticated structural equation models [21, 24] can also be used to partition the effects on function that are not mediated by biodiversity into their constituents. In addition, methods based on versions of the Price equation that do not require monoculture data but only need species contributions to function before and after environmental change can be used to separate the effects of species loss and gain that is random and non-random with respect to the effects species have on function from all other effects environmental change can have on function [42].
Box 2. Separating biodiversity-mediated effects on ecosystem functioning.
Structural equation models (SEMs) can be used to compare biodiversity-mediated effects on ecosystem functioning with the other effects environmental change can have on function. A SEM is described as “the use of two or more structural [cause-effect] equations to model multivariate relationships”, which allows for an intuitive graphical representation of complex causal networks [62, 63]. Most notably, a SEM cannot only be used to isolate biodiversity-mediated effects on ecosystem functioning, but also to investigate the partial contributions of correlated explanatory variables to test alternative hypotheses [62].
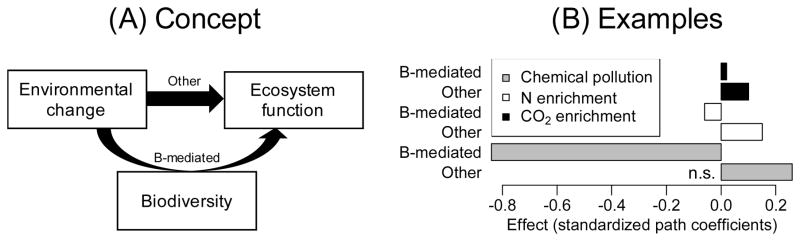
For illustrative purposes, we analysed data from a previously published microcosm study evaluating the effects of chemical stress (a mixture of insecticides) on aquatic invertebrate richness and decomposition in a ditch community [78, 79] with a simple structural equation model. We also present previously published effects of nitrogen and carbon dioxide enrichment on plant richness and biomass production in grasslands [23]. These analyses show that richness-mediated effects on function are negative for environmental change drivers that have negative effects on richness, and that these richness-mediated effects can be partly compensated by other effects of environmental change. Many examples in the literature support the conclusion that environmental change studies can be successfully analysed with SEMs, including SEMs with more extended effect pathways [21, 24]. In more replicated experimental setups [62], different biodiversity and community metrics could be tested in parallel to extract the most relevant biodiversity metric causing alterations in ecosystem functioning.
Figure I.
A: Environmental change drivers can affect functions by altering biodiversity or through other mechanisms [23]. B: Structural equation models for three environmental change drivers. All effects are significant (P < 0.05) except when indicated (n.s.). The variance of diversity and function explained by the model (R2) for the case of chemical stress was 68% and 65%, respectively. Effects are standardized path coefficients [63]. Details on the analysis for the other two drivers can be found in the original publication [23].
Post-hoc analyses are a useful first step to quantify biodiversity-mediated effects on function. However, we recommend combining direct and indirect biodiversity manipulations as separate treatments in a single experiment. In a first design, we recommend using a well-known environmental change driver to non-randomly manipulate a community, while setting up a second treatment where the same community is manipulated directly. Importantly, the direct manipulation should be done in the absence of the environmental change driver but aim to match the community resulting from the application of the environmental change driver, as observed in the first treatment, and should therefore be non-random. For example, in Fig. 1B, applying a level of change of 0.1 would constitute an indirect biodiversity manipulation that excludes species 1. Higher levels would exclude species 2, 3, and so on. Thus, the direct biodiversity manipulation treatments should represent the same gradient of community compositions, by consecutively excluding species 1, 2, 3, and so on. Next, the B-EF relationship resulting from the indirect manipulation (e.g. Fig. 1B, ‘resulting B-EF’ panel) could be compared to the one resulting from direct species removal. If both were not significantly different, this would suggest that the chosen type of environmental change mainly acts upon ecosystem functioning through compositional effects. If B-EF relationships do differ, follow-up studies could examine in more detail the potential mechanisms explaining this difference, for example by inspecting the magnitude of effects on per-capita contributions to function [25], or by considering effects on community structure. However, we recognize that this design can be challenging because, as mentioned in section 3, certain community compositions are impossible to reconstruct without the use of environmental variables. This problem could be addressed by statistically testing if per-capita contributions to function (functional contribution of a species, e.g. its total biovolume divided by its population density) differ between the direct and indirect biodiversity treatment. If the inferred values of per-capita contributions to function do not differ between both treatments, this suggests that the selected type of environmental change impacts on ecosystem functioning through other mechanisms than effects on per-capita contributions to function.
A second design consists of a factorial experiment where the presence or absence of a direct biodiversity manipulation that aims to match the community structure resulting from the indirect biodiversity manipulation is crossed with the presence and absence of an environmental change driver [64]. If all the effects of the driver on ecosystem functioning are mediated by biodiversity changes, then the combination of direct biodiversity manipulation and the environmental change treatment should display the same level of ecosystem functioning as both the direct manipulation alone and the environmental change treatment alone. If this were not the case, then it would suggest non-biodiversity-mediated effects on ecosystem functioning. Interestingly, the same design has been recently proposed by Vellend [65], yet motivated by a different objective. Vellend proposed to use this design to test if a community structure shaped by environmental change maximizes function under that same type of environmental change, a prediction based on the analogy between community ecology and population genetics.
Challenges of re-introducing environmental change drivers in B-EF research
Although we advocate re-introducing environmental change drivers in B-EF research, there are at least two challenges that need to be addressed for successful application. First, in the approach we advocate, we implicitly assume that environmental change does not affect per-capita species interactions (the in Box 1). In our model, the effects of species interactions on a focal species are only altered through changes in the density of species with which it interacts. This assumption has been shown to prevail in some systems [66], but not in others [67, 68]. Arguably the best-known example of environmental effects on per-capita interactions is the ‘stress gradient hypothesis’, where there is a shift from competitive (i.e. negative) to facilitative (i.e. positive) interactions as the level of stress increases [67, 68].
Such effects can lead to a variety of effects of stress on community structure and composition and ecosystem function, depending on the type of stress factor and species traits [69]. Suttle et al. [48] found that sustained increased precipitation eventually caused negative interactions among plant species that were not apparent before the treatment. In alfalfa communities, Barton and Ives [70] found that reduced precipitation changed interactions between spotted aphids and their ladybeetle predators through dietary shifts of the latter. These examples make clear that species interactions prevailing in the pre-change system cannot always be used to predict the chain of secondary and higher-order effects occurring after the change. In such cases, knowledge about shifts of per-capita species interactions is needed to gain control over community structure and composition in experiments (Box 3, ‘Outstanding questions’), and to correctly interpret the observed effects of environmental change on biodiversity and ecosystem functioning.
Second, we have discussed environmental change drivers eliciting either positive or negative responses that change monotonically as the level of environmental change increases, and stay constant through time. However, many environmental change drivers can elicit positive responses in some species but negative responses in others (e.g. temperature [38]), and many responses are non-monotonic, with the sign of the response depending on the level of environmental change (e.g. [47]). In addition, depending on the life history of the considered species, populations can genetically adapt [49], which can alter their response to environmental change through time. While these features do not threaten the general principle of our thesis, they do indicate that community structure and composition can be harder to interpret and predict, and therefore also more difficult to control in experiments, for certain combinations of environmental change drivers and ecosystem types.
Opportunities for ecosystem assessment and management
Novel tools for biological monitoring will substantially increase the amount of biodiversity data [71, 72]. However, linking monitored biodiversity trends to ecosystem functions remains a major difficulty for ecosystem assessment, as has been discussed in the framework of several environmental regulations worldwide [73, 74]. Re-introducing environmental change drivers in B-EF research could help ecosystem assessors by realistically translating observed biodiversity trends to trends of ecosystem function for a suite of well-studied environmental change drivers. Studies compiling and comparing different types of environmental change [22, 75] will be instrumental to ask if knowledge about one type of environmental change can be transposed to other types of environmental change (Box 3, ‘Outstanding questions’). Following ecosystem assessments, predicted changes of ecosystem functions could be used to inform management as well, for example by triggering mitigating measures if needed. In addition, ecosystem managers could propose critical levels of biodiversity change that, when exceeded, lead to unacceptable loss of ecosystem functioning. The connection of B-EF research to applied science has often been debated [76]. Re-introducing the use of environmental change drivers to B-EF research can reinforce this connection.
Concluding remarks
We have identified two reasons why environmental change drivers should be re-introduced in B-EF research. First, the amount of ecosystem function loss or gain following biodiversity change depends on the type of underlying environmental change driver(s). Second, environmental change drivers can serve as experimental agents to control various aspects of biodiversity and community composition and structure. These features facilitate studying to what extent changes in ecosystem function are caused by biodiversity change and which aspects of biodiversity are most important to ecosystem function.
Re-introducing environmental change drivers into B-EF research can be realised by analysing existing data of well-known environmental change drivers and through novel experimental designs. Designs combining direct and indirect biodiversity manipulations constitute a particularly useful research avenue as they allow to directly test how biodiversity, environmental change, and ecosystem function relate. However, unexpected effects of environmental change on per-capita species interactions and the variety of species’ responses to such change are two main challenges to the use of environmental change drivers in B-EF research. Opportunities include an improved capacity to assist ecosystem assessment and management, by translating monitored biodiversity trends to trends of ecosystem function, which are rarely monitored. We conclude that re-introducing environmental change drivers in B-EF research is a prerequisite for predicting shifts of ecosystem function in a changing world, facilitates understanding the mechanisms causing these shifts, and strengthens the connections between B-EF research and applied ecology.
Trends.
In the 1990s, critiques on early biodiversity-ecosystem function (B-EF) research pushed the field towards direct and random biodiversity manipulations.
This evolution allowed establishing causal relationships between ecosystem functioning and biodiversity, a main research gap at that time.
A main research gap today is to predict and mechanistically understand shifts of ecosystem functioning following real-world biodiversity shifts caused by different types of environmental change.
Data from direct and random biodiversity manipulations do not predict functioning of ecosystems that experience biodiversity shifts, as these shifts are often non-random and combine with a series of other effects such as changes in per-capita functioning and density.
Environmental change drivers are useful as they offer experimental control over (a) the relative magnitude of the different facets of biodiversity change, and (b) food-web composition. These two features facilitate inference of the mechanisms connecting environmental change with ecosystem functioning.
Acknowledgments
This paper is a joint effort of the working group sEcoToxDiv and an outcome of a workshop kindly supported by sDiv, the Synthesis Centre of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG FZT 118). This research used resources of the “Plateforme Technologique de Calcul Intensif (PTCI)” (http://www.ptci.unamur.be) located at the University of Namur, Belgium, which is supported by the FRS-FNRS under the convention No. 2.4520.11. The PTCI is member of the “Consortium des Équipements de Calcul Intensif (CÉCI)” (http://www.ceci-hpc.be). JRR was supported by grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499, R01TW010286), US Department of Agriculture (NRI 2006-01370, 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801). FDL is indebted to the University of Namur (FSR Impulsionnel 48454E1).
Glossary
- Environmental change driver
An environmental variable that exhibits long-term changes, often as a result of anthropogenic activities. Examples include nutrient deposition, climate warming, habitat fragmentation, and chemical pollution.
- Direct biodiversity manipulation
If biodiversity is manipulated directly, communities with different biodiversity levels are composed, e.g. by taking different subsets of a species pool in case of richness.
- Indirect biodiversity manipulation
If biodiversity is manipulated indirectly, one applies different levels of an environmental change driver to create a biodiversity gradient. Indirect biodiversity manipulations are by definition non-random with respect to species responses to environmental change.
- Random biodiversity manipulation
If biodiversity is manipulated randomly, community composition or structure is varied within a diversity level. By doing so, one can statistically control for effects of community composition or structure on ecosystem function.
- Non-random biodiversity manipulation
Non-random biodiversity manipulations are done based on known or presumed extinction or colonization orders (non-random with respect to species responses to environmental change), or based on the contribution of species to function (non-random with respect to species effects on ecosystem functions).
- Biodiversity-mediated effect of environmental change on ecosystem function
Effects occurring through changes in any aspect of biodiversity (mostly richness or evenness).
- Other effects of environmental change on ecosystem function
Effects occurring through mechanisms other than biodiversity changes. Examples include changes of community composition or structure, of total density (community size), of per-capita contributions to function (fi(l) in Box 1, e.g. physiological responses to warming), or of the bioavailability of macronutrients such as carbon, nitrogen, or phosphorous [80].
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frederik De Laender, Email: Frederik.delaender@unamur.be.
Jason R. Rohr, Email: jasonrohr@gmail.com.
Roman Ashauer, Email: roman.ashauer@york.ac.uk.
Donald J. Baird, Email: djbaird@unb.ca.
Uta Berger, Email: uta.berger@tu-dresden.de.
Nico Eisenhauer, Email: nico.eisenhauer@idiv.de.
Volker Grimm, Email: volker.grimm@ufz.de.
Udo Hommen, Email: udo.hommen@ime.fraunhofer.de.
Lorraine Maltby, Email: l.maltby@sheffield.ac.uk.
Carlos J. Meliàn, Email: Carlos.Melian@eawag.ch.
Francesco Pomati, Email: Francesco.Pomati@eawag.ch.
Ivo Roessink, Email: ivo.roessink@wur.nl.
Viktoriia Radchuk, Email: radchuk.victoria@gmail.com.
Paul J. Van den Brink, Email: paul.vandenbrink@wur.nl.
References
- 1.Bellard C, et al. Impacts of climate change on the future of biodiversity. Ecology Letters. 2012;15(4):365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 3.Malaj E, et al. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9549–54. doi: 10.1073/pnas.1321082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar R, et al. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters. 2006;9(8):968–80. doi: 10.1111/j.1461-0248.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 5.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–8. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 6.Worm B, et al. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 7.Tilman D, et al. Biodiversity and Ecosystem Functioning. Annual Review of Ecology, Evolution, and Systematics. 2014;45(1):471–493. [Google Scholar]
- 8.Zavaleta ES, Hulvey KB. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science. 2004;306(5699):1175–7. doi: 10.1126/science.1102643. [DOI] [PubMed] [Google Scholar]
- 9.Suding KN, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Global Change Biology. 2008;14(5):1125–1140. [Google Scholar]
- 10.Cardinale BJ, et al. The functional role of producer diversity in ecosystems. American Journal of Botany. 2011;98(3):572–92. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 11.McNaughton SJ. Biodiversity and function of grazing ecosystems. In: Schulze ED, Mooney HA, editors. Biodiversity Ecosystem Functioning. Springer; 1993. pp. 361–384. [Google Scholar]
- 12.Tilman D, Downing JA. Biodiversity and stability in grasslands. Nature. 1994;367:363–365. [Google Scholar]
- 13.Voigt W, et al. Trophic levels are differentially sensitive to climate. Ecology. 2003;84:2444–2453. [Google Scholar]
- 14.Hector A, Hooper R. Darwin and the First Ecological Experiment. Science. 2002;295(5555):639–640. doi: 10.1126/science.1064815. [DOI] [PubMed] [Google Scholar]
- 15.Huston MA. Hidden treatments in ecological experiments: re-evalutating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- 16.Aarssen LW. High productivity in grassland ecosystems: effected by species diversity or productive species? Oikos. 1997;80:183–184. [Google Scholar]
- 17.Grime JP. Biodiversity and ecosystem function: the debate deepens. Science. 1997;277:1260–1261. [Google Scholar]
- 18.Givnish TJ. Does diversity beget stability? Nature. 1994;371(6493):113–114. [Google Scholar]
- 19.Eisenhauer N, et al. Biodiversity–ecosystem function experiments reveal the mechanisms underlying the consequences of biodiversity change in real world ecosystems. Journal of Vegetation Science 2016 in press. [Google Scholar]
- 20.Wardle DA. Do experiments exploring plant diversity–ecosystem functioning relationships inform how biodiversity loss impacts natural ecosystems? Journal of Vegetation Science. 2016;27(3):646–653. [Google Scholar]
- 21.Halstead NT, et al. Community ecology theory predicts the effects of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters. 2014;17(8):932–41. doi: 10.1111/ele.12295. [DOI] [PubMed] [Google Scholar]
- 22.Hautier Y, et al. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348(6232):336–40. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- 23.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(29):11911–6. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon TA, et al. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecology Letters. 2012;15(7):714–22. doi: 10.1111/j.1461-0248.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- 25.Mensens C, et al. Stressor-induced biodiversity gradients: revisiting biodiversity-ecosystem functioning relationships. Oikos. 2015;124(6):677–684. [Google Scholar]
- 26.Dornelas M, et al. Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- 27.McGill BJ, et al. Fifteen forms of biodiversity trend in the Anthropocene. Trends in Ecology & Evolution. 2015;30(2):104–113. doi: 10.1016/j.tree.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Sax DF, Gaines SD. Species diversity: from global decreases to local increases. Trends in Ecology & Evolution. 18(11):561–566. [Google Scholar]
- 29.Gonzalez A, et al. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology. 2016;97(8):1949–1960. doi: 10.1890/15-1759.1. [DOI] [PubMed] [Google Scholar]
- 30.Diaz S, et al. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecology and Evolution. 2013;3(9):2958–75. doi: 10.1002/ece3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalifour A, Juneau P. Temperature-dependent sensitivity of growth and photosynthesis of Scenedesmus obliquus, Navicula pelliculosa and two strains of Microcystis aeruginosa to the herbicide atrazine. Aquatic Toxicology. 2011;103:9–17. doi: 10.1016/j.aquatox.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Vitousek PM, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecology. 1997;7(3):737–750. [Google Scholar]
- 33.Hillebrand H, et al. Consequences of dominance: a review of eveness effects on local and regional ecosystem processes. Ecology. 2008;89:1510–1520. doi: 10.1890/07-1053.1. [DOI] [PubMed] [Google Scholar]
- 34.Winfree R, et al. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecology Letters. 2015;18(7):626–35. doi: 10.1111/ele.12424. [DOI] [PubMed] [Google Scholar]
- 35.Wedin DA, Tilman D. Influence of Nitrogen Loading and Species Composition on the Carbon Balance of Grasslands. Science. 1996;274(5293):1720–1723. doi: 10.1126/science.274.5293.1720. [DOI] [PubMed] [Google Scholar]
- 36.Ledger ME, et al. Drought alters the structure and functioning of complex food webs. Nature Clim Change. 2013;3(3):223–227. [Google Scholar]
- 37.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313(5790):1072–7. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 38.Brose U, et al. Climate change in size-structured ecosystems. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2012;367(1605):2903–2912. doi: 10.1098/rstb.2012.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García Molinos J, et al. Climate velocity and the future global redistribution of marine biodiversity. Nature Clim. 2015 Change advance online publication. [Google Scholar]
- 40.Woodward G, et al. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1549):2093–2106. doi: 10.1098/rstb.2010.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahill AE, et al. How does climate change cause extinction? Proceedings of the Royal Society of London B: Biological Sciences. 2012;280:20121890. doi: 10.1098/rspb.2012.1890. (1750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox JW, Kerr B. Analyzing the effects of species gain and loss on ecosystem function using the extended Price equation partition. Oikos. 2012;121(2):290–298. [Google Scholar]
- 43.Loreau M. Linking biodiversity and ecosystems: towards a unifying ecological theory. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2010;365(1537):49–60. doi: 10.1098/rstb.2009.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crutsinger GM, et al. Plant Genotypic Diversity Predicts Community Structure and Governs an Ecosystem Process. Science. 2006;313(5789):966–968. doi: 10.1126/science.1128326. [DOI] [PubMed] [Google Scholar]
- 45.Hughes AR, et al. Ecological consequences of genetic diversity. Ecology Letters. 2008;11(6):609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 46.Hillebrand H, Matthiessen B. Biodiversity in a complex world: consolidation and progress in functional biodiversity research. Ecology Letters. 2009;12(12):1405–19. doi: 10.1111/j.1461-0248.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 47.Pomati F, Nizzetto L. Assessing triclosan-induced ecological and trans-generational effects in natural phytoplankton communities: a trait-based field method. Ecotoxicology. 2013;22(5):779–94. doi: 10.1007/s10646-013-1068-7. [DOI] [PubMed] [Google Scholar]
- 48.Suttle KB, et al. Species Interactions Reverse Grassland Responses to Changing Climate. Science. 2007;315(5812):640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 49.Doi H, et al. Genetic diversity increases regional variation in phenological dates in response to climate change. Global Change Biology. 2010;16(1):373–379. [Google Scholar]
- 50.Sax DF, Gaines SD. Species diversity: from global decreases to local increases. Trends in Ecology & Evolution. 2003;18(11):561–566. [Google Scholar]
- 51.Eklof A, Ebenman BO. Species loss and secondary extinctions in simple and complex model communities. Journal of Animal Ecology. 2006;75(1):239–246. [Google Scholar]
- 52.Naeem S, et al. Empirical evidence that declining species diversity may alter the performance of terrestrial ecosystems. Philos Trans R Soc Lond B Biol Sci. 1995;347:249–262. [Google Scholar]
- 53.Petchey OL, et al. Environmental Warming Alters Food-Web Structure and Ecosystem Function. Nature. 1999;402(6757):69–72. [Google Scholar]
- 54.Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515(7528):505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 55.Radchuk V, et al. Biodiversity and ecosystem functioning decoupled: invariant ecosystem functioning despite non-random reductions in consumer diversity. Oikos. 2016;125(3):424–433. [Google Scholar]
- 56.Brock TCM, et al. Fate and effects of the insecticide Dursban®4E in indoor Elodea-dominated and macrophyte-free freshwater model ecosystems: I. Fate and primary effects of the active ingredient chlorpyrifos. Archives of Environmental Contamination and Toxicology. 1992;23:69–84. doi: 10.1007/BF00225998. [DOI] [PubMed] [Google Scholar]
- 57.Brock TCM, et al. Fate and effects of the insecticide Dursban®4E in indoor Elodea-dominated and macrophyte-free freshwater model ecosystems: II. Secondary effects on community structure. Archives of Environmental Contamination and Toxicology. 1992;23:391–409. doi: 10.1007/BF00203801. [DOI] [PubMed] [Google Scholar]
- 58.Cuppen JG, et al. Sensitivity of macrophyte-dominated freshwater microcosms to chronic levels of the herbicide linuron. II. Community metabolism and invertebrates. Ecotoxicology and Environmental Safety. 1997;38(1):25–35. doi: 10.1006/eesa.1997.1556. [DOI] [PubMed] [Google Scholar]
- 59.Van den Brink PJ, et al. Sensitivity of Macrophyte-Dominated Freshwater Microcosms to Chronic Levels of the Herbicide Linuron. I: Primary producers. Ecotoxicology and Environmental Safety. 1997;38(1):13–24. doi: 10.1006/eesa.1997.1555. [DOI] [PubMed] [Google Scholar]
- 60.Roessink I, et al. Impact of triphenyltin acetate in microcosms simulating floodplain lakes. I. Influence of sediment quality. Ecotoxicology. 2006;15(3):267–93. doi: 10.1007/s10646-006-0058-4. [DOI] [PubMed] [Google Scholar]
- 61.Van Wezel AP, Opperhuizen A. Narcosis due to environmental pollutants in aquatic organisms: residue-based toxicity, mechanisms, and membrane burdens. Critical Reviews in Toxicology. 1995;25:255–279. doi: 10.3109/10408449509089890. [DOI] [PubMed] [Google Scholar]
- 62.Eisenhauer N, et al. From patterns to causal understanding: Structural equation modeling (SEM) in soil ecology. Pedobiologia. 2015;58(2–3):65–72. [Google Scholar]
- 63.Grace JB. Structural Equation Modeling and Natural Systems. 1. Cambridge University Press; New York: 2006. [Google Scholar]
- 64.Eisenhauer N, et al. No interactive effects of pesticides and plant diversity on soil microbial biomass and respiration. Applied Soil Ecology. 2009;42(1):31–36. [Google Scholar]
- 65.Vellend M. The Theory of Ecological Communities. Princeton: 2016. [Google Scholar]
- 66.Baert JM, et al. Per capita interactions and stress tolerance drive stress-induced changes in biodiversity effects on ecosystem functions. Nat Commun. 2016;7:12486. doi: 10.1038/ncomms12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertness MD, Callaway R. Positive interactions in communities. Trends in Ecology & Evolution. 1994;9(5):191–193. doi: 10.1016/0169-5347(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 68.Olsen SL, et al. From facilitation to competition: temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Global Change Biology. 2016;22(5):1915–1926. doi: 10.1111/gcb.13241. [DOI] [PubMed] [Google Scholar]
- 69.Maestre FT, et al. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology. 2009;97(2):199–205. [Google Scholar]
- 70.Barton BT, Ives AR. Species interactions and a chain of indirect effects driven by reduced precipitation. Ecology. 2014;95(2):486–494. doi: 10.1890/13-0044.1. [DOI] [PubMed] [Google Scholar]
- 71.Allan E, et al. Interannual variation in land-use intensity enhances grassland multidiversity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(1):308–13. doi: 10.1073/pnas.1312213111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baird DJ, Hajibabaei M. Biomonitoring 2.0: a new paradigm in ecosystem assessment made possible by next-generation DNA sequencing. Mol Ecol. 2012;21:2039–2044. doi: 10.1111/j.1365-294x.2012.05519.x. [DOI] [PubMed] [Google Scholar]
- 73.Birk S, et al. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators. 2012;18:31–41. [Google Scholar]
- 74.Aron JL, et al. Using watershed function as the leading indicator for water quality. Water Policy. 2013;15(5):850–858. [Google Scholar]
- 75.Tilman D, et al. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(26):10394–10397. doi: 10.1073/pnas.1208240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srivastava DS, Vellend M. Biodiversity-Ecosystem Function Research: Is It Relevant to Conservation? Annual Review of Ecology, Evolution, and Systematics. 2005;36(1):267–294. [Google Scholar]
- 77.Smith MD, et al. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology. 2009;90(12):3279–89. doi: 10.1890/08-1815.1. [DOI] [PubMed] [Google Scholar]
- 78.Cuppen JG, et al. Effects of a mixture of two insecticides in freshwater microcosms: I. Fate of chlorpyrifos and lindane and responses of macroinvertebrates. Ecotoxicology. 2002;11(3):165–80. doi: 10.1023/a:1015470731330. [DOI] [PubMed] [Google Scholar]
- 79.Van den Brink PJ, et al. Effects of a mixture of two insecticides in freshwater microcosms: II. Responses of plankton and ecological risk assessment. Ecotoxicology. 2002;11(3):181–97. doi: 10.1023/a:1015422815401. [DOI] [PubMed] [Google Scholar]
- 80.Kopáček J, et al. Nutrient cycling in a strongly acidified mesotrophic lake. Limnology and Oceanography. 2004;49(4):1202–1213. [Google Scholar]