Abstract
Cellular commitment to the mitochondrial pathway of apoptosis is accomplished when pro-apoptotic BCL-2 proteins compromise mitochondrial integrity through the process of mitochondrial outer membrane permeabilization (MOMP). For nearly three decades, intensive efforts focused on the identification and interactions of two key pro-apoptotic BCL-2 proteins: BAK and BAX. Indeed, we now have critical insights into which BCL-2 proteins interact with BAK/BAX to either preserve survival or initiate MOMP. In contrast, while mitochondria are targeted by BAK/BAX, a molecular understanding of how these organelles govern BAK/BAX function remains far less clear. Here, we integrate recent mechanistic insights of pro-apoptotic BCL-2 protein function in the context of mitochondrial environment, and discuss current and potential pharmacological opportunities to control MOMP in disease.
Keywords: Apoptosis, BCL-2 family, BH3 mimetics, Mitochondria, MOMP
The Mitochondrial Pathway of Apoptosis: Defining the Proteins
The mitochondrial pathway of apoptosis (see Glossary) is essential for metazoan development, tissue homeostasis, and cellular responses to therapeutics. The involvement of mitochondria in vertebrate apoptosis was first suggested when caspase activity resulted from Xenopus oocyte extract co-incubation with purified mitochondria. This activity was blocked by the addition of recombinant B-cell CLL/Lymphoma 2 (BCL-2) protein, suggesting that BCL-2 could prevent mitochondrial engagement of the cytosol by blocking cytochrome c release [1]. Since then, it was discovered that the adaptor protein APAF-1 (apoptotic protease activating factor-1) binds cytosolic cytochrome c, undergoes oligomerization into a heptameric complex, and recruit pro-caspase-9 to form the “apoptosome”. Dimerization of pro-caspase-9 within the apoptosome promotes downstream activation of executioner caspase activation (e.g., caspases-3 & -7) and ultimately commits a cell to apoptosis [2, 3].
After the discovery of BCL-2, the BCL-2 family quickly expanded to include almost twenty members that are divided into two functional classes of proteins: anti-apoptotic and pro-apoptotic (reviewed in [4]). Most cells express a variety of anti-apoptotic and pro-apoptotic BCL-2 proteins, and through the regulation of their interactions command survival or commitment to apoptosis.
Anti-apoptotic BCL-2 proteins are comprised of four BCL-2 homology domains (BH1-4) and are generally integrated within the outer mitochondrial membrane (OMM), but are present in other membranes like the endoplasmic reticulum (ER). BCL-2, BCL-xL, and MCL-1 are the major members of the anti-apoptotic BCL-2 repertoire that preserve OMM integrity by binding and inhibiting the pro-apoptotic BCL-2 proteins.
The pro-apoptotic BCL-2 members are divided into effectors (which also contain BH1-4) and the BH3-only proteins. The pro-apoptotic effector BCL-2 proteins BAK (BCL-2 antagonist killer 1) and BAX (BCL-2 associated x protein) homo-oligomerize into proteolipid pores within the OMM and are required to promote mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release. However, effectors require activation steps, upon which they oligomerize and gain the capacity to permeabilize membranes composed of mitochondrial lipids [5]. BAK and BAX activation occurs through interactions with so-called “direct activators” BH3-only proteins, or by physico-chemical effects of detergents, mild heat, or elevated pH.
In an issue of Trends in Cell Biology published approximately a decade ago [6], we discussed a highly controversial topic in the apoptosis field: Do BH3-only proteins bind BAK/BAX to induce conformational changes, revealing their membrane permeabilizing activity, or do BH3-only proteins solely promote apoptosis by neutralization of the anti-apoptotic BCL-2 proteins to release BAK/BAX? In essence, are BAK/BAX constitutively competent to promote MOMP - or - do BAK/BAX require interactions with BH3-only protein to activate apoptosis? [6] Since 2008, numerous biochemical, cellular, and structural studies provided evidence that BAK/BAX require direct interactions with BID/BIM to initiate membrane permeabilization and apoptosis. Furthermore, the vast majority of evidence supports that BH3-only proteins interact with both anti-apoptotic BCL-2 proteins and effector molecules to unify these once disparate hypotheses [7].
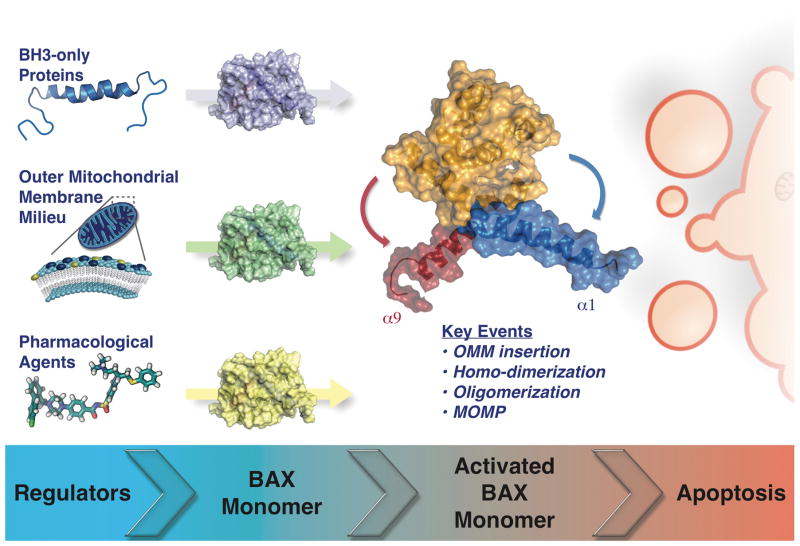
In the above article [6], we also proposed three questions pertinent for future investigations of pro-apoptotic BCL-2 family function and MOMP: (i) Will our understanding of the BH3-only proteins, as determined by synthetic BH3 domain peptides, be corroborated following full-length protein analyses? (ii) What mitochondrial components regulate BAK/BAX-dependent MOMP? And finally, (iii) Is it possible to pharmacologically regulate pro-apoptotic BCL-2 proteins as an effective therapy in human disease? These questions will be approached within the context of recent biochemical, cellular, and genetic evidence to provide an updated perspective of the physiological and pharmacological control of MOMP and apoptosis (Figure 1).
Figure 1. Key Figure. Pro-apoptotic effector BCL-2 proteins are activated via distinct regulators.
The pro-apoptotic BH3-only proteins (e.g., BID), the composition/shape of the outer mitochondrial membrane (e.g., cardiolipin), and the pharmacological agents mimicking the direct activator BH3-only proteins (e.g., BAM7), impact upon BAX activation. Upon activation, monomeric BAX undergoes structural rearrangements in which helix α1 and the C-terminal transmembrane helix α9 moves away from the BCL-2 globular core. Afterward, several key events take place (e.g., OMM insertion, homo-dimerization, oligomerization, and MOMP) to initiate apoptosis. An “inactive” BAX monomer is shown as a cartoon with surface representation in purple, green, and yellow. The conformationally active BAX monomer surface is depicted in orange representing the BCL-2 globular core; and helices α1 and α9 in blue and red, respectively (BAX PDB: 1F16). While BAX is the focus of this figure, BAK and BOK may share similar characteristics.
Are BH3 Domain Peptides Appropriate Surrogates to Study BH3-Only Protein Function?
A majority of mechanism-based studies involving the BCL-2 proteins defined the intra-family interactions with the BH3-only proteins via chemically-synthesized peptides derived from BH3 regions. These peptides are referred to as “BH3 domain peptides”, and due to the significant number of BH3-only proteins, these tools provided a rapid means to investigate key interactions and activities [8, 9].
BH3 domain peptides enabled rapid and insightful three-dimensional structures of anti-apoptotic BCL-2 family in complex with BH3 domains - along with providing the basis of drug discovery in the family [10]. Interactions between BH3 domains and the anti-apoptotic BCL-2 members are established through the cooperation between four hydrophobic residues (h1–h4) on the BH3 domain α-helix and the corresponding hydrophobic binding pocket of the anti-apoptotic BCL-2 protein (Figures 2 & 3) [11]. Variations in the BH3 domain sequence and hydrophobic grooves establish binding selectivity and affinity; and stapling BH3 domain peptides into stable helices increases their relative affinities for anti-apoptotic BCL-2 proteins [11]. But has the ease of this model system prevented us from fully understanding how BH3 domains regulate apoptosis?
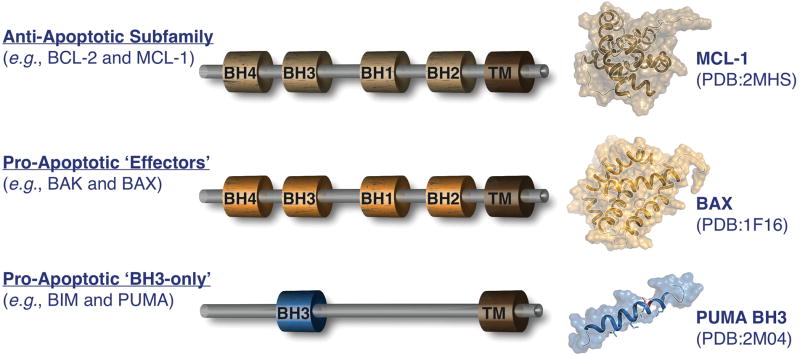
Figure 2. Overview of the BCL-2 family.
BCL-2 homology domain (BH) composition of the anti-apoptotic, pro-apoptotic ‘effectors’, and pro-apoptotic ‘BH3-only’ proteins in beige, orange, and blue, respectively. Corresponding three-dimensional structures of MCL-1 (PDB:2MHS), BAX (PDB:1F16), and the PUMA BH3 domain (generated by subtracting the BCL-xL from the complex structure PDB:2M04).
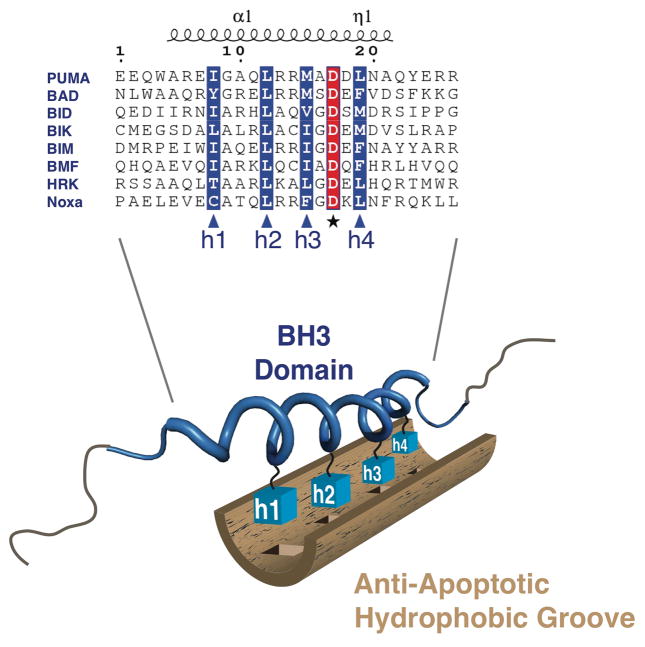
Figure 3. A BH3 domain - anti-apoptotic hydrophobic groove interaction.
Alignments of the α helical BH3 domains of BH3-only proteins are depicted. The hydrophobic (h) residues required for interactions with the hydrophobic groove of anti-apoptotic BCL-2 family members are highlighted in blue and indicated as h1 through h4. The conserved aspartic acid residue involved in salt-bridge formation is highlighted in red. A cartoon representation of the solution structure of the PUMA BH3 domain (PDB:2M04) is depicted in blue with the hydrophobic residues (h1–h4) as blue boxes. Motifs outside of the BH3 domain are likely regulatory regions necessary for cellular signaling modifications, stability/degradation, and secondary structure.
Early work suggested bifurcation of the BH3 domains into direct activators and sensitizers-de-repressors based on their abilities to activate BAX, or inhibit anti-apoptotic BCL-2 proteins [9, 12, 13]. Direct comparisons of recombinant proteins and BH3 domain peptides revealed that the majority of interactions and functions were maintained between these systems. For example, the BID-mediated BAX activation is potent for both protein and peptide [13]. A different study compared the full-length PUMA protein and its BH3 peptide counterpart. The findings revealed that full-length PUMA protein, like its BH3 peptide, induced MOMP through its de-repressor/sensitizer role by releasing sequestered direct activator proteins from the OMM, and by sensitizing mitochondria to future direct activator exposure [12]. A more recent study comparing BID and BIM proteins and peptides suggests that while BID/BIM can activate BAK/BAX, previously unrecognized differences are observed. In three complementary model systems (i.e., cells, mitochondria, & large unilamellar vesicles “LUVs”), distinct preferences for BID to activate BAK, and BIM to activate BAX was apparent [14].
Our understanding of BH3-only proteins, however, is not yet free from controversy. Several studies using both PUMA protein BH3 peptide domains suggest that PUMA is a direct activator. A stapled version of the PUMA BH3 peptide can potently inhibit anti-apoptotic BCL-2 proteins, and also directly bind and activate BAX and induce apoptosis [15]. Both the BH3 domain peptide and full-length PUMA protein are able to interact with BAK leading to BAK homo-oligomerization and membrane permeabilization, and in a cellular context, BAK-mediated apoptosis [16]. These results obtained with full-length protein and synthesized BH3 domain peptide provide strong evidence that PUMA, like BIM and BID, is able to act as a direct BAK/BAX activator. Curiously, the combined genetic loss of Bid, Bim, and Puma have also suggested that Noxa can have direct activator function in cells, yet the majority of data suggest that the Noxa BH3 domain peptide is a weak direct activator [9, 13, 17, 18]. Furthermore, a recent finding challenges the requirement for BH3-only protein in BAK/BAX activation by genome editing [19]. How do we reconcile these differences? Differences in protein and peptide preparations could be one explanation, but perhaps changes to the mitochondrial environment impact on successful activation by distinct BH3-only proteins and their BH3 domains? We should re-evaluate the role of mitochondrial shape and composition to determine if the differences observed are reconciled based on mitochondrial biology.
Alternatively, a recent structural study revealed a surprising function for PUMA in the mitochondrial apoptosis pathway [20]. The study showed that upon PUMA BH3 domain binding with BCL-xL, a partial unfolding within the BCL-xL structure was induced. Interestingly, residue Trp71 in the PUMA BH3 domain (which is not one of the hydrophobic h1-h4 residues) plays a critical role in mediating the partial unfolding in the BCL-xL structure. Via π-stacking with residue His113 of BCL-xL, this interaction unfolds α2–3 helices within BCL-xL disrupting the interface between BCL-xL and p53 and thereby releasing the bound p53 to engage BAX, and trigger apoptosis [21]. At present it is not known if full-length PUMA protein exhibits a similar disruptive behavior towards the BCL-xL·p53 interaction or how mitochondrial biology impacts on PUMA-mediated BCL-xL conformational changes.
Is it safe to assume that the BH3 peptides function like full-length counterparts in the regulation of BAK/BAX and anti-apoptotic proteins? We believe not, which then begs the question: what is the purpose of the region(s) outside the BH3 domain? The BH3-only protein subfamily members are intrinsically disordered proteins (except for BID) and contain both a BH3 and C-terminal transmembrane domain (Figure 2). The BH3 domain becomes ordered upon binding to a BCL-2 family member and folds into a α-helix. Perhaps, the rest of the disordered regions act as an interaction platform for cellular signaling modifications to positively and negatively regulate BH3 domain function, and confer secondary structure once the BH3 domain is bound by the hydrophobic groove.
What Mitochondrial Components Regulate BAK/BAX Dependent MOMP?
The vast majority of BCL-2 family literature focuses on the interactions between individual BCL-2 proteins as the major point of MOMP regulation. More recently, however, several key studies revealed that mitochondrial composition and shape directly control BAK/BAX function and sensitivity to apoptosis. There are at least three major factors that contribute to the mechanistic control of BAK/BAX-dependent MOMP: (i) a stress-specific combination of pro-apoptotic BH3-only proteins, (ii) an actively maintained and regulated lipid composition within the OMM, and more recently (iii) a specific mitochondrial shape/size. The first factor is briefly highlighted above, and more detailed discussions are available elsewhere. Here, we discuss the last two factors keeping in mind that this literature is still small, and at this point, many of the observations are not integrated into a cohesive mechanistic understanding (Figure 4). Recent discoveries reveal that mechanistic relationships between mitochondrial shape/size and the apoptosis machinery regulate stem cell identity, self-renewal, and cell fate decisions. This occurs via nuclear transcriptional responses and mitochondrial re-programming, and it is becoming evident that mitochondrial shape/size contribute a direct role in these processes [22, 23]. Indeed, earlier work first demonstrated that chronic mitochondrial fission promoted oncogenic transformation and tumor growth, suggesting that mitochondrial fission promotes apoptotic resistance and cancer signaling mechanisms [24–26].
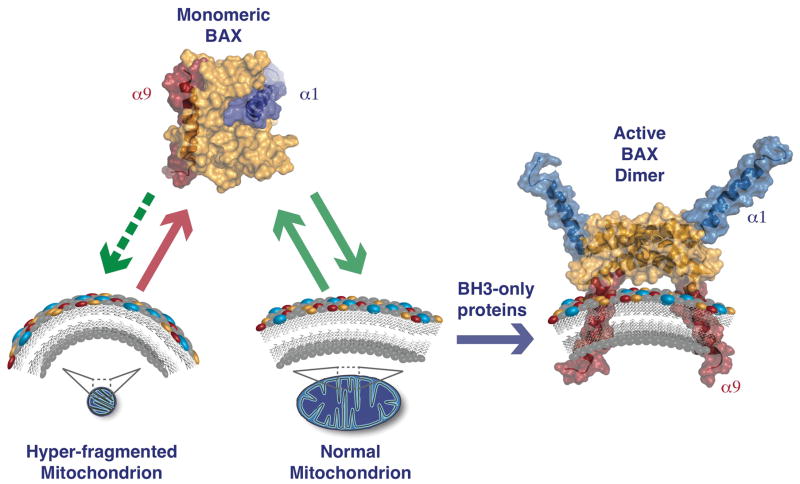
Figure 4. The shape and composition of the outer mitochondrial membrane governs BAX activation.
Monomeric BAX dynamically and reversibly interacts with the OMM, which causes transient exposure of both α1 and α9. When mitochondria are hyper-fragmented (i.e., high membrane curvature), the dynamics between BAX and the OMM are markedly reduced, which lowers the probability of stable activation by BH3-only proteins. In addition to shape, hydrophobic components within the OMM engage BAX to also regulate membrane interactions and successful activation by BH3-only proteins. Cholesterol, cardiolipin, and 2(E)-hexadecenal are depicted in yellow, blue, and red spheres, respectively. The BAX monomer and dimer are shown with surface representations: the BCL-2 globular core in orange, α1 helix in blue, and α9 helix in red (BAX monomer PDB:1F16 and BAX dimer PDB:4BDU).
The OMM environment is the predominant site for interactions between all pro-apoptotic BCL-2 proteins, including BH3-only·effector (e.g., BID·BAK) and effector·effector (e.g., BAX·BAX). Early insights into the mechanism of BAX-mediated pore formation suggested that mitochondrial lipids are involved in the activation of BAX, or alternatively, in the BAX-dependent membrane permeabilization process [27]. More specifically, data pointed to cardiolipin, a negatively charged phospholipid localized almost exclusively in the inner mitochondrial membrane (IMM). However, small amounts of cardiolipin are localized in the OMM, which produces a key structural junction between the IMM and OMM.
Various model systems (e.g., LUVs; outer membrane vesicles, OMVs) that mimic the OMM revealed a requirement of cardiolipin for BAX-induced permeabilization of membranes. Interestingly, LUVs composed of lipids that resemble the endoplasmic reticulum (ER) did not undergo BAX-mediated permeabilization unless cardiolipin was added [27, 28]. These data suggest that cardiolipin has the potential to regulate BAX-dependent MOMP by facilitating BAX insertion or nucleating BAX-dependent pore formation. However, deletion of cardiolipin synthase in yeast mitochondrial model systems expressing human BAX had no effect on BAX-dependent MOMP and the subsequent release of cytochrome c [29]. This has been confirmed in vitro, where BAX-mediated permeabilization of cardiolipin-free LUVs treated with specific proteins or lipids were added (discussed below) [30, 31]. In addition to BAX, the activated form of BID (cleaved by caspase-8; cBID) is also regulated by cardiolipin. Although cBID does not require cardiolipin for the initial binding to a membrane, cardiolipin may promote conformational changes in cBID that enhance BAX activation; Mtch2, a carrier protein localized to the OMM, may facilitate a similar and/or redundant mechanism [32, 33].
Mitochondrial cholesterol has also emerged as potential regulator of MOMP in multiple model systems [28, 32, 34]. Cholesterol hinders BAX-mediated membrane permeabilization due a combination of reduced interactions with BH3-only proteins within a membrane, and reduced kinetics of BAX insertion into membranes. The mechanism by which high levels of cholesterol block BAX-dependent membrane remains controversial. There are reports that cholesterol can reduce membrane fluidity that indirectly impacts on BAX dynamics with membranes. However, there is also a putative cholesterol-binding motif on BAX, which may also participate [35]. As cholesterol metabolism is often deregulated in cancer, it is tempting to speculate that high mitochondrial levels may contribute to both tumorigenesis and chemotherapeutic responses by disrupting BAX-dependent apoptosis [36].
Sphingolipids are another important class of lipids that regulate multiple aspects of BAK/BAX-dependent MOMP. Initial studies suggested that long chain ceramides form pores in defined membranes and isolated mitochondria, and that this pore-forming activity is regulated by anti-apoptotic BCL-2 proteins [37]. More recently, reports indicate that ceramide can promote BAX-mediated permeabilization [38]. While BAK on the other hand, contributes to the production of long-chain ceramides following genotoxic stress [39]. Connecting BAK to ceramide generation provides a novel link within the apoptotic pathways previously thought to function independently: Does pro-apoptotic signaling converge via the BCL-2 effector molecules to establish a BAK-regulated mitochondrial environment that is permissive for BAX-dependent MOMP?
One potential answer to the above question lies with the understanding that sphingolipids are metabolized into a myriad of lipid species that influence cellular sensitivity to apoptosis. For example, ceramides are often precursors for sphingoine-1-phosphate (S1P) generation, and S1P can be degraded into the fatty aldehyde, 2(E)-hexadecenal. Interestingly, a link between S1P and 2(E)-hexadecenal metabolism has also converged on BAK/BAX function. Using purified mitochondria deficient in S1P metabolism, biochemical reconstitution studies revealed that S1P and 2(E)-hexadecenal cooperated with BAK and BAX, respectively, to promote MOMP and apoptosis [31]. While direct binding between 2(E)-hexadecenal and BAX was observed and suggested to regulate activation-associated conformation changes within BAX, no further mechanistic insights are known.
The lipid composition of mitochondria impact on pro-apoptotic BCL-2 family function, but numerous reports suggest that mitochondrial membrane curvature and size also govern sensitivity to MOMP. Early work revealed that small LUVs (<0.2 micron diameter) resist BAX-dependent permeabilization [34]. These findings were corroborated and expanded in a recent study in which multiple OMM model systems were used to interrogate the functional contribution of mitochondrial shape in promoting BAX-mediated MOMP [26]. Specifically, in vitro reconstitutions of BID- and BIM-mediated BAX activation revealed that small LUVs, OMVs, and tiny mitochondria (<0.5 microns in diameter) failed to support the stable release and/or insertion of activated BAX monomers (specially, alpha helix 9) into membranes. Indeed, the insertion of activated BAX monomers into a membrane is a key step that precedes oligomerization and MOMP [40, 41].
While the majority of our discussions have focused on how mitochondrial membranes control BAK and BAX, several additional observations must be highlighted. First, mitochondrial lipids also facilitate several BH3-only proteins (i.e., BID & cardiolipin cooperate; and BIM & anionic lipids cooperate) and MCL-1 (regulated by cardiolipin and cholesterol) [32, 42]. Secondly, BAX and cBID can independently remodel membranes leading to alterations in tethering, stabilized membrane curvature, and the dissociation of lipid species --- all of which are inhibited by the addition of BCL-xL [43]. Finally, the large GTPases responsible for mitochondrial dynamics have been shown to interact with BAX for collateral regulation of both mitochondrial fission and apoptosis. An independent role for DRP1 (dynamin related protein 1: a large GTPase required for mitochondrial fission) was described to remodel the OMM via hemi-fusion to promote BAX oligomerization [44]. Considering multiple signaling pathways, environmental influences, and pathophysiological conditions directly alter mitochondrial composition and shape, it is becoming increasing clear that we must continue to interrogate and integrate these pathways.
Is It Possible To Pharmacologically Regulate Pro-Apoptotic BCL-2 Proteins as an Effective Therapy in Human Disease?
Over the last three decades, research on mitochondrial pathway of apoptosis has positioned this mechanism as a key regulator of tumorigenesis and chemotherapeutic success [45]. Three lines of evidence suggest that BCL-2 regulated apoptosis is an excellent therapeutic target for small molecule development to treat malignancies: (i) increased expression of anti-apoptotic BCL-2 proteins confers tumorigenesis and chemoresistance; (ii) tumors constitutively sequester functional direct activator proteins that can be unleashed to promote MOMP; and (iii) the in vitro treatment of cancer cells with a sensitizer BH3 domain peptide reveals which tumors are apoptosis-competent and likely to respond to chemotherapeutic interventions. [46, 47].
Chemically synthesized BH3 domain peptides represented a “proof of concept” that the hydrophobic groove of anti-apoptotic BCL-2 proteins is a rational drug target --- and significant efforts focused on discovering and refining small molecules that bind within groove [45]. In theory, these drugs would release constitutively sequestered direct activator BH3-only proteins to engage MOMP (i.e., functioning as a single agent), or alternatively, this class of molecules would also prevent chemotherapy-induced BH3 only proteins from being inhibited lowering the apoptotic threshold (i.e., functioning in combination strategies).
Structural analyses of BH3-only proteins in complex with anti-apoptotic BCL-2 members examined within the perspective of the above observations led to the development of “BH3-mimetics” [48]. In this final section we discuss the recent progress in the pharmacological modulation of individual BCL-2 family proteins to selectively promote MOMP. We are focused on cancer therapeutics, but BH3 mimetics may also influence other human diseases impacted by de-regulated apoptosis.
At present, there are approximately twenty small molecules defined as BH3-mimetics (Table 1). Some of these molecules (e.g., gossypol) were identified in high-throughput screens of natural products, while others (e.g., ABT-737) were developed using rational drug design strategies. Here, we will focus on the most specific BH3-mimetics: the Abbott compounds (ABT-199, ABT-263, & ABT-737), recent MCL-1 inhibitors (MIM1 & A-1210477), and a first in class small-molecule that activates BAX (BAM7).
Table 1.
Overview of Current Small Molecules Targeting the BCL-2 family of Proteins.
| Compound Name | Targets | Stage | Reference |
|---|---|---|---|
| ABT-737 | BCL-XL, BCL-2, BCL-W | Phase II completed | [49] |
| ABT-263, Navitoclax | BCL-XL, BCL-2, BCL-W | Phase I/II | [57, 58] |
| ABT-199, Venetoclax | BCL-2 | Phase I/II/III | [59] |
| GX15-070, Obatoclax | BCL-XL, BCL-2, MCL-1, BCL-W | Phase I/II | [60] |
| AT-101, R-(−)gossypol | BCL-XL, BCL-2, MCL-1, BCL-W | Phase I/II/III | [61] |
| S1 | BCL-XL, BCL-2, MCL-1 | Phase I/II/III | [62] |
| Apogossypol | BCL-XL, BCL-2, MCL-1, BCL-W | Preclinical | [63] |
| Apogossypolone, ApoG2 | BCL-XL, BCL-2, MCL-1 | Preclinical | [64] |
| BI-97C1, Sabutoclax | BCL-XL, BCL-2, MCL-1, BCL-W, A1 | Preclinical | [65] |
| BI-97D6 | BCL-XL, BCL-2, MCL-1, A1 | Preclinical | [66] |
| TW37 | BCL-XL, BCL-2, MCL-1, BCL-W | Preclinical | [67] |
| BH3-M6 | BCL-XL, BCL-2, MCL-1 | Preclinical | [68] |
| BM-1197 | BCL-XL, BCL-2 | Preclinical | [69] |
| JY-1-106 | BCL-XL, MCL-1 | Preclinical | [70] |
| Marinopyrrole A, Maritoclax | MCL-1 | Preclinical | [71] |
| MIM1 | MCL-1 | Preclinical | [54] |
| A1210477 | MCL-1 | Preclinical | [55] |
| Small molecule MCL-1 inhibitors | MCL-1 | Preclinical | [72] |
| BAM7 | BAX | Preclinical | [56] |
| WEHI-539 | BCL-XL | Preclinical | [73] |
| A-1155463 | BCL-XL | Preclinical | [74] |
| XXA1 | BCL-XL | Preclinical | [75] |
ABT-737 was discovered over a decade ago using nuclear magnetic resonance based screening, parallel synthesis, and structure-based design [49]. ABT-737 and its orally available derivative ABT-263 (Navitoclax) selectively bind to BCL-xL, BCL-2, and BCL-W (Table 1). ABT-737 possesses anti-tumor activity in many different cancer models including melanoma and prostate [50, 51]. ABT-737 is regarded as an experimental tool, but ABT-263 has potential clinical application. One major flaw of ABT-263 is dose-limiting thrombocytopenia, due to the dependence of platelets on BCL-xL [52]. To circumvent this limitation in tumor settings where BCL-2 is over-expressed, a BCL-2 specific antagonist was derived: ABT-199 [53]. ABT-199 has been highly successful in clinical trials and recently gained FDA approval for chronic lymphocytic leukemia.
Tumors often display dependence upon BCL-2/BCL-xL or MCL-1; so while the Abbott compounds represented a milestone in cancer therapy, successful treatment of MCL-1-dependent tumors remained a challenge. In 2012, the first MCL-1-specific inhibitor, MIM1 (MCL-1 inhibitor molecule 1), was identified an inducer of apoptosis in MCL-1 dependent leukemia [54]. More recently, another MCL-1 inhibitor was discovered, A-1210477, an indole-2-carboxylic acid based compound, which selectively inhibits MCL-1 at low nanomolar concentrations. It induces robust apoptosis in multiple human cancer cell lines, and potently synergizes with ABT-263 to ablate a cellular anti-apoptotic BCL-2 protein repertoire [55].
BH3 mimetics function by inhibiting anti-apoptotic BCL-2 proteins and subsequently lower the cellular apoptotic threshold. This is likely useful as a mechanism to increase primary responses to chemotherapeutics (both conventional and targeted), and to reduce subsequent resistance. A PubMed search for ABT-263 reveals that hundreds of studies examined the benefit of combining ABT-263 with a broad spectrum of cancer chemotherapeutics using in vitro and in vivo models of the most common human malignancies. While the pre-clinical results for these drugs is promising, clinical successes have been slow and few. Toxicity is one concern, but it appears that more specific BH3 mimetics --- molecules that inhibit a single anti-apoptotic BCL-2 protein --- may have solved that problem to produce a durable clinical benefit.
While our discussions focused exclusively on how to engage apoptosis by lowering the apoptotic threshold, another class of small molecules suggests alternative strategies may also hold clinical significance. Recently, a novel class of therapeutics has been described that directly engage BAX-dependent apoptosis. Utilizing computational screening, BAM7 (BAX activating molecule 7) was identified, which interacts with the amino terminal region of BAX to promote its oligomerization and MOMP [56]. Future investigations will likely describe more potent BAX activators, and will hopefully include compounds to selectively activate BAK.
Concluding Remarks
The utility of BH3 domain peptides as convenient and biochemically tractable surrogates for full-length BH3-only proteins has yielded critical and accurate insights into BCL-2 family function. Indeed, these tools have also enabled the exploration of how mitochondrial shape and composition impact upon the cellular decision to engage the mitochondrial pathway of apoptosis. As with all model systems, caveats pertaining to the specificity and function of BH3 domain peptides need to be recognized. While recent mechanistic insights are illuminating a path to apply the principles of MOMP to better appreciate disease etiology, prognosis, and treatment, much more needs to be done to gain a broader understanding of how this large family of complex proteins interact with each other, cellular lipids, and hydrophobic compartments (see Outstanding Questions). One of the most important questions in the field has persisted for years: What is the nature (i.e., proteinaceous, lipidic, both?) of the MOMP pore created by BAK and BAX? Furthermore, as we clarify the biochemistry and cell biology of the BCL-2 proteins, their impact in human disease will surely expand, and continued investment in small molecules to target specific proteins will ultimately provide a solid foundation for therapeutics to treat human disease.
Acknowledgments
This work was supported by: NIH grants CA157740 and CA206005, the JJR Foundation, the William A. Spivak Fund, the Fridolin Charitable Trust, an American Cancer Society Research Scholar Award, a Leukemia & Lymphoma Society Career Development Award, and an Irma T. Hirschl/Monique Weill-Caulier Trust Research Award. This work was also supported in part by two research grants (5-FY11-74 and 1-FY13-416) from the March of Dimes Foundation, and the Developmental Research Pilot Project Program within the Department of Oncological Sciences at the Icahn School of Medicine at Mount Sinai.
GLOSSARY
- Anti-apoptotic BCL-2 proteins
The anti-apoptotic B-cell lymphoma 2 (BCL-2) proteins are responsible for maintaining the integrity of the outer mitochondrial membrane (OMM). They are globular proteins containing BCL-2 homology domains 1 – 4 (BH1 - BH4). The members of this subfamily include A-1 (BCL-2 related gene A1), BCL-2, BCL-xL (BCL-2 related gene, long isoform), BCL-w, and MCL-1 (myeloid cell leukemia 1). These proteins are generally found at the OMM, but can also localize to the endoplasmic reticulum (ER) membrane and the cytosol. The anti-apoptotic BCL-2 proteins preserve OMM integrity by directly binding to both classes of pro-apoptotic BCL-2 proteins (i.e., the BH3-only and effector proteins), which prevents them from cooperating to induce MOMP and subsequent apoptosis.
- BAK and BAX activation
This phrase is used throughout the BCL-2 literature and it involves a highly regulated multi-step process: 1) structural rearrangement exposing N- and C-termini, 2) stable insertion into the OMM, 3) protein dimerization via the α2-α5 core, and 4) higher order protein oligomerization resulting in MOMP. Cellular and mitochondrial contributions to this process are not well defined, but the composition and shape of the OMM is critical for BAX and BAK activation. The multi-step activation process is specific to BAX, as BAK is constitutively localized and inserted into the OMM. Biochemical requirements for BOK have not been addressed (Box 1). BAK/BAX activation is often detected using conformation-specific antibodies recognizing hidden epitopes in the non-activated proteins.
- De-repressor/sensitizer BH3-only proteins
A subset of the BH3-only proteins, including: BAD (BCL-2 antagonist of cell death), BIK (BCL-2 interacting killer), BMF (BCL-2 modifying factor), HRK (Harakiri), Noxa, and PUMA (p53-upregulated modulator of apoptosis) that bind anti-apoptotic BCL-2 proteins, but do not efficiently activate BAK or BAX. These proteins can competitively displace direct activators from anti-apoptotic BCL-2 proteins, thus promoting MOMP. Also, this class of BH3-only proteins sensitize mitochondria and cells to low levels of direct activator proteins.
- Direct activator BH3-only proteins
A subset of the BH3-only proteins, including: BID (BCL-2-interacting domain death agonist) and BIM (BCL-2-interacting mediator of cell death) that transiently interact with BAK and BAX to induce their activation. PUMA and Noxa may also function as direct activators, but there are conflicting data. Direct activator proteins are often sequestered by anti-apoptotic BCL-2 proteins, which consequently inhibit their direct activator function until subsequent de-repressor BH3-only protein expression or activation.
- Mitochondrial outer membrane permeabilization (MOMP)
This event occurs immediately downstream of BAX/BAK/BOK activation and is responsible for the release of inter-membrane space (IMS) proteins, such as cytochrome c, SMAC/Diablo, EndoG, Omi/HtrA2, and AIF (but any protein residing in the IMM can be released, depending on its membrane association and solubility). Cytosolic proteins also gain access to the IMS and alter mitochondrial function; for example, cytochrome c activated caspases can cleave IMS proteins, which negatively impacts on ATP production. Importantly, the anti-apoptotic BCL-2 proteins inhibit MOMP by blocking both effector and BH3-only proteins.
- Mitochondrial pathway of apoptosis
A form of apoptosis that is engaged following cellular stress, such as DNA damage or nutrient deprivation, and is inhibited by anti-apoptotic BCL-2 proteins. In this pathway, pro-apoptotic effector BCL-2 proteins compromise the OMM, which causes mitochondria to release IMS proteins that initiate caspase activation, cellular disassembly, and phagocytosis.
- Pro-apoptotic effector BCL-2 proteins
BAK, BAX, and BOK are the pro-apoptotic ‘effector’ molecules of the BCL-2 family because they actively permeabilize the OMM and promote the release of IMS proteins. BAK and BAX activate, homo-oligomerize, and permeabilize the OMM in response to direct activator BH3-only protein interactions. BOK may not require activation, and may promote MOMP following after expression and protein stabilization.
TEXT BOX 1. BOK is a bona fide Pro-Apoptotic Effector Protein.
BCL-2 ovarian killer (BOK) is a pro-apoptotic member of the BCL-2 family that has puzzled the apoptotic community. BOK is considered a globular protein closely related to BAK/BAX sharing multiple BH domains, 70–80% sequence homology, and a carboxy-terminal transmembrane motif [76]. BOK was identified using a yeast two-hybrid screen using anti-apoptotic BCL-2 members as bait [76]. Exogenous BOK leads to apoptosis in numerous cell models [77]; and BOK is deleted in a subset of human malignancies suggesting a tumor suppressor function [78]. But what role does BOK play in the mitochondrial pathway of apoptosis?
Numerous genetic models of Bok deficiency have been generated, and each employed a different targeting strategy to eliminate Bok expression [79–81]. All Bok−/− murine models fail to display significant differences in development or fecundity compared with wild type counterparts. Most in vivo data suggest that BOK is not redundant with BAK or BAX. For example, Bok−/− mice do not demonstrate accelerated Eμ-Myc driven lymphomagenesis, which contrasts with the genetic loss of Bax [80].
Transformed liver fibroblasts from one of the genetic models of Bok deletion demonstrate impaired apoptotic responses to thapsigargin (TG, a SERCA pump inhibitor) and bortezomib (BTZ, a proteasome inhibitor) [79]. These in vitro results were extended with in vivo studies comparing Wt and Bok−/− mice for sensitivity to TG-induced apoptosis in the liver. This study also suggests that Bok deficiency leads to decreased ER stress signaling, potentially through the regulation of calcium release [82]. More recent findings support a selective and distinguishing role for BOK in regulating the apoptotic response to ER and proteasomal stressors, and show evidence that BOK is fully competent to promote MOMP in the absence of BAK/BAX and BH3-only proteins [83] [81].
In addition, proteomic analysis revealed that several components of the ER-associated degradation (ERAD) machinery interact with BOK, suggesting that BOK stability and pro-apoptotic function are responsive to signaling perturbations both upstream and downstream of ERAD. Remarkably, the pro-apoptotic activity of BOK does not seem to be negatively regulated by anti-apoptotic BCL-2 members [81].
While an apoptotic role for BOK was originally limited to the ovary, BOK is now generally accepted to have a broader impact in adult tissues. Recent insights into the chemotherapeutic responses of ovarian cancer cells also point to a BAK/BAX-independent role for BOK in mediating MOMP and apoptosis [83]. At present, we have neither structural information nor any details pertaining to how mitochondria may regulate BOK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newmeyer DD, et al. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 2.Hu Q, et al. Molecular determinants of caspase-9 activation by the Apaf-1 apoptosome. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16254–16261. doi: 10.1073/pnas.1418000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nature reviews Molecular cell biology. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, et al. The BCL-2 family reunion. Molecular cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luna-Vargas MP, Chipuk JE. The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. The FEBS journal. 2015 doi: 10.1111/febs.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in cell biology. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llambi F, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Molecular cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerflinger M, et al. BH3-only proteins: a 20-year stock-take. The FEBS journal. 2015;282:1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- 9.Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 10.Lessene G, et al. BCL-2 family antagonists for cancer therapy. Nature reviews Drug discovery. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 11.Petros AM, et al. Structural biology of the Bcl-2 family of proteins. Biochimica et biophysica acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Chipuk JE, et al. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Molecular cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Sarosiek KA, et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Molecular cell. 2013;51:751–765. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards AL, et al. Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chemistry & biology. 2013;20:888–902. doi: 10.1016/j.chembiol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai H, et al. Evaluation of the BH3-only protein Puma as a direct Bak activator. The Journal of biological chemistry. 2014;289:89–99. doi: 10.1074/jbc.M113.505701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HC, et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nature cell biology. 2015;17:1270–1281. doi: 10.1038/ncb3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du H, et al. BH3 domains other than Bim and Bid can directly activate Bax/Bak. The Journal of biological chemistry. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill KL, et al. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes & development. 2016;30:973–988. doi: 10.1101/gad.276725.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Follis AV, et al. Pin1-Induced Proline Isomerization in Cytosolic p53 Mediates BAX Activation and Apoptosis. Molecular cell. 2015;59:677–684. doi: 10.1016/j.molcel.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Follis AV, et al. PUMA binding induces partial unfolding within BCL-xL to disrupt p53 binding and promote apoptosis. Nature chemical biology. 2013;9:163–168. doi: 10.1038/nchembio.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khacho M, et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Buck MD, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serasinghe MN, et al. Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Molecular cell. 2015;57:521–536. doi: 10.1016/j.molcel.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashatus JA, et al. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Molecular cell. 2015;57:537–551. doi: 10.1016/j.molcel.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renault TT, et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Molecular cell. 2015;57:69–82. doi: 10.1016/j.molcel.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwana T, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 28.Lucken-Ardjomande S, et al. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell death and differentiation. 2008;15:929–937. doi: 10.1038/cdd.2008.9. [DOI] [PubMed] [Google Scholar]
- 29.Iverson SL, et al. Cardiolipin is not required for Bax-mediated cytochrome c release from yeast mitochondria. The Journal of biological chemistry. 2004;279:1100–1107. doi: 10.1074/jbc.M305020200. [DOI] [PubMed] [Google Scholar]
- 30.Schafer B, et al. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Molecular biology of the cell. 2009;20:2276–2285. doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chipuk JE, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamas-Din A, et al. Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax. The Biochemical journal. 2015;467:495–505. doi: 10.1042/BJ20141291. [DOI] [PubMed] [Google Scholar]
- 33.Raemy E, et al. Cardiolipin or MTCH2 can serve as tBID receptors during apoptosis. Cell death and differentiation. 2016 doi: 10.1038/cdd.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucken-Ardjomande S, et al. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell death and differentiation. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Abundis E, et al. A CRAC-like motif in BAX sequence: relationship with protein insertion and pore activity in liposomes. Biochimica et biophysica acta. 2011;1808:1888–1895. doi: 10.1016/j.bbamem.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Montero J, et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer research. 2008;68:5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 37.Perera MN, et al. Bax and Bcl-xL exert their regulation on different sites of the ceramide channel. The Biochemical journal. 2012;445:81–91. doi: 10.1042/BJ20112103. [DOI] [PubMed] [Google Scholar]
- 38.Ganesan V, et al. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis: an international journal on programmed cell death. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 39.Siskind LJ, et al. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. The Journal of biological chemistry. 2010;285:11818–11826. doi: 10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subburaj Y, et al. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nature communications. 2015;6:8042. doi: 10.1038/ncomms9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, et al. BH3-in-groove dimerization initiates and helix 9 dimerization expands Bax pore assembly in membranes. The EMBO journal. 2016;35:208–236. doi: 10.15252/embj.201591552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landeta O, et al. Minimalist Model Systems Reveal Similarities and Differences between Membrane Interaction Modes of MCL1 and BAK. The Journal of biological chemistry. 2015;290:17004–17019. doi: 10.1074/jbc.M114.602193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleicken S, et al. cBid, Bax and Bcl-xL exhibit opposite membrane remodeling activities. Cell death & disease. 2016;7:e2121. doi: 10.1038/cddis.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montessuit S, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vela L, Marzo I. Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside. Current opinion in pharmacology. 2015;23:74–81. doi: 10.1016/j.coph.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, et al. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Ni Chonghaile T, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartman ML, Czyz M. BH3 mimetics as a strategy to complement anticancer therapies. Postepy higieny i medycyny doswiadczalnej. 2012;66:67–77. [PubMed] [Google Scholar]
- 49.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 50.Parrondo R, et al. ABT-737, a small molecule Bcl-2/Bcl-xL antagonist, increases antimitotic-mediated apoptosis in human prostate cancer cells. PeerJ. 2013;1:e144. doi: 10.7717/peerj.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serasinghe MN, et al. Anti-apoptotic BCL-2 proteins govern cellular outcome following B-RAF(V600E) inhibition and can be targeted to reduce resistance. Oncogene. 2015;34:857–867. doi: 10.1038/onc.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudin CM, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peirs S, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124:3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 54.Cohen NA, et al. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chemistry & biology. 2012;19:1175–1186. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leverson JD, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell death & disease. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavathiotis E, et al. Direct and selective small-molecule activation of proapoptotic BAX. Nature chemical biology. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauck P, et al. Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737. Molecular cancer therapeutics. 2009;8:883–892. doi: 10.1158/1535-7163.MCT-08-1118. [DOI] [PubMed] [Google Scholar]
- 58.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 59.Souers AJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen M, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang WQ, et al. Gossypol-Induced Differentiation in Human Leukemia HL-60 Cells. International journal of biomedical science: IJBS. 2006;2:395–401. [PMC free article] [PubMed] [Google Scholar]
- 62.Song T, et al. Pan-BH3 mimetic S1 exhibits broad-spectrum antitumour effects by cooperation between Bax and Bak. Basic & clinical pharmacology & toxicology. 2013;113:145–151. doi: 10.1111/bcpt.12074. [DOI] [PubMed] [Google Scholar]
- 63.Kitada S, et al. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–3219. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y, et al. Apogossypolone, a nonpeptidic small molecule inhibitor targeting Bcl-2 family proteins, effectively inhibits growth of diffuse large cell lymphoma cells in vitro and in vivo. Cancer biology & therapy. 2008;7:1418–1426. doi: 10.4161/cbt.7.9.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei J, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. Journal of medicinal chemistry. 2010;53:4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarkar S, et al. Novel therapy of prostate cancer employing a combination of viral-based immunotherapy and a small molecule BH3 mimetic. Oncoimmunology. 2016;5:e1078059. doi: 10.1080/2162402X.2015.1078059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeitlin BD, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer research. 2006;66:8698–8706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 68.Kazi A, et al. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. The Journal of biological chemistry. 2011;286:9382–9392. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai L, et al. BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PloS one. 2014;9:e99404. doi: 10.1371/journal.pone.0099404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X, et al. The novel BH3 alpha-helix mimetic JY-1-106 induces apoptosis in a subset of cancer cells (lung cancer, colon cancer and mesothelioma) by disrupting Bcl-xL and Mcl-1 protein-protein interactions with Bak. Molecular cancer. 2013;12:42. doi: 10.1186/1476-4598-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doi K, et al. Discovery of marinopyrrole A (maritoclax) as a selective Mcl-1 antagonist that overcomes ABT-737 resistance by binding to and targeting Mcl-1 for proteasomal degradation. The Journal of biological chemistry. 2012;287:10224–10235. doi: 10.1074/jbc.M111.334532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friberg A, et al. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. Journal of medicinal chemistry. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lessene G, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nature chemical biology. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 74.Tao ZF, et al. Discovery of a Potent and Selective BCL-XL Inhibitor with in Vivo Activity. ACS medicinal chemistry letters. 2014;5:1088–1093. doi: 10.1021/ml5001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dutta S, et al. Potent and specific peptide inhibitors of human pro-survival protein Bcl-xL. Journal of molecular biology. 2015;427:1241–1253. doi: 10.1016/j.jmb.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu SY, et al. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inohara N, et al. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-XL. The Journal of biological chemistry. 1998;273:8705–8710. doi: 10.1074/jbc.273.15.8705. [DOI] [PubMed] [Google Scholar]
- 78.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carpio MA, et al. BCL-2 family member BOK promotes apoptosis in response to endoplasmic reticulum stress. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7201–7206. doi: 10.1073/pnas.1421063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ke F, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell death and differentiation. 2012;19:915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llambi F, et al. BOK Is a Non-canonical BCL-2 Family Effector of Apoptosis Regulated by ER-Associated Degradation. Cell. 2016;165:421–433. doi: 10.1016/j.cell.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulman JJ, et al. The Bcl-2 protein family member Bok binds to the coupling domain of inositol 1,4,5-trisphosphate receptors and protects them from proteolytic cleavage. The Journal of biological chemistry. 2013;288:25340–25349. doi: 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Einsele-Scholz S, et al. Bok is a genuine multi-BH-domain protein that triggers apoptosis in the absence of Bax and Bak and augments drug response. Journal of cell science. 2016 doi: 10.1242/jcs.193946. [DOI] [PubMed] [Google Scholar]