Abstract
Objective
Parental monitoring of adolescents’ diabetes self-care is associated with better adherence and glycemic control (A1c). A number of parent-level factors are associated with higher levels of parental monitoring, including lower levels of parental distress (depressive symptoms, stress, anxiety), as well as higher levels of parental self-efficacy for diabetes management and authoritative parenting. Often studied in isolation, these factors may be best considered simultaneously as they are interrelated and are associated with parental monitoring and youth adherence.
Methods
Structural equation modeling with a cross-sectional sample of 257 parent/youth (aged 11-14) dyads: 1) examined a broad model of parental factors (i.e., parental distress, parental diabetes self-efficacy, authoritative parenting) and 2) assessed their relation to parental monitoring, youth adherence, and A1c. Post-hoc ANOVAs evaluated clinical implications of daily parental monitoring.
Results
Parental distress was not related directly to parental monitoring. Instead less distress related indirectly to more monitoring via higher parental self-efficacy and more authoritative parenting which in turn related to better adherence and A1c. Higher parental self-efficacy also related directly to better youth adherence and then to better A1c. Clinically, more parental monitoring related to more daily blood glucose checks and to better A1c (8.48% v. 9.17%).
Conclusions
A broad model of parent-level factors revealed more parental distress was linked only indirectly to less monitoring via lower parental self-efficacy and less authoritative parenting. Behaviorally, more parental monitoring related to better adherence and to clinically better A1c in adolescents. Further study of parent-level factors that relate to parental distress and monitoring of adherence appears warranted.
Type 1 diabetes (T1D) is one of the most common pediatric chronic illnesses with a prevalence of 1 in every 400 youth (CDC, 2011). Adolescents are at-risk for poorer adherence and glycemic control due to unique biological and behavioral challenges associated with this developmental period (Amiel, Sherwin, Simonson, Lauritano, & Tamborlane, 1986; Rausch et al., 2012). While adolescents can complete most diabetes tasks independently, parental monitoring is indicated for support with organization, decision-making, and reliable completion of tasks (Wysocki et al., 1992; Wiebe et al., 2005). In adolescence, parental monitoring is instrumental to prevent deterioration in adherence and glycemic control (Ellis et al., 2007; Grey, Davidson, Boland, & Tamborlane, 2001; Holmes, Chen, Mackey, Grey, & Streisand, 2014). As such, understanding parent-level factors that can hinder or facilitate parental monitoring has received increasing scrutiny (Carcone, Ellis, & Naar-King, 2012; Jaser & Grey, 2010; Whittemore, Jaser, Chao, Jang, & Grey, 2012). Disruptive factors include parental distress such as depressive symptoms, parenting stress, and anxiety about hypoglycemia, while facilitative factors involve parental self-efficacy for diabetes care and authoritative parenting. Although parental distress has been directly related to lower levels of parental monitoring (Mackey et al., 2014; Streisand, Braniecki, Tercyak, & Kazak, 2001; Wiebe et al., 2011) consideration of naturally co-occurring facilitative factors that enhance monitoring may offset disruptive factors and provide a more realistic understanding of how parental distress and monitoring are related.
Parental distress is conceptualized as psychological factors that often inhibit parenting practices (Abidin, 1992) and specifically are detrimental to caring for an adolescent with T1D. Parental distress in the current study encompasses depressive symptoms, parenting stress, and anxiety about acute complications of T1D (i.e., hypoglycemia). Parents of youth with T1D are more at risk for depressive symptoms than the general population (Driscoll et al., 2010; Helgeson, Siminerio, Escobar & Becker, 2009) with a maternal prevalence rate of 10 to 33% which exceeds the national incidence of 5 to 9% (Eckshtain, Ellis, Kolmodin, & Naar-King, 2009; Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2008; Mackey et al., 2014). Maternal depression is directly associated with poorer youth adherence (Carcone et al., 2012; Jaser & Grey, 2010; Whittemore et al., 2012) and indirectly related to poorer glycemic control (Anderson, Vangness, Connell, Butler, & Laffel, 2002; Eckshtain et al., 2009; McGrady, Laffel, Drotar, Repaske, & Hood, 2009). These associations appear linked by less parental monitoring of diabetes management (Mackey et al., 2014; Wiebe et al., 2011).
In addition to parental depressive symptoms, parental stress and anxiety contribute to parental distress and disrupt parental monitoring (Helgeson, Becker, Escobar, & Siminerio, 2012; Patton, Dolan, Smith, Thomas, & Powers, 2011). Stress can compromise cognitive functioning (Gillis, 1993), relates to less monitoring of disease care tasks (Streisand et al., 2001), and is associated with poorer glycemic control (Helgeson et al., 2012; Streisand, Swift, Wickmark, Chen, & Holmes, 2005). Unique to parents of youth with T1D, anxiety about acute, severe complications of hypoglycemia, also contributes to parental distress. Many parents fear that their youth will experience hypoglycemia and as a result may maintain higher blood glucose levels, resulting in poorer glycemic control (Clarke, Gonder-Frederick, Snyder, & Cox, 1998; Patton, Dolan, Henry, & Powers, 2007). Parental anxiety about hypoglycemia also can exacerbate parental depression and parenting stress (Irvine, Cox, & Gonder-Frederick, 1992; Marrero, Guare, Vandagriff, & Fineberg, 1997; Streisand et al., 2005).
In contrast to the disruptive effects of distress on monitoring, parental self-efficacy facilitates monitoring and youth adherence. However, parental distress may reduce feelings of diabetes self-efficacy and in turn may relate to less monitoring. Bandura (1977) postulates that parental self-efficacy fosters constructive parenting practices and ultimately positive child behaviors (Jones & Prinz, 2005). In youth with T1D, parental self-efficacy includes the extent to which a parent feels competent to manage a youth's daily disease care and is related to effective parental diabetes monitoring and ultimately to better youth adherence (Johnston & Marsh, 1989). Parents who believe they can execute diabetes tasks are more likely to monitor their youths’ diabetes care (Streisand, et al., 2005) and to have youth who are better at self-management (Evans & Hughes, 1987; Leonard, Skay, & Rheinberger, 1998). Thus, greater parental self-efficacy may help offset the adverse effects of distress on monitoring.
Like self-efficacy, authoritative parenting facilitates more parental monitoring and may help offset the adverse relation between parental distress and monitoring. Belsky (1984) posits that authoritative parenting fosters constructive parenting and youth behaviors. An authoritative parent maintains consistent but flexible limits coupled with high levels of nurturance (Baumrind, 1967). This general parenting style serves as a protective factor against poorer diabetes adherence and glycemic control during adolescence (Greene, Mandleco, Roper, Marshall, & Dyches, 2010; Shorer et al., 2011; Monaghan, Horn, Alvarez, Cogen, & Streisand, 2012). Like parental self-efficacy, authoritative parenting can be disrupted by parental distress (Eckshtain et al., 2009; Jaser & Grey, 2010). Interestingly, authoritative parenting may prove to be a necessary precursor to successful parental monitoring of diabetes care to ensure that monitoring behaviors are implemented effectively.
The current study proposes a structural equation model (SEM) to examine parent-level factors of distress, parental self-efficacy for diabetes management, and authoritative parenting that can either disrupt or facilitate parental monitoring. Less parental distress should relate to both higher parental self-efficacy and more authoritative parenting. These parenting factors should then relate to more diabetes monitoring, better youth adherence, and better glycemic control.
Method
Participants
Participants were 257 primary caregivers and their youth recruited from pediatric endocrinology clinics at two academic medical centers. Inclusion criteria required youth to be aged 11 to 14 at time of recruitment and to have a diagnosis of T1D for at least one year, without significant medical comorbidities. Sufficient fluency in reading English also was necessary.
Procedure
Data were collected as part of a multi-site, randomized clinical trial for a treatment program designed to prevent deterioration of parental involvement in adolescent adherence. Parents and youth were blinded to the study intent and were provided nonspecific information that the interventions would help with self-care behaviors. Approval was obtained by institutional review boards at both sites. Potential participants were identified from clinic schedules and received a recruitment letter. Of 395 eligible families successfully contacted by phone, 281 provided verbal consent to participate (71%). Assessments were scheduled in conjunction with a youth's medical appointment. After written informed consent and assent were obtained, research staff interviewed and administered questionnaires to parents and youth. Complete baseline data were provided by 257 dyads (91%). Families received a $25 gift card.
Measures
Demographic and medical information
Demographic data were provided by parents and socioeconomic status (SES) was calculated using parental education and occupation data (Hollingshead, 1975). Medical data were verified with record review.
Parental distress
The Distress construct was comprised of three separate components: depressive symptoms, parenting stress, and anxiety about hypoglycemia. The Beck Depression Inventory, Second Edition (BDI-II; Beck, Steer & Brown, 1996) asked parents to rate the severity of 21 symptoms in the prior two weeks along a four-point scale (from “least severe” to “most severe”). Parents who scored above a 29 were provided with a psychological referral. The BDI-II has high internal consistency (α = .92-.93). Acceptable internal consistency was detected within the current sample (α = .91). Total scores were analyzed.
Parenting stress was measured by The Pediatric Inventory for Parents (PIP; Streisand et al., 2001), a 42-item questionnaire that measures frequency of stressful events and perceived difficulty. Items are rated along a five-point scale according to Frequency (from “never” to “very often”) and Difficulty (from “not at all” to “extremely”). Internal consistency (Frequency, α = .95; Difficulty, α = .96) for this measure is excellent. Within the current sample high internal consistency was detected (Frequency, α = .93; Difficulty, α = .95). Frequency and Difficulty scores were analyzed.
The Hypoglycemic Fear Survey-Parent (HFS-P; Clarke et al., 1998) measured parental anxiety about hypoglycemia. The Worry Subscale of 13 items was administered. Items are rated on a five-point scale from “never” to “always” for statements that reflect anxiety (e.g., “My child having a reaction while alone,” “ My child blacking out”). Internal consistency is adequate (α = .96). In the current sample, internal consistency was .88. Total scores were analyzed.
Parental self-efficacy for diabetes management
The Self-Efficacy for Diabetes Self-Management Scale-Parent (SEDSM-P; Iannotti et al., 2006b) assessed perceived self-efficacy to perform diabetes care behaviors (e.g., blood glucose checks). Parents responded to 10 items along a 10-point rating scale that ranged from “not sure at all” to “completely sure”. Internal consistency (α = .90) is adequate. Internal consistency in the present sample was high (α = .85). Total scores were analyzed.
Authoritative parenting
The Psychological Autonomy-Granting subscale of The Parenting Style Index (PSI; Steinberg, Lamborn, Darling, Mounts, & Dornbusch, 1994) was administered to youth. The nine items were measured on a five-point scale from “never” to “always”. Internal consistency ranged from .72 to .82. In the current sample, reliability was .66. Total scale scores were analyzed.
Youth adherence and parental monitoring
A multi-method, multi-source assessment of the observable study constructs of youth adherence and parental monitoring was employed. This approach controlled measurement error and source error bias and counterbalanced weaknesses of one approach with the strengths of the other (Holmbeck, Li, Schurman, Friedman, & Coakley, 2002). The multi-method approach incorporated data from both self-report questionnaires and semi-structured interviews. Parent- and youth- reports provided multi-source data which were later combined statistically into latent constructs for analysis.
Youth adherence
Diabetes self-care was assessed in two ways, a global self-report questionnaire and a semi-structured interview. Parents and youth each completed the Diabetes Behavior Rating Scale (DBRS; Iannotti, 2006a), a 37-item self-report measure in which respondents report the frequency of completed diabetes tasks over the prior week along a five-point scale (from “never” to “always”) or a six-point scale (from “none” to “five times”). The DBRS has good internal consistency (α = .84 for both parent and youth report). In this sample, the reliability coefficient was adequate (pump: parent report α = .69, youth report α = .81; non-pump: parent report α = .79, youth report α = .80). Total scores for parent and youth report were analyzed.
Data collected within the 24-Hour Diabetes Interview (DI; Holmes et al., 2006, adapted from Johnson, Silverstein, Rosenbloom, Carter, & Cunningham, 1986) were used in a non-traditional manner as a proxy for and a second indicator of adherence. An a priori decision was made to evaluate number of blood glucose checks per day. Of the diabetes tasks recalled in the 24-Hour DI, diet, exercise, and insulin administration vary across patients and regimens, whereas recommendations for blood glucose checks are consistent (Chiang, Kirkman, Laffel, & Peters, 2014). Number of checks has been used previously as a proxy measure of diabetes adherence both alone (Hilliard, et al., 2011) or in combination with other adherence measures (Ellis et al., 2008; Hilliard et al., 2013) and is reliably associated with glycemic control (Johnson et al., 1992). Parents and youth each completed a 24-Hour DI that was insulin pump compatible (S. B. Johnson, personal communication, August 7, 2007). One set of semi-structured interviews was conducted in clinic and a second was completed in a follow-up phone call. Parents and youth were interviewed separately and asked to report the previous day's diabetes tasks in temporal sequence. Youth and parents referenced blood glucose meters throughout the interview to aid accuracy. Average number of blood glucose checks reported by parents and by youth were analyzed.
Parental monitoring
Two measures of parental diabetes monitoring were employed, a global self-report questionnaire of parenting behaviors and the 24-Hour DI. Both measures elicited parent- and youth-reports, obtained separately. The Parental Monitoring of Diabetes Care scale (PMDC; Ellis et al., 2007; Ellis et al., 2008) asked respondents to rate the frequency with which parents typically monitored 18 diabetes tasks during the previous month. A sample item is: “How often do you check your child's insulin vials to see if the expected amount has been used?” Responses were scored along on a five-point scale (e.g., “more than once a day,” “once a day,” “several times a week,” “once a week,” “less than once a week”). Internal consistency is adequate (α = .81). Internal consistency was .75 in the current sample. Total scores for parent and youth report were analyzed.
Data also were collected within the 24-Hour DI (Holmes et al., 2006) for a novel measure of parental monitoring. During the interview, participants were asked if a parent observed or discussed each blood glucose check. Percentage of blood glucose checks observed/discussed was calculated separately for the parent and youth interviews. Percentages were then averaged within individuals across the two interview days. This monitoring measure has been used previously and is related to better adherence and glycemic control (Hilliard et al., 2013; Mackey et al., 2014). Percentage of checks observed/discussed reported by parents and youth was analyzed.
Glycemic control
Glycemic control was measured with the glycosylated hemoglobin (A1c) assay which yields an indicator of average blood glucose concentration over the previous three months. Recommended A1c levels for youth are < 7.5% (Chiang et al., 2014). A1c was analyzed via blood assay (DCA 2000, Bayer Inc.; Tarrytown, NY, USA) collected at a diabetes clinic visit. Values were extracted later from medical records. Lower A1c values indicate better glycemic control. A1c values from the baseline assessment and three months following baseline were used in the current study to establish greater stability in this primary outcome variable. Both data collections occurred prior to the start of intervention.
Data Analytic Plan
Descriptive analyses were conducted with SPSS 21 (IBM Corp., 2012). Pearson's correlation coefficients evaluated relations among demographic factors, parental distress variables, parental self-efficacy, authoritative parenting, parental monitoring variables, adherence variables, and glycemic control. SEM analyses were conducted with Mplus 6 software (Muthen & Muthen, 1998-2010). The full information maximum likelihood procedure was used to include participants who had missing individual data points, presumed to be missing at random. This procedure, which is the default in Mplus 6, imputes missing data values based on the current estimate of known parameters and then re-estimates the parameters based on known and imputed data (Collins, Schafer, & Kam, 2001). This method is a preferred way to handle missing data, as it includes all available data in statistical analyses (Collins et al., 2001). Demographic data were not estimated.
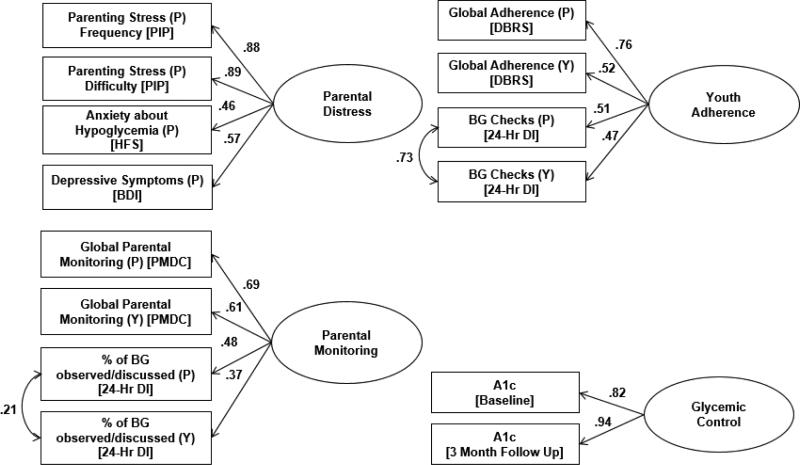
The fit and indicator factor loadings of each latent variable were examined using confirmatory factor analysis. The final measurement model consisted of four latent variables: parental distress (BDI-II, PIP, HFS), parental monitoring (parent- and youth-reported PMDC, parent- and youth-reported percentage of blood glucose checks observed/discussed on the 24-Hour DI), youth adherence (parent- and youth-reported DBRS, parent- and youth-reported blood glucose checks on the 24-Hour DI), and glycemic control (A1c at baseline and 3 months). Two observed variables also were included: authoritative parenting (PSI) and parental self-efficacy for diabetes management (SEDSM-P). See Figure 1 for the measurement model.
Figure 1.
Latent construct measurement models with standardized loadings. Source of data for each construct is provided within parentheses: P=Parent, Y=Youth, P+Y= Parent and Youth report, BG = blood glucose. Glycemic control values were obtained from medical charts.
With multiple reporters and multiple measures of observable behavior a latent construct can account more accurately for the data as a whole. Inclusion of ratings from multiple reporters allows analysis of family-level data, accounts for within-family non-independence and more accurately measures each construct (Kenny, 1995). Further, inclusion of parent- and youth-report reduces rater bias and improves the reliability of each construct (Kline, 2011).
Model fit and standardized path loadings were examined within the SEM (Kline, 2011; MacCallum & Austin, 2000). To account for contextual factors that may affect the constructs of interest, correlated medical and demographic variables were considered in the model. Mediation was tested through analysis of direct and indirect effects, or path coefficients, among the latent and observed variables in the model (MacKinnon, 2008). Mediated, or indirect, effects were calculated in Mplus as the product of direct effects (standardized coefficients) among independent, mediation, and dependent variables (Kline, 2011).
Overall model fit was assessed using five empirically established value indicators. A chi-square value closer to zero with a p value greater than .05 indicates good fit (Hu & Bentler, 1999). However, due to the large sample size of the current study, the chi-square statistic is not the best assessment of fit because it is closely related to sample size. A root-mean-square error of approximation (RMSEA) value below .06 indicates good fit (Hu & Bentler, 1998, 1999) and a standardized root mean square residual (SRMSR) value less than .08 indicates acceptable fit (Kline, 2011). Additionally, a comparative fit index (CFI) and Tucker-Lewis index (TLI) value above .90 indicate acceptable fit (Hu & Bentler, 1998).
Results
Descriptive Results
Participants included 257 youth (51% male) aged 11 to 14 years (M = 12.84, SD = 1.24) with T1D and their primary caregivers (91% mothers). A majority of youth were Caucasian (69%) and from middle-class families (42% upper-middle, 39% middle). Mean A1c was 8.81% (SD = 1.63) at baseline and 8.93% (SD = 1.54) at 3 months, higher than recommended by the American Diabetes Association (ADA) for this age (Chiang et al., 2014). Forty-four percent of youth used an insulin pump and 20% had an intensive basal/bolus regimen.
Parents reported levels of depressive symptoms similar to those found in other samples of parents of adolescents with T1D (Driscoll et al., 2010; Horsch, McManus, Kennedy, & Edge, 2007). Levels of frequency and difficulty of parenting stress (PIP; Streisand et al., 2001) and anxiety about hypoglycemia (HFS; Monaghan, Hilliard, Cogen, Streisand, 2009) also were normative. Normative data for parental self-efficacy and authoritative parenting were not available. Comparable levels of parental monitoring of diabetes care were observed in the present sample as in the test standardization sample (PMDC; Ellis et al., 2007; Ellis et al., 2008). Average item response for parents in the current v. test sample was 4.3 (range 2.9-5.0) v. 4.1, respectively, and for youth 4.3 (range 2.6-5.0) v. 4.0, respectively. The distribution of combined parent/youth scores reflects that most families perceived relatively frequent parental monitoring: 2% endorsed a M score of 5.0 (“more than once daily”); 72% had a M score 4.0-4.9 (“once/day”); 20% had a M score 3.0-3.9 (“3 or 4 times/week”); 1% had a M score of 2.0-2.9 (“once/week”); and none had a M score 1.0-1.9 (“less than once/week”).
Parents and youth reported somewhat low, though normative, levels of diabetes adherence on a global questionnaire (DBRS; Iannotti et al., 2006). Data from the 24-Hour DI revealed an average of 4.3 blood glucose checks per day. This falls below the six blood glucose checks per day recommended by the ADA but is comparable to that of other adolescent samples (Chiang et al., 2014; Holmes, et al., 2006). Demographic and disease characteristics of the sample are reported in Table 1. Bivariate correlations among primary study and demographic variables are reported in Table 2 (available online as supplemental material).
Table 1.
Participant characteristics; N = 257.
| Percentage or M (SD) | Parent Report M (SD) | Youth Report M (SD) | Sample Range | |
|---|---|---|---|---|
| Age (years) | 12.8 (1.2) | 11.0-14.9 | ||
| Age at Disease Onset (years) | 7.7 (3.2) | .3-13.6 | ||
| Disease Duration (years) | 5.1 (3.1) | .3-13.6 | ||
| A1c (%) | ||||
| Baseline | 8.81 (1.63) | 6.30-14.00 | ||
| 3 Months Post-baseline | 8.93 (1.54) | 5.90-14.00 | ||
| Hollingshead Index of SESa | 46.6 (11.7) | 12.0-66.0 | ||
| Gender: Male (%) | 51 | |||
| Ethnicity (%) | ||||
| Caucasian | 69 | |||
| African American | 19 | |||
| Hispanic | 6 | |||
| Asian/Asian American | 2 | |||
| Other | 4 | |||
| Insulin Regimen (%) | ||||
| Pump Therapy | 44 | |||
| Basal/bolus | 20 | |||
| Relationship to Child: Mother (%) | 91 | |||
| Two-Parent Families (%) | 77 | |||
| Parental Distress | ||||
| Parenting Stress Frequency (PIP) | 90.5 (24.6) | 43.0-189.9 | ||
| Parenting Stress Difficulty (PIP) | 83.7 (26.6) | 42.0-161.0 | ||
| Anxiety about Hypoglycemia (HFS-P) | 18.4 (8.9) | 0-50.0 | ||
| Depressive Symptoms (BDI) | 7.9 (7.7) | 0-44.0 | ||
| Parental Self-Efficacy (SEDSM-P) | 8.1 (1.3) | 3.6-10.0 | ||
| Authoritative Parenting (PSI) | 2.67 (.53) | 1.11-3.78 | ||
| Parental Monitoring | ||||
| % BG Checks Observed/Discussed (DI) | 60.0 (23.6) | 57.7 (21.8) | 0-100 (P+Y) | |
| Global Parental Monitoring (PMDC) | 78.0 (7.9) | 77.1 (8.5) | 52.3-90.0 (P) | |
| 47.0-90.0 (Y) | ||||
| Adherence | ||||
| Number of BG Checks per Day (DI) | 4.3 (1.5) | 4.3 (1.5) | 1.0-9.5 (P) | |
| 1.0-10.0 (Y) | ||||
| Global Adherence (DBRS) | .70 (.11) | .63 (.13) | .27-.92 (P) | |
| .28-.98 (Y) |
Note. BG = blood glucose, P=Parent, Y=Youth, P+Y= Parent and Youth report
Parental education level and occupation were transformed into scores that ranged from 8-66. Scores of 8-17 indicate “Lower Class,” 18-28 indicate “Lower-Middle Class,” 29-47 indicate “Middle Class,” 40-59 indicate “Upper-Middle Class,” and 60-66 indicate “Upper Class” (Hollingshead, 1975).
Latent Variable Measurement Model
Four latent variables were tested for use in the final structural equation model. The parental distress latent variable included four indicators: depressive symptoms, frequency and difficulty of parenting stress, and anxiety about hypoglycemia. This model fit the data well [χ2 (2) = .96, p = .618, CFI = 1.00, TLI = 1.01, RMSEA = .00, SRMR = .01]. The measurement construct of parental monitoring consisted of parent- and youth-report of global parental monitoring from the PMDC and parent- and youth-reported percentage of blood glucose checks that were observed by or discussed with a parent from the 24-Hour DI. With the inclusion of one covariance error between parent- and youth-report of observed/discussed blood glucose checks, the model achieved good fit [χ2 (1) = .92, p = .923, CFI = 1.00, TLI = 1.06, RMSEA = .00, SRMR = .01]. The latent variable for adherence included parent- and youth-report of global adherence from the DBRS and parent- and youth-report of the average number of blood glucose checks per day from the 24-Hour DI. The four indicators fit the data well with the inclusion of one covariance error between the parent- and youth-report of number of blood glucose checks [χ2 (1) = .56, p = .562, CFI = 1.00, TLI = 1.01, RMSEA = .00, SRMR = .00]. For all three measurement models, indicators sufficiently loaded onto the hypothesized latent constructs (β > .37, p < .001). Finally, given that the latent variable for glycemic control consisted of only two indicators, a significant bivariate correlation was sufficient to construct a latent variable (r = .78, p < .001). See Figure 1 for the standardized measurement model of the latent constructs.
Structure Equation Model
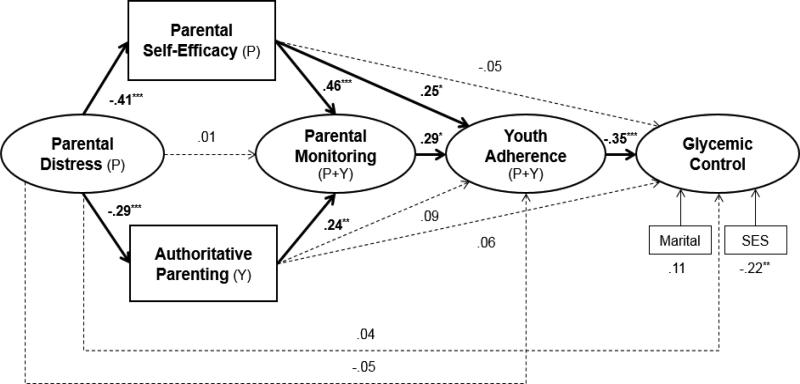
The proposed model fit the data well [χ2 (121) = 209.24, p < .001, CFI = .93, TLI = .91, RMSEA = .06, SRMR = .08; see Figure 2]. Less parental distress related to higher parental self-efficacy for diabetes management (β = -.41, p < .001) and to more authoritative parenting (β = -.29, p < .001). Higher parental self-efficacy (β = .46, p < .001) and more authoritative parenting (β = .24, p = .003) each were associated directly with more parental monitoring although parental distress only was associated indirectly with monitoring via self-efficacy and authoritative parenting. More parental monitoring related to better adherence (β = .29, p = .022) which in turn related to better glycemic control (β = -.35, p < .001). Finally, higher self-efficacy for diabetes management directly related to better adherence (β = .25, p = .011) and indirectly related to better adherence via more monitoring (β = .13, p < .05). The total indirect path between lower parental distress and better glycemic control was significant (β = .09, p = .044). Demographic variables significantly related to glycemic control were tested in the model as covariates. These included SES (β = -.22, p = .002) and marital status (β = .11, p = .111). Overall, the model accounted for 30% of the variance in parental monitoring, 27% of the variance in adherence, and 22% of the variance in glycemic control.
Figure 2.
Final structural equation model. Values shown are standardized regression coefficients. Source of data for each construct is provided within parentheses: P=Parent, Y=Youth, P+Y=Parent and Youth report. *p <.05. **p <.01, ***p<.001
Post-hoc analyses of variance (ANOVAs) were conducted to illuminate the clinical context of more versus less parental monitoring in daily diabetes management and glycemic control. A median split of combined parent/youth parental monitoring scores on the PMDC was used to evaluate dependent variables by groups with more parental monitoring (M Item Response = 4.6, SD = .2) versus less monitoring (M Item Response = 3.9, SD = .3). Bonferroni correction controlled for multiple comparisons, p-value <.008 (Bland & Altman, 1995). More parental monitoring was associated with more daily parental observation/discussion of blood glucose monitoring (M = 64.3%, SD = 18.2) compared to less monitoring [(M = 53.1%, SD = 17.3); F(1,253) = 25.49, p < .001]. More monitoring also was associated with better global adherence scores over the prior week (M DBRS = .70, SD = .11) compared to less monitoring [(M DBRS = .63, SD = .10); F(1,255) = 7.96, p = .005]. Further, more daily monitoring related to significantly more blood glucose checks per day (M = 4.6, SD = 1.4) compared to less monitoring [(M = 4.0, SD = 1.4); F(1,253) = 14.53, p < .001]. Finally, more parental monitoring related to statistically and clinically better glycemic control (M A1c = 8.48%, SD = 1.38) compared to less monitoring [(M A1c = 9.17%, SD = 1.80); F(1,255) = 11.94, p = .001]. Two additional ANOVAs were conducted to rule out the possibility that monitoring differences in A1c were due to underlying group differences in regimen type or SES which were not significant.
Discussion
A broad SEM model simultaneously evaluated disruptive (i.e., parental distress) and facilitative factors (i.e., parental self-efficacy, parenting style) on parental monitoring of diabetes care, youth adherence, and glycemic control. Most hypothesized interrelations among the parental factors and diabetes outcomes were supported. However, contrary to expectation, lower parental distress related to more parental monitoring only indirectly via facilitative factors of greater self-efficacy and more authoritative parenting. Interestingly, parental self-efficacy linked both directly and indirectly to better youth adherence, the latter via more parental monitoring of diabetes care. Authoritative parenting had only a direct relation to parental monitoring. As expected, more parental monitoring was associated with better youth adherence which in turn related to better glycemic control. Overall, the model demonstrated that parental distress, self-efficacy, and authoritative parenting accounted for approximately one-third of the variance in parental monitoring of diabetes care and approximately one-fourth of the variance in youth adherence behaviors and glycemic control.
As expected, less parental distress had a favorable relation with more authoritative parenting and with higher parental self-efficacy for diabetes management. These findings indicate two distinct avenues by which lower distress is related indirectly to more parental monitoring, to better youth adherence and ultimately, to better glycemic control. First, less distressed parents reported more authoritative parenting which was associated with more frequent monitoring. Authoritative parents are more likely to set expectations and to exhibit warmth and supportive communication, qualities that likely have a positive association with more parental monitoring. Previous research finds authoritative parenting related to better youth adherence directly, however these earlier studies did not evaluate the concomitant role of parental monitoring (Greene et al., 2010; Mlynarczyk, 2013). New to the literature then, the present results suggest authoritative parenting is related to youth adherence only indirectly via more monitoring. Further, less distressed parents reported more self-efficacy which related to more monitoring. Previously, depressive symptoms when studied in relative isolation were associated with less self-efficacy (Jones & Prinz, 2005) and less parental monitoring (Mackey et al., 2014). Current findings confirm that parents who are less distressed are more likely to believe that they can affect positive diabetes outcomes in youth. New to the literature, however, higher self-efficacy is also a mechanism by which lower parental stress and depressive symptoms relate to more frequent monitoring. That is, parents who are less distressed are more confident in their ability to manage diabetes and therefore are more likely to monitor youth's daily self-care tasks. Thus, parental stress, depressive symptoms, and anxiety about hypoglycemia should trigger further clinical attention because of their adverse relation with multiple health-related parenting behaviors.
Parental self-efficacy displayed direct associations with diabetes monitoring, as predicted, and with youth adherence, as found by others. More efficacious parents engage in more monitoring and also have youth with better adherence, regardless of the level of parental monitoring. Others also find parental self-efficacy directly relates to better youth adherence (Evans & Hughes, 1987) such as better insulin administration (Leonard et al., 1998). Although higher parental self-efficacy has beneficial associations with more monitoring and better youth adherence, cross-sectional data cannot determine the direction or transaction of effects. Plausibly, more parental monitoring or better youth adherence may promote higher parental self-efficacy that further enhances each individually. Importantly, in contrast to authoritative parenting, higher parental self-efficacy had multiple direct and indirect beneficial relations with better youth adherence while more authoritative parenting related only to more monitoring. Of these two mechanisms, the disease-specific indicator of higher parental diabetes efficacy had a stronger (β = .46, p < .001) relation to monitoring, double in magnitude to that of the more general parenting indicator (i.e., authoritative parenting, β = .23, p < .01). Unfortunately, adding to the general decline of adherence and parental monitoring in this early adolescent age, parental self-efficacy also tends to decline during adolescence (Glatz & Buchanan, 2015). Interventions that increase parental efficacy for diabetes management could have multiple emanative benefits on youth adherence.
Surprisingly, less parental distress did not relate directly to more parental monitoring. Instead, the current model indicates only indirect paths via the mechanisms of more parental self-efficacy and more authoritative parenting. Nevertheless, consistent with other reports (Mackey et al., 2014; Patton et al., 2007; Streisand et al., 2005), direct associations were found in the simple correlations (Table 2, available online) among individual components of parental distress (i.e., depressive symptoms, parenting stress, and anxiety about hypoglycemia) and individual elements of youth diabetes care (i.e., parental monitoring, youth adherence, and glycemic control). For example, the simple correlations showed parental depressive symptoms were significantly related to less parental monitoring. However, when parental depressive symptoms were examined in the present study along with other co-occurring symptoms of parental distress and within the context of parental self-efficacy and general parenting style, a more nuanced understanding of parental functioning in youth diabetes care emerged, as hypothesized. Consideration of co-occurring parental characteristics within this SEM provides a more thorough portrait of direct and indirect routes by which parental distress may be expressed in youths’ diabetes care.
Finally, a high level of parental monitoring was found in the current sample; three-fourths of families reported monitoring occurred daily or more than once per day. Nevertheless, against this backdrop of relatively frequent diabetes monitoring, the daily implications or clinical context of more monitoring are evident. Youth with relatively more parental monitoring had parents who observed or discussed daily blood glucose checks almost two-thirds of the time compared to a little over half of the time for those with less monitoring. Further, more parental monitoring related to better overall adherence reported in the prior week, to more blood glucose checks/day, and ultimately to A1c levels that were approximately .7% lower. An A1c difference of this magnitude is both statistically and clinically significant and approaches the degree of A1c change found with an intensification of insulin regimen (Johnson, Cooper, Jones, & Davis, 2013), although findings can vary by sample (Wiebe et al., 2010). Importantly, level of parental monitoring did not differ in relation to SES or type of insulin regimen, well known correlates of better A1c. Global reports of more parental monitoring related to a straightforward behavioral difference in more daily parental observation and discussion of blood glucose checks and to better glycemic control. These additional findings underscore the importance of parental monitoring in adolescents aged 11 to 14 even in a group of primarily middle-class youth. Correspondingly important then is a more nuanced understanding of the wide-range of parent-level factors that relate to parental monitoring since these factors accounted for 30% variance in the understanding of parental monitoring in this SEM.
Continued parental monitoring in diabetes management throughout adolescence is consistently related to better glycemic control (Grey et al., 2001; King, Berg, Butner, Butler, & Wiebe, 2014); however, this study represents one of the first attempts to evaluate multiple different parent factors and practices that relate to diabetes management. The validity of this SEM model is supported by replication of established demographic and disease relations. Higher SES and two-parent family status each were associated with better adherence and glycemic control, as well as with lower levels of parental distress. Inclusion of SES and family structure status in the model also helps establish generalizability of findings.
Limitations and Strengths
Study limitations include use of cross-sectional data, which do not allow causal inferences. Future longitudinal research is necessary to determine causality. Additionally, data were from the baseline assessment of a larger randomized clinical trial. Participants who enroll in a longitudinal study may be inherently different than families who do not. Further, the sample consisted of primarily middle-class families. In future studies, inclusion of additional parent-level factors, such as assessment of parental coping, work-related stress or marital support, might further illuminate the host of factors related to more parental monitoring.
Methodological strengths of the current study include use of multi-source, multi-method data from a large, ethnically diverse, multi-site sample of adolescents with T1D. Use of SEM allowed consideration of simultaneous interrelations among parent- and youth-report of behaviors and diabetes outcomes to better portray naturalistic functioning. Post-hoc analyses support the SEM findings and provide an indication of the clinical significance of the reported associations. However, while multi-source data are preferred, low informant agreement can occur as was the case in parent- and youth- report of parenting style in the present study. Less is known about parenting style in this age group of youth with T1D, therefore reporter bias was weighed in variable selection for the final measurement model (Holmbeck et al., 2002). Ceiling effects on the parent-completed questionnaire led to adoption of youth-report alone. Use of a single observed value of authoritative parenting should be weighed in the consideration of study findings.
Clinical Implications
Findings may inform further development of psychological interventions that target adherence in adolescents with T1D. Many family-based interventions aim to bolster parental monitoring during the transition to adolescence to prevent deterioration in glycemic control. The current study highlights parent-level factors that relate to more parental monitoring and to greater youth adherence. Providers may wish to consider additional components of parental functioning beyond depressive symptoms in an intervention program targeted at monitoring and adherence. Finally, given the multiple and adverse implications of parental distress, mental health screening could be incorporated into routine clinical care.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (Grant 5R01DK070917) awarded to CH.
Contributor Information
Elizabeth M. Robinson, Department of Psychology, Virginia Commonwealth University
Patrick Weaver, Department of Psychology, Eastern Michigan University
Rusan Chen, Center For New Designs In Learning & Scholarship, Georgetown University
Randi Streisand, Children's National Medical Center and George Washington University, Washington, DC.
Clarissa S. Holmes, Department of Pediatrics, Virginia Commonwealth University and Department of Psychiatry, Georgetown University.
References
- Abidin RR. The determinants of parenting behavior. Journal of Clinical Child Psychology. 1992;21:407–412. doi: 10.1207/s15374424jccp2104_12. [Google Scholar]
- Amiel S, Sherwin R, Simonson D, Lauritano A, Tamborlane W. Impaired insulin action in puberty: A contributing factor to poor glycemic control in adolescents with diabetes. New England Journal of Medicine. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. doi:10.1056/198607243150402. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Vangness L, Connell A, Butler D, Laffel LMB. Family conflict, adherence, and glycemic control in youth with short duration type 1 diabetes. Diabetic Medicine. 2002;19:635–642. doi: 10.1046/j.1464-5491.2002.00752.x. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. doi: 10.1016/0146-6402(78)90002-4. [DOI] [PubMed] [Google Scholar]
- Baumrind D. Child care practices anteceding three patterns of preschool behavior. Genetic Psychology Monographs. 1967;75:43–88. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Belsky J. The determinants of parenting: A process model. Child Development. 1984;55:83–96. doi: 10.1111/j.1467-8624.1984.tb00275.x. doi: 10.2307/1129836. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. British Medical Journal. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcone AI, Ellis DA, Naar-King S. Linking caregiver strain to diabetes illness management and health outcomes in a sample of adolescents in chronically poor metabolic control. Journal of Developmental and Behavioral Pediatrics. 2012;33:343–351. doi: 10.1097/DBP.0b013e31824eaac8. doi: 10.1097/DBP.0b013e31824eaac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. U.S. Department of Health and Human Services; Atlanta, GA: 2011. [Google Scholar]
- Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034–2054. doi: 10.2337/dc14-1140. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. Journal of Pediatric Endocrinology and Metabolism. 1998;11:189–194. doi: 10.1515/jpem.1998.11.s1.189. doi:10.1515/JPEM.1998.11.S1.189. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam C. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- Driscoll KA, Johnson SB, Barker D, Quittner AL, Deeb LC, Geller DE, Silverstein JH. Risk factors associated with depressive symptoms in caregivers of children with type 1 diabetes or cystic fibrosis. Journal of Pediatric Psychology. 2010;35:814–822. doi: 10.1093/jpepsy/jsp138. doi: 10.1093/jpepsy/jsp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckshtain D, Ellis DA, Kolmodin K, Naar-King S. The effects of parental depression and parenting practices on depressive symptoms and metabolic control in urban youth with insulin dependent diabetes. Journal of Pediatric Psychology. 2009;35:426–435. doi: 10.1093/jpepsy/jsp068. doi: 10.1093/jpepsy/jsp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Podolski TN, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact of regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. doi:10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Templin TN, Podolski CL, Frey MA, Naar-King S, Moltz K. The parental monitoring of diabetes care scale: Development, reliability and validity of a scale to evaluate parental supervision of adolescent illness management. Journal of Adolescent Health. 2008;42:146–153. doi: 10.1016/j.jadohealth.2007.08.012. doi: 10.1016/j.jadohealth.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Evans CL, Hughes IA. The relationship between diabetic control and individual and family characteristics. Journal of Psychosomatic Research. 1987;31:367–374. doi: 10.1016/0022-3999(87)90057-2. doi: 10.1016/0022-3999(87)90057-2. [DOI] [PubMed] [Google Scholar]
- Gillis JS. Effects of life stress and dysphoria on complex judgments. Psychological Reports. 1993;72:1355–1363. doi: 10.2466/pr0.1993.72.3c.1355. doi: 10.2466/pr0.1993.72.3c.1355. [DOI] [PubMed] [Google Scholar]
- Glatz T, Buchanan CM. Over-time associations among parental self-efficacy, promotive parenting practices, and adolescents' externalizing behaviors. Journal of Family Psychology. 2015;29:427–437. doi: 10.1037/fam0000076. doi: 10.1037/fam0000076. [DOI] [PubMed] [Google Scholar]
- Greene MS, Mandleco B, Roper SO, Marshall ES, Dyches T. Metabolic control, self-care behaviors, and parenting in adolescents with type 1 diabetes: A correlational study. The Diabetes Educator. 2010;36:326–336. doi: 10.1177/0145721710361270. doi:10.1177/0145721710361270. [DOI] [PubMed] [Google Scholar]
- Grey M, Davidson M, Boland EA, Tamborlane WV. Clinical and psychosocial factors associated with achievement of treatment goals in adolescents with diabetes mellitus. Journal of Adolescent Health. 2001;28:377–385. doi: 10.1016/s1054-139x(00)00211-1. doi:10.1016/S1054-139X(00)00211-1. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Becker D, Escobar O, Siminerio L. Families of children with diabetes: Implications of parent stress for parent and child health. Journal of Pediatric Psychology. 2012;37:467–478. doi: 10.1093/jpepsy/jsr110. doi: 10.1093/jpepsy/jsr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A four year longitudinal study. Journal of Pediatric Psychology. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Prediction of adolescents’ glycemic control 1 year after diabetes-specific family conflict. Archives of Pediatrics and Adolescent Medicine. 2011;165:624–629. doi: 10.1001/archpediatrics.2011.86. doi:10.1001/archpediatrics.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M, Holmes C, Chen ,R, Maher K, Robinson EM, Streisand R. Disentangling the role of family conflict in adolescents’ management of type 1 diabetes. Health Psychology. 2013;32:388–396. doi: 10.1037/a0027811. doi:10.1037/a0027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Holmbeck GN, Li ST, Schurman JV, Friedman D, Coakley RM. Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:5–18. doi: 10.1093/jpepsy/27.1.5. doi:10.1093/jpepsy/27.1.5. [DOI] [PubMed] [Google Scholar]
- Holmes CS, Chen R, Mackey E, Grey M, Streisand R. Randomized clinical trial of clinic-integrated, low-intensity treatment to prevent deterioration of disease care in adolescents with type 1 diabetes. Diabetes Care. 2014;37:1535–1543. doi: 10.2337/dc13-1053. doi:10.2337/dc13-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CS, Chen R, Streisand R, Marschall DE, Souter S, Swift EE, Peterson CC. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. doi:10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- Horsch A, McManus F, Kennedy P, Edge J. Anxiety, depressive, and posttraumatic stress symptoms in mothers of children with type 1 diabetes. Journal of Traumatic Stress. 2007;20:881–891. doi: 10.1002/jts.20247. doi: 10.1002/jts.20247. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. doi: 10.1037/1082-989X.3.4.424. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Iannotti RJ, Nansel TR, Schneider S, Haynie DL, Simons-Morton B, Sobel DO, Clark L. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006a;29:2263–2267. doi: 10.2337/dc06-0685. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Iannotti RJ, Schneider S, Nansel TR, Haynie DL, Plotnick LP, Clark LM, Simons-Morton B. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with T1D. Developmental and Behavioral Pediatrics. 2006b;27:98–105. doi: 10.1097/00004703-200604000-00003. doi: 10.1186/1479-5868-10-125. [DOI] [PubMed] [Google Scholar]
- Irvine AA, Cox DJ, Gonder-Frederick LA. Fear of hypoglycemia: Relationship to glycemic control and psychological factors in IDDM patients. Health Psychology. 1992;11:135–138. doi: 10.1037//0278-6133.11.2.135. doi: 10.1037/0278-6133.11.2.135. [DOI] [PubMed] [Google Scholar]
- Jaser S, Grey M. A pilot study of observed parenting and adjustment in adolescents with type 1 diabetes and their mothers. Journal of Pediatric Psychology. 2010;35:738–747. doi: 10.1093/jpepsy/jsp098. doi:10.1093/jpepsy/jsp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser S, Whittemore R, Ambrosino J, Lindemann E, Grey M. Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychology. 2008;33:509–519. doi: 10.1093/jpepsy/jsm104. doi:10.1093/jpepsy/jsm104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Kelly M, Henretta JC, Cunningham WR, Tomer A, Silverstein JH. A longitudinal analysis of adherence and health status in childhood diabetes. Journal of Pediatric Psychology. 1992;17:537–553. doi: 10.1093/jpepsy/17.5.537. doi:10.1093/jpepsy/17.5.537. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management of childhood diabetes. Health Psychology. 1986;5:545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case–control study. Diabetologia. 2013;56:2392–2400. doi: 10.1007/s00125-013-3007-9. doi: 10.1007/s00125-013-3007-9. [DOI] [PubMed] [Google Scholar]
- Johnston C, Mash EJ. A measure of parenting satisfaction and efficacy. Journal of Clinical Child Psychology. 1989;18:167–175. doi: 10.1037/0278-6133.5.6.545. [Google Scholar]
- Jones TL, Prinz RJ. Potential roles of parental self-efficacy in parent and child adjustment. Clinical Psychology Review. 2005;25:341–363. doi: 10.1016/j.cpr.2004.12.004. doi:10.1016/j.cpr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Kenny DA. The effect of nonindependence on significance testing in dyadic research. Personal Relationships. 1995;2:67–75. doi: 10.1111/j.1475-6811.1995.tb00078.x. [Google Scholar]
- King PS, Berg CA, Butner J, Butler JM, Wiebe DJ. Longitudinal trajectories of parental involvement in type 1 diabetes and adolescents' adherence. Health Psychology. 2014;33:424–432. doi: 10.1037/a0032804. doi: 10.1037/a0032804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd ed. Guilford Press; New York: 2011. [Google Scholar]
- Leonard BJ, Skay CL, Rheinberger MM. Self-management development in children and adolescents with diabetes: The role of maternal self-efficacy and conflict. Journal of Pediatric Nursing. 1998;13:224–233. doi: 10.1016/S0882-5963(98)80049-3. doi:10.1016/S0882-5963(98)80049-3. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- Mackey ER, Streumph K, Powell P, Chen R, Streisand R, Holmes C. Maternal depressive symptoms and disease care status in youth with type 1 diabetes. Health Psychology. 2014;33:783–791. doi: 10.1037/hea0000066. doi: 10.1037/hea0000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Erlbaum; New York, NY: 2008. [Google Scholar]
- Marrero DG, Guare JC, Vandagriff JL, Fineberg NS. Fear of hypoglycemia in the parents of children and adolescents with diabetes: Maladaptive or healthy response? The Diabetes Educator. 1997;23:281–286. doi: 10.1177/014572179702300306. doi:10.1177/014572179702300306. [DOI] [PubMed] [Google Scholar]
- McGrady M, Laffel LM, Drotar D, Repaske D, Hood KK. Depressive symptoms and glycemic control in adolescents with type 1 diabetes: Mediational role of blood glucose monitoring. Diabetes Care. 2009;32:804–806. doi: 10.2337/dc08-2111. doi: 10.2337/dc08-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarczyk SM. Adolescents' perspectives of parental practices influence diabetic adherence and quality of life. Pediatric Nursing. 2013;39:181–189. [PubMed] [Google Scholar]
- Monaghan MC, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with type 1 diabetes: Associations with illness characteristics and parent functioning. Families, Systems, and Health. 2009;27:28–38. doi: 10.1037/a0014770. [DOI] [PubMed] [Google Scholar]
- Monaghan M, Horn IB, Alvarez V, Cogen FR, Streisand R. Authoritative parenting, parenting stress, and self-care in pre-adolescents with type 1 diabetes. Journal of Clinical Psychology in Medical Settings. 2012;19:255–261. doi: 10.1007/s10880-011-9284-x. doi: 10.1007/s10880-011-9284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: Young children treated with continuous subcutaneous insulin infusion. Pediatric Diabetes. 2007;8:362–368. doi: 10.1111/j.1399-5448.2007.00242.x. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Smith LB, Thomas IH, Powers SW. Pediatric parenting stress and its relation to depressive symptoms and fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings. 2011;18:345–352. doi: 10.1007/s10880-011-9256-1. doi: 10.1007/s10880-011-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JR, Hood KK, Delamater A, Shroff Pendley J, Rohan JM, Reeves G, Drotar D. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care. 2012;35:1219–1224. doi: 10.2337/dc11-2163. doi: 10.2337/dc11-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorer M, David R, Schoenberg-Taz M, Levavi-Lavi I, Phillip M, Meyerovitch J. Role of parenting style in achieving metabolic control in adolescents with type 1 diabetes. Diabetes Care. 2011;34:1735–1737. doi: 10.2337/dc10-1602. doi: 10.2337/dc10-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Lamborn S, Darling N, Mounts N, Dornbusch S. Over-time changes in adjustment and competence among adolescents from authoritative, authoritarian, indulgent, and neglectful families. Child Development. 1994;65:754–770. doi: 10.1111/j.1467-8624.1994.tb00781.x. doi:10.2307/1131416. [DOI] [PubMed] [Google Scholar]
- Streisand R, Braniecki S, Tercyak KP, Kazak AE. Childhood illness-related parenting stress: The Pediatric Inventory for Parents. Journal of Pediatric Psychology. 2001;26:155–162. doi: 10.1093/jpepsy/26.3.155. doi:10.1093/jpepsy/26.3.155. [DOI] [PubMed] [Google Scholar]
- Streisand R, Swift E, Wickmark T, Chen R, Holmes C. Pediatric parenting stress among parents of children with type 1 diabetes: The role of self-efficacy, responsibility, and fear. Journal of Pediatric Psychology. 2005;30:513–521. doi: 10.1093/jpepsy/jsi076. doi:10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: A systematic mixed studies review. The Diabetes Educator. 2012;38:562–579. doi: 10.1177/0145721712445216. doi: 10.1177/0145721712445216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe DJ, Berg CA, Gelfand D, Butler J, Korbel C, Fortenberry KT, McCabe J. Longitudinal associations of maternal depressive symptoms, maternal involvement, and diabetes management across adolescence. Journal of Pediatric Psychology. 2011;36:837–846. doi: 10.1093/jpepsy/jsr002. doi: 10.1093/jpepsy/jsr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe DJ, Berg CA, Korbel C, Palmer DL, Beveridge RM, Upchurch R, Donaldson DL. Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30:167–178. doi: 10.1093/jpepsy/jsi004. doi:10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Croom A, Fortenberry KT, Butner J, Butler J, Swinyard MT, Berg CA. Parental involvement buffers associations between pump duration and metabolic control among adolescents with type 1 diabetes. Journal of Pediatric Psychology. 2010;35:1152–1160. doi: 10.1093/jpepsy/jsq012. doi:10.1093/jpepsy/jsq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T, Meinhold PA, Abrams KC, Barnard MU, Clarke WL, Bellando BJ, Bourgeois MJ. Parental and professional estimates of self-care independence of children and adolescents with IDDM. Diabetes Care. 1992;15:43–52. doi: 10.2337/diacare.15.1.43. doi:10.2337/diacare.15.1.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.