Abstract
Chronic lithium treatment effectively reduces behavioral phenotypes of mania in humans and rodents. The mechanisms by which lithium exerts these actions are poorly understood. Preclinical and clinical evidence have implicated increased mesolimbic dopamine (DA) neurotransmission with mania. We used fast-scan cyclic voltammetry to characterize changes in extracellular DA concentrations in the nucleus accumbens (NAc) core evoked by 20 and 60 Hz electrical stimulation of the ventral tegmental area (VTA) in C57BL6/J mice treated either acutely or chronically with lithium. The effects of chronic lithium treatment on the availability of DA for release were assessed by depleting readily releasable DA using short inter-pulse train intervals, or administering d-amphetamine acutely to mobilize readily releasable DA. Chronic, but not acute, lithium treatment decreased the amplitude of DA responses in the NAc following 60 Hz pulse train stimulation. Neither lithium treatment altered DA release or reuptake kinetics. Chronic treatment did not impact the progressive reduction in the amplitude of DA responses when, using 20- or 60 Hz pulse trains, the VTA was stimulated every six seconds to deplete DA. Specifically, the amplitude of DA responses to 60 Hz pulse trains was initially reduced compared to control mice, but by the fifth pulse train there was no longer a treatment effect. However, chronic lithium treatment attenuated d-amphetamine induced increases in DA responses to 20 Hz pulse trains stimulation. Our data suggest that long-term administration of lithium may ameliorate mania phenotypes by normalizing the readily releasable DA pool in VTA axon terminals in the NAc.
Keywords: lithium, mood stabilizer, dopamine, nucleus accumbens, fast scan cyclic voltammetry, amphetamine, mania
Graphical abstract
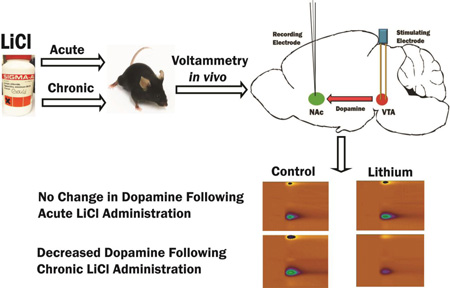
Three weeks’ treatment with the anti-manic drug, lithium, reduced dopamine release in mouse nucleus accumbens evoked by 60 Hz, but not 20 Hz, electrical stimulation of the ventral tegmentum, and attenuated increases in 20 Hz-evoked dopamine release following acute amphetamine administration. These data suggest that lithium stabilizes mood because it attenuates dopamine release only when it is abnormally high.
INTRODUCTION
Lithium is a mood stabilizer used in the treatment of bipolar disorders and the reduction of suicidal behaviors, and has both anti-manic and anti-depressant properties (Severus et al. 2014, Lewitzka et al. 2015). Although the therapeutic actions of lithium are well established and its effects on intracellular signaling pathways have been extensively characterized, the mechanisms by which it acts at the systems level are not understood (Can et al. 2014, Chiu & Chuang 2010, Quiroz et al. 2010). This gap in knowledge is a significant impediment to the development of improved therapies for mood disorders that could be based upon the mechanisms of lithium’s actions.
Several lines of evidence indicate that the mesolimbic dopamine (DA) projections, from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), are crucial for the expression of endophenotypes of mania and suicide, such as aggression and impulsivity (Ryding et al. 2008, Basar et al. 2010b). Drugs that acutely increase release, or reduce reuptake of DA result in mania phenotypes in humans (Drevets et al. 2001, Leyton et al. 2002, Anand et al. 2000, Murphy et al. 1971). Antipsychotics, as well as other treatments that impede DAergic neurotransmission, diminish mania in humans (McTavish et al. 2001, Perlis et al. 2006).
The clinical effectiveness of lithium therapy for mood disorders requires chronic treatment over multiple weeks (Gelenberg et al. 1989, Gershon et al. 2009). Chronic lithium treatment, at doses that do not change baseline locomotor activity levels, reduces d-amphetamine induced increases in locomotor activity in rodents (Gould et al. 2007, Cox et al. 1971, Borison et al. 1978). Rodents that are treated with lithium also manifest diminished impulsivity (Halcomb et al. 2013, Ohmura et al. 2011). Some studies have also found that lithium attenuates the behavioral and/or neurobiological effects of DAergic drugs in humans (van Kammen et al. 1985, Van Kammen & Murphy 1975, Huey et al. 1981, Bell et al. 2005). In vivo microdialysis has been used previously to study the effect of chronic lithium treatment on extracellular DA concentrations in the rodent NAc (Gambarana et al. 1999, Ferrie et al. 2008, Ferrie et al. 2005). However, the low temporal resolution of microdialysis makes it impossible to determine the relative contributions to those findings of changes in basal extracellular DA concentrations, DA release by axon terminals or the dynamics of DA reuptake. Here, we address this by using fast-scan cyclic voltammetry (FSCV), which has 100 msec temporal resolution, to study the effects of chronic and acute lithium treatment on the magnitude and temporal dynamics of DA release, and the reuptake of extracellular DA, in the NAc core. We tested the hypothesis that chronic administration of lithium attenuates electrically- and d-amphetamine-evoked changes in extracellular DA concentrations. We further assessed whether lithium’s actions require chronic administration, and if lithium’s actions are selective for the releasable or storage reserves of DA.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice, aged 11–12 weeks old at the beginning of experimentation were obtained from The Jackson Laboratories, Bar Harbor, Maine. Mice were housed five per cage at a constant temperature (22±1°C), with a 12-h light/dark cycle (lights on/off at 07:00–19:00) and free access to food and water. Experiments were performed in the light phase of the cycle. Separate cohorts of mice were used in each experiment. All experimental procedures were approved by the University of Maryland, Baltimore Animal Care and Use Committee, and were conducted in full accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Lithium Treatments
For chronic lithium administration, regular chow was removed from all cages and lithium chow containing 4 g/kg lithium chloride or a control chow (identical except for the lack of lithium chloride; both from Bioserv, Frenchtown, NJ) was provided ad libitum for a minimum of 3 weeks (typically 3–5 weeks) prior to the FSCV procedures. In C57BL/6J mice, this regimen results in brain lithium levels of approximately 1mM, which is similar to human therapeutic levels of ~0.8–1 mmol/l required for effective treatment (Can et al. 2011, Gelenberg et al. 1989). Mice of both groups had access to a water bottle, and a 0.9% saline bottle to reduce ion imbalances caused by lithium treatment (Can et al. 2011). In pilot studies we found that mice treated for 4 weeks with lithium chloride and then injected with urethane did not have brain lithium levels different from saline injected mice (saline 0.90 ± 0.11, urethane 0.93 ± 0.11; 5 hours after administration).
Acutely treated mice received injections of lithium chloride (300 mg/kg in 0.9% saline, i.p., 4 ml/kg, Sigma, Saint Louis, MO) or as a control, saline (0.9%, i.p., 4 ml/kg). FSCV recordings began 5–7h post-injection. The acute lithium dose and the interval between the injection and the recording session were chosen on the basis of an earlier study in which we found that these parameters resulted in the maintenance of brain levels of lithium similar to those produced by chronic administration, and blood plasma levels below the high levels observed at earlier time points (Can et al. 2011).
Surgical and FSCV Procedures
Electrodes for measuring extracellular DA concentration were constructed by inserting a carbon fiber (7 µm diameter, Goodfellow, UK) into a glass capillary tube (1.2mm diameter o.d., A-M Systems, Sequim, WA), pulled with a micropipette puller (Narishige, Japan). Carbon fibers were then cut at approximately 100 µM past the glass tip (Heien et al. 2004). Mice were anesthetized with urethane (1.5 g/kg, i.p.) and their heads were positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Body temperature was regulated with a rectal thermoregulator (CMS Instruments) and maintained at 37 °C during surgery. Burr holes were drilled in the skull for the implantation of three electrodes (recording, stimulating, and reference) in the brain. The recording electrode was placed at the level of the NAc core (+1.2 AP, +1.1 ML, and −3.4 DV). A bipolar steel stimulation electrode (Plastics1, Roanoke, VA) was positioned ipsilaterally in the VTA (−3.1 AP, +0.7 ML, and +4.8 DV). An Ag/AgCl reference electrode (0.5mm diameter; Acros, NJ) was placed in the cortex contralateral to the recording and stimulating electrodes. Both recording and stimulating electrodes were slowly lowered into target locations until evoked DA release was maximized. Electrode placements were finalized once maximal evoked DA output was reached and the locations of electrodes were kept unchanged throughout the remainder of the experiment. Recording electrodes were conditioned by applying an inverted V waveform (−0.4V to +1.3V to −0.4V, 400 V/s) at 60 Hz for 10 minutes, after which the frequency of the waveform was changed to 10 Hz and kept constant during the subsequent procedures. In all experiments, we recorded the “background currents” produced by the inverted V waveform applied to the recording electrode (Heien et al. 2004). This background current was subtracted from the “faradic currents” recorded after each stimulation, to derive the current attributable to DA release. Cyclic voltammograms were recorded and analyzed with TarHeel CV and Demon Voltammetry software (UNC, Chapel Hill, NC and Wake Forest University, Winston-Salem, NC, respectively).
VTA Stimulation Parameters
DA release was evoked by electrical stimulation of the VTA using a constant current isolator (A-M Systems, Sequim, WA). Table 1 shows the stimulation parameters used in each of the four experiments. In Experiments 1 and 2, at each pulse amplitude, the VTA was stimulated with a train of 60 rectangular, biphasic pulses (2 ms/phase, 60 Hz, 1 sec.). Stimulation began at 100 µA pulse amplitude and was increased in 100 µA steps after each pulse train, up to a maximum of 800 µA with 3 min. intervals between pulse trains. DA responses following 600–800 µA were excluded from the analysis as DA release diminished at these amplitudes in some mice.
TABLE.
Experimental conditions used in each experiment
| EXPERIMENT | Pulse Amplitude (µA) |
Pulse Frequency |
Pulse Train Duration |
Number of PulseTrains |
Inter-Train Interval |
|---|---|---|---|---|---|
| 1) Chronic lithium treatment | 100 – 500 (100 µA steps) |
60 Hz | 1 sec | One at each pulse amplitude |
3 min |
| 2) Acute lithium treatment | 100 – 500 (100 µA steps) |
60 Hz | 1 sec | One at each pulse amplitude |
3 min |
| 3) DA depletion (chronic lithium) |
500 | 20 Hz; then 60 Hz |
1 sec | 60 @ 20 Hz 40 @ 60 Hz |
6 sec |
| 4A) Baseline (chronic lithium) | 500 | 20 Hz | 1 sec | One | NA |
| 4B) Following d-amphetamine | 500 | 20 Hz | 1 sec | 40 | 6 sec |
In Experiments 3 and 4, pulse amplitude was always 500 µA and pulse train duration was always 1 sec. In Experiment 3, we depleted readily releasable DA by repeated VTA stimulation at short intervals. We recorded DA release evoked by VTA stimulation with sixty, 20 Hz pulse trains repeated at 6 sec. intervals. The mice were then given a 20 min recovery period, followed by forty 60 Hz pulse trains repeated at 6 sec. intervals. We used either 20 Hz or 60 Hz frequency stimulation to model physiological and supraphysiological neural activity levels, respectively (Yavich 1996).
In Experiment 4, we used a similar DA depletion paradigm to investigate the effects of chronic lithium treatment on changes in DA release after d-amphetamine administration. We first measured baseline DA release for each mouse by electrically stimulating the VTA with one pulse train at 20 Hz. We then injected d-amphetamine (2 mg/kg, 4 ml/kg injection volume, Sigma, Saint Louis, MO). Ten minutes after the injection, we recorded DA release evoked by stimulating the VTA with forty pulse trains (20 Hz, 1 sec. duration, repeated at 6 sec. intervals).
Data Analysis
Following data collection, recording electrodes were calibrated by placing them in known concentrations of DA and measuring oxidation currents induced by the inverted V waveform used during the recording sessions. This allowed us to report the peak extracellular DA concentration ([DA]max) evoked by each pulse train. The “rise time” of each evoked DA release was calculated as the time for DA concentrations to rise from baseline to [DA]max. The time constant of decay (τ) was calculated by fitting the falling phase of each DA transient to a single exponential decay function (Yorgason et al. 2011). In experiments 1 and 2, we analyzed evoked DA release by each pulse train, whereas in experiments 3 and 4, we analyzed DA release evoked by the first and fifth pulse train and every fifth pulse train thereafter. In all experiments, data are presented as the mean ±SEM. We used two-way repeated measures ANOVA to analyze the main effects and interactions of fixed factors (treatment and stimulation amplitude in experiments 1 and 2; treatment and train number in experiments 3 and 4). Posthoc comparisons were made by Fisher’s LSD test. The criterion for statistical significance was p<0.05.
RESULTS
Experiment 1: Effects of chronic lithium treatment on DA release
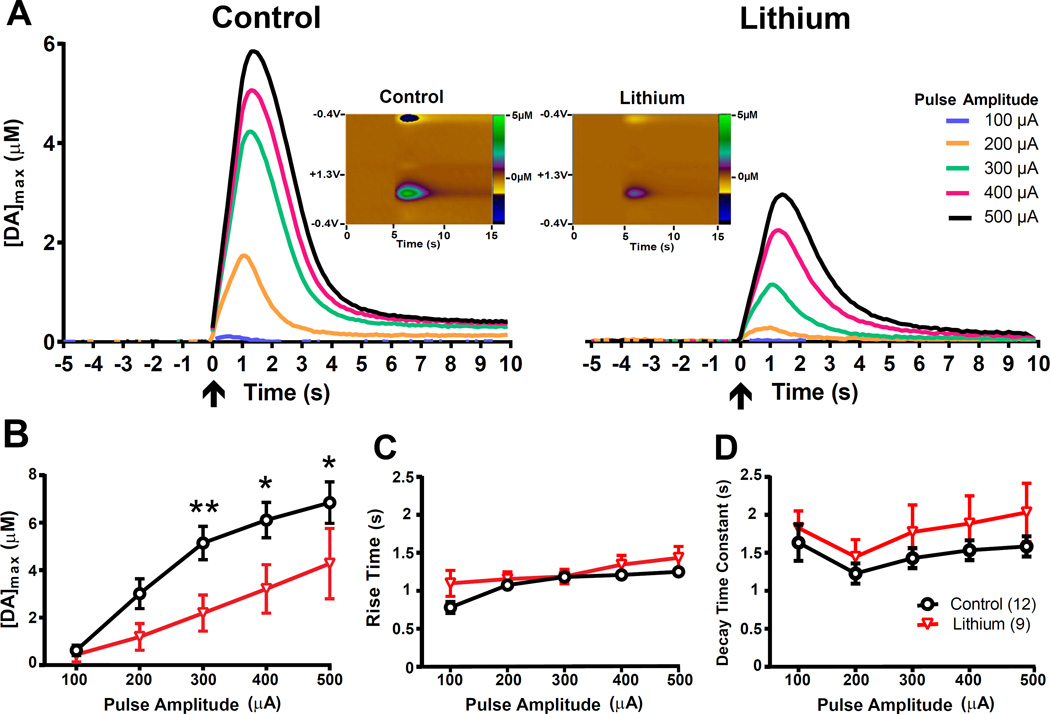
We determined the effects of chronic lithium treatment on DA release in the NAc core at different pulse amplitudes. We focused on the core as this NAc subregion has been implicated etiology of pathological impulsivity (Basar et al. 2010a) and d-amphetamine-induced hyperactivity (Sellings & Clarke 2003, Boye et al. 2001), which are both behaviors modified by lithium treatment. Representative voltammograms (color plots) and extracellular DA responses obtained from control and lithium-treated mice are shown in Figure 1A. The results shown in Figures 1A and 1B indicate that chronic lithium treatment significantly lowered [DA]max (F1,19 = 4.74, p<0.05), stimulation with larger pulse amplitudes significantly increased [DA]max (F4,76 = 40.87, p<0.0001) and there was a significant interaction between treatment and pulse amplitude (F4,76 = 3.37, p<0.05). Post hoc comparisons revealed that [DA]max was significantly lower in the lithium treated mice at pulse amplitudes of 300 µA (p<0.01), 400, and 500 µA (p<0.05). Rise time (Figure 1C) was significantly affected by pulse amplitude (F4,76 = 15.17, p<0.0001) but not by chronic lithium treatment (F1,19 =2.04, p>0.05) and there was not a significant interaction (F4,76 = 2.15, p>0.05). The time constant of decay (Figure 1D) was significantly affected by pulse amplitude (F4,76= 4.13, p<0.01) but not by lithium treatment (F1,19 =1.15, p>0.05) and the interaction was not significant (F4,133<1).
Figure 1. Chronic lithium treatment reduces stimulation-evoked extracellular DA levels.
(A) Electrical stimulation-evoked extracellular DA concentrations from representative mice that were chronically fed with either 0.4% lithium chloride-containing or control chow. Voltammetric recordings began 5 seconds prior to electrical stimulation. Arrows at t=0 indicate the onset of VTA stimulation. Each 1 sec. pulse train consisted of 60 pulses, with varying levels of pulse amplitude (100–500 µA). Inset: Color plots showing DA signals represented by the signal in the approximate center (~0.6 V) of the rising phase of the voltage ramp, from representative mice stimulated at 300 µA pulse amplitude. X-Axis: Time (seconds), Y-Axis: Applied potential, Z-Axis (in pseudocolor): DA concentration levels. Group averages of the (B) amplitude, (C) rise time and (D) decay time constants of evoked DA levels. At stimulation amplitudes from 300 µA to 500 µA, chronic lithium treatment decreased the magnitude ([DA]max) of DA release. For panels B–D, error bars indicate mean ± SEM. *p<0.05; **p<0.01; n=9–12/group.
Experiment 2: Effects of acute lithium treatment on electrically evoked DA release
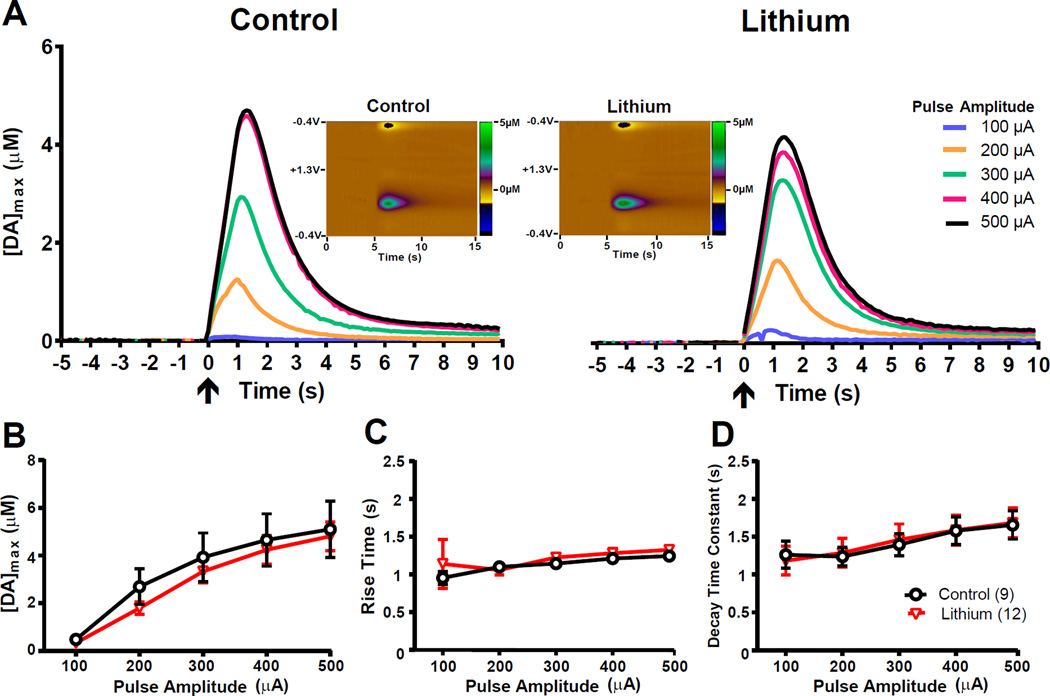
Representative voltammograms and extracellular DA responses obtained from mice acutely treated with saline or lithium are presented in Figure 2A. Acute lithium administration had no statistically significant effects on [DA]max (F1,19 <1), rise time (F1,19 <1) or decay time constant (F1,19 <1) (Figures 2B, C, D, respectively). Increasing pulse amplitude significantly increased [DA]max (Figure 2B; F4,76 = 45.18, p<0.0001), had no significant effect on rise time (Figure 2C; F4,76 = 1.67, p>0.05) and significantly increased the time constant of decay (Figure 2D; F4,76= 15.21, p<0.01), and there were no significant interactions with acute lithium treatment for any of the outcomes (F4,133 <1).
Figure 2. Acute lithium treatment does not change stimulation-evoked, extracellular DA levels.
(A) Stimulation-evoked extracellular DA concentration from representative mice that were acutely treated 5 hours earlier with lithium chloride (300 mg/kg) or vehicle. Voltammetric recordings began 5 seconds prior to electrical stimulation. Arrows at t=0 indicate the onset of VTA stimulation. Each electrical stimulation consisted of 60 pulses, 1 sec. in duration, with varying levels of pulse amplitude (100–500 µA). Inset: Color plots showing DA signals represented by the signal in the approximate center (~0.6 V) of the rising phase of the voltage ramp, from representative mice stimulated at 300 µA pulse amplitude. X-Axis: Time (seconds), Y-Axis: Applied potential, Z-Axis (in pseudocolor): DA concentration levels. Group averages of the (B) amplitude, (C) rise time and (D) decay constants of evoked DA levels. Acute treatment with lithium chloride does not affect the magnitude of ([DA]max), rise time of DA concentrations, or decay time constants. For panels B–D, error bars indicate mean ± SEM. n=12/group.
Experiment 3: Effects of chronic lithium treatment on short inter-train interval-induced DA depletion
Only a small portion of DA contained in presynaptic terminals is ready for immediate release (readily releasable pool), but the remaining majority is stored in synaptic vesicles that are not immediately available for exocytosis, and this DA constitutes a reserve pool (Alabi & Tsien 2012, Yavich & MacDonald 2000). When DAergic VTA neurons are stimulated at short inter-train intervals, there is an initial increase in DA release. This is followed by a depletion of DA reserves because they become releasable and undergo exocytosis, at a faster rate than they can be replaced by new DA synthesis (Yavich 1996, Yavich & MacDonald 2000). Thus, we used short inter-pulse train intervals to study the effect of chronic lithium treatment on the distribution of DA between the readily releasable and reserve pools following chronic lithium treatment (Yavich 1996, Venton et al. 2006).
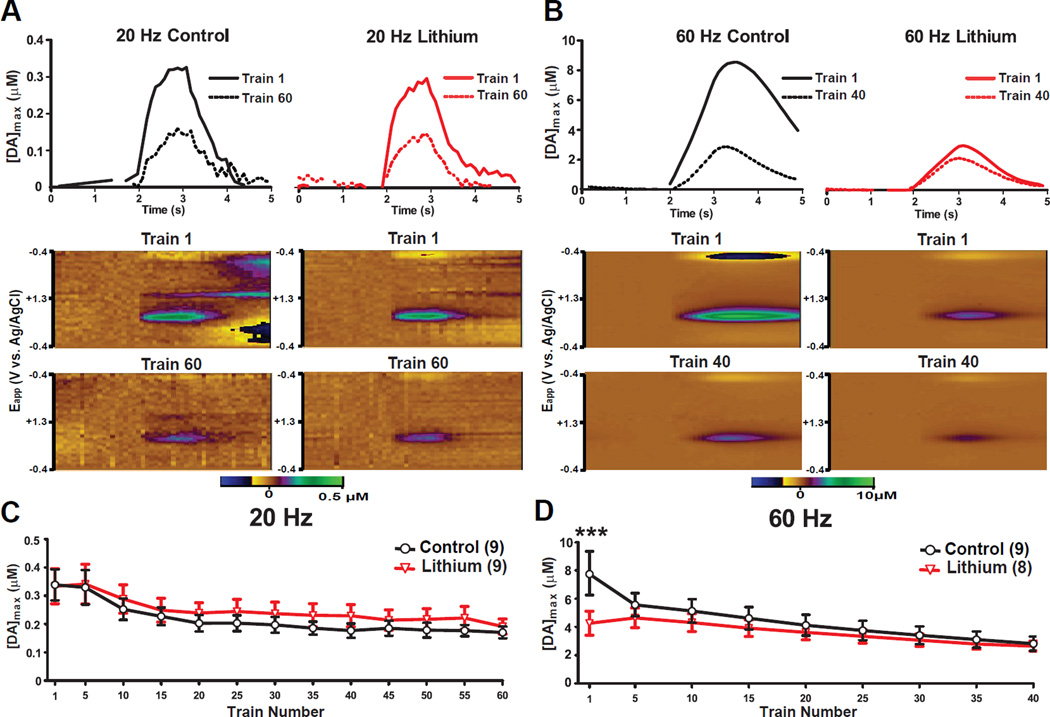
Representative extracellular DA responses and voltammograms obtained from control and lithium treated mice exposed to either 20 Hz or 60 Hz pulse frequencies are presented in Figures 3A and B. There were no significant effects of chronic lithium treatment on [DA]max in response to 20 Hz frequency trains (Figure 3C; F1,16 <1), or its interaction with the effects of repeated stimulation (F12,192 <1). However, there was a significant reduction in [DA]max with repeated stimulation (F12,192 = 22.02, p<0.0001). In contrast, at 60 Hz stimulation frequencies, there was a main effect of repeated stimulation (Figure 3D; F8,120 = 29.99, p<0.0001), no effect of chronic lithium administration (F1, 15 <1), and a significant interaction between the effects of lithium treatment and repeated stimulation (F8,120 = 6.64, p<0.0001). Post hoc analysis revealed that chronic lithium treatment reduced [DA]max for the first of the 60 Hz pulse trains (consistent with Experiment 1), but by the fifth pulse train, no significant differences remained between the groups.
Figure 3. Effects of chronic lithium treatment on repeated electrical stimulation induced depletion of DA.
Stimulation-evoked extracellular DA levels from representative mice that were chronically fed with either 0.4% lithium chloride-containing or control chow following (A) 20 Hz, 500 µA and (B) 60 Hz, 500 µA electrical stimulation of the VTA each 1 sec. in duration. Color plots indicate DA signals represented by the signal in the approximate center (~0.6 V) of the rising phase of the voltage ramp from representative mice. X-Axis: Time (seconds) same as above panels, Y-Axis: Applied potential, Z-Axis (in pseudocolor): DA concentration levels. Peak ([DA]max) levels of electrically evoked DA concentrations during (C) 20 Hz and (D) 60 Hz 500 µA pulse trains. For panels C and D, error bars indicate mean ± SEM. ***p<0.001; n=8–9/group.
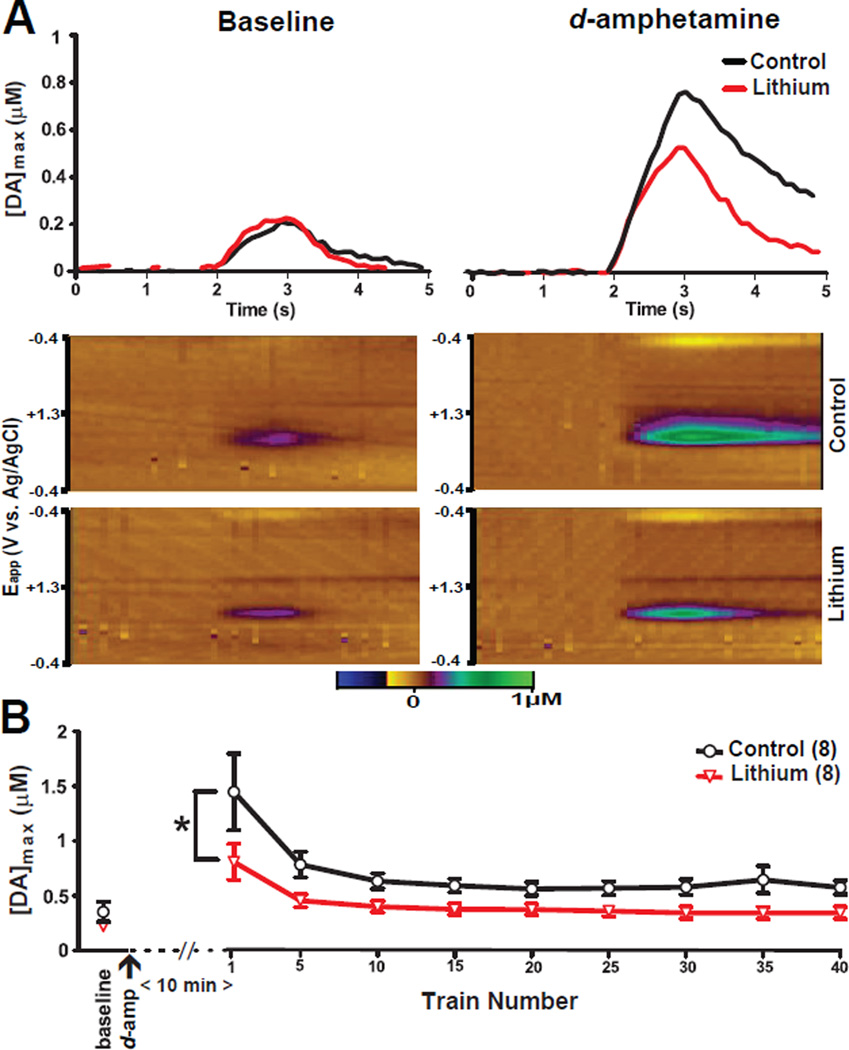
Experiment 4: Effects of chronic lithium treatment on d-amphetamine-induced DA release
In addition to its actions on the DA transporter, d-amphetamine increases vesicular DA release from the readily releasable pool of DA and partially depletes the reserve pool (Covey et al. 2013, Ramsson et al. 2011, Daberkow et al. 2013). We stimulated the VTA using short inter-train intervals (as in Experiment 3) under 20Hz pulse frequency conditions, to assess the effect of chronic lithium administration on extracellular DA concentrations following acute d-amphetamine treatment.
Representative extracellular DA responses and voltammograms obtained from control and lithium treated mice are presented in Figure 4A. Chronic lithium (F1,14 = 6.1, p<0.05) and repeated stimulation (F8,112 = 14.42, p<0.0001) both significantly altered [DA]max evoked by 20 Hz pulse trains but their interaction was not significant (F8,112= 1.49, p>0.05) (Figure 4B). However, consistent with the results of Experiment 3 (20 Hz pulse trains) an independent measures t-test revealed that there was no significant difference between control and chronically lithium treated mice at baseline before d-amphetamine administration (t14= 1.39, p>0.05). Thus, chronic lithium treatment attenuated d-amphetamine induced increases in evoked DA release during stimulation with 20 Hz pulse trains.
Figure 4. Effects of chronic lithium on d-amphetamine induced DA concentration changes.
Stimulation-evoked extracellular DA levels from representative mice chronically fed with either 0.4% lithium chloride-containing chow or control chow following (A) VTA electrical stimulation (500 µ, 60 Hz, 1 sec. in duration) prior to (baseline) and 10 minutes after administration of 2 mg/kg d-amphetamine. Color plots showing DA signals represented by the signal in the approximate center (~0.6 V) of the rising phase of the voltage ramp from representative mice. X-Axis: Time (seconds) same as above panels, Y-Axis: Applied potential, Z-Axis (in pseudocolor): DA concentration levels. [DA]max levels of electrically evoked DA concentrations during (B) baseline and pulse train. Error bars indicate mean ± SEM. *p<0.05; n=8/group.
DISCUSSION
We found that chronic, but not acute, lithium treatment diminished the amplitude of extracellular DA responses to VTA stimulation in the NAc core evoked by electrical stimulation of the VTA with 60 Hz pulse trains. Chronic lithium had no significant effect on the rise time or the decay constant of responses, thus demonstrating that the decreased amplitude of the DA response is due to reduced release and not to enhanced DA reuptake. We additionally investigated the effects of chronic lithium treatment on the distribution of DA into readily releasable and reserve vesicle pools by repeatedly stimulating the VTA at short inter-stimulus intervals that do not allow enough time for replenishment of DA stores (Yavich & MacDonald 2000, Yavich 1996, Rizzoli & Betz 2005). Under these conditions, the amplitude of extracellular DA responses to stimulation with 20 Hz pulse trains decreased with repeated stimulation in both groups of mice, but lithium treatment had no significant main effect on response amplitude. As observed in our earlier experiment, chronic lithium administration significantly reduced the amplitude of DA responses to the stimulation with first 60 Hz pulse train but by the fifth 60 Hz pulse train DA response amplitudes were no longer significantly different from those in the control group.
It is well established that chronic lithium treatment attenuates d-amphetamine-induced hyperlocomotion in rodents (Gould et al. 2007, Cox et al. 1971, Borison et al. 1978). d-amphetamine increases the readily releasable pool of DA (Covey et al. 2013). Based upon these findings, as well as our results using short inter-pulse train intervals at 60 Hz stimulation, we hypothesized that chronic lithium would attenuate increased DA release in response to d-amphetamine. Indeed, chronic lithium treatment did not affect baseline [DA]max elicited at 20 Hz simulation as we had shown earlier, but did diminished [DA]max values after the administration of d-amphetamine. These findings provide evidence for our hypothesis that chronic lithium treatment attenuates DA release only when DA release is abnormally high. Together, these results suggest that lithium diminishes the readily releasable DA pool, on which responses to the earliest pulse trains respond, but has less or no impact on the reserve pool, on which responses to later stimuli depend.
Our results extend the conclusions of an earlier microdialysis study that identified no effect of chronic lithium on basal DA levels but revealed attenuation of potassium-evoked DA release (Ferrie et al. 2005). It may be that lithium treatment affects overall production or storage of DA, which does not affect release under normal conditions, but modifies release under conditions of abnormally high neuronal stimulation. This interpretation of our results explains the apparent discrepancies found in the previous literature concerning the interactions between lithium treatment and overall DA levels. Chronic lithium treatment has been found to elevate, reduce, or not change tissue levels of DA in the striatum depending upon length of treatment and study conditions (Dziedzicka-Wasylewska et al. 1996, Otero Losada & Rubio 1985, Hesketh et al. 1978). Our findings indicate that chronic lithium’s relevant effects may be limited to the mechanisms of immediate release of DA without necessarily affecting the overall tissue levels of DA, or even overall levels found in the synaptic terminals of the NAc.
The absence of significant effects of acute (single administration) lithium treatment on evoked DA release in the NAc core observed in our experiments seems to be contradictory to the results of a recent study, using similar voltammetry methods, in which acute lithium treatment diminished electrical stimulation of the VTA-induced DA release in the NAc core (Fortin et al. 2015). These discrepancies might be due to procedural differences between the two studies. Fortin et al. monitored DA for one hour after acute lithium treatment. We began our acute FSCV measurements five hours after the administration of lithium. Our rationale for this approach was based on our previous findings, which indicated that while brain lithium levels reach steady levels within one to two hours after i.p. administration of lithium, plasma levels of lithium are very high immediately after injection, and subsequently decrease becoming approximately equal with brain levels at the three hour time point and thereafter (Can et al. 2011). Considered within the context of the pharmacokinetics of acute lithium treatment, the acute lithium effects observed in Fortin et al. may be mediated by its peripheral actions. Indeed, these authors reported that the immediate effects of acute lithium are dependent on signaling via glucagon-like peptide 1 (GLP-1), which is a peptide hormone mainly produced in the intestines and also in the hindbrain in response to consumption of food (Holst 2007). Central or systemic administration of GLP-1 receptor antagonists counters taste aversion and anorexia following systemic treatment with lithium (Seeley et al. 2000, Rinaman 1999, Fortin et al. 2015). It is possible that GLP-1 mediated effects of acute lithium on DA release are transient and dependent on high plasma lithium levels that are only present immediately after systemic injection. Our finding that chronic but not acute lithium administration diminishes the release of DA is in accordance with human studies that demonstrate it takes weeks of lithium treatment to observe its full therapeutic effects on mania (Gershon et al. 2009). Indeed, chronic but not acute lithium treatment is results in a wide array of plastic changes in mood-relevant regions of the forebrain (Quiroz et al. 2010).
Mesolimbic DA circuitry originating from the VTA and projecting to the NAc has a crucial role for the expression of mania. Drugs that elevate DAergic neurotransmission, either through increasing release or preventing reuptake, produce states similar to mania in humans (Drevets et al. 2001, Leyton et al. 2002, Anand et al. 2000, Murphy et al. 1971). Similarly, drugs or treatments that impede DAergic transmission or block DA production by depleting DA precursor amino acid tyrosine diminish mania in humans (McTavish et al. 2001, Perlis et al. 2006). As mentioned earlier, chronic lithium treatment, which does not typically change baseline activity levels, reduces d-amphetamine induced increases in locomotor activity in rodents (Gould et al. 2007, Cox et al. 1971, Borison et al. 1978). In mice, the lithium-induced reduction of d-amphetamine induced hyperlocomotion is prevented by preadmininistration of the DA precursor L-DOPA (Berggren et al. 1978). Similarly, the euphoric and/or activating effects of amphetamine, methyphenidate, and L-DOPA in humans are attenuated by the chronic administration of lithium (van Kammen et al. 1985, Van Kammen & Murphy 1975, Huey et al. 1981, Bell et al. 2005).
One of the most validated rodent models of mania are mice with a dysfunctional circadian gene (Clock-Δ19). These mice manifest hyperactivity in novel environments, and increased preference for cocaine, and NAc phase-signaling dysfunction (Roybal et al. 2007, Dzirasa et al. 2010, Easton et al. 2003, McClung et al. 2005). Many of the phenotypes in Clock-Δ19 mice are reversed by chronic administration of lithium (Roybal et al. 2007, Dzirasa et al. 2010). Clock-Δ19 mice have higher DA levels in the NAc and chronic lithium treatment decreases the NAc tissue DA levels in these mice (Coque et al. 2011). While we tested our mice in the light phase, extracellular DA in the NAc is higher during the dark phase (Castaneda et al. 2004). Clock-Δ19 mice also display differences in DAergic activity dependent upon phase of the cycle (Sidor et al. 2015). The robustness of the electrical stimulation effect and the depletion of readily releasable DA by d-amphetamine suggest that a similar effect would be observed during the dark phase when DA synthesis by VTA neurons proceeds at a lower rate, but this was not specifically tested in our study.
Taken together, our results support the hypothesis that lithium acts to reduce the symptoms of mania by “toning down” DA release in the context of hyperactivity of the mesolimbic DA system. Indeed, our results indicate that lithium only modified DA release in the context of abnormal (high-frequency stimulation or d-amphetamine) DA release. Thus, a critical next step will be to conduct similar in rodent models of mania such as the Clock-Δ19 mice. While our results suggest that lithium’s effects may be due to actions on specific synaptic pools of DA, the exact mechanism by which lithium results these this effect is not clear. As the behavioral effects of DAergic signaling are tightly coupled with the timing of phasic DA release, it is essential that future studies investigate lithium-induced changes in DA release in behaving animals.
Acknowledgments
Research was supported by NIMH grant MH091816 to TDG. We thank Paul Shepard for comments on the manuscript and experimental results.
Dr. Gould reports receiving consulting fees from Sunovion Pharmaceuticals and Janssen Pharmaceuticals, and research funding from Janssen Pharmaceuticals and Roche Pharmaceuticals during the preceding three years.
Footnotes
FINANCIAL DISCLOSURES
Drs. Can, Frost, Cachope, and Cheer declare no competing financial interests.
REFERENCES
- Alabi AA, Tsien RW. Synaptic vesicle pools and dynamics. Cold Spring Harbor perspectives in biology. 2012;4:a013680. doi: 10.1101/cshperspect.a013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010a;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HWM, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Progress in Neurobiology. 2010b;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Asghar SJ, Silverstone PH. Lithium and valproate attenuate dextroamphetamine-induced changes in brain activation. Hum Psychopharmacol. 2005;20:87–96. doi: 10.1002/hup.665. [DOI] [PubMed] [Google Scholar]
- Berggren U, Tallstedt L, Ahlenius S, Engel J. The effect of lithium on amphetamine-induced locomotor stimulation. Psychopharmacology (Berl) 1978;59:41–45. doi: 10.1007/BF00428028. [DOI] [PubMed] [Google Scholar]
- Borison RL, Sabelli HC, Maple PJ, Havdala HS, Diamond BI. Lithium prevention of amphetamine-induced 'manic' excitement and of reserpine-induced 'depression' in mice: possible role of 2-phenylethylamine. Psychopharmacology (Berl) 1978;59:259–262. doi: 10.1007/BF00426631. [DOI] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O'Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes, Brain and Behavior. 2011;10:434–443. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacology Biochemistry and Behavior. 2014;123:3–16. doi: 10.1016/j.pbb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Chiu C-T, Chuang D-M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacology & Therapeutics. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology. 2011;36:1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Juliano SA, Garris PA. Amphetamine Elicits Opposing Actions on Readily Releasable and Reserve Pools for Dopamine. PLoS ONE. 2013;8:e60763. doi: 10.1371/journal.pone.0060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C, Harrison-Read PE, Steinberg H, Tomkiewicz M. Lithium attenuates drug-induced hyperactivity in rats. Nature. 1971;232:336–338. doi: 10.1038/232336a0. [DOI] [PubMed] [Google Scholar]
- Daberkow D, Brown H, Bunner K, Kraniotis S, Doellman M, Ragozzino M, Garris P, Roitman M. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. The Journal of Neuroscience. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Mackowiak M, Fijat K, Wedzony K. Adaptive changes in the rat dopaminergic transmission following repeated lithium administration. J Neural Transm. 1996;103:765–776. doi: 10.1007/BF01273357. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, McClung CA, Nicolelis MA. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. The Journal of Neuroscience. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Ferrie L, A HY, McQuade R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int J Neuropsychopharmacol. 2005:1–7. doi: 10.1017/S1461145705006243. [DOI] [PubMed] [Google Scholar]
- Ferrie LJ, Gartside SE, Martin KM, Young AH, McQuade R. Effect of chronic lithium treatment on D2/3 autoreceptor regulation of dopaminergic function in the rat. Pharmacology Biochemistry and Behavior. 2008;90:218–225. doi: 10.1016/j.pbb.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Fortin SM, Chartoff EH, Roitman MF. The Aversive Agent Lithium Chloride Suppresses Phasic Dopamine Release Through Central GLP-1 Receptors. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C, Ghiglieri O, Masi F, Scheggi S, Tagliamonte A, De Montis MG. The effects of long-term administration of rubidium or lithium on reactivity to stress and on dopamine output in the nucleus accumbens in rats. Brain Res. 1999;826:200–209. doi: 10.1016/s0006-8993(99)01286-x. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. New England Journal of Medicine. 1989;321:1489–1493. doi: 10.1056/NEJM198911303212201. [DOI] [PubMed] [Google Scholar]
- Gershon S, Chengappa KNR, Malhi GS. Lithium specificity in bipolar illness: a classic agent for the classic disorder. Bipolar Disorders. 2009;11:34–44. doi: 10.1111/j.1399-5618.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- Halcomb ME, Gould TD, Grahame NJ. Lithium, but not Valproate, Reduces Impulsive Choice in the Delay-Discounting Task in Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien MLAV, Johnson MA, Wightman RM. Resolving Neurotransmitters Detected by Fast-Scan Cyclic Voltammetry. Analytical Chemistry. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Hesketh JE, Nicolaou NM, Arbuthnott GW, Wright AK. The effect of chronic lithium administration on dopamine metabolism in rat striatum. Psychopharmacology (Berl) 1978;56:163–166. doi: 10.1007/BF00431843. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The Physiology of Glucagon-like Peptide 1. Physiological Reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Huey LY, Janowsky DS, Judd LL, Abrams A, Parker D, Clopton P. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73:161–164. doi: 10.1007/BF00429209. [DOI] [PubMed] [Google Scholar]
- Lewitzka U, Severus E, Bauer R, Ritter P, Muller-Oerlinghausen B, Bauer M. The suicide prevention effect of lithium: more than 20 years of evidence-a narrative review. International journal of bipolar disorders. 2015;3:32. doi: 10.1186/s40345-015-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C] raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTavish S, McPherson M, Harmer C, Clark L, Sharp T, Goodwin G, Cowen P. Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. The British Journal of Psychiatry. 2001;179:356–360. doi: 10.1192/bjp.179.4.356. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Brodie HK, Goodwin FK, Bunney WE., Jr Regular induction of hypomania by L-dopa in "bipolar" manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Tsutsui-Kimura I, Kumamoto H, Minami M, Izumi T, Yamaguchi T, Yoshida T, Yoshioka M. Lithium, but not valproic acid or carbamazepine, suppresses impulsive-like action in rats. Psychopharmacology. 2011:1–12. doi: 10.1007/s00213-011-2496-9. [DOI] [PubMed] [Google Scholar]
- Otero Losada ME, Rubio MC. Striatal dopamine and motor activity changes observed shortly after lithium administration. Naunyn Schmiedebergs Arch Pharmacol. 1985;330:169–174. doi: 10.1007/BF00572429. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Welge JA, Vornik LA, Hirschfeld RM, Keck PE., Jr Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67:509–516. doi: 10.4088/jcp.v67n0401. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Machado-Vieira R, Zarate CA, Jr, Manji HK. Novel insights into lithium's mechanism of action: neurotrophic and neuroprotective effects. Neuropsychobiology. 2010;62:50–60. doi: 10.1159/000314310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. Journal of Neurochemistry. 2011;117:937–948. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1999;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nature Reviews Neuroscience. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, et al. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryding E, Lindström M, Träskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression. Progress in brain research. 2008;172:307–315. doi: 10.1016/S0079-6123(08)00915-1. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The Role of CNS Glucagon-Like Peptide-1 (7–36) Amide Receptors in Mediating the Visceral Illness Effects of Lithium Chloride. The Journal of Neuroscience. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severus E, Taylor MJ, Sauer C, Pfennig A, Ritter P, Bauer M, Geddes JR. Lithium for prevention of mood episodes in bipolar disorders: systematic review and meta-analysis. International journal of bipolar disorders. 2014;2:15. doi: 10.1186/s40345-014-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry. 2015;20:1406–1419. doi: 10.1038/mp.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, Docherty JP, Marder SR, Rosenblatt JE, Bunney WE., Jr Lithium attenuates the activation-euphoria but not the psychosis induced by d-amphetamine in schizophrenia. Psychopharmacology (Berl) 1985;87:111–115. doi: 10.1007/BF00431789. [DOI] [PubMed] [Google Scholar]
- Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44:215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L. Two simultaneously working storage pools of dopamine in mouse caudate and nucleus accumbens. British Journal of Pharmacology. 1996;119:869–876. doi: 10.1111/j.1476-5381.1996.tb15753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, MacDonald E. Dopamine release from pharmacologically distinct storage pools in rat striatum following stimulation at frequency of neuronal bursting. Brain Research. 2000;870:73–79. doi: 10.1016/s0006-8993(00)02403-3. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]