Abstract
In quadrupeds, acute lateral hemisection of the spinal cord (LHS) severely impairs postural functions, which recover over time. Postural limb reflexes (PLRs) represent a substantial component of postural corrections in intact animals. The aim of the present study was to characterize the effects of acute LHS on two populations of spinal neurons (F and E) mediating PLRs. For this purpose, in decerebrate rabbits, responses of individual neurons from L5 to stimulation causing PLRs were recorded before and during reversible LHS (caused by temporal cold block of signal transmission in lateral spinal pathways at L1), as well as after acute surgical (Sur) LHS at L1. Results obtained after Sur-LHS were compared to control data obtained in our previous study.
We found that acute LHS caused disappearance of PLRs on the affected side. It also changed a proportion of different types of neurons on that side. A significant decrease and increase in the proportion of F- and non-modulated neurons, respectively, was found. LHS caused a significant decrease in most parameters of activity in F-neurons located in the ventral horn on the lesioned side and in E-neurons of the dorsal horn on both sides. These changes were caused by a significant decrease in the efficacy of posture-related sensory input from the ipsilateral limb to F-neurons, and from the contralateral limb to both F- and E-neurons. These distortions in operation of postural networks underlie the impairment of postural control after acute LHS, and represent a starting point for the subsequent recovery of postural functions.
Keywords: spinal cord injury, spinal neurons, postural reflexes, cat, spinal networks
In quadrupeds, a major motor deficit caused by the lateral hemisection of the spinal cord (LHS) at the low thoracic level is paralysis of the ipsilateral hindlimb, while the contralateral hind limb suffers much less. A dramatic left-right asymmetry in the muscular tone and limb reflexes prevents normal operation of spinal and supraspinal mechanisms responsible for standing and walking in the hindquarters. However, these functions mostly recover in a few weeks post-lesion (Helgren and& Goldberger, 1993; Hultborn and Malmsten, 1983; Kuhtz-Buschbeck et al., 1996; Frigon and Rossignol, 2006). Our studies on rabbits balancing on a tilting platform have shown that LHS at T12 caused a loss of balance in the hindquarters, but close-to-normal postural reactions to tilts recovered in 2–3 weeks after LHS (Lyalka et al., 2005).
In terrestrial quadrupeds, the dorsal-side-up trunk orientation is maintained due to the activity of the postural system driven mainly by somatosensory information from limb mechanoreceptors (Inglis and Macpherson, 1995; Deliagina et al., 2000, 2006, 2012; Beloozerova et al., 2003; Stapley and Drew, 2009; Horak and Macpherson, 1996). It was shown that organization of the system responsible for stabilization of the trunk orientation in the transverse plane is similar in the cat and rabbit. In both animals the nervous mechanisms controlling orientation of the anterior and posterior parts of the body can operate independently of each other (Beloozerova et al., 2003; Deliagina et al., 2006).
Earlier, in decerebrate rabbits, we studied postural limb reflexes (PLRs). It was suggested that PLRs in intact animals substantially contribute to postural corrections generated during standing (Musienko et al., 2008, 2010; Deliagina et al., 2012). Recently, in decerebrate cat, we demonstrated that PLRs contribute also to maintenance of lateral stability of the hindquarters during locomotion (Musienko et al., 2014). We have shown that PLRs are generated mainly in response to somatosensory inpits from the ipsilateral limb (Musienko et al., 2010). It was also found that the spinal cord contains neuronal networks generating spinal PLRs. However, their efficacy is low, and supraspinal influences substantially contribute to generation of PLRs (Musienko et al., 2010, Deliagina et al., 2014). Recently, two populations of spinal neurons (F and E) contributing to generation of PLRs have been characterized (Hsu et al., 2012; Zelenin et al., 2015). During PLRs, F-neurons were excited in phase with extensors of the ipsilateral limb, while E-neurons – in antiphase.
The overall goal of our current research is to reveal the changes in postural networks underlying the recovery of postural functions after LHS. The aim of the present study was to characterize in detail the starting point for these changes that is the state of the postural networks at the acute stage of LHS. For this purpose, decerebrate rabbits were used. First, in experiments with reversible (Rev) LHS (that is a temporal cold block of signal transmission in spinal pathways on one side), which allowed recording the same spinal neurons before and during their deprivation of unilateral supraspinal drive, we demonstrated that it did not change the phase of the response to stimulation causing PLRs in majority of F- and E-neurons. Thus, F- and E-neurons recorded after acute surgical (Sur) LHS are neurons of spinal postural networks. Second, to characterize in detail the changes in activity of spinal neurons of postural networks caused by LHS, their activity in L5 was recorded in rabbits with Sur-LHS at T12 during somatosensory stimulation causing PLRs in intact subjects. The results were compared with control data obtained in our previous study (Zelenin et al., 2015).
We found drastic right-left asymmetry in PLRs after LHS, which explains the lateral instability observed after the injury. We have delineated the gray matter areas, in which LHS caused reduction in activity of PLR-related neurons, and demonstrated specific changes in the efficacy of posture-related sensory inputs to them.
A brief account of this study was published in abstract form (Deliagina et al., 2013).
Methods
Two types of experiments (with Rev-LHS, N=12, and with Sur-LHS, N=7) were carried out on adult New Zealand rabbits (weighing 2.5–3.0 kg). All experiments were conducted in accordance with NIH guidelines and were approved by the local ethical committee (Norra Djurförsöksetiska Nämden) in Stockholm. The main methods were similar to those used in our previous studies (Zelenin et al., 2013, 2015). They are briefly described below. The data obtained in experiments with Sur-LHS are compared with the control data taken from the database of our previous study. The experimental subjects, as well as all methods used in the present study in experiments with Sur-LHS were similar (except for Sur-LHS) to those used in the control study (Zelenin et al., 2015). The control data for F-, E- and non-modulated neurons were published earlier (Zelenin et al., 2015 and Zelenin et al., 2016, respectively).
Surgical procedures
The animal was injected with propofol (average dose, 10 mg/kg, administrated intravenously) for induction of anaesthesia, which was continued on isoflurane (1.5–2.5%) delivered in O2. The trachea was cannulated. The spinal cord was exposed by laminectomy at T12-L2 (for the subsequent Sur-LHS or for placing of the cooling element), as well as at L5 (for recording of neurons). In experiments with Sur-LHS, at L1, the dura mater was removed for subsequent spinal cord lesion. Small holes (~1 mm2) were made in the dura mater at L5 to insert the recording microelectrode. Bipolar EMG electrodes were inserted bilaterally into two representative extensors: gastrocnemius lateralis (ankle extensor) and vastus lateralis (knee extensor).
The animal was then decerebrated at the precollicular-postmammillary level (Musienko et al., 2008). After decerebration, the anaesthesia was discontinued. In 1 h after cessation of anesthesia, to examine the functional state of the preparation, PLRs were tested (see below). Only preparations with rather stable extensor tonus and pronounced PLRs, which are important attributes of postural activity (Musienko et al., 2008, 2010) were used for experiments. Then, in experiments with Sur-LHS, left or right half of the spinal cord was transected using micro scissors. During the experiment, the rectal temperature and mean blood pressure of the animal were continuously monitored and were kept at 37–38°C and at greater than 90 mmHg, respectively. Recordings of neurons began 30–40 min after hemisection.
Experimental design
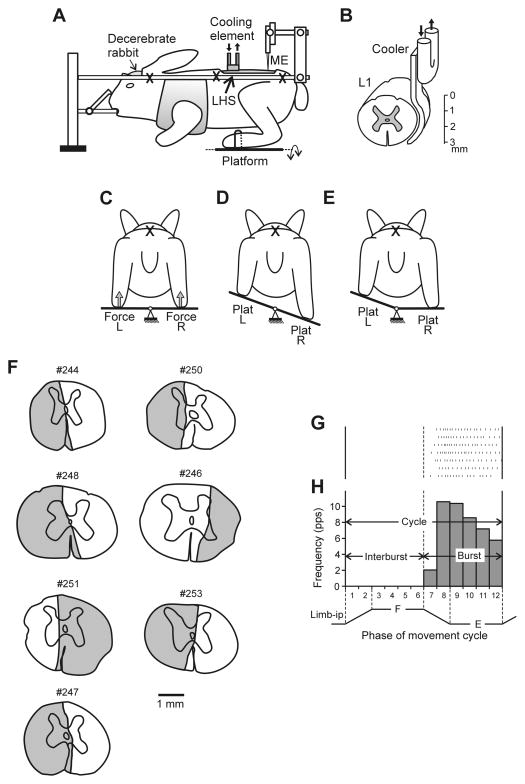
The head and vertebral column of the decerebrate rabbit were rigidly fixed; the forelimbs were suspended in a hammock (Fig. 1A). The method of induction of PLRs was similar to that described earlier (Musienko et al., 2010; Hsu et al., 2012). In short, the hindlimbs of the rabbit were positioned on the horizontal platform, with limb configuration and the inter-feet distance similar to that observed in freely standing rabbits (Beloozerova et al., 2003). The platform as a whole, or its right or left parts separately, could be tilted periodically by rotation around the medial axis with the amplitude ±20° (Fig. 1C–E). Since the vertebrate column and pelvis were fixed, tilts of the whole platform led to flexion/extension movements of the hip, knee and ankle joints and close-to-vertical displacements of the distal point of the limb. A time trajectory of tilting the platform and, therefore, a time trajectory of the foot displacement was trapezoidal with a period of ~6 s (Fig. 2A,B), transition between extreme positions lasted for ~0.5 s, and each extreme position was maintained for ~2.5 s. The tilt angle of each platform was monitored with a mechanical sensor. The contact forces under the limbs were measured by means of force sensors (Force in Fig. 1C). In subjects with intact spinal cord, the tilt-related somatosensory stimulation (caused by loading and flexion of the limb on the platform side moving up, and unloading and extension of the limb on the platform side moving down) evoked PLRs (Fig. 2A, 3A, before cooling), which included activation of extensors in the flexing limb and an increase in its contact force, as well as inactivation of extensors in the extending limb and a decrease in its contact force (Musienko et al., 2010).
Fig. 1. Experimental design.
(A) the decerebrate rabbit was fixed in a rigid frame (points of fixation are indicated by X). In a part of experiments surgical lateral hemisection of the spinal cord (LHS) was performed at L1 (indicated by red arrow). In a part of experiments the cooling element was positioned on the lateral surface of the spinal cord at L1. (B) Position of the cooler on the lateral surface of the spinal cord. To evoke postural limb reflexes (PLRs), the hind limbs were positioned on a platform (C), which was periodically tilted in the transversal plane either as a whole (D), or its left (Plat L) and right (Plat R) parts were tilted separately (E). The contact forces under the left and right limbs were measured by the force sensors (Force L and Force R in C). Activity of spinal neurons from L5 was recorded by means of the microelectrode (ME in A). (F) Extent of the spinal cord damage in rabbits with lateral lesions. The total extent of the lesion is projected on a spinal cord section taken more rostrally after inspecting several consecutive sections. (G) A raster of responses of E-neuron in 7 sequential movement cycles of the ipsilateral limb. (H) A histogram of spike activity in different phases (1–12) of the cycle of movement (F, flexion; E, extension) of the ipsilateral limb (Limb-ip). The halves of the cycle with higher (E, bins 7–12) and lower (F, bins 1–6) neuronal activity were designated as “Burst” and “Interburst” periods, respectively. A,C–D is modified from Fig. 2A–D in Musienko et al., 2010.
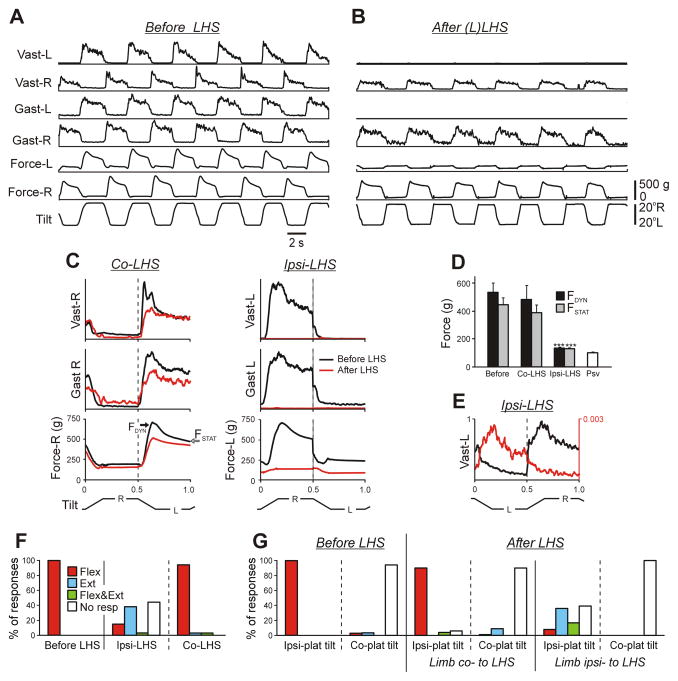
Fig. 2. Effect of LHS on PLRs.
(A,B) Reflex electromyographic (EMG) and force responses to the whole platform tilts before (A) and after (B) left LHS at L1 in Rabbit #244. EMGs of m. gastrocnemius lateralis (Gast) and m. vastus lateralis (Vast) were recorded. (C) Averaged EMG and force responses recorded before and after LHS in the limb ipsilateral (Ipsi-LHS) and in the limb contralateral (Co-LHS) to LHS. Under each condition, responses in 10 sequential tilt cycles were averaged. (D) Mean values (±SE) of the dynamic and static force responses (indicated in C) before and after LHS on the lesioned (Ipsi-LHS) and on the intact (Co-LHS) side (N=7; n=134, before LHS; n=117, after LHS), as well as the passive force (Psv, N=4, n=20). (E) An example of the residual incorrectly phased response in Vast-L on the lesioned side. There was 30x increase in EMG amplification after LHS. (F) Proportion of different types of EMG responses to the whole platform tilts in Vast and Gast before LHS and after LHS in the limb contralateral to the lesion (Co-LHS) and in the limb ipsilateral to the lesion (Ipsi-LHS). (Flex, activation with ipsi-limb flexion; Ext, activation with limb extension; Flex&Ext, activation with both movements; No resp, EMG not responding). Number of animals and analysed cycles: N=7, n=134 (before LHS), n=117 (after LHS). (G) Proportion of different types of EMG responses to separate tilts of the left and right platform before and after LHS. Ipsi-plat tilts, tilts of the platform under the limb; Co-plat tilts, tilts of the platform under contralateral limb. Number of animals and analysed cycles: N=7, n=96 and n=95 during ipsi- and co-plat tilts, respectively, under each condition.
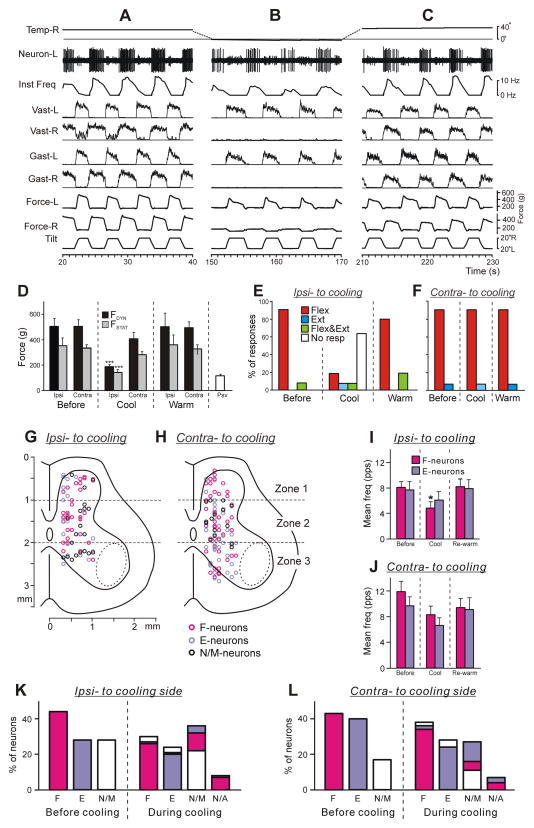
Fig. 3. Effect of lateral cooling (Rev-LHS) on PLRs and on activity of spinal neurons.
(A–C) Activity of a spinal neuron recorded on the left side of L5 before (A), during right Rev-LHS (B) and after re-warming (C). Note that disappearance of the contact force and EMG responses to tilts during cooling on the side of cooling and two-fold decrease in neuronal reactions, while re-appearance of EMG responses and restoration of the value of neuronal reactions after re-warning. (D) Mean values (±SE) of the dynamic and static force responses in the limb ipsilateral to cooling (Ipsi) and in the limb contralateral to cooling (Contra) before cooling, during the unilateral cold block, after re-warming (N=9, n=80 for each condition), as well as the passive force (Psv, N=4, n=20). (E,F) Proportion of different types of EMG responses (elicited by the whole platform tilts) in Vast and Gast before cooling (Before), during the cold block (Cool), and after re-warming (Warm) on the cooled side (E) and on the opposite side (F). Number of animals and tests: N=12, n=96 for each condition. (G,H) Position of all F-, E- and non-modulated neurons recorded on the side of cooling (G, Ipsi-to coolig, n=69) and on the side contralateral to the cooling side (H, Contra- to cooling, n=95) on the cross-section of the spinal cord. The area of motor nuclei is indicated by a dotted line. Three zones of the grey matter are shown: the dorsal (1), intermediate (2) and ventral (3) ones. (I,J) The mean and SE values of the mean frequency of F- and E-neurons recorded on the cooled (I) and opposite side of the spinal cord (J) before and during Rev-LHS, as well as after re-warming. Number of F- and E-neurons on the cooled side before cooling, during cooling and after re-warming: n=31 and 19, 31 and 19, 23 and 17, respectively. Number of F- and E-neurons on the side opposite to the cooled one before cooling, during cooling and after re-warming: n=41 and 38, 41 and 38, 37 and 31, respectively. (K,L) Effects of Rev-LHS on the phase of the neuronal response to tilt. Relative number of F-, E- and non-modulated (N/M) neurons recorded on the side of Rev-LHS (K) and on opposite side (L) before cooling, as well as relative number of F-, E-, N/M and completely inactivated (N/A) neurons during Rev-LHS. See text for further explanations. Designations as in Fig. 2.
Reversible LHS
Experiments with Rev-LHS were based on the technique of blocking the spike propagation in spinal pathways by means of cooling; this technique was adapted from our previous study, where it was described in detail (Deliagina et al., 1983; Zelenin et al., 2013). In short, for better thermo-conductance, the cooler was made of a silver plate, and its shape replicated the shape of the spinal cord. A cooling agent was pumped through a tube soldered to the plate. The cooler was positioned on the lateral aspect of L1, as shown schematically in Fig. 1B. By pumping a cooling agent through the cooler, we decreased the temperature of adjacent tissues to below the threshold for spike generation and spike propagation (Brooks, 1983), which led to inactivation of neurons and to abolition of signal transmission in the spinal pathways mainly on the cooled side of the segment (Deliagina et al., 1983). Thus, the effect of lateral cooling at L1 on activity of neurons recorded in L5 was caused by deprivation of unilateral supraspinal drive as well as of descending drive transmitted by propriospinal neurons located in L1. Re-warming of the spinal cord led to the restoration of neuronal activity and spike propagation. The temperature on the surface of the cooler was monitored by a miniature thermo-resistor. See Results for further details. The method of Rev-LHS has some limitations. First, during cooling one can expect a progressive inactivation of nervous tissue in latero-medial direction. We defined the condition of Rev-LHS as a period when effect of cooling on PLRs was similar to that after Sur-LHS. However, one cannot exclude that during this period, more than a half of spinal pathways were inactivated. Second, considerable difficulty was encountered in maintaining stable neuronal recording because the preparation often underwent a series of locomotor-like movements during cooling. As a result, a limited population of spinal neurons was recorded during Rev-LHS. Episodes of movements caused by local cooling of dorso-lateral spinal pathways was also observed by Chen et al (2001). Third, the duration of the condition of Rev-LHS was rather short (40–60 s), which did not allow any detailed characterization of the postural networks affected by LHS. That is why we used Rev-LHS only for revealing the changes in reactions of individual neurons to tilts caused by their deprivation of unilateral supraspinal drive. For more detailed characterization of the activity of spinal postural networks at the acute stage of LHS, we recorded spinal neurons after acute Sur-LHS and compared the obtained data with control data obtained in the previous study (Zelenin et al., 2015).
Recording of neurons and data analysis
Neurons were recorded extracellularly from the spinal segment L5 by means of commercially available varnish-insulated tungsten electrodes (75 μm shaft diameter; FHC, Bowdoin ME). The impedance of the electrodes was 4–7 MΩ. We tended to explore systematically the whole cross-section of the left and right grey matter except for the areas of motor nuclei, which are indicated by the dotted line in Fig. 4A,B (Portal et al., 1991). The lateral and vertical co-ordinates of each neuron were marked on the map of the spinal cord cross-section (Shek et al., 1986; Hsu et al., 2012; Zelenin et al., 2013).
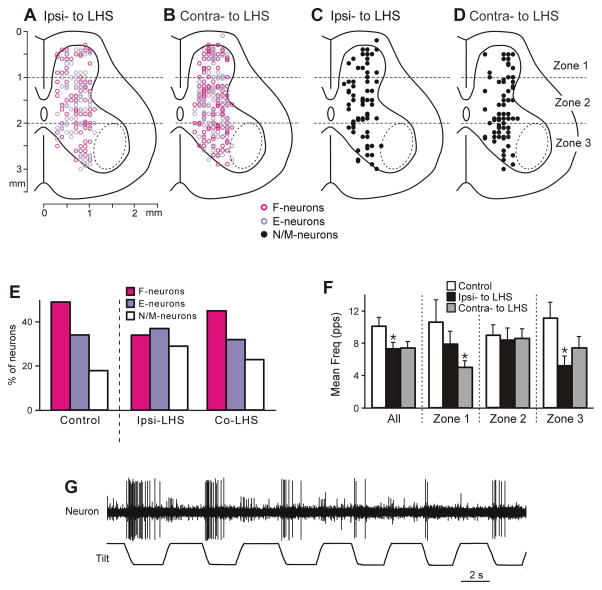
Fig. 4. Neurons recorded during Sur-LHS.
(A,B) Position of all F- and E-neurons on the cross-section of the spinal cord recorded on the side ipsilateral to LHS (A, n=155) and on the side contralateral to LHS (B, n=209). (C,D) Position of all non-modulated neurons on the cross-section of the spinal cord recorded on the side ipsilateral to LHS (C, n=63) and on the side contralateral to LHS (D, n=63). (E) Relative number of F-, E- and non-modulated neurons in control and after LHS (on the side ipsilateral to LHS and on the side contralateral to LHS). (F) Effect of LHS on activity of non-modulated spinal neurons. The mean and SE values of the mean frequency of non-modulated neurons recorded in control and in rabbits with LHS (on the side ipsilateral to the lesion and on the intact side), are shown for the entire population of non-modulated neurons (All), as well as for its sub-populations located in different zones (1–3) of the grey matter. The numbers of non-modulated neurons recorded in zones 1–3 in control were n=14, 27, 23, respectively. The numbers of non-modulated neurons recorded in zones 1–3 in rabbits with LHS on the lesioned and intact side were n=16, 27, 20, and n=13, 26, 24, respectively. (G) Example of a neuron with inconsistent responses to tilt.
In experiments with Rev-LHS, individual neurons were recorded during PLRs caused by whole platform tilts under three conditions: (i) before cooling (control), (ii) during cooling, and (iii) during re-warming. In experiments with Sur-LHS, individual neurons were recorded during PLRs caused by the whole platform tilts. In addition, to reveal tilt-related somatosensory inputs from the left and right limbs to individual neurons, the majority of neurons were also recorded during separate tilts of the right and left platform (Fig. 1E). Under each condition, the neuronal activity was recorded along with the EMGs and ground reaction forces in many (~10) sequential tilt cycles.
Signals from the microelectrode (neuronal activity), from the EMG electrodes, and from the position, force, and temperature sensors were amplified, digitised with a sampling frequency of 30 kHz (neuron), 5 kHz (EMGs) and 1 kHz (sensors), and recorded on a computer disk using the data acquisition and analysis system (Power1401/Spike2, Cambridge Electronic Design, Cambridge, UK). This system also allowed the waveform analysis to discriminate and identify the spikes of a single neuron using the waveform-matching algorithm. Only neurons with a stable recordings and spike shape were used for analysis. The EMG signals were rectified and smoothed (time constant, 100 ms).
When processing the recorded data, we considered the activity of each neuron in the movement cycle of the ipsilateral limb, since the phase of modulation in the majority of neurons was determined by the tilt-related sensory input from the ipsilateral limb (Hsu et al., 2012; Zelenin et al., 2015). For each individual neuron, the raster of activity in sequential movement cycles was obtained. The raster for one of the neurons is shown in Fig. 1G. The cycle was divided into 12 bins; the onset of flexion of the ipsilateral limb was taken as the cycle onset. Bins 1–2 corresponded to flexion of the limb; bins 3–6, to maintenance of the flexed position; bins 7–8, to extension of the limb; bins 9–12, to maintenance of the extended position. The firing frequency in each bin was calculated and averaged over the identical bins in all cycles at a given condition, and the phase histogram was generated (Fig. 1H). The mean frequency during flexion of ipsilateral limb (bins 1–6) and that during extension (bins 7–12) was compared. The larger and the smaller values were named the burst frequency (FBURST) and the interburst frequency (FINTER), respectively. A neuron was considered to be modulated by tilts if the difference between the mean burst frequency and mean interburst frequency was statistically significant (two-tailed Student’s t-test, P<0.05). Then we calculated the mean frequency (average value over bins 1–12) and the depth of modulation M=FBURST − FINTER.
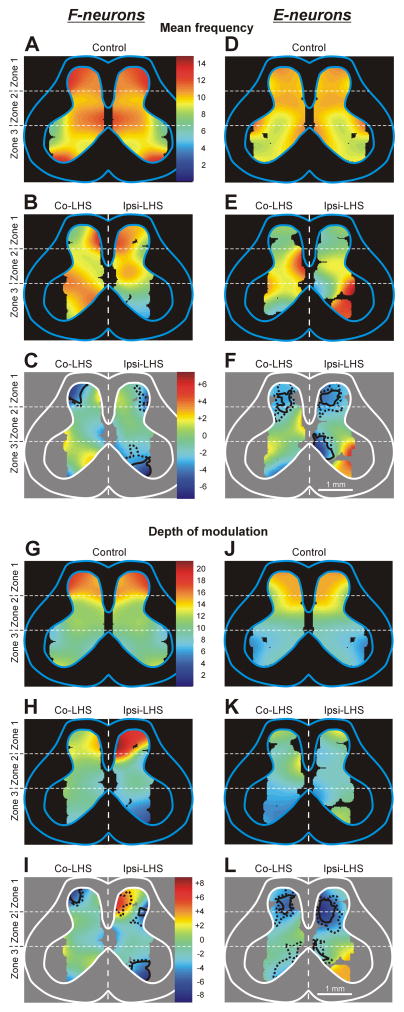
To characterize the effects of Sur-LHS on the activity of local populations of neurons in different areas of the grey matter, “heatmaps” were generated for two parameters of neuronal activity – the mean frequency and the depth of modulation. In these maps, each value of a parameter was coded by a specific colour (see Figs. 6,7). To calculate a value in the heatmap point with coordinates (x,y), we used measured values of the parameter for the neurons that were recorded in the vicinity of the point. The measured values were weighted depending on the distance d from (x,y) to the recording point (Gaussian weighting w(d)=exp(−d2/D2) with the spatial constant D=0.4 mm). Areas of significant local changes (t-test, P=0.01 and P=0.05) were delimited by solid and hatched lines, correspondingly (e.g. Figs. 3C,F, and 4C,F).
Fig. 6. Effect of Sur-LHS on the mean frequency and the depth of modulation of local populations of F- and E-neurons.
(A,B,D,E) Averaged distributions of the mean frequency of F-neurons (A,B) and E-neurons (D,E) on the cross-section of the spinal cord in rabbits with intact spinal cord (A,D, Control) and in rabbits with LHS (B,E). (G,H,J,K) Averaged distributions of the depth of modulation of local populations of F-neurons (G,H) and E-neurons (J,K) in different areas of the gray matter in animals with intact spinal cord (G,J, Control) and in animals with LHS (H,K). For animals with LHS the distributions on the lesioned side (Ipsi-LHS) and on the intact side (Co-LHS) are shown. The difference between the distributions in LHS animals and in control control animals (subtraction of Control from LHS) is shown for F- and E-neurons in C,I and F,L, respectively. The mean frequency and the depth of modulation values are presented as heatmaps (see Materials and Methods). In C,F and I,L the areas of significant changes of the local mean frequency and the local depth of modulation, respectively, are delimited by a solid line (t-test, P<0.01) and a hatched line (t-test, P<0.05).
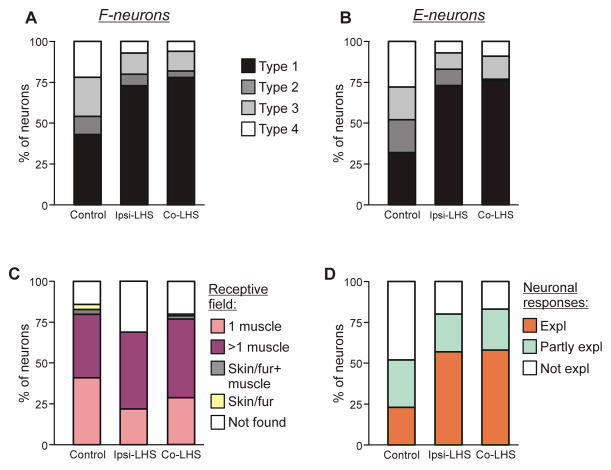
Fig. 7. Effect of Sur-LHS on sources of modulation and on receptive fields of F- and E-neurons.
(A,B) Percentage of F- and E-neurons (A and B, respectively) receiving different combinations of tilt-related somatosensory inputs from the limbs (Types 1–4) in control and after LHS. See text for explanation. (C) Proportion of neurons receiving sensory inputs from different sources, i.e., from receptors of only one muscle (1 muscle), from receptors of more than one muscle (>1 muscle), from cutaneous and muscle receptors (Skin/fur+muscle), from cutaneous receptors only (Skin/fur), and with no receptive field found (Not found) in rabbits with an intact spinal cord (Control) and in rabbits with LHS. See text for explanation. (D) Proportion of neurons in which response to tilts could be completely explained (Expl), partly explained (Partly expl) and could not be explained (Not expl) by input from their receptive field, in rabbits with an intact spinal cord (Control) and in rabbits with LHS. In (A–D) the data for neurons recorded on the side of LHS (Ipsi-LHS) and the data for neurons recorded on the intact side (Co-LHS) are shown separately.
All quantitative data in this study are presented as mean±s.e.m. Student’s t-test (two-tailed) was used to characterize the statistical significance when comparing different means; the significance level was set at P=0.05. To evaluate the statistical significance of the effects of LHS on the proportion of different functional groups of neurons, we used Pearson’s χ2 test; the significance level was set at P=0.05.
Peripheral receptive fields were examined in some of the neurons. Stimuli included light brushing of the hairs, light tapping of hair skin, firm tapping on and palpation of muscle bellies (flexors and extensors of ankle, knee and hip, and abductors and adductors of hip) and tendons, pinching the skin with fingers, and in a few cases manual movements of joints. If a cutaneous input was observed then the responses from the underlying muscles were not taken into account. Stimuli that could potentially activate nociceptors (i.e. pinching or poking with sharp instruments) were not used.
Histological procedures and evaluation of the extent of spinal lesions
At the end of experiment, reference electrolytic lesions were made in the spinal cord. Peaces of the spinal cord with electrolytic lesions as well as those containing Sur-LHS were fixed with 10% formalin solution. Frozen sections of 30 μm thickness were cut in the region of recording and in the region of Sur-LHS. The tissue was stained for Nissl substance with Cresyl violet. Positions of recording sites were estimated in relation to the lesions. The position and extent of lesions were verified by observation of a series of magnified digital images of sections.
Figure 1F shows the reconstructed lesion sites from seven rabbits with lateral lesions. In all rabbits with surgical lesions (N=7), the lesion occupied about half of the spinal cord cross-section (Fig. 1F). The medial border of cutting was close to the sagittal plane, with some “under-cut” in three rabbits (#246, 250, 253), and “over-cut” in four rabbits (#244, 247, 248, 251).
Results
Effects of surgical and reversible LHS on postural limb reflexes
Before Sur-LHS and Rev-LHS, in all preparations, the whole platform tilts (Fig. 1D) evoked PLRs similar to those described in detail in our earlier studies (Musienko et al., 2008, 2010; Hsu et al., 2012; Zelenin et al., 2015). They included activation of extensors in the flexing limb and an increase in its contact force, as well as inactivation of extensors in the extending limb and a decrease in its contact force, as illustrated in Figs. 2A, 3A. A separate tilt of the left or right platform evoked PLRs mainly in the ipsilateral limb (not illustrated).
Sur-LHS
Figure 2, A and B, shows motor responses to whole platform tilts recorded before and after left Sur-LHS, respectively, in Rabbit #244. One can see that after hemisection, PLRs on the right side persisted, though the values of EMGs and forces were somewhat reduced (Fig. 2B). A difference between the effects of Sur-LHS on the two sides is clearly seen in Fig. 2C.
Similar results, i.e., a dramatic reduction in PLRs on the Sur-LHS side and their small reduction on the opposite side were obtained in all rabbits with Sur-LHS (N=7). Figure 2D shows the average values of the dynamic and static components of the force response (indicated in Fig. 2C) recorded before (Before) and after Sur-LHS, on the damaged side (Ipsi-LHS) and on the intact side (Co-LHS). After Sur-LHS, the force responses on the damaged side were 4–5 times smaller than in control and did not differ from the passive force (Psv) measured after sacrificing the animal. By contrast, the force responses on the intact side were similar to those observed before Sur-LHS.
On the damaged side, weak residual EMG responses to tilts of the whole platform sometimes were observed. The phase of these responses could differ from that observed in control, as illustrated in Fig. 2E (there was a 30x increase in EMG amplification for the Sur-LHS condition). To characterize the residual responses, we used four categories: (i) activation of the muscle with flexion of the ipsilateral limb; (ii) activation with limb extension; (iii) activation with both flexion and extension; (iv) no response. Figure 2F shows the proportion of different types of responses observed in Vast and Gast before Sur-LHS (Before LHS) and after Sur-LHS, on the damaged side (Ipsi-LHS) and on the intact side (Co-LHS). After Sur-LHS, on the damaged side, weak residual responses were observed in 58% of cases; more than half of them (38%) were caused not by the limb flexion (as in control and as on intact side) but by the limb extension.
To estimate the role of tilt-related sensory inputs from different limbs in the generation of different patterns of residual responses observed on the damaged side after Sur-LHS, we recorded motor responses to separate tilts of the left and right platform. Figure 2G shows the proportion of responses with different patterns evoked by tilts of either ipsilateral (Ipsi-plat tilt) or contralateral platform (Co-plat tilt), performed before (Before LHS) and after Sur-LHS (After LHS) on the damaged (Limb ipsi- to LHS) and intact (Limb co- to LHS) side. One can see that all incorrectly phased responses in the limb ipsilateral to Sur-LHS are generated on the bases of sensory input from the same limb, suggesting severe distortions in the processing of sensory information from this limb. By contrast, in the limb contralateral to Sur-LHS, sensory input from this limb evoked mainly correct responses, similar to those observed before Sur-LHS.
Rev-LHS
Effects of unilateral cold block of spinal pathways on PLRs are shown in Fig. 3A–C. The cooler was positioned on the right side of L1. Initially, the cooler had the temperature close to that of the surrounding tissues (~35°C) (trace Temp-R in Fig. 3A). Then pumping of a cooling agent (−5°C) through the cooler was turned on and the temperature on the surface of the cooler plate started to decrease from its initial value and, usually in 30–40 s, the surface temperature reached a plateau (−1°C) (trace Temp-R in Fig. 3B). A decrease in the cooler temperature caused a gradual decrease of PLRs on the right side (not illustrated) and, in ~110 s after switching on cooling, the activity of Vast-R and Gast-R was practically absent, while Force-R was dramatically reduced (Fig. 3B). On the left side (opposite to cooler), cooling also caused a decrease in PLRs, but of a much smaller value than on the right side (compare Fig. 3, A and B). When pumping of the cooling agent was turned off, and pumping of the warming agent (35°C) was started instead, the surface temperature returned to its initial value (trace Temp-R in Fig. 3C). Re-warming resulted in recovery of PLRs on both sides (Fig. 3C). Similar dynamics of PLRs during unilateral cooling was observed in all rabbits (N=12). The period when effect of cooling on PLRs was similar to that observed after Sur-LHS (i.e., disappearance of PLRs on the affected side and less than 40% decrease in PLRs on the opposite side) was considered as a condition of Rev-LHS. We suggested that during this period, cooling blocked signal transmission in almost entire lateral half of the spinal pathways. Figure 3D–F shows effects of Rev-LHS on average force (D) and EMG (E,F) responses to tilts. One can see, that they were reversible and similar to those caused by Sur-LHS (compare Fig. 3D with Fig. 2D, and Fig. 3E,F with Fig. 2F). In particular, as after Sur-LHS, weak residual correctly and incorrectly phased EMG responses to tilts were sometimes observed on the side of the cooling during Rev-LHS (compare Ipsi-LHS in Fig. 2F with Cool in Fig. 3E).
Effect of reversible LHS on spinal neurons
In total, 164 individual neurons were tested under condition of Rev-LHS. 69 of them were recorded on the side of cooling and 95 on the opposite side. Figure 3G,H shows location of individual F-, E- and non-modulated neurons on the cross section of the spinal cord ipsilateral (G) and contralateral (H) to cooling. Lateral cooling affected the activity of neurons of both sides of the spinal cord. This is not surprising since unilateral fibers of many descending pathways affect spinal interneurons bilaterally (Aoyama et al., 1971; Stecina et al., 2008a,b). The effects of lateral cooling on individual neurons were different. In majority (56%) of neurons on each of two sides it caused inactivation of different degree. An example of the E-neuron inhibited during cooling is shown in Fig. 3A–C. This neuron exhibited a twofold decrease in the instantaneous frequency (compare A and B) during cooling of the contralateral side of the spinal cord, and the recovery of the activity level during re-warming (C). Lateral cooling caused activation of 16% and 17%, while did not affect activity of 29% and 27% of neurons on the cooling side and on the opposite side, respectively. Thus, the proportions of inactivated, activated and not affected by lateral cooling neurons were similar on two sides of the spinal cord.
Figure 3I,J shows the mean frequency averaged separately over whole population of F-neurons and E-neurons recorded on the side ipsilateral (I) and on the side contralateral to cooling (J) before cooling, during cooling, as well as after re-warming. Under condition of Rev-LHS, one can see a decrease in the activity of F- and E-neurons on both sides of the spinal cord. However, this decrease was significant only in F-neurons located on the side of cooling. Re-warming resulted in recovery of the mean frequency of F- and E-neurons on both sides of the spinal cord.
Figure 3K,L shows relative number of F-, E- and NM-neurons recorded before and during Rev-LHS on the cooled side (K) and on the opposite side (L). One can see that the majority of F- and E-neurons active during Rev-LHS did not change the phase of their response to tilts (purpure and violet parts of F and E bars, respectively, in K and L, During cooling). Only a few F- and a few E-neurons changed the phase of their responses to tilts during cooling (purpure and violet parts of E and F bars, respectively, in K and L, During cooling), as well as a few non-modulated neurons started to respond to tilts and became F- and E-neurons (white parts of E and F bars in K and L, During cooling). One can also see that during cooling a part of F- and E-neurons lost their modulation (purpure and violet parts of N/M bars, in K and L, During cooling). Finally, a few F- and E-neurons on each side of the spinal cord were completely inactivated by the lateral cooling (purpure and violet parts of N/A bars, in K and L, During cooling). These changes in reactions of neurons to tilts caused by lateral cooling resulted in some changes in proportion of F-, E- and non-modulated neurons in population of active neurons during Rev-LHS as compared to the proportion observed before Rev-LHS. The relative number of non-modulated neurons was increased on both sides of the spinal cord, as well as a substantial decrease was observed in the relative number of F-neurons on the cooling side and in relative number of E- neurons on the side contralateral to cooling (Fig. 3K,L).
Effect of surgical LHS on spinal neurons
In decerebrate rabbits subjected to acute Sur-LHS at T12, 490 neurons in total were recorded during tilts of the whole platform. 218 neurons were recorded on the damaged side of the spinal cord and 272 neurons – on the intact side. To reveal the effects of acute Sur-LHS on spinal postural networks, the data obtained in the present study were compared to control data from our previous study on decerebrate rabbits with intact spinal cord, in which a large sample of neurons (n=499) was analysed (Zelenin et al., 2015). As in control, F-, E- and non-modulated neurons were found in all rabbits both on the intact and damaged sides of the spinal cord.
Proportion of different types of neurons recorded on intact and damaged side
Figure 4A–D shows location of individual F- and E-neurons (A,B), as well as non-modulated neurons (C,D) on the cross section of the damaged (A,C, Ipsi- to LHS) and intact (B,D, Contra- to LHS) side of the spinal cord. As in control (Zelenin et al., 2015), F-, E- and non-modulated neurons were distributed over the grey matter and intermixed on both sides. However, in the population of neurons recorded on the damaged side, the relative number of F-neurons was significantly smaller, and the relative number of non-modulated neurons – significantly larger than those in control population (75 out of 219 vs 175 out of 360 in control, χ2 test, P<0.0001, and 64 out of 219 vs 64 out of 360 in control, χ2 test, P <0.0001, respectively, Fig. 4E). By contrast, the relative numbers of F- and non-modulated neurons on intact side of the spinal cord did not differ from those in control (122 out of 272 vs 175 out of 360 in control, χ2 test, P=0.4, and 63 out of 272 vs 64 out of 360 in control, χ2 test, P=0.1, respectively). There was no significant difference between the proportion of E-neurons in the population of neurons recorded after LHS on the intact side and that in control (87 out of 272 vs 121 out of 360 in control, χ2 test, P=0.7), as well as between the proportion of E-neurons in the population of neurons recorded on the damaged side and that in control (80 out of 218 vs 121 out of 360 in control, χ2 test, P=0.5). In about 20% of modulated neurons recorded on each of two sides we observed either inconsistent modulation or large variability in the burst duration or frequency (as the neuron in Fig. 4G). Such neurons were never observed in control.
Activity of neurons on intact and damaged side
The activity of F- and E-neurons was characterized by four parameters – the mean frequency of a neuron, burst frequency, interburst frequency, and depth of modulation (see Methods). To characterize quantitatively the activity of F- and E-neurons, each parameter was averaged over the entire population of F- or E-neurons or separately over F- or E-neurons in each of three zones (zone 1 corresponds to the dorsal part of the dorsal horn, zone 3 – to the ventral horn, and zone 2 – to the intermediate area of the gray matter). To reveal the effect of LHS on the activity of local populations of neurons in different areas of the grey matter, we used the averaged distribution of two parameters of the neuronal activity, the mean frequency and the depth of modulation, across the gray matter of the spinal cord (heatmaps, see Materials and Methods).
F-neurons
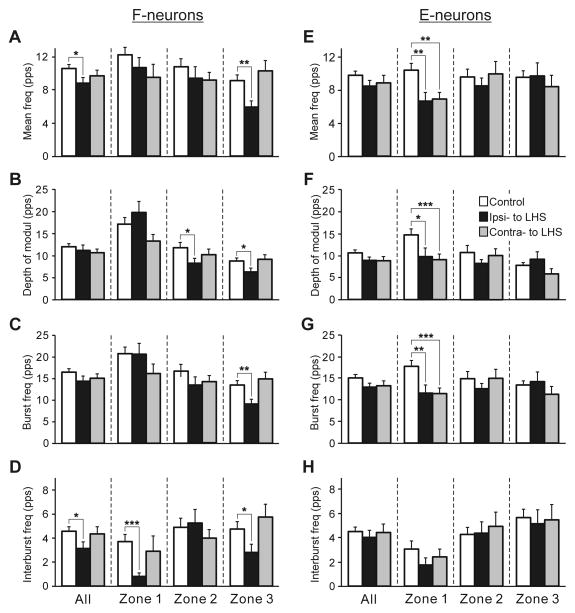
The effects of LHS on the activity of F-neurons located on the damaged side of the spinal cord (Ipsi-LHS) and on the intact side (Contra-LHS) are shown in Fig. 5A–D. One can see that LHS affected mainly activity of F-neurons located on the damaged side. The mean frequency and the interburst frequency averaged over all F-neurons of the damaged side (All in Fig. 5, A and D, respectively) were significantly decreased as compared with control. The most dramatic reduction in activity was exhibited by F-neurons located in zone 3. In these neurons, about 30% decrease in the mean frequency (6.0±0.7 pps against 9.1±0.7 pps in control), burst and interburst frequencies (9.2±1.0 pps against 13.5±1.0 pps in control and 2.8±0.7 pps against 4.8±0.6 pps in control, respectively), as well as in the depth of modulation (6.3±0.9 pps against 8.7±0.7 pps in control) were observed. By contrast, small changes (as compared to control) in values of different parameters of activity of F-neurons located on the intact side of the spinal cord were insignificant.
Fig. 5. Effects of Sur-LHS on the activity of F-neurons (A–D) and E-neurons (E–H) during tilts of the whole platform.
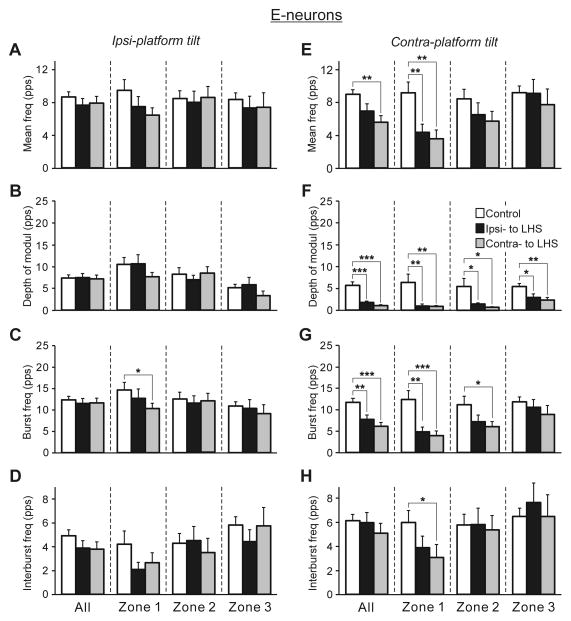
The mean and SE values of the mean frequency (A,E), the depth of modulation (B,F), the burst frequency (C,G), and the interburst frequency (D,H) of neurons recorded in rabbits with intact spinal cord (Control) and in rabbits with LHS on the side of lesion (Ipsi- to LHS) and on the intact side (Contra- to LHS). These values are shown for the entire population of F- and E-neurons (All), as well as for its sub-populations located in different zones (1–3) of the gray matter (Fig. 4A,B). The total number of F-neurons recorded in control were n=249 and in rabbits with LHS on the lesioned and intact side were n=75, 122, respectively. The numbers of F-neurons recorded in zones 1,2,3 in control were n=62, 94, 93. The numbers of F-neurons recorded in zones 1,2,3 in rabbits with LHS on the lesioned and intact side were n=23, 29, 23 and n=34, 46, 42, respectively. The total number of E-neurons recorded in control were n=186, and in rabbits with LHS on the lesioned and intact side were n=80, 122, respectively. The numbers of E-neurons recorded in zones 1,2,3 in rabbits with LHS on the lesioned and intact side were n=18, 36, 26 and n=22, 45, 20, respectively. Indication of significance level: *P<0.05; **P<0.01; ***P<0.001.
Figure 6A,B shows heatmaps for the mean frequency of F-neurons in control and after LHS, respectively. One can see that LHS caused a reduction in the activity of neuronal populations in different areas of the grey matter on both intact (Co-LHS) and damaged (Ipsi-LHS) sides of the spinal cord. This reduction was maximal in the neuronal populations located in the ventral half of zone 3 on the damaged side and in the lateral part of the dorsal horn on both damaged and intact sides, as shown by subtracting Control from LHS in Fig. 6C. The local populations with significant decrease of the mean frequency are delineated in Fig. 6C by solid and interrupted lines (for P=0.01 and P=0.05, respectively).
Figure 6G–I shows heatmaps for the depth of modulation of F-neurons in control (G) and after LHS (H), as well as the changes in the depth of modulation after LHS (I). One can see that LHS caused a significant reduction in the depth of modulation of the neuronal populations located in the lateral part of the dorsal horn on both damaged and intact sides, as well as in the ventral part of zone 3. Thus, the areas, in which neuronal populations exhibited a maximal reduction in the depth of modulation and in the mean frequency, practically coincided. In addition, LHS caused a significant increase of the depth of modulation in population of F-neurons located on the medial part of the dorsal horn on the side of the lesion (Fig. 6I, Ipsi-LHS).
E-neurons
The effects of LHS on the activity of E-neurons located on the damaged side of the spinal cord (Ipsi-LHS) and on the intact side (Contra-LHS) are shown in Fig. 5E–H. As one can see, LHS significantly affected, E-neurons located in zone 1 on both intact (Ipsi-LHS) and damaged (Co-LHS) sides of the spinal cord. They exhibited about 35% decrease in the mean frequency (6.7±1.0 pps against 10.4±0.8 pps in control, and 7.0±0.8 pps against 10.4±0.8 pps in control, on the damaged and intact sides, respectively), burst frequency (11.6±1.9 pps against 17.8±1.3 pps in control, and 11.5±1.3 pps against 17.8±1.3 pps in control, on the damaged and intact sides, respectively) and depth of modulation (9.8±1.9 pps against 14.7±1.3 pps in control, and 9.1±1.3 pps against 14.7±1.3 pps in control, on the damaged and intact sides, respectively). By contrast, small changes (as compared to control) in values of different parameters of activity of E-neurons located in zones 2 and 3 on the damaged and on the intact side of the spinal cord were insignificant.
Figure 6, D,E and J,K shows heatmaps for the mean frequency and the depth of modulation of E-neurons in control and after LHS, respectively. One can see that LHS caused an increase and decrease in the mean frequency of neuronal populations located in different parts of the grey matter on both the damaged and intact side (compare Fig. 6, D and E), but it caused mainly a decrease in the depth of modulation of neuronal populations on both the intact and damaged sides of the spinal cord (compare Fig. 6, J and K). However, a significant change that is a reduction in the mean frequency and in the depth of modulation was observed only in E-neurons located in the central part of the dorsal horn on both damaged and intact sides, as well as in the medial part of the ventral horn on the damaged side (Fig. 6, F and L, respectively). In addition, E-neurons located in the medial part of the ventral horn on the intact side also exhibited a significant decrease in the depth of modulation (Fig. 6L).
Non-modulated neurons
The effects of LHS on the activity of non-modulated neurons located on the damaged side of the spinal cord (Ipsi-LHS) and on the intact side (Contra-LHS) are shown in Fig. 4F. The mean frequency averaged over all non-modulated neurons, as well as over those located in zones 1 and 3 was substantially decreased. However, this decrease was significant only for all neurons of the damaged side and those located in zone 3 of the damaged side.
Processing of tilt-related sensory information
Neuronal responses to tilts are driven by the somatosensory input from the limbs. To characterize the effects of Sur-LHS on the processing of tilt-related sensory information, in 70 F-neurons and in 74 E-neurons on the lesioned side, as well as in 112 F-neurons and in 79 E-neurons on the intact side, we recorded responses not only to tilts of the whole platform, but also to tilts of its right or left part (Fig. 1D), and compared the obtained results to control.
Sources of modulation of F- and E-neurons
By tilting either the left or right platform alone, we determined the sources of sensory input to individual neurons. As in control (Zelenin et al., 2015), in spinal animals four types of neurons differing in the combination of tilt-related somatosensory inputs from the ipsilateral and contralateral limb, as well as in the direction of imposed movement (limb flexion or extension) activating the neuron, were found. Type 1 neurons were activated by ipsi-limb flexion or extension; Type 2, by contra-limb flexion or extension; Type 3, by ipsi-limb flexion and contra-limb extension, or ipsi-limb extension and contra-limb flexion; Type 4, by ipsi-limb and contra-limb flexion, or ipsi-limb and contra-limb extension.
Figure 7A,B shows the relative number of neurons of different types in control and after Sur-LHS on the lesioned (Ipsi-LHS) and on the intact (Co-LHS) sides, separately for F-neurons (A) and E-neurons (B). After Sur-LHS, about a two-fold increase in the relative number of Type 1 neurons on both sides of the spinal cord (73% on the lesioned side and 78% on the intact side vs 43% in control for F-neurons, and 73% on the lesioned side and 76% on the intact side vs 32% in control for E-neurons) was observed. Correspondingly, the relative number of neurons receiving input from the contralateral limb (Types 2–4), decreased (27% on the lesioned side and 22% on the intact side vs 57% in control for F-neurons, and 27% on the lesioned side and 24% on the intact side vs 68% in control for E-neurons). These changes in proportions of Type 1 neurons and Types 2, 3 and 4 neurons were significant both for F- and E-groups (χ2 test for the number of neurons receiving or not receiving inputs from the contralateral limb, in intact animals and after spinalization, P<0.0001).
Efficacy of sensory inputs to F- and E-neurons from different limbs
Inputs to F-neurons
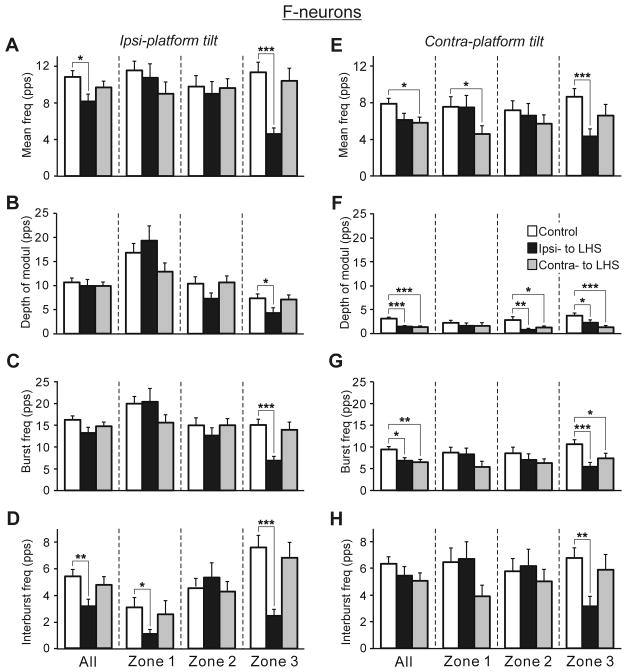
Figure 8, A–D and E–H shows the effect of Sur-LHS on the efficacy of sensory inputs to F-neurons on the damaged (Ipsi-LHS) and on the intact (Co-LHS) side of the spinal cord from the ipsilateral and from the contralateral limb, respectively. Sur-LHS caused the most dramatic decrease in efficacy of inputs from both ipsilateral and contralateral limb to neurons located in zone 3 on the damaged side. During ipsilateral platform tilts (Fig. 8A–D) and during contralateral platform tilts (Fig. 8E–H), one can see more than two-fold decrease in the mean frequency (A and E, respectively), in the burst frequency (C and G, respectively), in the interburst frequency (D and H, respectively), and a significant decrease in the depth of modulation (B and F, respectively) as compared to control. In addition, during contralateral platform tilts (Fig. 8E–H), in neurons of the intact side, a significant decrease in the mean frequency of zone 1 neurons (E), in the burst frequency of zone 3 neurons (G), and in the depth of modulation of zones 2 and 3 neurons (F) was observed. During ipsilateral platform tilts (Fig. 8A–D), a significant decrease in the interburst frequency of zone 1 neurons (D) was also observed. Thus, Sur-LHS caused a significant decrease in the efficacy of input from ipsilateral limb to neurons of the damaged side only. By contrast, a decrease in efficacy of input from contralateral limb to neurons of both damaged and intact side was observed after Sur-LHS.
Fig. 8. Effects of Sur-LHS on the efficacy of tilt-related sensory inputs from the ipsilateral (A–D) and contralateral (E–H) limbs to F-neurons.
The mean and SE values of the mean frequency (A,E), the depth of modulation (B,F), the burst frequency (C,G), and the interburst frequency (D,H) of F-neurons recorded in rabbits with an intact spinal cord (control, n=175) and in rabbits with LHS on the side of damage (Ipsi-to LHS, n=70) and on the intact side (Contra- to LHS, n=112). The numbers of F-neurons from zones 1,2,3 subjected to these tests were: in control animals - n=42, 62, 71, respectively; on the damaged side and on the intact side of animals with LHS - n=21, 27, 22 and n=30, 41, 41, respectively. Designations and indication of significance level are the same as in Fig. 5.
Inputs to E-neurons
Figure 9, A–D and E–H shows the effects of Sur-LHS on the efficacy of sensory inputs from the ipsilateral and from the contralateral limb to E-neurons, respectively. The most dramatic decrease in the efficacy of inputs from the contralateral limb to neurons located in zone 1 on both damaged and intact side was observed (Fig. 9E–H). In these neurons, one can see a two-three fold decrease (as compared to control) in most of activity parameters. In addition, during contralateral platform tilts, zone 2 and 3 neurons located on both damaged and intact side, and zone 2 neurons located on the intact side exhibited a significant decrease in the depth of modulation (Fig. 9F), and zone 2 neurons located on the intact side – a significant decrease in the burst frequency (Fig. 9G). By contrast, during ipsilateral platform tilts, only in zone 1 neurons located on the intact side a significant decrease in the burst frequency was observed (Fig. 9C). Thus, in contrast to F-neurons (Fig. 8), Sur-LHS caused mainly a significant decrease in efficacy of input from contralateral limb to E-neurons of both intact and damaged side.
Fig. 9. Effects of Sur-LHS on the efficacy of tilt-related sensory inputs from the ipsilateral (A–D) and contralateral (E–H) limbs to E-neurons.
The mean and SE values of the mean frequency (A,E), the depth of modulation (B,F), the burst frequency (C,G), and the interburst frequency (D,H) of E-neurons recorded in rabbits with an undamaged spinal cord (control, n=132) and in rabbits with LHS on the side of damage (Ipsi-to LHS, n=74) and on the intact side (Contra- to LHS, n=79). The numbers of E-neurons from zones 1,2,3 subjected to these tests were: in control animals - n=32, 45, 55, respectively; on the damaged side and on the intact side of animals with LHS - n=17, 33, 24 and n=19, 43 17, respectively. Designations and indication of significance level are the same as in Fig. 5.
Relation between responses to tilts and receptive fields of neurons
After Sur-LHS, somatosensory receptive fields were found in 97 out of 140 and in 144 out 180 tested modulated neurons on the damaged and on the intact sides, respectively. The proportion of such neurons on the damaged side significantly decreased as compared to control (69% vs 86%, respectively, χ2 test, P<0.0001) and was almost not changed on the intact side (80% vs 86% in control, χ2 test, P=0.09) (Fig. 7C). As in control, in the majority of neurons on the damaged and on the intact side, the receptive fields were “deep”: the neurons responded to palpation of muscles or to movements of joints, but not to stimulation of the fur or skin alone (Fig. 8C). Sur-LHS did not affect the percentage of neurons with multiple (from more than 1 muscle) deep receptive fields on the damaged and on the intact side (46% and 48% vs 38% in control, respectively, χ2 test, P>0.05), but it caused some decrease in the relative number of neurons with receptive field from one muscle (24% and 29% vs 42% in control on the damaged and on the intact side, respectively, χ2 test, P<0.01).
For 75 modulated neurons on the damaged side and for 114 modulated neurons on the intact side with deep receptive fields, we compared responses of a neuron to tilts with afferent signals that the neuron presumably receives from its receptive field during tilts. One could expect that tilt of the platform would activate stretch and load receptors in extensors of the flexing limb, and those in flexors of the extending limb.
It was found that in rabbits with Sur-LHS, more than half of neurons on the damaged side and on the intact side (43 out of 75, 57%, and 66 out of 114, 58%, respectively) had “corresponding” receptive fields, i.e., their response to tilts could be fully explained by receptive field inputs, for example, an F-neuron with excitatory inputs only from one or several extensor muscles (Expl in Fig. 7D, Ipsi-LHS and Co-LHS, respectively). 17 neurons (23%) on the damaged side and 29 neurons (25%) on the intact side had afferent inputs that could be responsible for their reaction to tilts, but they also had mismatching inputs, for example, excitatory inputs from the antagonistic muscles of one limb (Partly expl in Fig. 7D, Ipsi-LHS and Co-LHS, respectively). Finally, modulation of 15 neurons (20%) on the damaged side and of 19 neurons (17%) on the intact side could not be explained by input from the receptive field (Not expl in Fig. 7D, Ipsi-LHS and Co-LHS, respectively). Figure 7D also shows the proportions of neurons with different role of receptive field input in their modulation in control animals. One can see that Sur-LHS caused more than two-fold increase in the relative number of neurons on both damaged and intact side in which receptive field input could explain the response to tilts (57% and 58% on the damaged and on the intact side, respectively, vs 23% in control), and the corresponding decrease in the relative number of neurons in which response could not be explained by input from the receptive field (20% and 17% on the damaged and intact side, respectively, vs 48% in control).
Discussion
In the rabbit, a lateral hemisection of the spinal cord in the mid-body area causes a dramatic impairment of the postural control, and the animal is not able to keep balance of its hindquarters during standing on a laterally tilting platform. The balance control recovers in a few weeks post-lesion (Lyalka et al., 2005). Similar initial postural deficits and following recovery after LHS were observed in cats (Hultborn and Malmsten, 1983; Kuhtz-Buschbeck et al., 1996), rats (Leszczyńska et al., 2015), and mice (Rank et al., 2015). In the present study on decerebrate rabbits, we analysed the origin of postural deficits after acute LHS.
Effect of LHS on PLRs
Earlier (Deliagina et al., 2006) we have demonstrated that a substantial part of the limb corrective movement caused by disturbance of the body orientation in the transverse plane is generated on the basis of sensory inputs from the same limb. PLRs are important component of this mechanism (Musienko et al., 2008, 2010; Deliagina et al., 2012). One can expect that impairment of postural functions by LHS is well reflected in the PLRs pattern. This was confirmed in the present study, which demonstrated disappearance of PLRs on the damaged side, whereas only a small decrease in PLRs was observed on the undamaged side (Fig. 2). This drastic right-left asymmetry in PLRs is apparently one of the reasons for lateral instability in acute LHS-rabbits (Lyalka et al., 2005). After LHS, weak inconsistent residual EMG responses (either correctly or incorrectly phased in relation to platform tilts) were observed in a part of the tilt cycles (Fig. 2F). We demonstrated that they are generated in response to tilt-related sensory input from the limb ipsilateral to LHS suggesting severe distortions in processing of tilt-related sensory information from this limb. Weak residual correct and incorrect responses to tilts were also observed after acute spinalization (Musienko et al., 2010; Zelenin et al., 2013; Zelenin et al., 2016). We found that during lateral cooling small proportion of non-modulated neurons on both sides of the spinal cord became E and F-neurons (Fig. 3K,L) indicating a distortion in processing of sensory information. One can suggest that these neurons were responsible for appearance of incorrectly phased EMG responses to tilts observed in the limb ipsilateral to Rev-LHS or to Sur-LHS (Fig. 3E and Fig. 2F, respectively). Transformation of small proportion of non-modulated neurons to F- and E-neurons was also observed during complete reversible spinalization (Zelenin et al., 2013). A hypothesis explaining the mechanism underlying generation of inconsistent weak correctly and incorrectly phased EMG responses to tilts observed after spinal cord injury is described below.
A number of factors could be responsible for disappearance of PLRs on the damaged side after LHS: (i) a decrease in excitability of spinal neurons after elimination of unilateral supraspinal drive; (ii) a decrease in efficacy of sensory input from limb mechanoreceptors due to distortions in processing of this input; (iii) a decrease in the value of sensory input from the limb. The latter factor was due to a dramatic reduction in the forces developed by extensor muscles on the damaged side (Fig. 2B,D) and monitored by load receptors, as well as due to inactivation of gamma-motoneurons [which receive significant supraspinal influences (Pompeiano, 1972; Granit, 1979; Hulliger, 1984)], leading to a decrease in signals from muscle spindles. The present study has demonstrated that a change in proportion of F-, E- and non-modulated neurons, a decrease in their activity, a change in processing of sensory information from limbs are among those factors.
Effects of LHS on proportion of different types of neurons on intact and damaged side
We demonstrated that the majority of F- and E-neurons on both sides of the spinal cord did not change the phase of their response to tilts during Rev-LHS (Fig. 3K,L) suggesting that the majority of F- and E-neurons recorded after Sur-LHS contributed to generation of PLRs before lesion, and thus they belonged to postural networks.
We found that Sur-LHS caused a significant change (as compared to control) in proportions of F- and non-modulated neurons in the population recorded on the damaged side of the spinal cord (Fig. 4E). The relative number of F-neurons was decreased and the relative number of non-modulated neurons was increased. Experiments with Rev-LHS had shown that about 30% of F-neurons on the cooling side became non-modulated or completely inactivated after their deprivation of ipsilateral supraspinal drive (Fig. 3K). This could explain a significant decrease in the relative number of F-neurons observed after Sur-LHS. During Rev-LHS not only a part of F-neurons, but also a part of E-neurons on the cooled side became non-modulated (Fig. 3K). Disappearance of modulation in a part of F- and E-neurons after Sur-LHS could explain an increase in the relative number of non-modulated neurons. Similar changes in proportions of F- and non-modulated neurons were observed after acute spinalization (Zelenin et al., 2014). After Sur-LHS the proportion of F-, E- and non-modulated neurons on the intact side was similar to that in control.
Effects of LHS on activity of spinal neurons of postural networks located on the damaged and intact side
We found that effects of Sur-LHS on activity of F- and E-neurons differed to some extent. They are summarized schematically in Fig. 10A,C. On average, as compared to control, a significant decrease in all parameters of activity of F-neurons in zone 3 on the damaged side was observed (Zone 3 in Fig. 5A–D, 10A). By contrast, on the intact side, they were similar to those in control. In contrast to F-neurons, Sur-LHS caused a significant decrease (as compared to control) in the majority of activity parameters of E-neurons located on both sides of the spinal cord in zone 1 (Zone 1 in Fig. 5E–H and Fig. 10C). However, the positions of the local populations of F- and E-neurons exhibiting a substantial decrease in activity after Sur-LHS were mostly similar. They were located in both dorsal horns and in the ventral horn on the side of lesion (compare Fig. 6, C,F and I,L, respectively). Tilt-related somatosensory signals causing modulation of F- and E-neurons are most likely transmitted by group I and II afferents from the limb muscles. Spinal interneurons (including commissural interneurons) with inputs from group II muscle afferents and from corticospinal system were found in Rexed laminae IV, which is a part of Zone 1. Zone 3 contains a part of laminae VII and laminae VIII in which interneurons (including commissural interneurons) with inputs from group I and II afferents and from vestibulospinal, reticulospinal and rubrospinal systems were described (Bannatyne et al., 2003, 2006, 2009; Jankowska and Edgley, 2010).
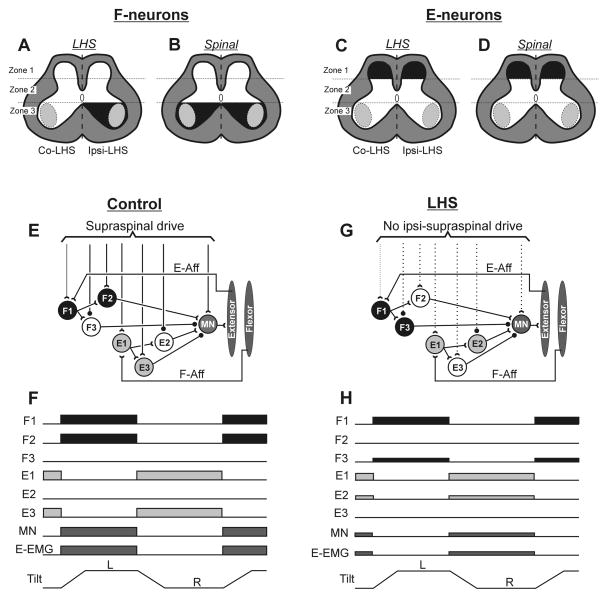
Fig. 10. Changes in spinal postural networks underlying effects of acute LHS and spinalization.
(A–D) Effects of LHS (A,C) and spinalization (B,D) on activity of F-neurons (A,B) and E-neurons (C,D). Zones of the grey matter, in which neurons exhibited a significant decrease in majority of activity parameters are shown in black. The data related to the effects of acute spinalization on F- and E-neurons are taken from our previous study (Zelenin et al., 2014). (E,G) A hypothetical spinal circuit generating motor responses to platform tilts in control (E) and after right LHS (G). Only a circuit related to the right limb is shown. The circuit includes three sub-groups of F-neurons and three sub-groups of E-neurons, differing in their input and output connections. The proposed connections could be mono- or polysynaptic. Designations: interneurons activated by left tilt, by right tilt, and not activated by any tilt are shown in black, grey and white, respectively. Open triangles show excitatory synapses, small filled circles – inhibitory synapses. Continuous and dotted lines indicate connections that persisted or were abolished after spinalization, respectively. F1 and E1 interneurons receive tilt-related afferent input from the ipsilateral limb, from afferents of extensors (E-Aff) or flexors (F-Aff), respectively. F1 and E1 neurons can affect motoneurons of extensor muscles (MNs) through excitatory (F2 and E3) or inhibitory (F3 and E2) interneurons, respectively. (F,E) Responses of neurons of the circuit to tilts in control (F) and after LHS (H). Horizontal bars show the phases of neuronal activity, as well as extensor muscle activity (E-EMG); reduction in the width of bars (H vs F) indicates a decrease in activity after LHS.
Thus surprisingly, despite a strong left/right asymmetry in PLRs, LHS created much less left/right asymmetry in the activity of PLR-related spinal interneurons. One of possible explanations is that spinal neurons on the intact side, exhibiting a decrease in their activity after Sur-LHS, contribute to generation of PLRs on the damaged side. It is noteworthy that in the areas of the grey matter affected by Sur-LHS, the maximal reduction in the activity of F- and E-neurons was less than two-fold. However, one should take into account that elimination of unilateral supraspinal drive caused by Rev-LHS resulted in a substantial decrease (by about 30%) in the relative number of neurons with activity modulated by tilts. One can conclude that, among other factors, both a decrease in the number of modulated neurons and a reduction in the activity of still modulated neurons, as well as a decrease in the excitability of spinal motoneurons due to the loss of ipsilateral direct supraspinal influences (Barnes et al., 1962; Walmsley and Tracey, 1983), contribute to PLRs loss observed on the damaged side.
In Fig. 10 effects of acute Sur-LHS (A,C) and acute spinalization (B,D) on activity of F-neurons (A,B) and E-neurons (C,D) are shown schematically. A significant decrease in most activity parameters of F-neurons from zone 3 and in most activity parameters of E-neurons from zone 1 was observed after acute spinalization (Zelenin et al., 2016). Taken into account the results of the present study one can suggest that abolishment of ipsilateral supraspinal drive largely contributes to inactivation of F-neurons in zones 3 after acute spinalization. By contrast, both ipsilateral and contralateral supraspinal drive most likely equally contributes to inactivation of E-neurons located in zone 1. One cannot also rule out that LHS cause distortions in activity of intact supraspinal neurons, which can contribute to the changes in operation of spinal postural networks. To reveal effect of LHS on activity of intact supraspinal neurons is one of the questions for the future studies.
Since in control F- and E-neurons are modulated in-phase and in anti-phase with extensor motoneurons, it was suggested that at least some of them are pre-motor interneurons that excite and inhibit extensor motoneurons, respectively (Zelenin et al., 2015). Such pre-motor interneurons with inputs from group I and II afferents and with ipsilateral supraspinal inputs were found in zone 3 of the lumbosacral spinal cord (Cavallari et al., 1987; Jankowska et al., 2005; Bannatyne et al., 2006, 2009; Stecina et al., 2008b; Jankowska, 2008; Jankowska et al., 2009; Jankowska and Edgley, 2010). In the same area the majority of almost completely inactivated by reversible spinalization F-neurons was found (Zelenin et al., 2013). Most likely inactivation of premotor F-neurons in zone 3 caused by their deprivation of ipsilateral supraspinal drive largely contributes to disappearance of PLRs on the damaged side after SR-LHS.
Thus, in the present study we have delineated the gray matter areas strongly affected by LHS. To reveal the identity of the affected spinal neurons and to determine their specific role in generation of PLRs are questions for the future studies.
Effect of LHS on processing of sensory information
The present study has shown that Sur-LHS affected the contribution of sensory inputs from the ipsilateral and contralateral limbs to modulation of F- and E-neurons. We have found an almost two-fold increase in the proportion of neurons modulated by sensory input from the ipsilateral limb only (Type 1), and a corresponding decrease in the proportion of neurons with a contribution of input from the contralateral limb (Types 2–4, see Fig. 7A,B) on both intact and damaged side of the spinal cord. This was caused by a significant reduction in the efficacy of tilt-related sensory inputs from the contralateral limb to both F- and E-neurons located on both intact and damaged side of the spinal cord (Figs. 8F and 9F, respectively). One explanation could be that commissural interneurons transmitting signals from the contralateral limb are inactivated bilaterally by LHS. Another explanation could be that only a decrease in the efficacy of the contralateral input from the limb of the damaged side is caused by inactivation of commissural neurons, and a decrease in the efficacy of the contralateral input from the limb of the intact side is caused by inactivation of premotor interneurons located on the damaged side. Commissural neurons, with inputs from group I and II afferents and with excitatory inputs from ipsilateral descending pathways had been described (Jankowska, 2008; Stecina et al., 2008; Jankowska et al., 2009; Jankowska and Edgley, 2010). Some of them are located in zones 1 and 3, which were mostly affected by LHS (Fig. 10A,C).
Sur-LHS affected differently the efficacy of sensory inputs from the ipsilateral limb to F- and E-neurons. The efficacy of these inputs to E-neurons located on the intact and on the damaged side remained almost unchanged (Fig. 9B), but it was significantly decreased for F-neurons located in zone 3 on the damaged side (Fig. 8B). Since there were differences in the strength of tilt-related sensory inputs from the ipsilateral and contralateral limb to F- and E-neurons in control (compare Fig. 9, B,F and Fig. 10, B,F, respectively), one can suggest that a decrease in response of F- and E-neurons to whole platform tilts after Sur-LHS was caused mainly by a decrease in input from the ipsilateral limb to F-neurons of the damaged side, and by a decrease in input from the contralateral limb to E-neurons located on both sides of the spinal cord. A decrease in the efficacy of sensory inputs from both ipsilateral and contralateral limb to F-neurons in zone 3 and a decrease in the efficacy of sensory inputs from contralateral limb to E-neurons in zone 1 were observed also after acute spinalization (Zelenin et al., 2016). Results of the present study also suggest that mainly an ipsilateral supraspinal drive controls the efficacy of sensory inputs from ipsilateral and from contralateral limb to F-neurons of zone 3, whereas a bilateral supraspinal drive controls the efficacy of inputs from contralateral limb to E-neurons of zone 1.
Origin of inconsistent correct and incorrect residual EMG responses to tilts after spinal cord injury
As demonstrated in the present study, after both Rev-LHS and acute Sur-LHS, weak inconsistent residual EMG responses, either correctly or incorrectly phased in relation to platform tilts, were observed in a part of the tilt cycles in the limb on the side of the damage (Figs. 3B and 2F, respectively). Similar residual EMG responses were observed after acute spinalization in our previous studies (Musienko et al., 2010; Zelenin et al., 2013, 2016). Figure 10E–H shows one of possible hypothetical spinal circuits, which allows us to explain the origin of these responses, as well as their inconsistency. The circuit is based on the following results obtained in the present study, as well as in our previous studies (Musienko et al., 2010; Zelenin et al., 2013; Zelenin et al., 2016): (i) PLRs in intact subjects, as well as residual EMG responses in subjects with an injured spinal cord were generated mainly in response to sensory inputs from the ipsilateral limb. (ii) In the majority of F- and E-neurons, their deprivation of the whole or unilateral supraspinal drive did not affect the phase of their responses to tilts. (iii) After spinal cord injury, most F- and E-neurons exhibited only moderate changes in the magnitude of their responses to tilts as compared with control, suggesting that they receive a rather weak supraspinal excitatory drive, or no drive. (iv) In some neurons, spinal cord injury caused inconsistent tilt-related modulation. (v) In some neurons complete elimination of the supraspinal drive, as well as its unilateral elimination resulted in appearance of tilt-related modulation.
The proposed circuit includes F and E groups of neurons; each of the groups consists of three sub-groups (F1, F2, F3, and E1, E2, and E3, respectively) with different connectivity patterns (Fig. 10E). F1 and E1 neurons receive the tilt-related somatosensory input from the ipsilateral limb. They are activated by flexion and extension of this limb, respectively (Fig. 10F). F1 and E1 neurons can affect motoneurons of extensor muscles (MNs) of the ipsilateral limb through excitatory (F2 and E2) and inhibitory (F3 and E3) interneurons, respectively (Fig. 10E). In control, F3 and E2 neurons are inhibited by supraspinal drive, and platform tilts evoke correct EMG responses in extensors: they are activated during ipsilateral limb flexion (through the reflex chain E-Aff→F1→F2→MN) and inactivated during the limb extension (through the reflex chain F-Aff→E1→E3→MN) (Fig. 10E,F).
Spinal cord injury (Fig. 10G) causes (i) an increase in the excitability of F3 and E2 neurons (due to disinhibition), (ii) a decrease in the excitability of F2, E3 neurons and MNs (due to a loss of excitatory supraspinal drive), and (iii) only small changes in the excitability of E1 and F1 neurons (since the excitatory drive to these neurons is either weak or absent). Inconsistent correct and incorrect motor responses to tilts observed after spinal cord injury can be explained by spontaneous fluctuation of the excitability of F2, F3, E2, E3 neurons. If the excitability of F3 and E2 neurons is higher than that of F2 and E3 neurons, flexion/extension movements of the limb will activate the reflex chains E-Aff→F1→F3→MN and F-Aff→E1→E2→MN, respectively, resulting in the generation of incorrect responses (Fig. 10G,H). If the excitability of F2 and E3 neurons is higher than that of F3 and E2 neurons (as in control, Fig. 10E,F), the correct responses will be generated. Finally, there will be no responses if the excitability of F2, F3, E2 and E3 is low. We suggest that majority of F- and E-neurons that were recorded after acute spinalization in our previous study (Zelenin et al., 2016), as well as those recorded after acute Sur-LHS in the present study belonged to F1 and E1 sub-populations, respectively.
To conclude, in the present study the effects of acute LHS on PLRs and on activity of spinal PLR-related neurons has been characterized for the first time. A drastic right-left asymmetry in PLRs has been found, which underlies the lateral instability observed after acute LHS. The asymmetry was much weaker expressed in activity of PLR-related neurons. On both sides of the spinal cord the gray matter areas have been delineated in which LHS caused a significant reduction in the activity of these neurons, as well as specific changes in the efficacy of posture-related sensory inputs to them has been demonstrated. The obtained results suggest that LHS causes considerable distortions in the operation of postural networks on both sides of the spinal cord. These distortions underlie the impairment of postural control after acute LHS, and represent a starting point for the subsequent recovery of postural functions. To clarify if restoration of postural control after LHS is associated with recovery of normal activity of posture-related neurons in areas of the grey matter delineated in the present study is a question for the future studies.
Acute lateral hemisection (LHS) of the spinal cord causes right-left asymmetry in postural limb reflexes.
We examine the effects of acute LHS on two populations of spinal interneurons (F and E) mediating postural limb reflexes.
LHS causes a reduction in the proportion of F-neurons on the lesioned side.
LHS causes a reduction in activity of E- and F- neurons located in specific areas of the grey matter.
These activity changes are due to specific changes in the efficacy of posture-related sensory inputs to F- and E-neurons.
Acknowledgments
This work was supported by grants from NIH (R01 NS-064964), from Christopher & Dana Reeve Foundation, from Swedish Research Council (no.11554), Gösta Fraenckels Foundation to T.G.D.; by grant from Swedish Research Council (no.21076) to P.V.Z.
Abbreviations
- Co-
contralateral
- EMG
electromyogram
- Ipsi-
ipsilateral
- LHS
lateral hemisection of the spinal cord
- PLRs
postural limb reflexes
- Rev-LHS
reversible lateral hemisection of the spinal cord
- Sur-LHS
surgical lateral hemisection of the spinal cord
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama M, Hongo T, Kudo N, Tanaka R. Convergent effects from bilateral vestibulospinal tracts on spinal interneurons. Brain Res. 1971;35:250–253. doi: 10.1016/0006-8993(71)90612-3. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Different projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol (Lond) 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CD, Joynt RJ, Schottelius BA. Motoneuron resting potentials in spinal shock. Am J Physiol. 1962;203:1113–1116. doi: 10.1152/ajplegacy.1962.203.6.1113. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- Brooks VB. Study of brain function by local, reversible cooling. Rev Physiol Biochem Pharmacol. 1983;95:1–109. [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of mid-lumbar interneurons on motoneurons of hind-limb muscles in the cat. J Physiol. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DC, Theiss RD, Ebersole K, Miller JF, Rymer WZ, Heckman CJ. Spinal interneurons that receive input from muscle afferents are differentially modulated by dorsolateral descending systems. J Neurophysiol. 2001;85:1005–1008. doi: 10.1152/jn.2001.85.2.1005. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Pavlova GA. The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res. 1983;53:81–90. doi: 10.1007/BF00239400. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Popova LB, Sirota MG, Swadlow H, Grant G, Orlovsky GN. Role of different sensory inputs for maintenance of body posture in sitting rat and rabbit. Motor Control. 2000;4:439–452. doi: 10.1123/mcj.4.4.439. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol (Lond) 2006;573:211–224. doi: 10.1113/jphysiol.2006.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Physiological and circuit mechanisms of postural control. Curr Opin Neurobiol. 2012;22:646–652. doi: 10.1016/j.conb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Lyalka VF, Hsu L-J, Orlovsky GN. Effect of acute lateral hemisection of the spinal cord on neurons of spinal postural network. Soc Neurosci Abstr. 2013;830.09 doi: 10.1016/j.neuroscience.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Orlovsky GN, Zelenin PV. Contribution of supraspinal systems to generation of automatic postural responses. Front Integr Neurosci. 2014;8:1–20. doi: 10.3389/fnint.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R. Interpretation of supraspinal effects on the gamma system. Prog Brain Res. 1979;50:147–154. doi: 10.1016/S0079-6123(08)60815-8. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- Helgren ME, Goldberger ME. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp Neurol. 1993;123:17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Horak F, Macpherson J. Postural orientation and equilibrium. In: Shepard J, Rowell L, editors. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 255–292. Sect. 12. [Google Scholar]
- Hsu LJ, Zelenin PV, Orlovsky GN, Deliagina TG. Effects of galvanic vestibular stimulation on postural limb reflexes and neurons of spinal postural network. J Neurophysiol. 2012;108:300–313. doi: 10.1152/jn.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Malmsten J. Changes in segmental reflexes following chronic spinal cord hemisection in cat. I. Increased monosynaptic and polysynaptic ventral root discharges. Acta Physiol Scan. 1983;119:405–422. doi: 10.1111/j.1748-1716.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol. 1995;73:1181–1191. doi: 10.1152/jn.1995.73.3.1181. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurons. J Physiol (Lond) 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol (Lond) 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group 1b and II muscle afferents; an update. Eur J Neurosci. 2010;32:881–893. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Boczek-Funcke A, Mautes A, Nacimiento W, Weinhardt C. Recovery of locomotion after spinal cord hemisection: an X-ray study of the cat hindlimb. Exp Neurol. 1996;137:212–224. doi: 10.1006/exnr.1996.0020. [DOI] [PubMed] [Google Scholar]
- Leszczynska AN, Majczynski H, Wilczynski GM, Slawinska U, Cabaj AM. Thoracic hemisection in tats results in initial recovery followed by a late decrement in locomotor movements with changes in coordination correlated with serotonergic innervation of the ventral horn. PLoS ONE. 2015;10:e0143602. doi: 10.1371/journal.pone.0143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol. 2005;94:3677–3690. doi: 10.1152/jn.00538.2005. [DOI] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res. 2008;190:124–134. doi: 10.1016/j.bbr.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J Neurophysiol. 2010;103:1080–1092. doi: 10.1152/jn.00575.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Deliagina TG, Gerasimenko YP, Orlovsky GN, Zelenin PV. Limb and trunk mechanisms for balance control during locomotion in quadrupeds. J Neurosci. 2014;34:5704–5716. doi: 10.1523/JNEUROSCI.4663-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O. Vestibulospinal relations: vestibular influences on gamma motoneurons and primary afferents. Prog Brain Res. 1972;37:197–232. doi: 10.1016/S0079-6123(08)63904-7. [DOI] [PubMed] [Google Scholar]
- Portal JJ, Corio M, Viala D. Localization of the lumbar pools of motoneurons which provide hindlimb muscles in the rabbit. Neurosci Lett. 1991;124:105–107. doi: 10.1016/0304-3940(91)90832-e. [DOI] [PubMed] [Google Scholar]
- Rank MM, Flynn JR, Battistuzzo CR, Galea MP, Callister R, Callister RJ. Functional changes in deep dorsal horn interneurons following spinal cord injury are enhanced with different durations of exercise training. J Physiol. 2015;593:331–345. doi: 10.1113/jphysiol.2014.282640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek JW, Wen GY, Wisniewski HM. Atlas of the rabbit brain and spinal cord. New York: Karger; 1986. [Google Scholar]
- Stapley P, Drew T. The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat. J Neurophysiol. 2009;101:1334–1350. doi: 10.1152/jn.91013.2008. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson LG, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol. 2008a;586:557–574. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Slawinska U, Jankowska E. Ipsilateral actions from the feline red nucleus on hindlimb motoneurones. J Physiol (Lond) 2008b;586:5865–5884. doi: 10.1113/jphysiol.2008.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Tracey DJ. The effect of transection and cool block of the spinal cord on synaptic transmission between 1a afferents and motoneurons. Neuroscience. 1983;9:445–451. doi: 10.1016/0306-4522(83)90307-x. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Lyalka VF, Hsu L-J, Orlovsky GN, Deliagina TG. Effects of reversible spinalization on individual spinal neurons. J Neurosci. 2013;33:18987–18998. doi: 10.1523/JNEUROSCI.2394-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Lyalka VF, Hsu LJ, Orlovsky GN, Deliagina TG. Effects of acute spinalization on neurons of postural networks. Sci Rep. 2016;6:27372. doi: 10.1038/srep27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Hsu LJ, Lyalka VF, Orlovsky GN, Deliagina TG. Putative spinal interneurons mediating postural limb reflexes provide a basis for postural control in different planes. Eur J Neurosci. 2015;41:168–181. doi: 10.1111/ejn.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]